Abstract
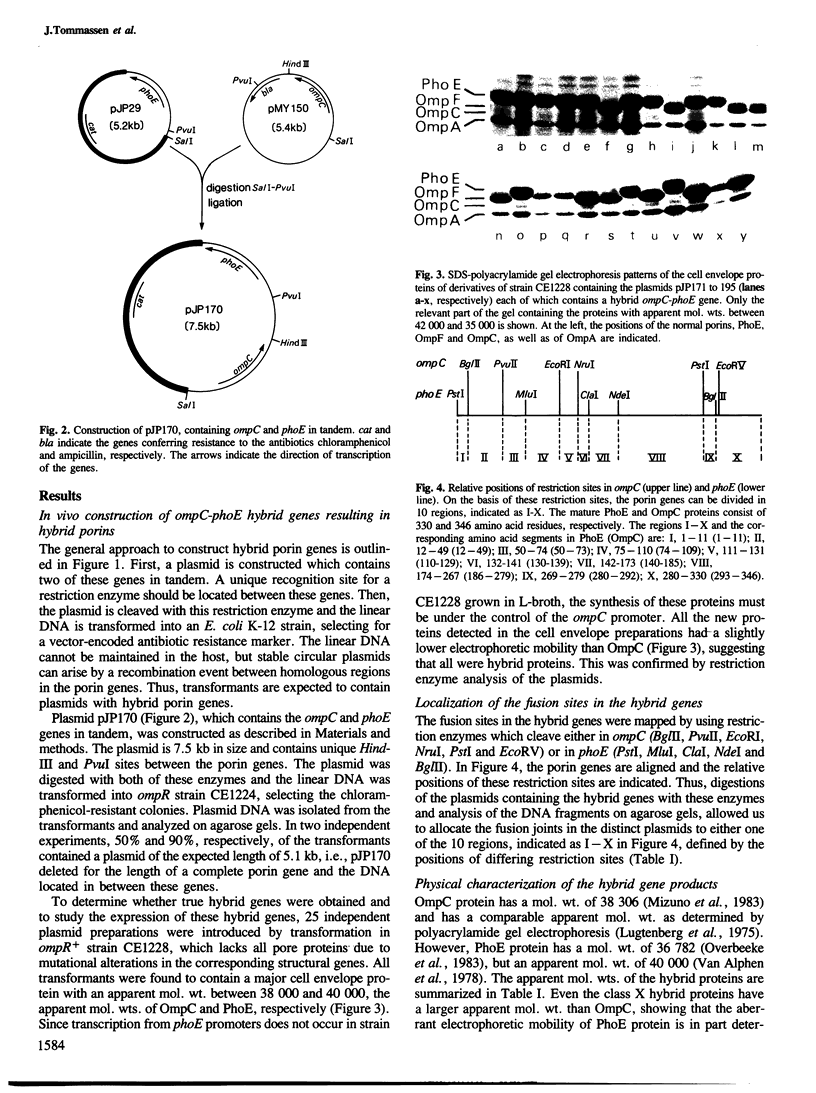
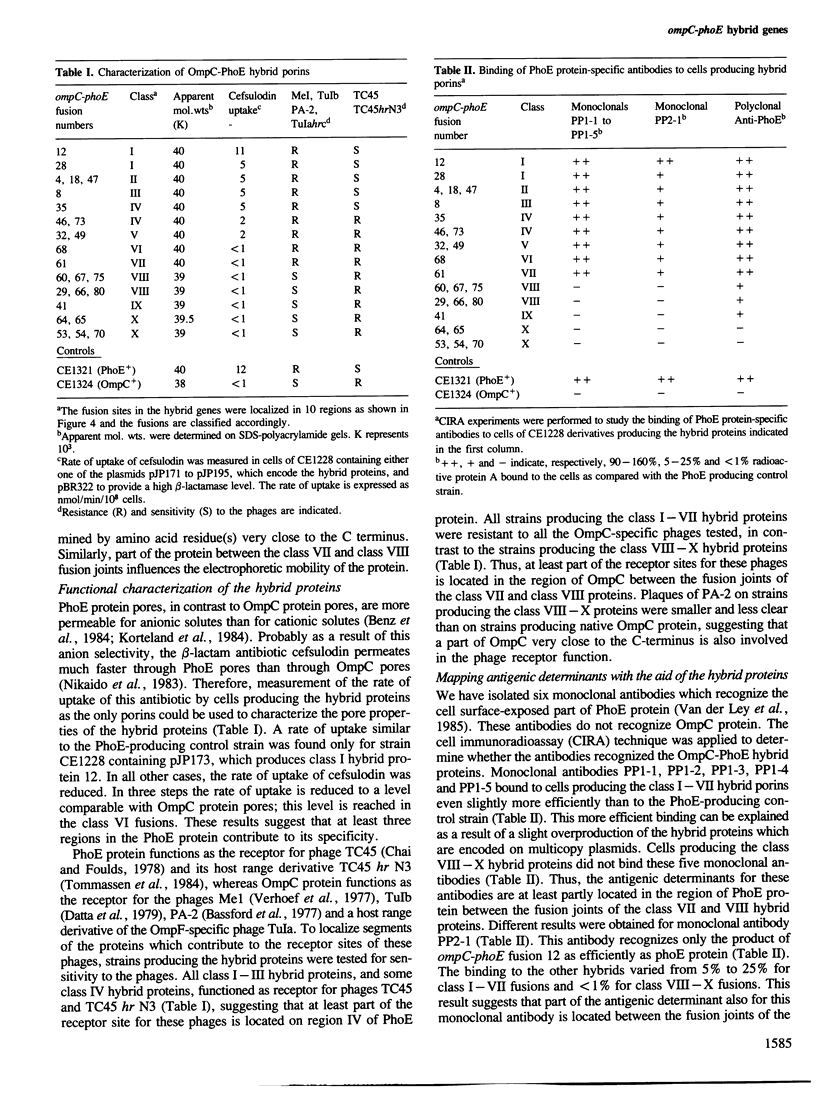
The genes ompC and phoE of Escherichia coli K-12 encode outer membrane pore proteins that are very homologous. To study the structure-function relationship of these proteins, we have constructed a series of ompC-phoE hybrid genes in which the DNA encoding part of one protein is replaced by the corresponding part of the other gene. These hybrid genes were easily obtained by using in vivo recombination. The fusion sites in the hybrid genes were localized by restriction enzyme mapping. The hybrid gene products were normally expressed and they were characterized with respect to functions and properties in which the native OmpC and PhoE proteins differ, such as pore characteristics, the receptor activity for phages and the binding of specific antibodies. Three regions within the N-terminal 130 amino acids were localized which determine pore characteristics and a segment between residues 75 and 110 contains amino acids which determine specificity for PhoE phages. A major cell surface-exposed region is located between residues 142 and 267. This region contains residues which are required for the binding of monoclonal antibodies directed against the cell surface-exposed part of PhoE and residues which determine specificity for OmpC phages.
Full text
PDF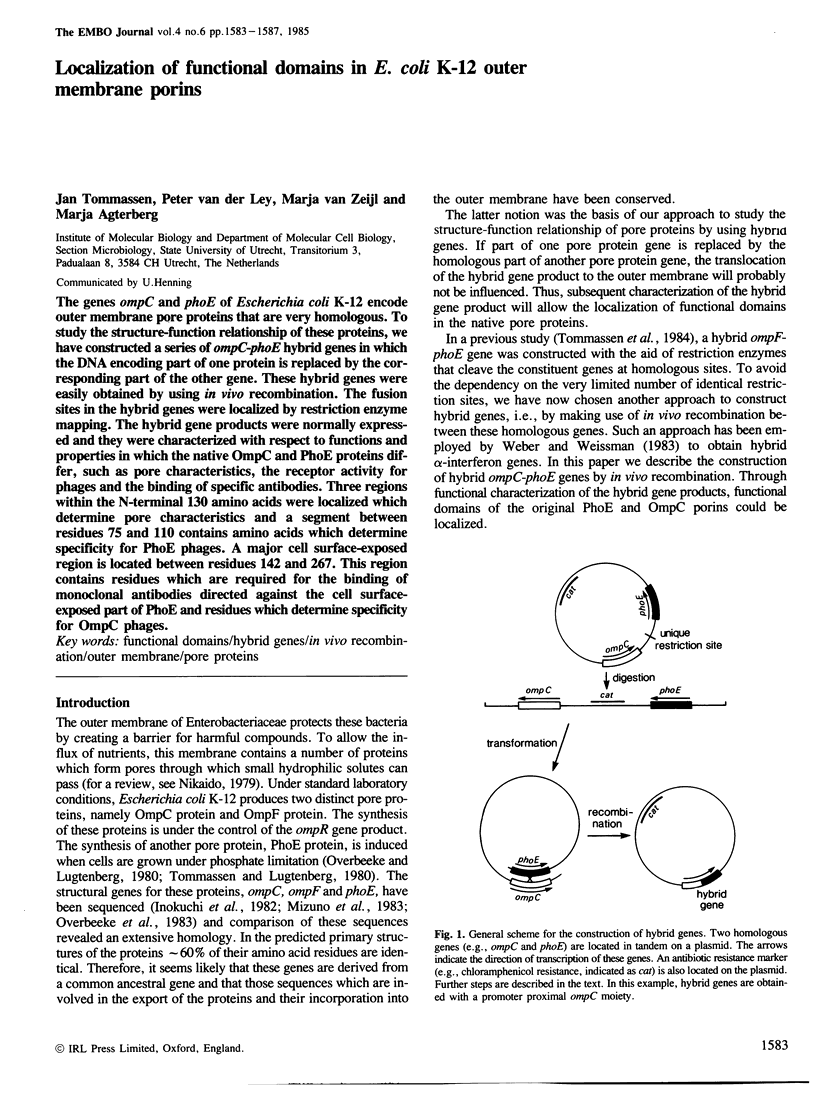


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Darveau R. P., Hancock R. E. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur J Biochem. 1984 Apr 16;140(2):319–324. doi: 10.1111/j.1432-1033.1984.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Two bacteriophages which utilize a new Escherichia coli major outer membrane protein as part of their receptor. J Bacteriol. 1978 Jul;135(1):164–170. doi: 10.1128/jb.135.1.164-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Expression of outer membrane protein e of Escherichia coli K12 by phosphate limitation. FEBS Lett. 1980 Apr 7;112(2):229–232. doi: 10.1016/0014-5793(80)80186-4. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Recognition site for phosphorus-containing compounds and other negatively charged solutes on the PhoE protein pore of the outer membrane of Escherichia coli K12. Eur J Biochem. 1982 Aug;126(1):113–118. doi: 10.1111/j.1432-1033.1982.tb06754.x. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Amino terminus of outer membrane PhoE protein: localization by use of a bla-phoE hybrid gene. J Bacteriol. 1984 Jan;157(1):327–329. doi: 10.1128/jb.157.1.327-329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Overduin P., Lugtenberg B., Bergmans H. Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J Bacteriol. 1982 Feb;149(2):668–672. doi: 10.1128/jb.149.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Pugsley A. P., Korteland J., Verbakel J., Lugtenberg B. Gene encoding a hybrid OmpF--PhoE pore protein in the outer membrane of Escherichia coli K12. Mol Gen Genet. 1984;197(3):503–508. doi: 10.1007/BF00329950. [DOI] [PubMed] [Google Scholar]
- Tommassen J., van Tol H., Lugtenberg B. The ultimate localization of an outer membrane protein of Escherichia coli K-12 is not determined by the signal sequence. EMBO J. 1983;2(8):1275–1279. doi: 10.1002/j.1460-2075.1983.tb01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef C., de Graaff P. J., Lugtenberg E. J. Mapping of a gene for a major outer membrane protein of Escherichia coli K12 with the aid of a newly isolated bacteriophage. Mol Gen Genet. 1977 Jan 7;150(1):103–105. doi: 10.1007/BF02425330. [DOI] [PubMed] [Google Scholar]
- Weber H., Weissmann C. Formation of genes coding for hybrid proteins by recombination between related, cloned genes in E. coli. Nucleic Acids Res. 1983 Aug 25;11(16):5661–5669. doi: 10.1093/nar/11.16.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen W., van Seim N., Lugtenberg B. Pores in the outer membrane of Escherichia coli K12: involvement of proteins b and e in the functioning of pores for nucleotides. Mol Gen Genet. 1978 Feb 7;159(1):75–83. doi: 10.1007/BF00401750. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Amesz H., Tommassen J., Lugtenberg B. Monoclonal antibodies directed against the cell-surface-exposed part of PhoE pore protein of the Escherichia coli K-12 outer membrane. Eur J Biochem. 1985 Mar 1;147(2):401–407. doi: 10.1111/j.1432-1033.1985.tb08764.x. [DOI] [PubMed] [Google Scholar]



