Abstract
Gap junctions are plasma membrane channels between neighboring cells. We previously described a powerful technique where gap junctional, intercellular communication (GJIC) of adherent cells can be examined by in situ electroporation on a slide, part of which is coated with electrically conductive and transparent indium‐tin oxide. An electric pulse is applied through an electrode placed on the cells in the presence of the tracking dye, Lucifer yellow (LY). The pulse causes LY's penetration into the cells growing on the conductive part of the slide, and the subsequent migration of the dye to the non‐electroporated cells growing on the non‐conductive area is microscopically observed under fluorescence illumination. Although this technique is adequate for a number of cell lines, the turbulence generated as the electrode is removed can cause cell detachment, which makes GJIC examination problematic. In this communication, we describe a slide configuration where junctional communication can be examined in the absence of an upper electrode: Cells are grown on two co‐planar electrodes separated by a barrier which diverts the electric field, rendering it vertical to the cell layer. The elimination of an upper electrode is especially valuable for the electroporation of sensitive cells, such as terminally differentiated adipocytes. This technique can also be used for the introduction of other non‐permeant molecules such as peptides or siRNA, followed by examination of the cellular phenotype or gene expression levels in situ.
Keywords: Electroporation, Gap junctions, Adherent cells, Adipocytes
1. Introduction
Gap junctions are plasma membrane channels that serve as conduits for the passage of small molecules between the interiors of neighboring cells. A reduction in gap junctional, intercellular communication (GJIC) is believed to lead to an increase in cell proliferation (reviewed in Vinken et al. (2006)). Interestingly, a loss of GJIC also accompanies the cessation of proliferation and differentiation of murine preadipocytes (Azarnia and Russell, 1985; Brownell et al., 1996a).
The investigation of junctional permeability is usually conducted through the introduction of a fluorescent dye such as Lucifer yellow (LY) by microinjection, scrape‐loading (el‐Fouly et al., 1987) or preloading with dye (Goldberg et al., 1995), followed by observation of its migration into neighboring cells, or by measuring the recovery of fluorescence after photobleaching (Wade et al., 1986). These methods are generally expensive and time‐consuming or introduce the potential complication of cellular damage. We previously developed a technique for GJIC measurement which can overcome these problems (Raptis et al., 1994, 2006). Cells are grown on a glass slide, half of which is coated with electrically conductive, optically transparent, indium‐tin oxide (ITO) (Raptis et al., 1994, 2000). A Lucifer yellow solution is added to the cells and an electrical pulse delivered through an electrode placed on top of the slide (Figure 1A). Cells growing on the conductive side of the slide are electroporated, while those on the adjoining, non‐conductive area do not receive any current, therefore they are not permeabilized (Raptis et al., 1994). The electrode set is subsequently removed, the unincorporated dye washed and the cells observed under phase contrast and fluorescence illumination. Tracer movement can be evaluated several minutes after the electrical pulse, by overlapping the phase contrast and fluorescence images of the cells (Raptis et al., 1994; Vultur et al., 2003; Tomai et al., 1998). As previously demonstrated, dye transfer through gap junctions can be precisely quantitated in this way, simultaneously and in a large number of cells, without any detectable disturbance to cellular metabolism (Brownell et al., 1996, 1998, 1994, 1998, 1999).
Figure 1.
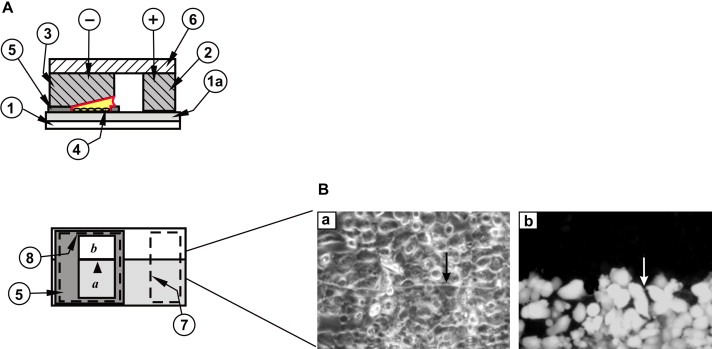
Electrode and slide assembly for the study of intercellular, junctional communication: standard slide. (A) Top panel: side view. A coating of ITO (1a) (shown greatly enlarged for clarity) on the upper surface of the glass slide (1) makes a conductive path from the positive contact bar (2), through the cells (4) and the electroporation solution to the bottom surface of the negative electrode (3). + and − denote connections to the positive and negative poles of the pulse source, respectively. (5), insulating Teflon frame, (6), electrode holder. Note that the negative electrode is inclined to compensate for the resistance of the coating (Raptis et al., 2006). Lower panel: top view. The outline of the positive (7) and negative (8) electrodes and their relative position on the slide in relation to the frame (5) and the window where the cells are grown are indicated. The lightly shaded area (a) represents the conductive coating. (b), area where the conductive coating has been removed. Arrowhead points to the transition line between conductive and non‐conductive areas. (B) Examination of gap junctional communication using the above apparatus. Human lung carcinoma A549 cells were plated in the window (a,b) as shown in (A) and electroporated in the presence of 5 mg/ml Lucifer yellow. After washing the unincorporated dye, cells from the same field were photographed under fluorescence (right panel) or phase contrast (left panel) illumination. Arrows point to the transition line between conductive and non‐conductive areas. Note the absence of fluorescence in cells growing on the nonconductive part of the slide (Tomai et al., 1999).
Although this technique has been employed extensively for GJIC examination, it is not adequate for a number of cell types which do not adhere well to their solid support. This is often especially acute if cells are grown to high confluences, when they may detach due to the turbulence and suction forces created as the top electrode is removed after electroporation, making it impossible to examine GJIC. Even detachment of a small part of the cell layer during removal of the electrode may allow the dye to penetrate under the cell sheet where it cannot be removed by washing, and this makes the examination of GJIC problematic. Coating the slide with CelTak™, fibronectin or collagen, although it improves cell adhesion (Anagnostopoulou et al., 2006), it does not eliminate the problem. Besides, these coatings can increase the voltage required for electroporation, so that a slight variation in the thickness of the coating may affect the uniformity of poration. For these reasons the ability to electroporate without the use of an upper electrode is highly desirable.
The induction of differentiation of preadipocytic cell lines such as 3T3 L1 is invariably conducted by treating confluent cultures with inducers of adipogenesis for approximately 10days, at which time differentiation is manifested by a change in morphology from a fibroblastoid to a rounder one with accumulation of cytoplasmic lipid droplets, which is the hallmark of terminal adipocytic differentiation (Raptis et al., 1997a). The adipocytic differentiation process requires that the cells remain undisturbed and attached onto their substratum; if the cells detach then differentiation is abolished, and this makes the examination of gap junctional communication problematic. The elimination of an upper electrode would greatly simplify the introduction of Lucifer yellow or other tracer molecules into differentiated adipocytes. This communication describes an electrode configuration to this effect.
2. Results and discussion
To examine gap junctional communication, it is important to be able to reliably distinguish cells that received Lucifer yellow by electroporation, from cells that have taken in dye from neighboring cells through gap junctions. To achieve this, a sharp transition in electrical field intensity between electroporated and non‐electroporated areas must be established. When an upper electrode is used, the electric field follows a path that is straight up from the conductive ITO surface, through the cells that are growing on it, so that no electroporation takes place on the adjacent glass surface from which the ITO has been removed (Figure 1A and B; Raptis et al., 2006).
In initial experiments involving electroporation without an upper electrode (Figure 2A) we attempted to use a slide where a 3‐mm‐wide strip of ITO had been removed (c), leaving two co‐planar electrodes (a,b and e,f), supported by the same glass slide substrate (1). A containing wall (4) made of hydrophobic plastic and bonded to the slide held the cells and growth medium over this region. When the two sections of ITO coating were connected to a power source and short pulses alternating in polarity delivered, cells growing on the ITO surfaces were electroporated (Figure 2C). However, electroporation using this approach produced a gradient of fluorescence at the edges of the ITO coating (Figure 2C), even for A549 cells which do not have gap junctions (Figure 1B and Tomai et al., 1999). This indicated a gradient of actual field strength, which makes this approach unsuitable for the examination of junctional communication. This gradient could be the result of a combination of the coplanar relationship of the ITO electrodes and the electrical characteristics of the membranes of the cells growing in this region. In the absence of cells, based on the principle of electricity tending to flow preferentially along the path of least resistance, it is expected that the flow of current would be from one ITO surface to the other, laterally in a diffuse straight line. However, when cells are growing on the ITO surfaces and on the plain glass between them, the impedance of the cell membranes would affect the rate and pattern of electrical charge flow (Ghosh et al., 1993; Wegener et al., 2000). As previously described (Wegener et al., 2000), the cell membrane is essentially insulating, so that current leaving the electrode has to flow in the narrow space underneath the cells before it can escape into the bulk electrolyte above them. Thus, as the charge carriers escaping from the edge of each ITO electrode are attracted to the opposite pole, they concentrate in the region immediately beyond the edge of the ITO coating, such that a sufficient charge across the cell membrane builds up as to cause electroporation of these cells (Ghosh et al., 1993). At the same time, the electroporation event causes a sudden lowering of impedance across these cells, allowing electrical current to flow more easily through the cells into the bulk electrolyte surrounding them (Ghosh et al., 1993). The net effect is that several rows of cells are electroporated at progressively weaker voltages, until the intensity of the electric field drops below the threshold necessary for electroporation in cells farther away from the edges of the coating. This results in a gradient of fluorescence, in a pattern indistinguishable from the gradient seen following dye transfer through gap junctions (Figure 2B and C) (Tomai et al., 1999), which makes this approach unsuitable for GJIC examination.
Figure 2.
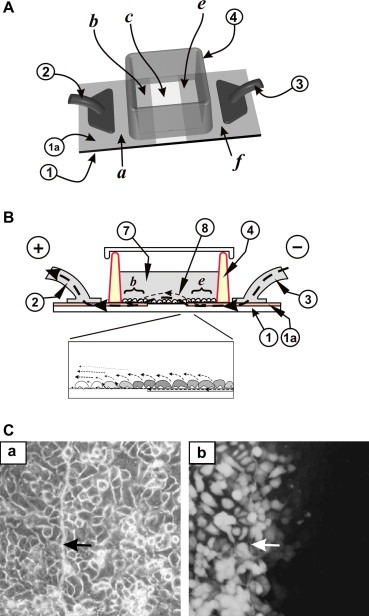
Electroporation in the absence of an upper electrode. (A) Top view. Cells were grown on an ITO‐coated slide from which the coating was removed in a strip (c) as shown. The two conductive sides, (b, e), serving as electrodes, continue outside the cell growth area (a, f), and are connected to the positive (2) and negative (3) poles of the pulse generator. (B) Side view. The slide with the cells growing on the ITO‐coated and the bare glass regions is schematically shown. Current from the pulse generator connected to contact point (3) passes into the thin conducting ITO layer (1a), under the wall of the chamber (4) to area (e) to the opposite electrode (b) as shown. (8), electric field lines. An electrical potential is created across the cells growing on (e) and those on the glass area (c) (Fig. 2A), immediately next to the edge as current passes into the electroporation buffer, which acts as an electrolyte, around and through the electroporated cells. Bottom panel, enlargement of the edge of the conductive coating. Dotted lines with arrows indicate the electric field lines along the surface of the slide, around the cells and through the surrounding fluid. The gray‐scale represents the relative intensity of Lucifer yellow fluorescence; darker gray cells have been electroporated with higher fields than their lighter neighbors. (C) A549 cells were plated in a chamber as in (A) above and electroporated in the presence of Lucifer yellow (30 V, 0.2 µF, 6 times, with the polarity reversed on alternate pulses). After washing the unincorporated dye, cells fromthe same field were photographed under fluorescence (b) or phase contrast (a) illumination. Note the gradient of fluorescence in cells growing on the non‐conductive part of the slide, despite the fact that these cells do not have gap junctions, as shown in Fig. 1B (Tomai et al., 1999). Arrows point to the transition line between conductive and non‐conductive areas.
In an attempt to ensure that only cells in contact with the ITO surface are electroporated, a 0.5mm barrier of non‐conductive plastic was inserted in the middle of the glass area, bonded onto the glass and attached to the inner sides of the wall (Figure 3A and B (5)). The two ITO electrodes ((2) and (3)) were connected to the pulse source. However, they were electrically isolated until sufficient electroporation medium was added to cover the barrier (5), thus establishing an electrical path between the fluid pools on either side of the barrier. The results of this simple change were dramatic and consistent. Apparently, the path of the electric current during the electroporation pulses is diverted through the layer of fluid above the barrier so that it now consists of a diffuse field that is substantially above the ITO and cell layer, rather than adjacent to them (Figure 3B). Extensive experimentation demonstrated that in this setup, cells such as A549 do not display a gradient of fluorescence, indicating a sharp transition in electroporation intensity (Figure 3C).
Figure 3.
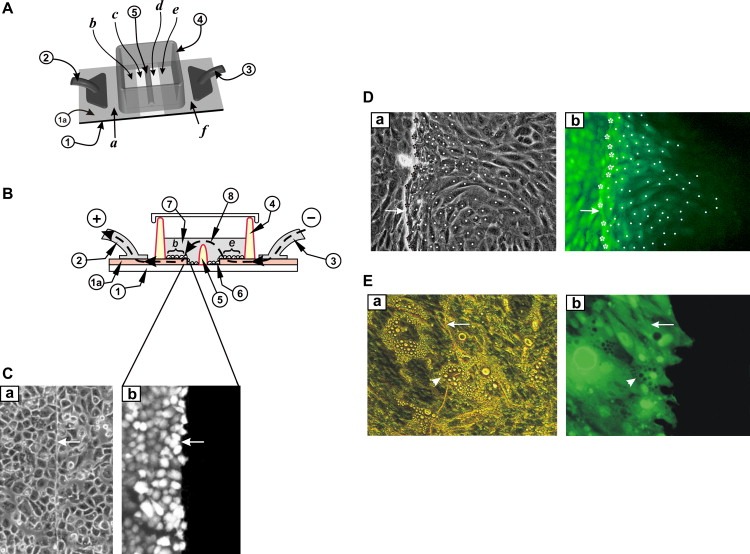
Electroporation on two co‐planar ITO electrodes, with a barrier separating the two areas. (A) Top view. Cells were grown on an ITO‐coated slide from which the coating was removed in a strip as shown. The two conductive sides (a, f), serving as electrodes, were connected to the positive and negative poles of the pulse generator (2) and (3). A non‐conductive barrier (5) divides the strip of bare glass in half and separates the chamber into two sections. (B) Side view. The slide with the cells growing on the ITO coated and the bare glass regions is shown. When electroporation buffer is added to the chamber to a level above the height of the barrier (5) then an electrical path between the electrodes (e and b) is formed. Note that the ITO layer (1a) is shown with dramatically exaggerated thickness for clarity, although its actual thickness is much less than the thickness of the cells. (C) A549 cells were plated on the slide in (A) above and electroporated in the presence of Lucifer yellow as in Fig. 2 above. After washing the unincorporated dye, cells from the same field were photographed under fluorescence (b) or phase contrast (a) illumination. Note the absence of fluorescence in cells growing on the non‐conductive part of the slide. Arrows point to the transition line between conductive and non‐conductive areas. (D) 3T3 L1 preadipocytes were plated in the window shown in (A) above and electroporated in the presence of Lucifer yellow. After washing the unincorporated dye, cells from the same field were photographed under fluorescence (b) or phase‐contrast (a) illumination. Note the gradient of fluorescence, indicating extensive dye transfer through gap junctions (Raptis et al., 2006; Tomai et al., 1999). To quantitate gap junctional communication, electroporated cells growing at the border with the non‐conductive zone (stars), and fluorescing cells growing on the non‐conductive side of the slide, into which the dye had transferred through gap junctions (dots) were identified. The number of cells into which the dye has transferred per electroporated border cell can be calculated by dividing the total number of fluorescing cells on the non‐conductive side by the number of cells growing at the border with the conductive coating. Arrows on the conductive side point to the transition line between conductive and non‐conductive areas. Magnification: 200×. (E) 3T3L1 preadipocytes were plated in the window shown in (A) above and induced to differentiate by the addition of IBMX, insulin and dexamethasone at confluence (see Section 3). Ten days later, terminally differentiated adipocytes were electroporated in the presence of Lucifer yellow. Note the absence of dye transfer through gap junctions. Arrows point to the transition line between conductive and non‐conductive areas. Arrowheads point to a terminally differentiated adipocyte growing on the transition line between conductive and non‐conductive areas. Magnification: 200×.
The results using this approach for the electroporation of 3T3 L1 preadipocytes are shown in Figure 3D and E. Cells were plated in a chamber as in Figure 3A and electroporated in the presence of Lucifer yellow (see Section 3). Pulses were delivered with alternating polarity to minimize the build‐up of ions at either edge of the ITO coating. As shown in Figure 3D, undifferentiated, confluent 3T3L1 cells display extensive junctional communication. In stark contrast however, 10days following confluence and induction of differentiation, no transfer from the terminally differentiated cells to their neighbors is observed, even if the latter are not completely differentiated yet (Figure 3E).
In conclusion, we describe a method which greatly facilitates electroporation of adherent cells. The elimination of an upper electrode is especially valuable for the non‐traumatic examination of junctional communication of cells that do not adhere well, such as NIH 3T3 cells overexpressing the epidermal growth factor receptor (Anagnostopoulou et al., 2006), a variety of neoplastically transformed lines, primary tumor cells, insect cells or very sensitive cells such as terminally differentiated adipocytes, whose physiology may be disturbed by placing and removing an electrode. Moreover, the possibility of using identical conditions regarding pulse intensity and LY solution in the two chambers offers the opportunity for a direct and precise comparison of the effect of different treatments to the same cell type, upon junctional communication. This is especially important considering that the apparent GJIC measured may be greatly affected by dye concentration, the exact pulse conditions and time after the pulse when the cells are observed. However, if GJIC in two different types of cells is measured, the fact that these cells may require slightly different voltages for optimum permeation must also be taken into account. An added advantage is that since there is no need for an upper electrode, and the electrical contacts are outside the cell growth chamber, sterility can be easily maintained. The possibility of observing intercellular communication between large numbers of cells simultaneously, permits a precise quantitation of the degree of intercellular communication (Figure 3D). Most importantly, the above approach can also be employed for the introduction of a variety of non‐permeant molecules, such as quantum dots, peptides or siRNA, followed by detection of changes in gene expression by immunocytochemistry or other techniques in situ. In this case, the assessment of the effect of the introduced material can be greatly facilitated by a slide configuration providing non‐electroporated cells side by side with the electroporated ones as a control and this can be especially valuable in signal transduction studies (Raptis et al., 2000a).
3. Experimental procedures
3.1. Cell lines and culture techniques
Tissue culture media and sera were from Life Technologies Inc. A549 human lung carcinoma cells and 3T3 L1 preadipocytes (both from ATCC) were grown in petri dishes in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% fetal calf serum in a 37°C CO2 incubator. Differentiation was induced by the addition of 0.2μM dexamethasone, 10μg/ml insulin and 0.5mM 3‐isobutyl‐1‐methyl‐xanthine (IBMX) in 10% fetal calf serum (FCS) at confluence for 48h, followed by 10% FCS and insulin until the onset of differentiation, approximately 10days later (Raptis et al., 1997b).
3.2. Gap junctional intercellular communication assays: standard procedure
In the standard configuration of the apparatus (Figure 1A) (Raptis et al., 2006), cells are grown on a glass slide (1) coated with electrically conductive, optically transparent indium‐tin oxide (1a) (ITO, Colorado Concept Coatings, Boulder CO, USA), in a window cut from electrically insulating teflon (5). The thickness of the coating is 800Å, for a surface resistivity of 20Ω/sq (Raptis et al., 2006). In order to study the transfer of material from cell to cell, the conductive coating was removed lengthwise from half the slide, including the area within the window (Figure 1A, (b)) by etching with acids (Raptis et al., 1994). Cells growing on this non‐conductive surface do not receive any pulse, therefore they are not permeated. The slide is placed inside a 3‐cm petri dish and gas sterilized. Prior to pulse application, the growth medium is removed, the cells washed twice with prewarmed, calcium‐free DMEM and the same medium, supplemented with 5mg/ml Lucifer yellow (HPLC‐purified, dipotassium salt, Biotium) added to the cells. A series of short electric pulses (25–45V, 0.1μF, 6 times, for a conductive area of 4×4mm) are delivered by placing an electrode set on top of the slide as shown in Figure 1A. During the pulse, current flows through the positive contact bar (2) along the conductive surface of the slide, up through the electroporation solution and the cells growing in this space (4), to the bottom surface of the negative electrode (3) and back to the pulse source. This treatment opens pores on the plasma membrane which rapidly reclose without any disruption in cellular metabolism (Brownell et al., 1998). Unincorporated Lucifer yellow is washed with calcium‐free DMEM supplemented with 10% dialyzed calf serum which facilitates pore closure (Bahnson and Boggs, 1990). Tracer movement is evaluated 5min after the pulse by overlapping phase contrast and fluorescence images of the cells taken with an Olympus IX70 microscope.
Supporting information
Appendix Supplementary data
Supplementary data
Acknowledgements
The authors would like to thank Marilyn Garrett for excellent technical assistance. AA was supported by a Queen's University Graduate Award (QGA) and a predoctoral traineeship award from the Department of Defense Breast Cancer Research Program (BCRP‐CDMRP, award number W81XWH‐05‐1‐0224). AV was supported by studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Ontario graduate studentship program (OGS) and QGA. JC was supported by a Queen's University post‐doctoral fellowship. The financial assistance of CIHR, NSERC, the Canadian Breast Cancer Research Alliance, the Clare Nelson Bequest Fund, the Breast Cancer Action Kingston, the Cancer Research Society Inc. and the Ontario Centers of Excellence through grants to LR is gratefully acknowledged.
Appendix A. Supplementary data 1.
1.1.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.molonc.2007.06.002.
Anagnostopoulou Aikaterini, Cao Jun, Vultur Adina, Firth Kevin, Raptis Leda, (2007), Examination of gap junctional, intercellular communication by in situ electroporation on two co‐planar indium‐tin oxide electrodes, Molecular Oncology, 1, doi:10.1016/j.molonc.2007.06.002.
References
- Anagnostopoulou, A. , Vultur, A. , Arulanandam, R. , Cao, J. , Turkson, J. , Jove, R. , Kim, J.S. , Glenn, M. , Hamilton, A.D. , Raptis, L. , 2006. Differential effects of Stat3 inhibition in sparse vs confluent normal and breast cancer cells. Cancer Lett.. 242, 120–132. [DOI] [PubMed] [Google Scholar]
- Azarnia, R. , Russell, T.R. , 1985. Cyclic AMP effects on cell-to-cell junctional membrane permeability during adipocytic differentiation of 3T3-L1 fibroblasts. J. Cell Biol.. 100, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnson, A.B. , Boggs, S.S. , 1990. Addition of serum to electroporated cells enhances survival and transfection efficiency. Biochem. Biophys. Res. Commun.. 171, 752–757. [DOI] [PubMed] [Google Scholar]
- Brownell, H.L. , Narsimhan, R. , Corbley, M.J. , Mann, V.M. , Whitfield, J.F. , Raptis, L. , 1996. Ras is involved in gap junction closure in mouse fibroblasts or preadipocytes but not in differentiated adipocytes. DNA Cell Biol.. 15, 443–451. [DOI] [PubMed] [Google Scholar]
- Brownell, H.L. , Whitfield, J.F. , Raptis, L. , 1996. Cellular Ras partly mediates gap junction closure by the polyoma virus middle Tumor antigen. Cancer Lett.. 103, 99–106. [DOI] [PubMed] [Google Scholar]
- Brownell, H.L. , Lydon, N. , Schaefer, E. , Roberts, T.M. , Raptis, L. , 1998. Inhibition of epidermal growth factor-mediated ERK1/2 activation by in situ electroporation of nonpermeant [(alkylamino)methyl]acrylophenone derivatives. DNA Cell Biol.. 17, 265–274. [DOI] [PubMed] [Google Scholar]
- el-Fouly, M.H. , Trosko, J.E. , Chang, C.C. , 1987. Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res.. 168, 442–430 [DOI] [PubMed] [Google Scholar]
- Ghosh, P. , Keese, C.R. , Giaever, I. , 1993. Monitoring electropermeabilization in the plasma membrane of adherent mammalian cells. Biophys. J.. 64, 1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, G.S. , Bechberger, J.F. , Naus, C.C. , 1995. A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 18, 490–497. [PubMed] [Google Scholar]
- Raptis, L. , Brownell, H.L. , Firth, K.L. , MacKenzie, L.W. , 1994. A novel technique for the study of intercellular, junctional communication; electroporation of adherent cells on a partly conductive slide. DNA Cell Biol.. 13, 963–975. [DOI] [PubMed] [Google Scholar]
- Raptis, L. , Brownell, H.L. , Lu, Y. , Preston, T. , Narsimhan, R.P. , Anderson, S. , Schaefer, E. , Haliotis, T. , 1997. v-Ras and v-Raf block differentiation of transformable C3H10T1/2-derived preadipocytes at lower levels than required for neoplastic transformation. Exp. Cell Res.. 235, 188–197. [DOI] [PubMed] [Google Scholar]
- Raptis, L. , Yang, J. , Brownell, H.L. , Lai, J. , Preston, T. , Corbley, M.J. , Narsimhan, R.P. , Haliotis, T. , 1997. Rasleu61 blocks differentiation of transformable 3T3 L1 and C3HT1/2-derived preadipocytes in a dose- and time-dependent manner. Cell Growth Differ. 8, 11–21. [PubMed] [Google Scholar]
- Raptis, L. , Brownell, H.L. , Vultur, A.M. , Ross, G. , Tremblay, E. , Elliott, B.E. , 2000. Specific inhibition of growth factor-stimulated ERK1/2 activation in intact cells by electroporation of a Grb2-SH2 binding peptide. Cell Growth Differ. 11, 293–303. [PubMed] [Google Scholar]
- Raptis, L. , Tomai, E. , Firth, K.L. , 2000. Improved procedure for examination of gap junctional, intercellular communication by in situ electroporation on a partly conductive slide. Biotechniques. 29, 222–226. [DOI] [PubMed] [Google Scholar]
- Raptis, L. , Vultur, A. , Brownell, H.L. , Firth, K.L. , 2006. Dissecting pathways; in situ electroporation for the study of signal transduction and gap junctional communication. In Celis J.E., Cell Biology: A Laboratory Handbook. Academic Press; San Diego, CA: 341–354. [Google Scholar]
- Tomai, E. , Brownell, H.L. , Tufescu, T. , Reid, K. , Raptis, S. , Campling, B.G. , Raptis, L. , 1998. A functional assay for intercellular, junctional communication in cultured human lung carcinoma cells. Lab. Invest.. 78, 639–640. [PubMed] [Google Scholar]
- Tomai, E. , Brownell, H.L. , Tufescu, T. , Reid, K. , Raptis, L. , 1999. Gap junctional communication in lung carcinoma cells. Lung Cancer. 23, 223–231. [DOI] [PubMed] [Google Scholar]
- Vinken, M. , Vanhaecke, T. , Papeleu, P. , Snykers, S. , Henkens, T. , Rogiers, V. , 2006. Connexins and their channels in cell growth and cell death. Cell Signal. 18, 592–600. [DOI] [PubMed] [Google Scholar]
- Vultur, A. , Tomai, E. , Peebles, K. , Malkinson, A.M. , Grammatikakis, N. , Forkert, P.G. , Raptis, L. , 2003. Gap junctional, intercellular communication in cells from urethane-induced tumors in A/J mice. DNA Cell Biol.. 22, 33–40. [DOI] [PubMed] [Google Scholar]
- Wade, M.H. , Trosko, J.E. , Schindler, M. , 1986. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 232, 525–528. [DOI] [PubMed] [Google Scholar]
- Wegener, J. , Keese, C.R. , Giaever, I. , 2000. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res.. 259, 158–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Supplementary data
Supplementary data


