Abstract
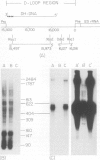
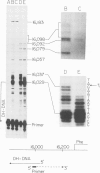
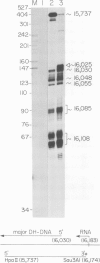
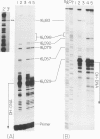
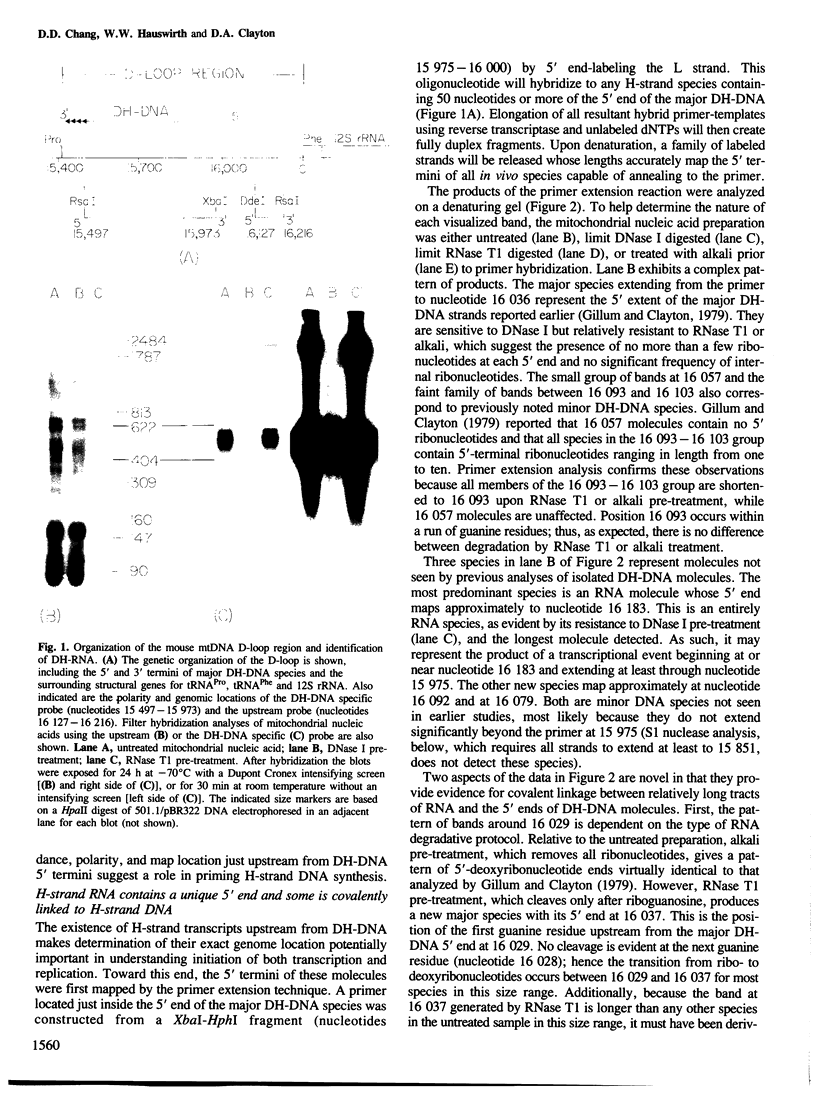
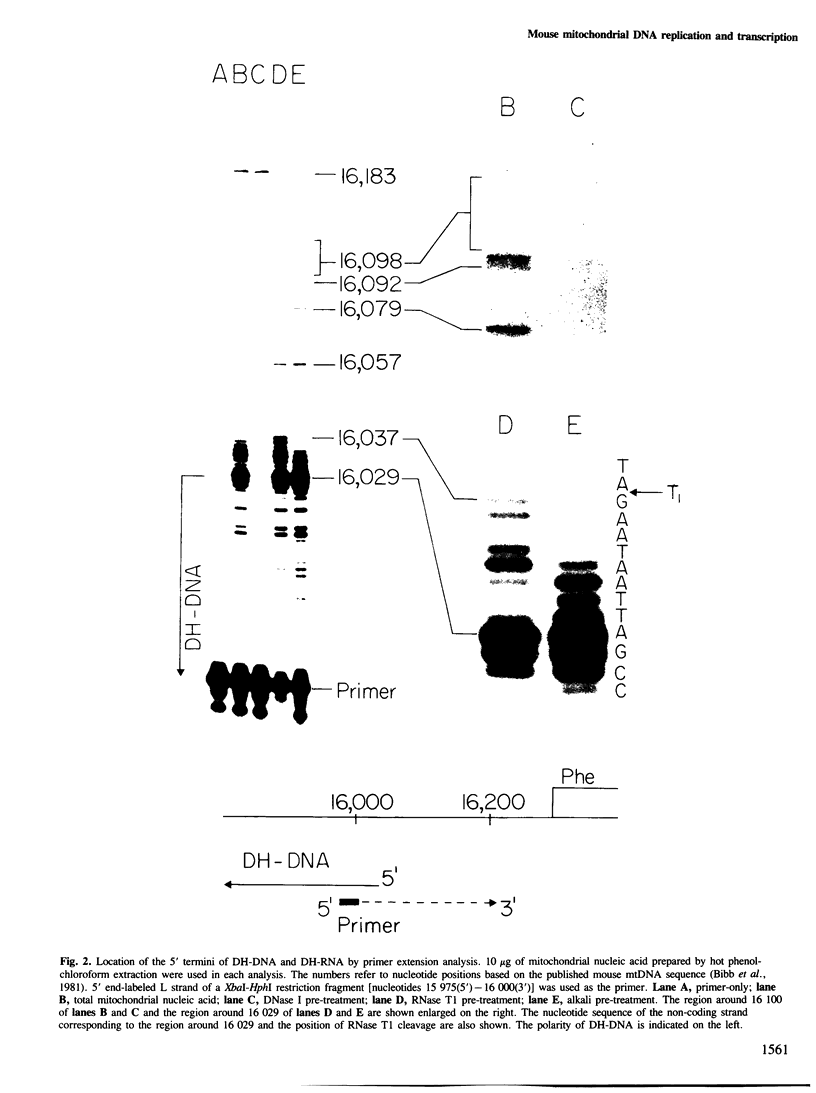
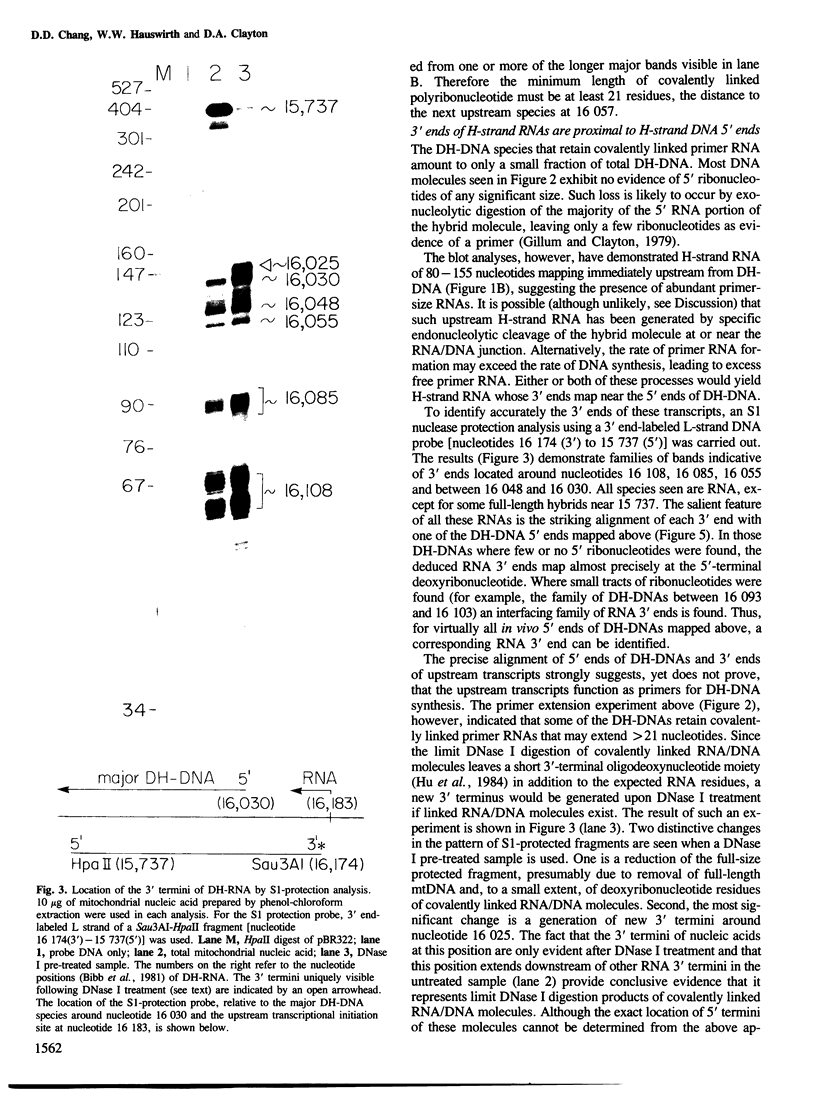
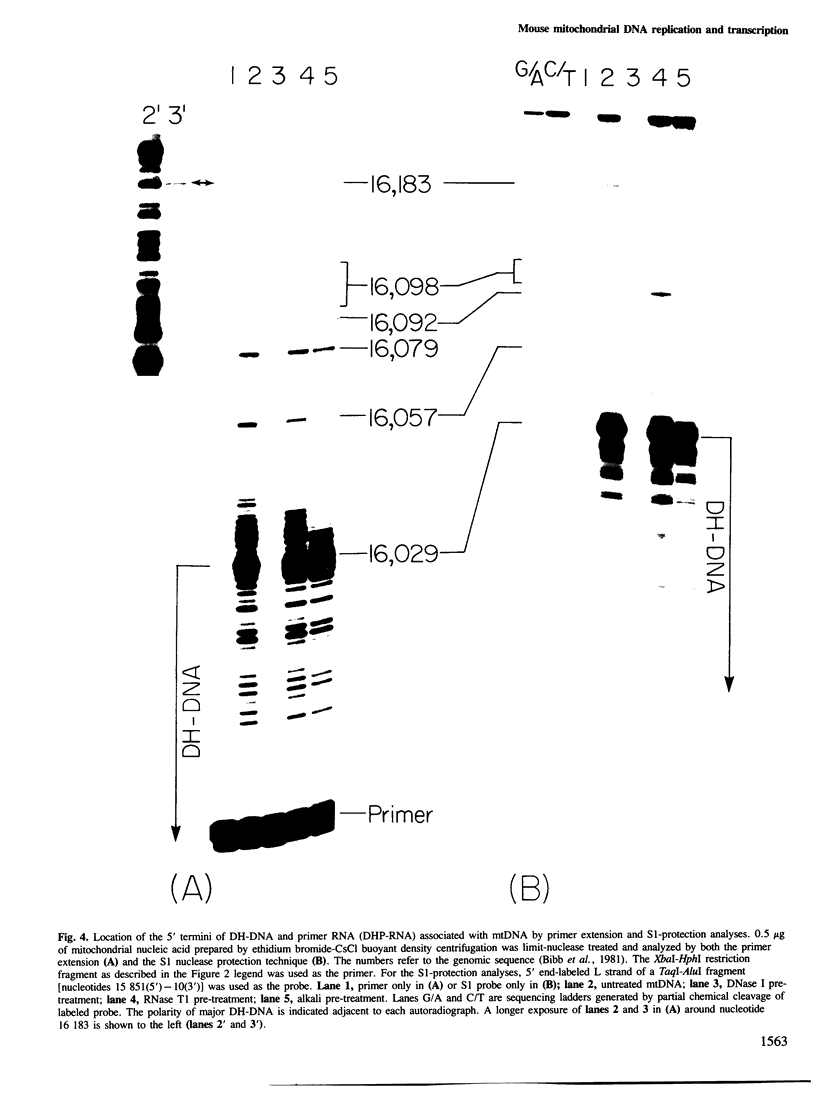
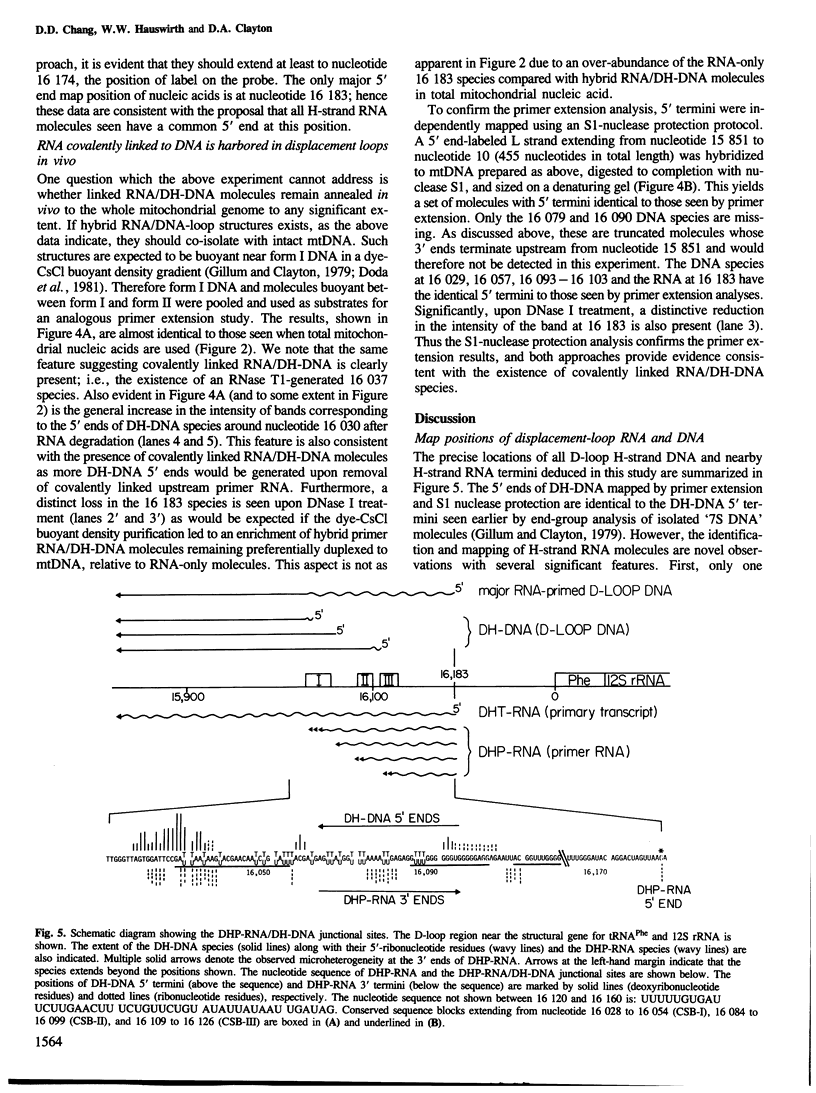
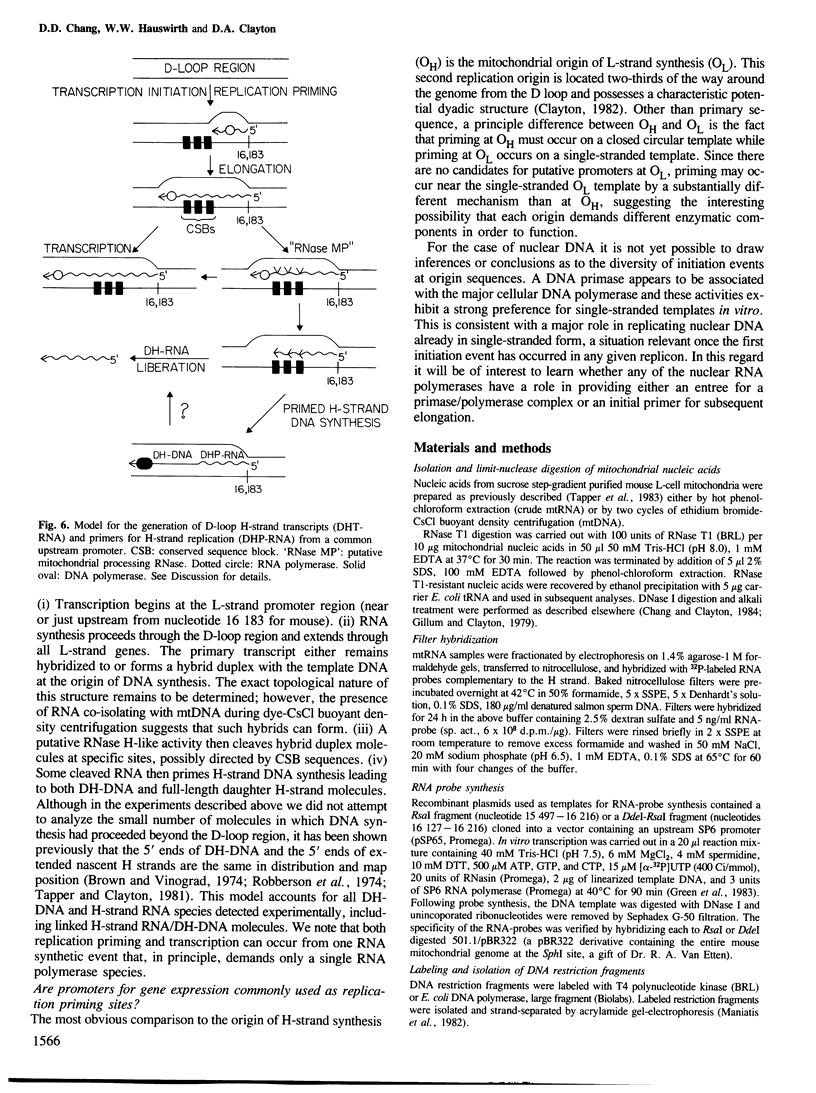
Mammalian mitochondrial DNA maintains a novel displacement-loop region containing the major sites of transcriptional initiation and the origin of heavy strand DNA replication. Because the exact map positions of the 5' termini of nascent mouse displacement-loop strands are known, it is possible to examine directly a potential relationship between replication priming and transcription. Analyses of in vivo nucleic acids complementary to the displacement-loop region reveal two species with identical 5' ends at map position 16 183. One is entirely RNA and the other is RNA covalently linked to DNA. In the latter the transition from RNA to DNA is sharp, occurring near or within a series of previously identified conserved sequences 74-163 nucleotides downstream from the transcriptional initiation site. These data suggest that the initial events in replication priming and transcription are the same and that the decision to synthesize DNA or RNA is a downstream event under the control of short, conserved displacement-loop template sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell. 1984 Mar;36(3):635–643. doi: 10.1016/0092-8674(84)90343-x. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Doda J. N., Wright C. T., Clayton D. A. Elongation of displacement-loop strands in human and mouse mitochondrial DNA is arrested near specific template sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6116–6120. doi: 10.1073/pnas.78.10.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller C. W., Beauchamp B. B., Engler M. J., Lechner R. L., Matson S. W., Tabor S., White J. H., Richardson C. C. Mechanisms for the initiation of bacteriophage T7 DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):669–679. doi: 10.1101/sqb.1983.047.01.078. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: RNA priming during the initiation of heavy-strand synthesis. J Mol Biol. 1979 Dec 5;135(2):353–368. doi: 10.1016/0022-2836(79)90441-8. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Hu S. Z., Wang T. S., Korn D. DNA primase from KB cells. Evidence for a novel model of primase catalysis by a highly purified primase/polymerase-alpha complex. J Biol Chem. 1984 Feb 25;259(4):2602–2609. [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Effects of point mutations on formation and structure of the RNA primer for ColE1 DNA replication. Cell. 1984 Feb;36(2):513–522. doi: 10.1016/0092-8674(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G. T., Tabak H. F. Initiation of transcription in yeast mitochondria: analysis of origins of replication and of genes coding for a messenger RNA and a transfer RNA. Nucleic Acids Res. 1984 Feb 24;12(4):1889–1900. doi: 10.1093/nar/12.4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A., Morrow J. F. Cleavage of replicating forms of mitochondrial DNA by EcoRI endonuclease. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4447–4451. doi: 10.1073/pnas.71.11.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano L. J., Tamanoi F., Richardson C. C. Initiation of DNA replication at the primary origin of bacteriophage T7 by purified proteins: requirement for T7 RNA polymerase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4107–4111. doi: 10.1073/pnas.78.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., Clayton D. A. Mechanism of replication of human mitochondrial DNA. Localization of the 5' ends of nascent daughter strands. J Biol Chem. 1981 May 25;256(10):5109–5115. [PubMed] [Google Scholar]
- Tapper D. P., Van Etten R. A., Clayton D. A. Isolation of mammalian mitochondrial DNA and RNA and cloning of the mitochondrial genome. Methods Enzymol. 1983;97:426–434. doi: 10.1016/0076-6879(83)97153-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Itoh T. The importance of RNA secondary structure in CoIE1 primer formation. Cell. 1982 Dec;31(3 Pt 2):575–583. doi: 10.1016/0092-8674(82)90313-0. [DOI] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. F., Ma D. P., Wilson R. K., Roe B. A. DNA sequence of the Xenopus laevis mitochondrial heavy and light strand replication origins and flanking tRNA genes. Nucleic Acids Res. 1983 Jul 25;11(14):4977–4995. doi: 10.1093/nar/11.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]