Abstract
Aim:
Assess the comparative effectiveness of two blood pressure (BP) control interventions for black patients with uncontrolled hypertension.
Patients & methods:
A total of 845 patients were enrolled in a three-arm cluster randomized trial. On admission of an eligible patient, field nurses were randomized to usual care, a basic or augmented intervention.
Results:
Across study arms there were no significant 12 months differences in BP control rates (primary outcome) (25% usual care, 26% basic intervention, 22% augmented intervention); systolic BP (143.8 millimeters of mercury [mmHg], 146.9 mmHG, 143.9 mmHG, respectively); medication intensification (47, 43, 54%, respectively); or self-management score (18.7, 18.7, 17.9, respectively). Adjusted systolic BP dropped more than 10 mmHg from baseline to 12 months (155.5–145.4 mmHg) among all study participants.
Conclusion:
Neither the augmented nor basic intervention was more effective than usual care in improving BP control, systolic BP, medication intensification or patient self-management. Usual home care yielded substantial improvements, creating a high comparative effectiveness threshold.
Clinical Trial Registration:
Keywords: : blood pressure, comparative effectiveness, home care, hypertension, nurse-led interventions
Background
Uncontrolled hypertension (HTN), a major cardiovascular risk factor, is a continuing public health problem marked by wide racial disparities in morbidity and mortality [1–3]. Black men and women have disproportionately high rates of HTN [4,5], HTN-related coronary heart disease, stroke and renal disease [1,6]. A few large randomized clinical trials have achieved high rates of blood pressure (BP) control in multiethnic hypertensive populations. However, black participants in the landmark ALLHAT trial [7,8], conducted primarily in community-based practices, were 31% less likely than whites to achieve control. A variety of multifaceted, team-based and nurse-led HTN interventions also have demonstrated promising results [9–12]. Nevertheless, the most effective ways to organize HTN care for high-risk patients remain unclear. Moreover, there is a dearth of evidence on how to optimize HTN service delivery to address disparate outcomes among high-risk black patients in community-care settings.
Hypertension is the most prevalent diagnosis among 3 million Medicare beneficiaries receiving home healthcare [13]. This population, with multiple comorbid conditions, is at high risk of HTN-related morbidity and mortality. However, HTN has not been a priority for quality improvement or comparative effectiveness (CE) research in home care.
We conducted a three-arm cluster randomized CE study that used home health care as a springboard for improving HTN outcomes in high-risk black patients whose BP on home care admission was not controlled to levels recommended by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (hereafter referred to as ‘JNC7’) [14], the national guideline in effect throughout the study. We tested, relative to usual home care, the addition of either: a basic intervention, consisting of emailed guidelines for home care nurses and home BP monitors for patients; or an augmented intervention, delivered by a master's level prepared nurse and a health educator, that provided focused review of HTN medications and extended patient self-management support tailored to the patient's HTN stage over a 12-month period during and beyond the index home care admission. The study was designed in response to an initiative of the National Heart Lung and Blood Institute (NHLBI) seeking ‘clinically feasible interventions to effect changes in medical care delivery’ [3] leading to an increase in the proportion of treated hypertensive black patients whose BP was controlled to levels specified by the JNC7.
We hypothesized that at 12 months both the basic and augmented interventions would yield higher BP control rates than usual care and that the augmented intervention's relative benefit would be greater than that of the basic intervention. We hypothesized further that the augmented intervention would have a greater relative impact on systolic blood pressure (SBP) and on two process measures, HTN medication intensification and patient self-management.
Findings from our interim 3-month post-randomization evaluation were promising. Although relative to usual care neither intervention had significantly improved 3-month BP control among the entire study population of black patients with uncontrolled HTN at baseline, the augmented had a sizeable effect on a prespecified population of patients who had ‘severe’ stage 2 HTN (SBP >160 mmHg [millimeters of mercury]) as defined by JNC7. At the 3-month point 17.6% of stage 2 patients in the augmented group achieved BP control compared to 8.9% in usual care (p = 0.01) [15].
Here, we report the results of the full 12-month CE trial for the entire study population (primary outcome) as well as for the prespecified patient subgroup with stage 2 HTN at baseline. Our 12-month results, in contrast to those at 3 months, showed no significant comparative intervention benefit. We discuss the possible reasons for this finding and the implications of our findings both for BP control and for CE research.
Patients & methods
Study design
We conducted a prospective 3-arm cluster randomized trial that randomized home care field nurses and their patients to one of two interventions or to usual care only. Research assistants blinded to the patient's randomization status enrolled patients and completed the BP measurements, and collected all the medication and process measures in the patient's homes. The study was approved by the Institutional Review Boards of the Visiting Nurse Service of New York and Weill Cornell Medical College, and monitored by a Data Safety and Monitoring Board. Additional details about the study design have been published previously [16]. Clinical Trial Registration: NCT00139490 [17].
Setting & participants
The study was conducted at the Visiting Nurse Service of New York (VNSNY), a large urban home healthcare organization serving over 30,000 black patients yearly, of whom an estimated a third (10,000) entered care with BP above then-recommended targets. All field nurses in the organization's four main regions (Manhattan, Brooklyn, the Bronx and Queens) were study-eligible, based on identification of an eligible patient. Eligible patients were: black, English-speaking, aged 21–80, with uncontrolled HTN determined by a primary, secondary or tertiary HTN admission diagnosis (ICD9-CM401, 402, 403 or 404) in the electronic health record (EHR) and a BP at the time of the study recruitment interview of ≥140 mmHg/90 mmHg (≥130/80 mmHg for patients with diabetes or kidney disease). Within the entire study population we also identified a prespecified subpopulation of patients with stage 2 HTN as defined by JNC7. JNC7 recommended that this subpopulation, defined by a baseline BP of ≥160/100 mmHg, receive more intensive intervention due to heightened risk of an HTN-related cardiovascular event [14]. Research interviewers completed the informed consent process and forms with patients who met eligibility.
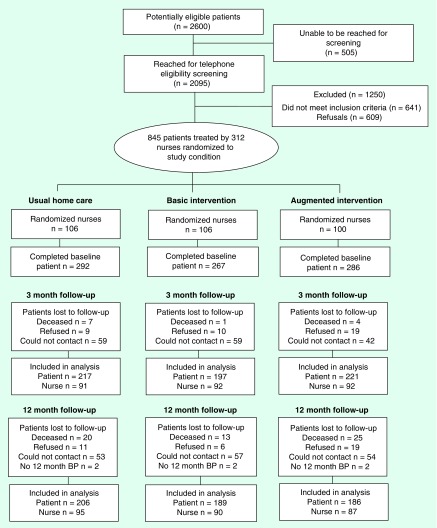
An electronic algorithm was used to randomize field nurses. To avoid contamination, a nurse's initial random assignment determined the status for all new patients allocated to that particular nurse's care for the study's duration (see Figure 1).
Figure 1. . Patient flow diagram.
BP: Blood pressure.
Usual home care
Regardless of study arm, all patients received usual home health services: a physician-ordered plan of care plus patient education, monitoring and hands-on care delivered by a field nurse. All field nurses used tablet computers, electronic messaging and EHRs. Prior to randomization all field nurses received a study-issued email with a web-link to the JNC7 guidelines.
Basic intervention
Field nurses randomized to the basic intervention received two additional automated e-mails targeted to a specific HTN patient on their caseload shortly after the patient had been enrolled in the study. The emails relayed information on key nursing-specific practices to support JNC7 recommendations. In addition to usual home care, basic intervention patients received the JNC7 guide ‘Lowering your Blood Pressure’, a BP monitor with instructions and a BP log with the recommendation to review their BP readings with their home care nurse or primary care physician.
Augmented intervention
The augmented intervention incorporated all components of the basic intervention and of usual home care. Additionally, concurrent with usual care and extending over 12 months, a study-trained ‘HTN support’ nurse and a health educator, following a ‘stepped-intensity’ protocol (specifying greater nurse involvement and more frequent health educator contact for stage 2 patients), provided HTN medication assessment, monitoring, education and self-management support that incorporated motivational interviewing techniques to promote behavior change [16,18]. Additional details on the augmented intervention have been published elsewhere [16].
Outcome measures
BP control at 12 months was the primary outcome. We examined this outcome for the entire study population as well as for stage 2 patients. In accord with JNC7 guidelines, patients were considered controlled if their BP was <140/90 mmHg (<130/80 mmHg for patients with diabetes or kidney disease). Two secondary patient-level outcomes also were examined: 12-month systolic blood pressure (SBP); and achievement of a 20 mmHg or greater reduction in SBP, overall and in the subgroup of patients with stage 2 HTN at baseline. SBP rather than DBP was the focus because ALLHAT found that in patients older than age 50 years, SBP >140 mmHg was a more important cardiovascular disease risk factor than DBP [14] and because very few patients started the study with elevated DBP. Achieving a 20-point SBP reduction was selected because meta-analyses have found each difference of 20 mmHg in SBP to be associated with a twofold difference in death rates from stroke, ischemic heart disease and other vascular causes [19]. All BP outcome measurements were based on the average of 3 readings taken by a blinded research interviewer using a Microlife Model 3AA1–2, validated using British Hypertension Society criteria, at baseline, 3-month and 12-month in-home interviews.
We also examined treatment impact on two process measures: HTN medication intensification, and patient-reported HTN self-management. Intensification was calculated from medication inventories taken at the in-home patient interviews. Intensification was defined as either an additional HTN medication or a new class of HTN medication. Patients’ self-reported self-management behavior was measured using the Hill-Bone Compliance to High Blood Pressure Therapy scale [20]. This is a 14-item scale comprised of three 4-point subscales measuring reduced sodium intake (three items), appointment keeping (two items) and medication taking (nine items) as reported by the respondent. Lower scores on this continuous measure signify greater compliance.
Data analyses
Bivariate probit specifications were used to model attrition-corrected treatment effects on binary dependent variables (e.g., BP control, medication intensification), while a conditional linear regression with selection was used to model continuous variables (SBP and logarithmic transformation of Hill-Bone scale).
In addition to the main variables of interest – membership in the basic or augmented treatment group – all multivariate analyses controlled for clustering (multiple patient observations for each randomized nurse) and the nurse's caseload size. The analyses also controlled for patient-level sociodemographic and clinical characteristics that might influence the probability of attrition or confound the relationship between interventions and outcomes. Interaction terms were used in analyses to examine the impact of the intervention on the prespecified patient subgroup with stage 2 HTN at baseline [21]. The parameter estimates of the models estimated with cluster and attrition correction were the basis for the results reported below. The magnitude of intervention effects was estimated by comparing regression-adjusted outcome probabilities for the three study groups (usual, basic, augmented).
Results
Sample characteristics
The study's CONSORT diagram appears in Figure 1. Of 2600 patients identified in the EHR as meeting initial study criteria, 19.4% could not be reached by telephone for eligibility screening. Of the 2095 potentially eligible patients reached for screening, 30.6% did not meet inclusion criteria during the in-person screening interview and 29% declined to participate, yielding a sample of 845 patients who consented to the study and completed baseline interviews. At the interim 3-month point, 1.4% of patients had died and 23.1% refused or could not be reached for the 3-month follow-up interview, leaving a sample of 635 patients. By the 12-month follow-up, 6.9% patients had died; 23.7% refused or could not be contacted for the research interview; and 0.7% did not have a BP measurement, leaving a final sample of 581 patients for whom complete baseline and 12 month data were complete.
Attrition attributable to nondiscretionary factors (e.g., hospitalization or death) was similar across study arms; however, the 12-month refusal rate of the augmented intervention patients was significantly higher than the usual care-only or basic patients. Patient adherence to the augmented intervention also was incomplete. Just over 80% of the patients in the augmented arm were considered ‘completers’ and participated at various levels through the 12 month intervention period, although only 50% of the augmented arm patients reached the implementation target of completing 80% or more of their scheduled contacts (on average 13 of 17 scheduled contacts were completed). Almost 5% of the augmented intervention patients did not have any contacts at all, with an additional 3.5% withdrawing before the end of the intervention period. Other ‘non-completers’ were those who died or were admitted to hospice or other long-term care facilities.
Table 1 presents selected demographic and health characteristics of enrolled patients and those included in the 12-month follow-up. There were no significant differences between enrolled patients and 12-month respondents. Nor were there significant differences among augmented, basic and usual care patients with respect to baseline comorbidity score, average BP values or number of HTN medications. The average baseline SBP of enrolled patients was 155.5 mmHg, and 54% had stage 2 HTN. Patients in the augmented arm were less likely than in the other arms to have less than a high school education and more likely than the basic intervention patients to be obese. Compared to patients in usual care-only, the basic arm patients were more likely to be elderly (65+) and to have diabetes.
Table 1. . Key sociodemographic and health characteristics of study patients, overall and by randomization status.
| Key characteristics | Enrolled (n = 845) | Included in 12-month analysis (n = 581) | Usual home care (n = 206) | Basic intervention (n = 189) | Augmented intervention (n = 186) |
|---|---|---|---|---|---|
|
Socio-demographic characteristics | |||||
| Age: | |||||
| – Mean age in years (SD) | 64.3 (10.9) | 64.2 (10.8) | 63.3 (10.5) | 65.1(10.4) | 64.4 (11.1) |
| – 65+ |
0.53 |
0.51 |
0.46 |
0.58† |
0.49 |
| Gender: female |
0.66 |
0.67 |
0.66 |
0.65 |
0.70 |
| Education: | |||||
| – Less than high school | 0.40 | 0.40 | 0.43 | 0.42 | 0.35†,‡ |
| – High school or more |
0.60 |
0.60 |
0.57 |
0.58 |
0.65 |
| Medicaid enrollee |
0.44 |
0.45 |
0.48 |
0.47 |
0.41 |
|
Baseline health status | |||||
| Comorbidity score, mean (SD) |
3.8 (2.8) |
3.7 (2.8) |
3.8 (3.0) |
3.6 (2.6) |
3.8 (2.6) |
| Overweight§ |
0.26 |
0.27 |
0.26 |
0.31 |
0.24 |
| Obese§ |
0.44 |
0.45 |
0.45 |
0.40 |
0.50‡ |
| Diabetes |
0.59 |
0.61 |
0.55 |
0.66† |
0.62 |
| Years with HTN dx, mean (SD) |
13.6 (12.2) |
13.6 (12.1) |
13.1 (12.0) |
14.0 (12.0) |
13.7 (12.6) |
|
Blood pressure status at baseline | |||||
| SBP, mmHg (SD) |
155.5 (20.4) |
154.8 (20.1) |
154.5 (19.1) |
155.7 (21.5) |
154.1 (19.9) |
| DBP, mmHg (SD) |
87.3 (13.0) |
87.0 (13.4) |
87.6 (13.3) |
86.5(14.3) |
87.0 (12.4) |
| JNC7-stage 2 |
0.54 |
0.53 |
0.52 |
0.56 |
0.51 |
| SBP among stage 2 (SD) |
168.4 (18.6) |
167.6 (18.8) |
166.6 (18.1) |
168.3 (20.2) |
167.9 (18.2) |
| DBP among stage 2 (SD) |
92.5 (13.2) |
93.0 (13.3) |
93.0 (13.9) |
93.0 (14.1) |
93.1 (11.9) |
| Number of HTN medications, mean (SD) | 2.27 (1.23) | 2.25 (1.20) | 2.21 (1.17) | 2.27 (1.25) | 2.28 (1.20) |
†Significantly different from usual care group at the p < 0.05 level.
‡Significantly different from basic intervention group at the p < 0.05 level.
§Height and weight were collected to calculate body mass index (BMI); overweight is defined as BMI between 25 and 30; obesity is defined as BMI >30.
DBP: Diastolic blood pressure; HTN: Hypertension; JNC7: Joint National Committee Report; SBP: Systolic blood pressure; SD: Standard deviation.
Impact of interventions on HTN outcomes
Table 2 presents the adjusted 12-month treatment effects of the interventions for the entire study population and for the subgroup with stage 2 HTN at baseline. Relative to usual care only, neither the basic nor the augmented intervention significantly improved the primary outcome, BP control, for the entire study population or for the stage 2 subgroup. BP control rates in usual care-only, basic and augmented arms were 25%, 26% (p = 0.78) and 22% (p = 0.43), respectively; in the stage 2 subgroup they were 16%, 15% (p = 0.79) and 13% (p = 0.57), respectively. Nor at 12 months did either intervention do significantly better than usual care-only in improving SBP or achieving a 20 mmHg or greater reduction in SBP for the entire study population or the stage 2 subgroup.
Table 2. . Adjusted treatment effects on 12-month hypertension outcomes.
|
Outcomes |
Usual |
Basic |
Augmented |
Usual |
Basic |
Augmented |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| BL | BL | Difference | BL | Difference | 12 month | 12 month | Difference | 12 month | Difference | |
|
SBP (mmHg) | ||||||||||
| Overall |
155.5 |
156.0 |
-0.5 (0.81) |
155.0 |
0.5 (0.75) |
143.8 |
146.9 |
4.3 (0.19) |
143.9 |
0.1 (0.98) |
| JNC7 stage 2 at baseline |
167.3 |
169.2 |
-1.9 (0.37) |
168.8 |
-1.5 (0.49) |
149.1 |
151.0 |
1.9 (0.67) |
147.1 |
-1.9 (0.69) |
|
Achieved BP control (%) | ||||||||||
| Overall |
Not applicable |
25.1 |
26.3 |
1.2 (0.78) |
21.6 |
-3.5 (0.43) |
||||
| JNC7 stage 2 at baseline |
|
16.0 |
14.6 |
-1.4 (0.79) |
13.0 |
-3.0 (0.57) |
||||
|
Experienced clinically meaningful reduction in SBP (%)† | ||||||||||
| Overall |
Not applicable |
26.2 |
30.8 |
4.6 (0.25) |
30.8 |
4.6 (0.30) |
||||
| JNC7 stage 2 at baseline | 44.0 | 37.6 | -6.4 (0.10) | 47.1 | 3.1 (0.56) | |||||
Adjusted probabilities are calculated based on underlying coefficients from bivariate probit models with selection that jointly estimate outcomes conditional on survey participation at the 12-month follow-up. All models control for patient's age and gender as well as baseline measures of health status (including measures of systolic and diastolic BP, presence of diabetes and number of other comorbidities); and the provider nurse's caseload. In addition to all variables included in the outcome equations, the retention equation included indicators for patent's borough of residence and whether the patient was hospitalized within 14 days from baseline home care admission. Numbers in brackets represent p-values based on adjusted estimates of treatment effects relative to usual care.
†Clinically meaningful reductions were defined as 20 mmHg for systolic BP relative to baseline levels.
Augmented: Augmented intervention; Basic: Basic intervention; BP: Blood pressure; BL: Baseline; JNC7: Joint National Committee Report; SBP: Systolic blood pressure; Usual: Usual home care.
Figure 2 displays patients’ adjusted 3-month and 12-month SBP. It illustrates the differential distribution of improvements in the study population over time, between patient groups and across intervention arms, leading to convergence of SBP at the 12-month point, despite the promising results of the augmented arm at 3 months. At baseline, the average adjusted SBP was 155.5 mmHg in the entire study population and 168.4 mmHg in the stage 2 subgroup. At the interim 3-month follow up, SBP in both the entire population and the stage 2 subgroup had declined across all three intervention arms (usual care-only, basic and augmented arms), with the largest, and statistically significant decline relative to usual care, occurring in the stage 2 augmented group (three-month SBP was 152.5 mmHg for augmented versus 160.8 mmHg for usual care-only [p < 0.01]). Over the 12-month study period, the stage 2 augmented patients continued to experience the largest reduction in SBP. However, by the end of 12 months, the difference across populations and intervention arms had closed substantially, with adjusted SBP across all intervention arms and in the entire study population as well as in the stage 2 subgroup converging in the range of 144–151 mmHg. The average adjusted 12-month decline for the entire population across all intervention arms was 10.1 mmHg (from 155.5 to 145.4 mmHg SBP); the stage 2 subgroup achieved an average adjusted 12-month reduction of 18.8 mmHg (from 168.4 mmHg SBP at baseline to an average of 149.6 mmHg SBP at 12 months) regardless of intervention group.
Figure 2. . Mean adjusted systolic blood pressure at baseline, 3 and 12 months (mmHg).
Mean systolic blood pressure adjusted for patients’ age, baseline measures of health status (presence of diabetes, and number of other comorbidities), whether the patient was on Medicaid, was hospitalized within 14 of home care admission; and the provider nurse's caseload.
Impact of interventions on process measures
Table 3 presents regression-adjusted estimates of the interventions’ 12-month impact on intensification of patients’ hypertension medications and on their self-reported HTN self-management. The table includes data on the average summary Hill-Bone score of each intervention group. Here, we report the summary scores because our augmented intervention focused on all three subscale domains (medication adherence, sodium intake and appointment keeping) included in the summary score. Further, study participants’ summary scores were closely correlated with their medication adherence subscale scores. The table shows that neither the basic nor the augmented intervention was significantly more effective than usual care only in yielding increases in medication intensification or self-reported self-management.
Table 3. . Adjusted treatment effects on process variables: medication intensification and behavioral change.
|
Process variables |
Usual home care |
Basic intervention |
Augmented intervention |
||
|---|---|---|---|---|---|
| Adjusted outcome | Adjusted outcome | Difference | Adjusted outcome | Difference | |
|
Medication intensification | |||||
| Overall |
46.9 |
43.0 |
-3.9 (0.46) |
54.6 |
7.7 (0.19) |
| JNC7 stage 2 at baseline |
56.9 |
48.4 |
-8.5 (0.31) |
64.7 |
7.8 (0.33) |
|
Hill-Bone HTN self-management (range:15–39) | |||||
| Overall |
18.7 |
18.7 |
0.0 (0.85) |
17.9 |
-0.8 (0.06) |
| JNC7 stage 2 at baseline | 21.4 | 21.3 | -0.1 (0.78) | 21.5 | 0.2 (0.90) |
Adjusted probabilities are calculated based on underlying coefficients from probit models that estimate outcomes conditional on survey participation at the 12-month follow-up. Medication intensification models control for patient's age, baseline measures of health status (presence of diabetes and number of other comorbidities), number of HTN medications and drug class of HTN medications (β- blockers, ace inhibitors, diuretics, angiotensin drugs, calcium channel blockers). Numbers in brackets represent p-values based on adjusted estimates of treatment effects relative to usual care. Lower scores on the Hill-Bone scale represent better self-reported self management. Bold indicates that the variable was marginally signficant.
HTN: Hypertension; JNC7: Joint National Committee Report.
Figure 3A & B graphically presents the range of 3- and 12-month intervention impacts on medication intensification for the entire sample and the stage 2 subgroup (because intensification was calculated in relation to a patient's baseline medication regimen, by definition none of the study sample could have experienced medication intensification at baseline). It shows that an average of 44–57% of study participants experienced medication intensification at 3 months depending on population and intervention arm; by 12 months, the range was 44–65%. At 12 months, 54.6% of all patients enrolled in the augmented arm had achieved a medication intensification compared to 46.9% of all patients in usual care-only (Figure 3A), a 7.7 percentage point relative advantage. Medication intensification rose to 64.7% in the stage 2 augmented group compared to 56.9% of stage 2 patients in usual care-only, a 7.8 percentage point relative advantage. However, these differences did not achieve statistical significance, and, in general, regardless of intervention arm, stage 2 patients were more likely to experience medication intensification (56.7% of the stage 2 subgroup compared to 48% of the entire population, an 8.7 percentage point difference) (Figure 3, both panels).
Figure 3. . Mean adjusted medication intensification at 3 and 12 months.
Adjusted for patients’ age, baseline measures of health status (presence of diabetes, and number of other comorbidities), whether the patient was on Medicaid, was hospitalized within 14 of home care admission.
Lastly, Figure 4, which graphically presents 3- and 12-month data on Hill-Bone Self-Management summary scores, shows no relative benefit of either intervention compared to usual care at either 3 or 12 months. Moreover, the modest improvement in self-management reported at 3 months across all study arms and both patient populations was erased by 12 months, with the greatest deterioration reported in the stage 2 population, increasing from 18.4 to 21.4 on a scale in which lower scores represent better reported self-management (p < 0.001).
Figure 4. . Mean adjusted Hill-Bone self-management score at baseline, 3 and 12 months.
Adjusted for patients’ age, baseline measures of health status (presence of diabetes, and number of other comorbidities), whether the patient was on Medicaid, was hospitalized within 14 of home care admission.
Discussion
Comparative effectiveness trials have tended to shy away from high cost, high-risk patients with multiple chronic conditions [22], and evidence is scarce about how best to organize and deliver HTN care in the community to help improve BP control among such patients. To our knowledge, this is the first reported CE study designed to improve BP control by targeting high-risk black patients in the home care setting. The study findings highlight both the opportunities and challenges presented for CE researchers seeking to identify improved care delivery modes for the increasingly older, multi-ethnic, chronically ill and vulnerable patient populations served in community-based care settings.
We had hypothesized that both the basic and the augmented interventions would significantly increase BP control relative to usual care and that the augmented intervention would result in greater relative benefit. Despite some promising 3-month results for the augmented intervention, at 12 months neither the basic nor the augmented intervention yielded significantly better HTN outcomes than usual care alone. One set of factors contributing to the lack of comparative intervention benefit may have been related to influences on the usual care-only group. Although there has been relatively little attention in the HTN literature to BP changes in the control group, one recent study suggested that most HTN study protocols, by employing obtrusive physical and psychosocial measures across study arms, may over time alert control group participants to the importance of BP control [23]. Furthermore, in this study both the basic and augmented interventions were added to usual home care, which is itself an ‘intervention’ of 29 days median duration. Usual care field nurses are specifically charged with, among other tasks, assessing all of a patient's medications and educating patients about the importance of adherence to prescribed medical and behavioral regimens. Thus the basic and augmented interventions had a higher benefit threshold to exceed than interventions in many other community-based trials, which generally are assessed relative to no intervention or to a ‘placebo’ intervention.
Our study results may have been attributable to several additional factors. First, the augmented intervention, which was the more proactive of the two interventions, nevertheless took an indirect approach to influencing physicians’ HTN management, including management of patients’ medications – a key process affecting BP outcomes. For the most part, the nurse/health educator team in the augmented intervention did not interact directly with physicians but rather with patients’ usual home care nurses (during the postacute care episode) or with patients themselves to help them understand their HTN risk factors and medication regimens and urge them to communicate their HTN concerns to their individual physicians. Only in the most serious cases in which the patient's BP was extremely high did the HTN support nurse directly try to speak to a patient's physician. In contrast, especially at the start of a patient's postacute care episode, the usual home care nurse could have had several direct contacts with a patient's physician or the physician's staff to clarify and/or modify the patient's overall medication regimen and obtain final approval of the patient's physician-ordered plan of care. Even though the usual care nurses’ medication review did not necessarily focus on HTN medications, it may well have included them, as suggested by the relatively similar rates of HTN medication intensification across study arms at both the 3- and 12-month follow-up. The highest level of medication intensification ultimately achieved across all study arms was the 65% rate achieved among stage 2 augmented intervention patients at the 12-month follow-up. The continued emphasis on intensification among stage 2 augmented arm patients in the stepped protocol – where the HTN support nurse continued to focus on medications, interact with the patient after the patient's discharge from routine home care and, on occasion, reach out to the patient's physician – suggests that both a vigilant nurse clinician and a physician motivated by a patient's continued BP in the >160 mmHg SBP range may be the optimum condition for effecting reduction in SBP.
Another factor that likely contributed to the lack of the interventions’ differential results was their lack of demonstrated positive impact on patient self-management. The minimal variation and relatively high rates of self-management reported when the Hill-Bone measurement instrument was administered to our study population at baseline, combined with the findings of two subsequent publications about the psychometric properties of the Hill-Bone [24,25], suggest that the tool may not be an ideal research instrument. The current literature indicates that about 50% of patients traditionally classified with resistant hypertension are effectively nonadherent [26,27]. In contrast, our study participants’ baseline compliance score on the Hill-Bone medication subscale range was 11.5–11.7 out of a potential range of 9–28 with lower scores indicating greater adherence, suggesting a far higher rate of medication compliance than probable by the fact that all patients were selected because their BP was not controlled.
Measurement issues notwithstanding, the deterioration of reported self-management in our study may have been attributable partly to patients’ increasing awareness overtime of the specific requirements of effective self-management, inculcated by usual care and augmented intervention staff, and their heightened realization of their own self-management short-comings. Alternatively, it may have been due partly to the relatively poor health status of the targeted population. Our black study participants were sicker than the typical volunteer research subject recruited to a community study. They were already patients dealing with 3–4 poorly managed chronic conditions in addition to an acute illness episode that led to home care services. On average, they had been diagnosed with HTN 14 years prior to study enrollment and had not achieved BP control. A significant number faced the risk factors of older age and higher baseline SBP, over 70% were overweight or obese and three in five had diabetes – all factors associated with significantly worse BP outcomes in the ALLHAT trial [8].
Examining pooled study data, Pavlik and colleagues recently suggested that well-accepted behavioral strategies used for highly selected community research volunteers may not be powerful enough to demonstrate large comparative advantages when added to routine care delivery for high-risk patients [23]. One implication is that the growing number of CE studies testing organizational interventions in real world care delivery systems may need to have larger sample sizes to detect smaller effects. Another implication is that more direct behavior interventions may be needed in such situations.
In addition to complex health issues, participants in this study faced a variety of structural barriers to adherence likely not faced by typical research volunteers in the community. These included unstable living conditions, acute financial distress, fragmented support systems and under-resourced/inattentive clinic physicians who were the patient's primary source of care [28]. Such barriers the augmented nurse/health educator team could but imperfectly address, especially considering the lower than expected number of completed patient contacts and the reliance on nurse/patient telephone calls as the principal contact vehicle. Recent evidence indicates that in-person contacts and direct three or four-way nurse/physician/patient/caregiver communication may be a more effective [29,30] means for enhancing the impact of chronic care interventions designed for complex patients than simple telephonic approaches and possibly could have enhanced patient self-management in the augmented arm of this study. More direct behavior interventions discussed in the literature have included electronic reminders and/or remote patient monitoring, allowing immediate three- or four-way feedback (including family caregivers) and timely intervention in a patient's home. In a study such as ours, for example, focused feedback on medication adherence, such as the use of ‘smart packages’ to compile patient-specific drug dosing data as a basis for specific discussion between patients and healthcare providers, might have enhanced the accuracy and immediacy of efforts to improve patients’ medication adherence in the augmented study arm [31].
Lastly, our study results may have been influenced by clinicians’ skepticism about the guidelines that were used to set the study's BP control targets. The JNC7 report recommended treating older adults to reach the same level of SBP, <140 mmHg, as younger people, even though it explicitly said there was no conclusive evidence, for example, from a large randomized controlled trial, to support such a recommendation for people aged 60 or over. Further, although the report acknowledged possible negative effects of intensively treating older hypertensive patients, including symptoms such as postural unsteadiness, dizziness or even fainting, these potential adverse effects were not judged to outweigh the benefits of recommending lower SBP for the older population in general. The report from the panel members appointed to the eighth Joint National Committee (JNC 8), which was issued after completion of this study, took a different tack [32] and addressed the issue of past skepticism about the evidence base for some of the JNC7 recommendations. The JNC8 report explicitly stated that there is ‘strong evidence to support treating hypertensive persons aged 60 years or older to a BP goal of less than 150/90 mmHg’ and essentially retracted the JNC7 recommendation to treat people older than 60 years to <140 mmHg SBP. Further, it specifically recommended that clinicians watch for adverse health or quality of life effects should an older person's BP fall <140 mmHg [32]. The majority of the patients enrolled in our study were older than 60 years of age. To the extent that our findings were attributable to clinicians’ concerns about the evidence base of JNC7 and the potential harms of overtreatment, they suggest that in the future CE research sponsors and CE research studies should be cautious in testing interventions designed to promote guidelines based on weak recommendations and with significant potential for adverse events.
Strengths & limitations
This study successfully addressed a veritable ‘checklist’ of challenges entailed in conducting CE research relevant to the increasingly diverse and rapidly aging American population: it mounted a cluster randomized trial to examine ‘head to head’ treatment effects in an older black patient population with uncontrolled HTN, multiple comorbid conditions and relatively high risk of poor outcomes in the absence of effective chronic care. It did so in a real world setting, a prototypical urban home health organization, where consent had to be obtained from home health patients who required skilled nursing services to get them through a period of postacute illness and where new interventions had to be grafted onto usual ongoing care and tested relative to usual care alone. Field nurses were randomized via a computer-administered algorithm and patients were assigned to nurses by operations staff blinded to the study and applying exogenous operational rules (patient's location and each nurse's overall caseload), which were controlled for in all analyses along with clustered assignment of patients to nurses. Thus procedures were employed to mitigate unmeasured selection bias.
One limitation of the study, however, was the evidence of differential attrition whereby augmented intervention participants were more likely to refuse the 12-month follow-up research visit than usual care or basic intervention patients (there were no significant differences in deaths or other unavoidable reasons such as hospitalization). We accounted for differential attrition in all analyses by employing the traditional statistical approach of estimating outcome models jointly with a sample retention equation to produce attrition-corrected estimates of the interventions on all outcomes [33–36]. Thus, we do not believe that differential attrition undermines the validity of our findings. The differential attrition, however, in conjunction with the incomplete intervention adherence of augmented arm participants, does raise questions about the extent of patient ‘saturation’ with the augmented intervention itself, which could have potentially important implications for the design of future CE studies with intensive behavioral interventions. An additional study limitation was the instrument used to measure patient self-management, in particular medication adherence, on which all patients reported quite high adherence despite their history of uncontrolled HTN. Barring electronic medication caps or interviewer-pill counts, both of which were beyond the resources of this study, a more sensitive medication adherence measure might have yielded a more accurate picture of participants’ medication adherence over time.
Conclusion
This study highlights several ongoing challenges of conducting real world CE research affecting vulnerable patients with complex care needs related to multiple chronic conditions confounded with cultural, personal and structural barriers to effective self-care management. The study's findings suggest that employing more direct behavior change and concrete socio-economic intervention strategies may be required to help these patients more effectively manage complicated medication and behavioral regimens. Establishing more effective three or four-way physician/patient/nurse communication and collaboration – a strategy found to have reduced rehospitalizations in a variety of chronic care management programs – should be tested as a potential strategy for improving HTN outcomes as well. Attention also must focus on reducing intervention burden and improving patient adherence to complex interventions in the chronically ill population and among specific subgroups for whom specific behavior and health targets may be especially challenging to attain. As part of this testing process, we recommend that CE studies such as ours more fully embrace the approach of patient-centeredness and patient engagement, two cornerstones of research elaborated by the Patient-Centered Outcomes Research Institute established in 2009 and funded by a provision of the Affordable Care Act. The introduction of these concepts early in the research process would not only enhance the relevance of CE studies to consumers but also could potentially reduce investment in intervention strategies that overburden some study participants and limit their CE.
Lastly, this study raises an important question about the relationship between clinicians’ decisions, the recommendations of national evidence-based guidelines and CE research studies intended to promote those recommendations. To the degree that the recommendations embodied in national guidelines rest on missing or explicitly problematic evidence, funders of CE studies may wish to modify program or Request for Application announcements and/or their expectations of the outcomes of associated research projects.
Executive summary.
Uncontrolled hypertension, a major cardiovascular risk factor, is a continuing public health problem marked by wide racial disparities in morbidity and mortality.
This is the first reported comparative effectiveness study designed to improve blood pressure control by targeting high-risk black patients in the home care setting.
The study enrolled 845 black home care patients with uncontrolled hypertension in a three-arm cluster randomized trial.
Despite some promising 3-month results for the augmented intervention, at 12 months neither the basic nor the augmented intervention yielded significantly better hypertension outcomes than usual home care.
Systolic blood pressure dropped greater than 10 mmHg from baseline to the 12 month measurement across all randomized groups.
One potential contributing factor to the lack of comparative intervention benefit may have been related to influences on the usual home care-only group.
More powerful behavior strategies in the augmented intervention group and more direct nurse/physician/patient communication might have yielded greater comparative benefit.
Study results may also have been influenced by clinicians’ skepticism about the guidelines that were used to set the study's blood pressure control targets; raising an important question about the relationship between clinicians’ decisions, the recommendations of national evidence-based guidelines and comparative effectiveness research studies intended to promote those recommendations.
Acknowledgements
The authors wish to acknowledge the significant contributions of the project manager, J Mongoven. The authors would also like to thank P Wilson, the intervention clinical coordinator and S Sridharan for her programming support. The authors also grateful to our Project Officer, P Einhorn, for her invaluable feedback on early drafts of this manuscript.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Financial & competing interests disclosure
This work was supported by grant R01 HL078585 from the National Heart, Lung and Blood Institute (NHLBI). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Redmond N, Baer HJ, Hick LS. Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension. 2011;57(3):383–389. doi: 10.1161/HYPERTENSIONAHA.110.161950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ. Cardiovasc. Qual. Outcomes. 2009;2(3):236–240. doi: 10.1161/CIRCOUTCOMES.109.850008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 5.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control – continued disparities in adults: United States, 2005–2006. NCHS Data Brief No. 3. National Center for Health Statistics; Hyattsville, MD, USA: 2008. www.cdc.gov/nchs/data/databriefs/db03.pdf [PubMed] [Google Scholar]
- 6.Howard G, Lackland DT, Kleindorfer DO, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern. Med. 2013;173(1):46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using data from a large national study (REGARDS), the authors provide robust linkages between race, uncontrolled blood pressure and disparities in stroke.
- 7.Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-Up Program Cooperative Group. JAMA. 1979;242(23):2562–2571. [PubMed] [Google Scholar]
- 8.Cushman WC, Ford CE, Cutler JA, et al. ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J. Clin. Hypertens. 2002;4(6):393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 9.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst. Rev. 2010;3:CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Clark C, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse, and teamwork in hypertension therapy. J. Clin. Hypertens. 2012;14(1):51–65. doi: 10.1111/j.1751-7176.2011.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill JL, Cunningham TL, Wiitala WL, Bartley EP. Collaborative hypertension case management by registered nurses and clinical pharmacy specialists within the patient aligned care teams (PACT) model. J. Gen. Intern. Med. 2014;29(Suppl. 2):S675–S681. doi: 10.1007/s11606-014-2774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey C, Sengupta M, Moss A, Harris-Kojetin L, Valverde R. National Health Statistics Reports. Hyattsville, MD, USA: Home health care and discharged hospice care patients: United States, 2000 and 2007.www.cdc.gov/nchs/data/nhsr/nhsr038.pdf no 38. [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Pezzin LE, Feldman PH, Mongoven JM, McDonald MV, Gerber LM, Peng TR. Improving blood pressure control: results of home-based post-acute care interventions. J. Gen. Intern. Med. 2011;26(3):280–286. doi: 10.1007/s11606-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman PH, McDonald MV, Mongoven JM, et al. Home-based blood pressure interventions for blacks. Circ. Cardiovasc. Qual. Outcomes. 2009;2(3):241–248. doi: 10.1161/CIRCOUTCOMES.109.849943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.Gov. http://clinicaltrials.gov/
- 18.Levensky ER, Forcehimes A, O'Donohue WT, Beitz K. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. Am. J. Nurs. 2007;107(10):50–58. doi: 10.1097/01.NAJ.0000292202.06571.24. [DOI] [PubMed] [Google Scholar]
- 19.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog. Cardiovasc. Nurs. 2000;15(3):90–96. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 21.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N. Engl. J. Med. 2011;364(26):2478–2481. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 23.Pavlik VN, Chan W, Hyman DJ, et al. Evaluating health systems level hypertension control interventions for African–Americans: lessons from a pooled analysis of three cluster randomized trials. Curr. Hypertens. Rev. 2015;11(2):123–131. doi: 10.2174/1573402111666150325234503. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides practical advice to consider when designing randomized-controlled trials investigation blood pressure interventions.
- 24.Koschack J, Marx G, Schnakenberg J, Kochen MM, Himmel W. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties. J. Clin. Epidemiol. 2010;63(3):299–306. doi: 10.1016/j.jclinepi.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Escamilla B, Franco-Trigo L, Moullin JC, Martínez-Martínez F, García-Corpas JP. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer. Adherence. 2015;13(9):569–578. doi: 10.2147/PPA.S76139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnier M, Schneider MP, Chioléro A, Stubi CL, Brunner HR. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J. Hypertens. 2001;19(2):335–341. doi: 10.1097/00004872-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Strauch B, Petrák O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J. Hypertens. 2013;31(12):2455–2461. doi: 10.1097/HJH.0b013e3283652c61. [DOI] [PubMed] [Google Scholar]; • Highlights the high extent of nonadherence to medications, how it may impact labeling a patient with resistant hypertension, along with the potential risks and waste of basing prescribing decisions without better adherence data.
- 28.McDonald MV, Pezzin LE, Peng TR, Feldman PH. Understanding the complexity of hypertensive African American home care patients: challenges to intervention. Ethn. Dis. 2009;19(2):148–153. [PMC free article] [PubMed] [Google Scholar]
- 29.Brown R, Peikes D, Peterson G, Schore J, Razafindrakoto C. Six features of Medicare coordinated care demonstrations programs that cut hospital admissions of high-risk patients. Health Aff. 2012;31(6):1156–1166. doi: 10.1377/hlthaff.2012.0393. [DOI] [PubMed] [Google Scholar]; • Provides concrete examples of successful program features that can make for a more successful care coordination program.
- 30.Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw. Fund.) 2014;19:1–19. [PubMed] [Google Scholar]
- 31.Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 33.Heckman J. Sample selection bias as a specification error. Econometrica. 1979;47(1):153–161. [Google Scholar]
- 34.Heckman J. Addendum to sample selection bias as a specification error. In: Stromsdorfer E, Farkas G, editors. Evaluation Studies (Vol. 5) Sage Publications; San Francisco, CA, USA: 1980. pp. 69–74. [Google Scholar]
- 35.Heckman J, Robb R. Alternative methods for evaluating the impact of interventions. In: Heckman J, Singer B, editors. Longitudinal Analysis of Labor Market Data. Cambridge University Press; Cambridge, NY, USA: 1985. pp. 156–246. [Google Scholar]
- 36.Angrist JD. Estimation of limited dependent variable models with dummy endogenous regressors: simple strategies for empirical practice. J. Bus. Econ. Stat. 2001;19:2–28. [Google Scholar]