ABSTRACT
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide, and immune checkpoint blockade therapy provides an opportunity for improving the outcome of CRC patients. Recent studies suggest that programmed death ligand-1 (PD-L1) is only expressed in 12% of CRCs. Here, we demonstrate that PD-L2 is expressed in approximately 40% CRCs, and its expression independently associates with poor survival of CRC patients. By detection of PD-L2 expression by immunofluorescence in 124 CRC cases with 10-y survival data, we found significant association between PD-L2 overexpression in cancer cells and worse overall survival (46.3 vs 69.1 mo; p = 0.0004). The association remained significant in multivariate COX regression analysis (hazard ratio = 2.778, 95% confidence interval [CI] = 1.668–4.627; p < 0.0001). In the validation CRC data set, significant association between PD-L2 overexpression and poor survival was supported by the univariate analysis (27.1 vs. 88.9 mo; p = 0.0002) and multivariate model (hazard ratio = 7.09, 95%CI 1.78–28.16; p = 0.005). Western Blot revealed strong induction of PD-L2 expression by interferon-γ (IFNγ) in CRC cells, and the mRNA levels of both genes were significantly correlated in CRC tissue samples. Suppression of glycosylation with tunicamycin caused a shift in molecular weight and significant decrease in the expression of PD-L2 protein. In conclusion, PD-L2 overexpression in CRC cells, under the regulation by IFNγ and glycosylation, associates with poor survival of patients with colorectal cancer. These findings highlight PD-L2 as a promising therapeutic target in CRC and suggest potential routes to control PD-L2 expression in CRC cells.
KEYWORDS: Colorectal cancer, glycosylation, overall survival, PD-L2, prognosis
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer worldwide, second to lung cancer.1,2 In the United States, the incidence of CRC has increased by 22% from 2000 to 2013, and the death rates have increased by 13% in patients aged <50 y.3 In metastatic CRC (mCRC), the outcomes of surgery and adjuvant therapy remain low.4 In comparison to conventional therapies, cancer immunotherapies generally display improved tolerability and more long-lasting effects.5,6 Among these, the novel checkpoint blockade therapy targeting programmed cell death 1 (PD-1) pathway has achieved unprecedented clinical effects in a wide range of tumors.7-9 In microsatellite instability-high (MSI-H) mCRC, the checkpoint inhibition therapy has displayed promising effects,10 leading to the approval by FDA for the Critical Path Initiative use in these patients. Even though, a subset of MSI-H and most microsatellite stable CRCs will not respond to checkpoint inhibitor alone.11 Therefore, it has been proposed to improve the therapeutic responses by careful patient selection and the combination of other therapeutic approaches.
The PD-1 pathway mainly contains two ligands, namely programmed death ligand-1 (PD-L1/B7-H1/CD274) and programmed death ligand-2 (PD-L2/B7-DC).12 Activated T cells expressing PD-1 may diminish their effector functions when PD-L1 or PD-L2 forms complex with PD-1 on cell surface. It is commonly accepted that PD-L1 expression on epithelial cancer cells and immune cells can inhibit T-cell antitumor response and facilitate cancer development.9,13 A recent study demonstrated that PD-L1 may also promote the generation of tumor-initiating cells (TICs) in melanoma and ovarian cancer.14 Consistently, a study based on immunohistochemistry (IHC) detection of PD-1, PD-L1 and PD-L2 expression in melanoma, lung cancer, kidney cancer and CRC suggested that PD-L1 expression by tumor cells may be most closely correlated with response to anti-PD-1 blockade.15 However, the study only included eight CRC subjects, thus limiting the applicability of the conclusions in CRC.16 Song and colleagues evaluated the prognostic significance of PD-L1 expression by IHC in 404 CRC subjects, but multivariate analysis did not support PD-L1 expression as an independent prognostic factor in CRC.17 In another research including 181 CRC subjects, PD-L1 expression was only detected in 16 cases (9%) and was not associated with survival in the entire cohort.18 A more recent study involving 454 CRC subjects reported PD-L1 expression in only 12% patients, and molecular characterizations suggested that PD-L1 positivity associates with tumors arising through the serrated neoplasia pathway.19 In summary, the expression of PD-L1 is only positive in a limited subset of CRCs (∼10%) and does not represent an independent prognostic factor for the survival of patients with CRC. Then comes a crucial question of how CRC cells arising from the adenoma-carcinoma route (up to 80% CRCs) may have evaded antitumor immune response, in the absence of PD-L1 expression.
In contrast to PD-L1, the expression of PD-L2 has been thought to be limited in macrophages and dendritic cells, and its expression in cancer cells has rarely been reported. Predominant PD-L2 expression was initially found only in primary mediastinal large B-cell lymphomna that is not solid tumor.20 Recently, PD-L2 was found to be moderately or strongly expressed in 51.7% of esophageal adenocarcinomas, while PD_L1 was expressed on only 2% of subjects.21 Moreover, PD-L2+ tumors had a higher average number of PD-1+ TILs than tumors without PD-L2 expression, suggesting its functional relevance for the tumor microenvironment. In their report, PD-L2 expression was found to be associated with early stage, smaller tumor size and well-differentiated grade, although no association with patient survival was found. To date, it is largely unclear if PD-L2 may be predominantly expressed in other epithelial cancer types, and thus the functional, prognostic, and therapeutic significance of PD-L2 in various cancers remain yet to be defined.
In the present study, we detected PD-L2 expression in a set of CRC subjects with long-term (10 y) survival data, to clarify the association between PD-L2 expression and the outcome of CRC. An independent validation data set was also tested to assess the prognostic significance in CRC. The mechanisms of PD-L2 regulation were also explored to provide potential routes for controlling the expression of PD-L2 in colorectal cancer.
Results
Differential expression of PD-L2 in colorectal cancer tissues
We first applied IHC detection of PD-L2 in human CRC tissues and evaluated the expression and subcellular localization of PD-L2 in these cases. A total number of 124 human CRC specimens immobilized in a tissue microarray were detected with differential expression of PD-L2 in tumor cells, which was scored into 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Representative staining results corresponding to these scores are shown in Fig. 1A. Among these samples, moderate to strong staining was found in 48 cases (38.7%), and only 14.5% cases were found negative for PD-L2 expression. These different staining patterns were also found in the Human Protein Atlas results using another antibody for PD-L2 (Fig. 1B), suggesting that PD-L2 is indeed differentially expressed in CRC. Moreover, a subset of tumors exhibited membranous expression of PD-L2, which was categorized as weak or strong pattern (Fig. 1C). The positivity of PD-L2 on inflammatory cells was identified in 23% of CRC subjects (Fig. 1D and E). Higher expression of PD-L2 in tumor cells was associated with an increased pattern of membrane localization (Fig. 1E).
Figure 1.
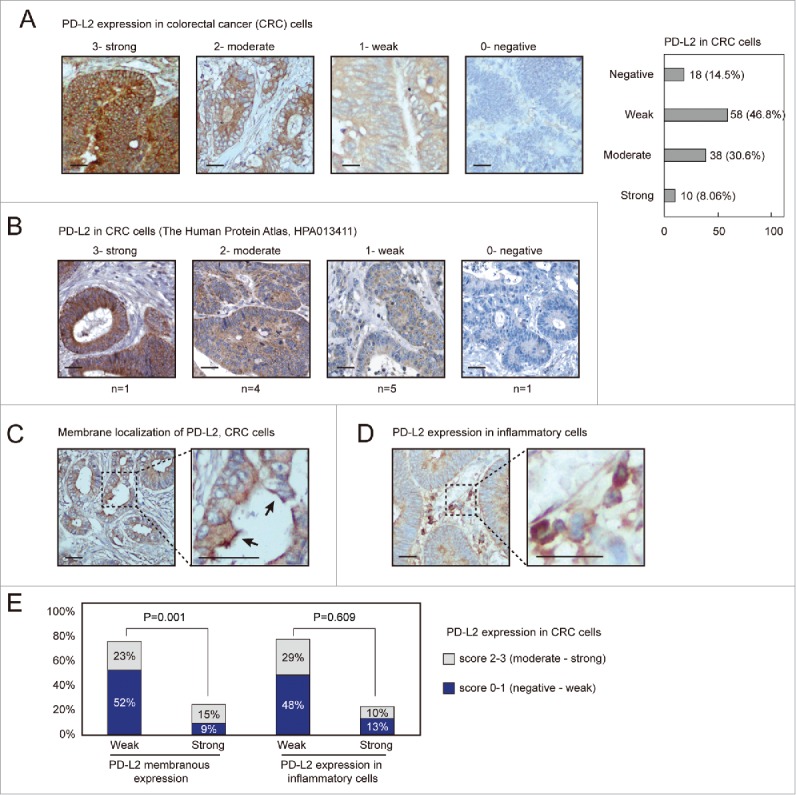
PD-L2 is differentially expressed in colorectal adenocarcinoma tissues. (A) Immunofluorescence (IHC) detection of PD-L2 in colorectal cancer tissues, with representative staining intensities scored as 0–3. Scale bars indicate 50 μm for panels. A numbers of cases with different scored expression levels are shown in the plot on the right (percentage indicated). (B) The representative IHC staining results as presented in the Human Protein Atlas data set. (C) Positive PD-L2 staining in the inflammatory cells adjacent to tumor cells. (D) Representative membranous distribution of PD-L2 in tumor cells. The black errors show membranous regions enriched for PD-L2. (E) The bar plot shows the percentage cases with PD-L2 expression in inflammatory cells and membranous distribution of PD-L2 in tumor cells. The chi-square test suggested that strong expression of PD-L2 associates with its membranous enriched pattern (p = 0.001), but not with its positivity in inflammatory cells.
Association between PD-L2 expression and clinicopathological features of CRC
The CRC patients were stratified according to PD-L2 expression patterns (moderate-strong vs. negative-weak in tumor cells; weak vs. strong membrane expression patter; weak vs. strong expression in inflammatory cells) and were compared for different pathological features (Table 1). PD-L2 positive tumors displayed slight increase with the mucinous histological type (14.6% vs. 7.2%, p = 0.043), although tubular and papillary histological types were not significantly lower in this subset (45.8% vs. 48.3%; 39.5% vs. 44.3%, respectively). Moreover, strong membranous expression pattern of PD-L2 associated with infiltrating ulcerative pathological type (p = 0.030) and advanced tumor stage (T4 stage, p = 0.011). The positivity of PD-L2 in inflammatory cells associated with mucinous histological type (0.044) and elevated pathological type (p = 0.003). However, none of these PD-L2 expression patterns displayed significant association with lymph node infiltration or metastasis (Table 1).
Table 1.
PD-L2 expression in relation to clinicopathologic characteristics of colorectal adenocarcinomas.
| Cancer cells |
Membrane enrichment |
Inflammatory cells |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Score 0–1 | Score 2–3 | p | Weak | Strong | p | Weak | Strong | p | ||
| Sex | |||||||||||
| Male | 70 | 40 | 30 | 0.280 | 50 | 20 | 0.195 | 57 | 13 | 0.224 | |
| Female | 54 | 36 | 18 | 44 | 10 | 39 | 15 | ||||
| Age (average) | 68 | 67.36 | 69.48 | 0.275 | 68.26 | 67.93 | 0.88 | 68.18 | 68.16 | 0.993 | |
| Tumor location | |||||||||||
| Transverse | 27 | 13 | 14 | 0.552 | 17 | 10 | 0.365 | 19 | 8 | 0.299 | |
| Descending | 14 | 9 | 5 | 11 | 3 | 13 | 1 | ||||
| Ascending | 42 | 27 | 15 | 35 | 7 | 30 | 12 | ||||
| Sigmoid | 39 | 25 | 14 | 29 | 10 | 33 | 6 | ||||
| Rectum | 2 | 2 | 0 | 2 | 0 | 1 | 1 | ||||
| Histological types | |||||||||||
| Tubular | 60 | 38 | 22 | 0.043* | 43 | 17 | 0.456 | 47 | 13 | 0.044 | |
| Papillary | 55 | 36 | 19 | 43 | 12 | 45 | 10 | ||||
| Mucinous | 9 | 2 | 7 | 8 | 1 | 4 | 5 | ||||
| Pathological types | |||||||||||
| Colloid type | 3 | 0 | 3 | 0.109 | 2 | 1 | 0.030 | 0 | 3 | 0.003 | |
| Infiltrating ulcerative | 38 | 24 | 14 | 24 | 14 | 30 | 8 | ||||
| Infiltrating | 40 | 23 | 17 | 29 | 11 | 34 | 6 | ||||
| Ulcerative | 21 | 12 | 9 | 18 | 3 | 19 | 2 | ||||
| Elevated | 22 | 17 | 5 | 21 | 1 | 13 | 9 | ||||
| Tumor grade (differentiated) |
|||||||||||
| Well | 5 | 4 | 1 | 0.576 | 3 | 2 | 0.164 | 3 | 2 | 0.562 | |
| Moderately | 87 | 54 | 33 | 63 | 24 | 69 | 18 | ||||
| Poorly | 32 | 18 | 14 | 28 | 4 | 24 | 8 | ||||
| Size (cm3) | 46 | 38 | 53 | 0.456 | 43 | 47 | 0.863 | 37 | 69 | 0.167 | |
| Tumor stage (AJCC7) | |||||||||||
| T1 | 1 | 1 | 0 | 0.132 | 1 | 0 | 0.011 | 1 | 0 | 0.063 | |
| T2 | 5 | 4 | 1 | 5 | 0 | 2 | 3 | ||||
| T3 | 90 | 58 | 32 | 72 | 18 | 70 | 20 | ||||
| T4 | 24 | 10 | 14 | 12 | 12 | 22 | 2 | ||||
| Node stage (AJCC7) | |||||||||||
| N0 | 70 | 42 | 28 | 0.609 | 53 | 17 | 0.332 | 54 | 16 | 0.886 | |
| N1 | 41 | 25 | 16 | 29 | 12 | 33 | 8 | ||||
| N2 | 12 | 9 | 3 | 11 | 1 | 9 | 3 | ||||
| Metastasis stage | |||||||||||
| M0 | 120 | 74 | 46 | 0.637 | 91 | 29 | 0.969 | 92 | 28 | 0.272 | |
| M1 | 4 | 2 | 2 | 3 | 1 | 4 | 0 | ||||
| Final AJCC7 stage group | |||||||||||
| Stage I | 6 | 5 | 1 | 0.552 | 6 | 0 | 0.552 | 3 | 3 | 0.263 | |
| Stage II | 64 | 37 | 27 | 47 | 17 | 51 | 13 | ||||
| Stage III | 49 | 32 | 17 | 37 | 12 | 38 | 11 | ||||
| Stage IV | 4 | 2 | 2 | 3 | 1 | 4 | 0 | ||||
Statistically significant differences are marked in bold and underlined font (p < 0.05).
PD-L2 overexpression independently associates with worse overall survival in CRC
To clarify the association between PD-L2 expression and the overall survival of CRC patients, we first applied the univariate Kaplan Meier (log rank) model to compare patient survival after surgery (Fig. 3A). As a result, patients expressing higher PD-L2 in CRC tumor cells exhibited significantly shorter overall survival than those with lower PD-L2 expression (mean survival time 46.3 vs. 69.1 mo; p = 0.0004). Interestingly, in both earlier CRC (AJCC stage I–II) and advanced CRC (stage III–IV), higher PD-L2 expression associated with worse overall survival (p = 0.012, and p = 0.002, Fig. 3B and C). Furthermore, we examined the prognostic effect of PD-L2 expression by multivariate COX regression model that included AJCC stage, gender, age, tumor grade (differentiation), and PD-L2 expression patters (expression in tumor cells, membranous localization, and immune cell positivity). As a result, higher PD-L2 expression in tumor cells (hazard ratio = 2.778, 95% confidence interval [CI] = 1.668–4.627; p < 0.0001) and AJCC stage (hazard ratio = 2.901, 95% CI 1.874–4.490; p < 0.0001) were the only factors that remained in the forward conditional model, suggesting their independent prognostic effects (Fig. 3D and E). However, membranous localization of PD-L2 and inflammatory cell positivity did not show significant association with OS in CRC in both multivariate (Fig. 3D) and univariate (Fig. S1A and B) models.
Figure 3.
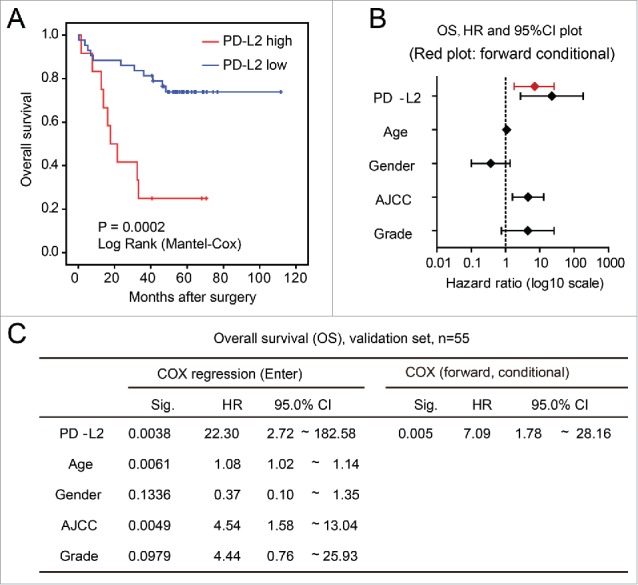
Validation of the association between worse overall survival and PD-L2 overexpression in tumor cells. (A) Kaplan–Meier (log rank) analysis showing the survival curves of patients stratified by PD-L2 expression (p value shown). (B) and (C) The multivariate COX regression model for the overall survival of CRC patients. The hazard ratios and 95% confidence interval (CI) of different factors in the COX regression model are shown in (B), with results of forward stepwise model marked in red. The detailed parameters in the model are shown in (C).
To test prognostic effect of PD-L2 in an independent data set, we obtained the PD-L2 mRNA expression data (measured by microarray) in CRCs from a published study with prognostic data.22 When CRC patients were stratified according to PD-L2 expression, PD-L2 overexpression significantly associated with worse overall survival (mean survival time 27.1 vs. 88.9 mo; p = 0.0002, Fig. 4A). In the multivariate COX regression model that included PD-L2 expression, age, gender, AJCC, and tumor grade (differentiation), only PD-L2 was remained in the forward conditional model (hazard ratio = 7.09, 95% CI 1.78–28.16; p = 0.005), suggesting its independent and strong prognostic effect for overall survival in CRC (Fig. 4B and C).
Figure 4.
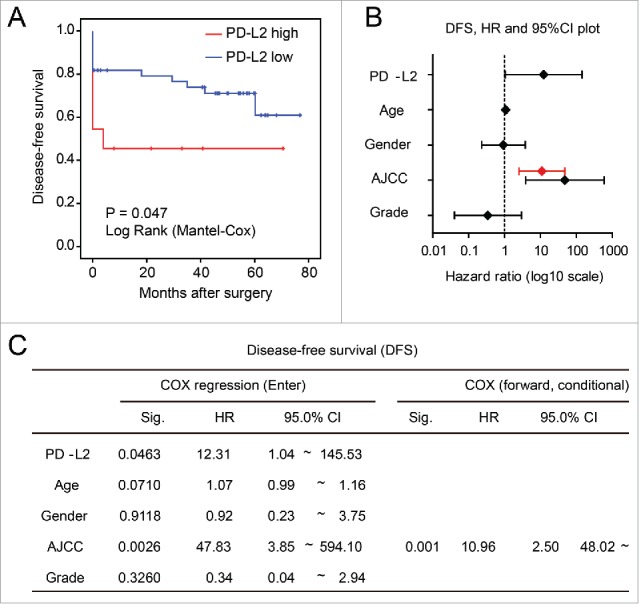
Association between worse disease-free survival (DFS) and overexpression of PD-L2 in tumor cells. (A) Univariate analysis of DFS in patients stratified according to the PD-L2 expression level. The p value is shown. (B) and (C) The multivariate COX regression model for the DFS of CRC patients, with hazard ratios plotted in (B). The hazard ratio and 95%CI in the forward stepwise model is plotted in red. Detailed model parameters in the COX regression models are shown in (C).
PD-L2 expression and disease-free survival (DFS) in CRC
To assess whether PD-L2 overexpression associates with earlier cancer relapse in CRC, we compared the disease-free survival of patients expressing high/low level of PD-L2 mRNA as determined by microarray in the GSE17537 data set. The univariate Kaplan–Meier model revealed significantly shorter DFS for patients with higher PD-L2 expression than those expressing low levels of PD-L2 (p = 0.047, Fig. 5A). Accordingly, multivariate COX regression (enter model) supported this association after adjustment of other factors such as age, gender, tumor grade, and AJCC stage (p = 0.046, Fig. 5B and C). However, in the COX regression using forward stepwise entry method (forward conditional), AJCC stage was the only significant factor remained in the model. These findings suggest that PD-L2 expression may moderately accelerate cancer relapse or metastasis and strongly associates with the refractory phenotypes of relapsed tumor and cause lethality.
Figure 5.
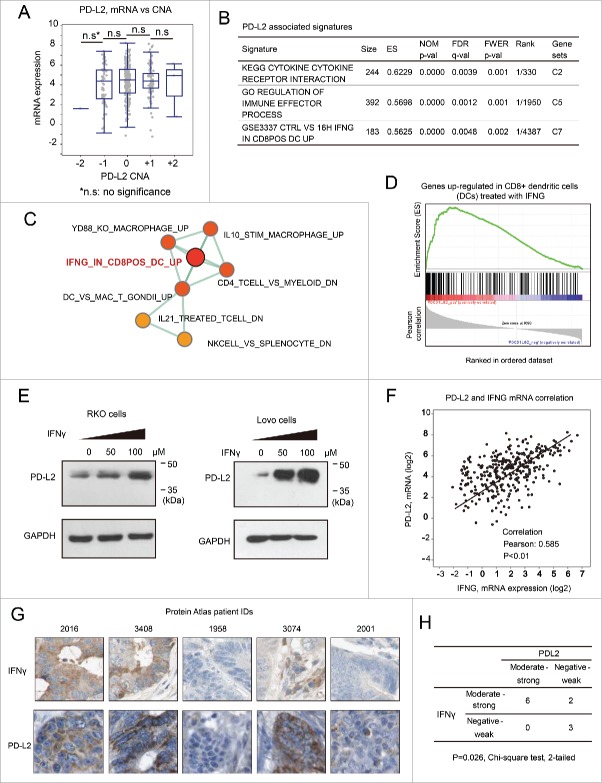
PD-L2 expression is inducible by IFNγ in colorectal cancer cells. (A) The relationship between gene copy number alterations of PD-L1/PD-L2 and their mRNA expression levels. No significant difference was found between the mRNA levels in tumors bearing different types of copy number alterations (two-sided student t-test). (B) The molecular signatures associated with PD-L2 in CRC as suggested by GSEA analysis. The C2 (curated gene sets), C5 (Gene Ontology gene sets), and C7 (immunologic signatures) gene sets were, respectively, used as reference gene sets, and the top hits are provided. (C) Immunologic (C7) signatures that associated with PD-L2 expression as determined by GSEA analysis. The network plots represent “enrichment maps” generated by Cytoscape program according to the similarity between the gene sets. Relatively more significant results are marked in red, with less significant gene sets in yellow. (D) The GSEA plot for the signature “IFNG_IN_CD8POS_DC_UP,” which represents a set of genes that can be upregulated in dendritic cells by treatment with IFNγ. (E) Western Blot using specific antibody for PD-L2 revealed significant upregulation of PD-L2 by treatment of IFNγ. The human colorectal cancer RKO and Lovo cells were incubated with IFNγ at the indicated concentrations for 12 h, followed by cell lysis and Western Blot analysis. GAPDH was detected as loading control. (F) Correlation between the mRNA expression of PD-L2 and IFNγ (IFNG) according to the microarray data of TCGA colorectal cancer cohort. (G) Immunohistochemistry staining of PD-L2 and IFNγ in the tissue specimen of the same patients, according to the Human Protein Atlas data set. Representative images in each column correspond to the same patient. (H) Statistical results of PD-L2 and IFNγ expression correlation using the Chi-square test. The Human Protein Atlas CRC subjects (with the IHC results of both PD-L2 and IFNγ) were included in the analysis, and the positivity of IFNγ was based on its expression in either the cancer cells or adjacent immune cells (higher expression is considered representative).
PD-L2 expression is strongly inducible by IFNγ in CRC cells
To assess the mechanism for PD-L2 differential expression in CRC, we first analyzed the effects of DNA copy number alteration (CNA) on PD-L2 expression. Although PD-L2 sits in a genomic region with considerable CNAs, the mRNA expression level of PD-L2 did not display significant association with CNA (Fig. 5A). To identify the signaling pathways that may control PD-L2 expression, we performed gene set enrichment analysis (GSEA) among different collections of gene sets (C2, curated gene sets; C5, Gene Ontology gene sets, and C7, immunologic signatures) based on the CRC data set of The Cancer Genome Atlas (TCGA). As a result, the C2 and C5 signatures were consistent with the known roles of PD-L2, being “cytokine: cytokine receptor interaction” and “regulation of immune effector process,” respectively (Fig. 5B). Among all the analyzed 4,872 immunologic signatures, PD-L2 most significantly associated with a gene set termed “genes upregulated in dendritic cells treated with interferon-γ (IFNγ)” (Fig. 5B–D), suggesting a potential role of IFNγ in regulating PD-L2 in CRC. Previous studies suggest that PD-L1 is strongly inducible by IFNγ, but PD-L2 only weakly respond to this cytokine in immune cells.21 It remains unknown whether PD-L2 expression may response to this cytokine in CRC cells. To test this, human CRC RKO and Lovo cells were treated with different concentrations of IFNγ (0, 50, or 100 μM) for 12 h, followed by Western Blot using antibody for PD-L2. Resultantly, the expression of PD-L2 was substantially induced by IFNγ in these CRC cells, displaying a dose-dependent effect (Fig. 3D). The extent of PD-L2 induction by IFNγ was highly comparable to that of PD-L1 (Fig. 3E). We analyzed the relationship between PD-L2 and IFNγ mRNA expression in the microarray data set of TCGA (CRC) cohort, and found significant positive correlation between the expression levels of both genes (Fig. 5F). Consistently, the analysis of PD-L2 expression in the Human Protein Atlas immunofluorescence data set also suggested that cancer cell expression of PD-L2 in CRC tissues significantly associated with positive expression of IFNγ in cancer cells or immune cells within the tumor site (p = 0.026, Chi-square test, Fig. 5G and H). These results consistently support the notion that PD-L2 expression in CRC cells may be induced by IFNγ expressed in CRC cells or adjacent immune cells.
PD-L2 protein is stabilized by glycosylated in CRC cells
It has recently been reported that PD-L1 is expressed in a glycosylated form,23 but it remains unclear whether PD-L2 may be glycosylated. Prediction with the N-GlycoSite tool suggested that multiple sites in PD-L2 that may be modified by N-linked glycosylation to a high fraction (Fig. 6A). The endogenous PD-L2 protein in human colorectal RKO and Lovo cells displayed molecular weight of ∼45 kDa in Western Blot assay (Fig. 2B), being greater than the predicted size of PD-L2. Treatment with different concentrations (0, 10, and 20 μM) of tunicamycin, a specific inhibitor of N-linked glycosylation, caused a decrease in molecular weight (MW) by ∼15 kDa in both CRC cell lines (Fig. 6B). The amount of total PD-L2 protein expression (quantified by the summary of both MW species) also decreased upon the treatment with tunicamycin (Fig. 6B). These changes resembled the deglycosylation process of PD-L1, which induced a comparable decrease in the molecular weight and expression level (Fig. 6B). It has been proposed that glycosylation of PD-L1 stabilizes this membrane protein by suppression of ubiquitination and degradation.23 Therefore, the glycosylation of PD-L2 may represent a crucial mechanism for the regulation of PD-L2 protein expression in CRC cells.
Figure 6.
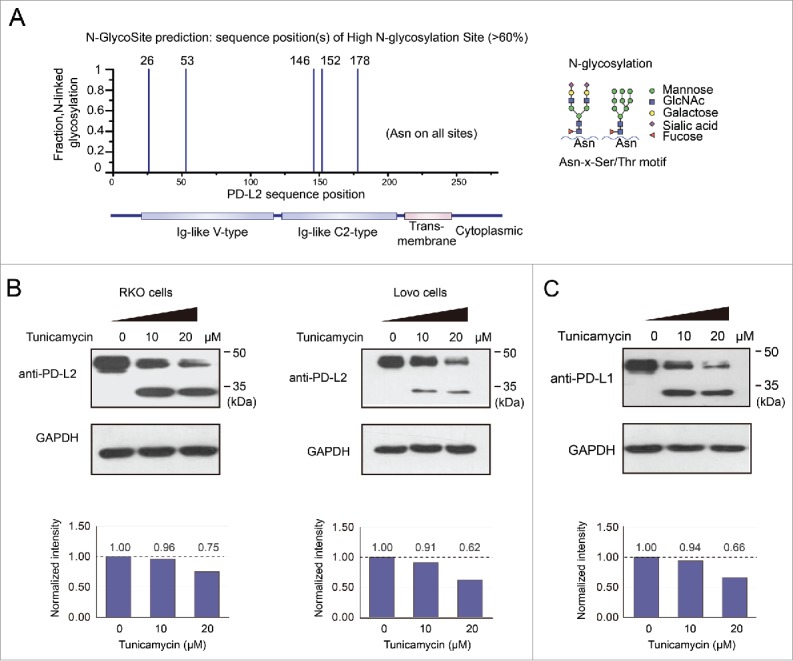
Glycosylation contributes to the stabilization of PD-L2 in colorectal cancer cells. (A) The predicted high glycosylation sites of PD-L2 by the N-GlycoSite algorithm. The x-axis indicates the amino acid position of the PD-L2 protein sequence, and the y-axis shows the predicted ratio of glycosylation in different Asparagine residues of PD-L2. The schematic diagram on the right represents the main subtypes of glycosylation found in PD-L1, the homologous protein of DP-L2. (B) Suppression of glycosylation decreased PD-L2 expression level in colorectal cancer cells. The inhibitor of N-linked glycosylation tunicamycin was used to treat Lovo cells at the indicated concentrations for 12 h, and the antibody for PD-L2 was used to detect the expression of PD-L2. Note the bands near 45 kDa represent glycosylated species, and the bands below 35 kDa were not glycosylated. The total levels of PD-L2 were quantified by the summary of both bands and normalization by GAPDH (quantification plots shown in the lower panels).
Figure 2.
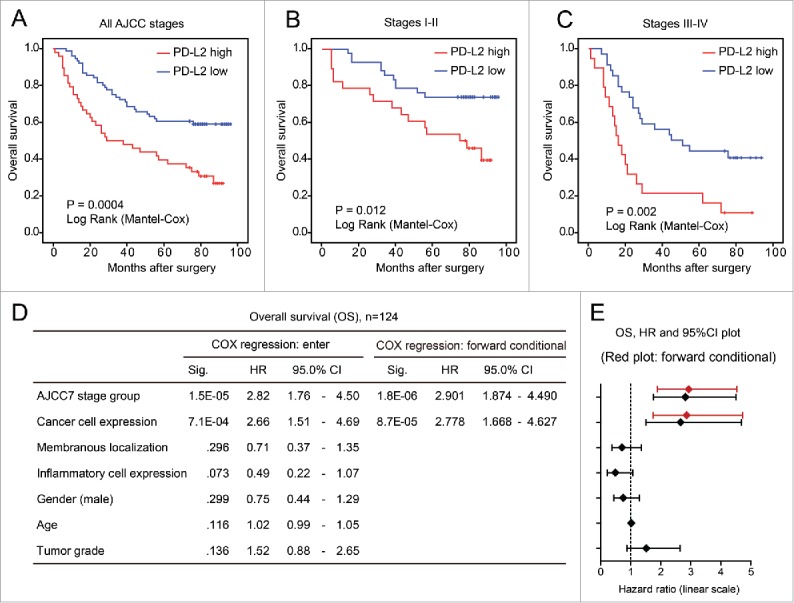
PD-L2 overexpression associates with poor overall survival of patients with colorectal cancer. (A–C) The univariate Kaplan–Meier analysis showing the survival curves of patients with high/low PD-L2 expression. The association between PD-L2 overexpression and poor overall survival is significant in (A) all patients, (B) earlier CRC patients with AJCC stage I–II, and (C) late CRC patients. The p values are indicated in the graph. (D) The multivariate COX regression model indicating the association of different factors with overall survival of CRC patients. The results of forward stepwise model are shown on the right. (E) The plot shows the hazard ratios and 95% confidence interval (CI) of different factors in the COX regression model. The result of forward stepwise model (forward conditional) is marked in red.
Discussion
Being the first study demonstrating a link between tumor cell expression of PD-L2 and poor survival of patients with colorectal cancer, our findings highlight the prognostic and therapeutic significances of PD-L2 in colorectal cancer. The regulation of PD-L2 by IFNγ induction and glycosylation-dependent stabilization may at least partially contribute to the differential expression of PD-L2 in CRC, and therefore provide potential venues for controlling PD-L2 expression in CRC.
Being the homologous ligands for PD-1 that regulate immune tolerance, PD-L1 and PD-L2 may play overlapping but differential roles in various lineages, tissues, and cancers.24 Previous studies have suggested that PD-L1 is expressed in a broad range of cancers and may help cancer cells to evade immune responses.25 Accordingly, antibodies against PD-L1 have been used to combat multiple types of cancers in clinical trials.26-28 However, the potential roles and expression of PD-L2 in various cancers have relatively less studied. Our results suggest that PD-L2 is differentially expressed in CRC, with moderate-strong staining of PD-L2 in tumor cells found in approximately 40% CRC subjects. In contrast, PD-L1 has been reported to be expressed in only 12% CRCs that may arise from the serrated neoplasia pathway. The expression of PD-L2 was strongly inducible by IFNγ in CRC cells, and such regulatory relationship was supported by the significant correlation of their mRNA and protein expression levels in CRC tissues. Moreover, suppression of glycosylation caused a shift in molecular weight of PD-L2 and reduced the expression of PD-L2 protein in CRC cells, supporting the notion that glycosylation may stabilize PD-L2 in CRC cells. These findings collectively demonstrate that PD-L2 is positively expressed in a considerable subset of CRCs, which is under the control of IFNγ induction and glycosylation-mediated stabilization.
The significant association between PD-L2 expression and poor survival of CRC has been found in both our samples and an independent data set. Such association remained significant in the multivariate model including other factors such as age, sex, AJCC stage, and tumor differentiation. In contrast, PD-L1 has been reported to lack independent prognostic significance in CRC.17 The difference in the prognostic effects of PD-L2 and PD-L1 in CRC may be relevant to their overlapping but distinct molecular functions. Although both proteins share the receptor PD-1, alternative secondary receptors for PD-L1 (CD80) and PD-L2 (RGMb) have been reported.29 The role of PD-L2 in suppressing T-cell responses has been supported by numerous studies, but the exquisite effects of PD-L2 still seemed to vary, potentially due to differences in the investigated tissue types and heterogeneity in the immune background. It is yet to be clarified why the expression of PD-L1 and PD-L2 may display such differences in CRC, and what transcription factors may dominantly drive the expression of PD-L1 and PD-L2 in CRC. It is also unclear to what extent the subcellular transportation and degradation pathways of PD-L1 and PD-L2 may overlap in CRC. Nonetheless, our results strongly suggest that PD-L2 may have more widespread and significant roles than PD-L1 in colorectal cancer. Accordingly, more attention should be paid to PD-L2 while evaluating molecular markers for patient selection with immune checkpoint blockade therapy.
The expression of PD-L2 in CRC cells may be induced by IFNγ produced by tumor cells or adjacent immune cells, suggesting an adverse effect of IFNγ in the tumor microenvironment. Although the functions of IFNγ in cancers have been largely controversial, an adverse effect of IFNγ has been reported in CRC. Accumulation of LAP+CD4+ T cells in the tumor microenvironment was found to produce significantly higher levels of IFNγ and associate with tumor progression in patients with CRC.30 Our results suggest that, at least in the context of PD-L2 regulation, IFNγ may play an unfavorable role in CRC. Of course, these may not preclude other mechanisms that may constitute a complex signaling network to express the outcome of IFNγ under various conditions.
In conclusion, PD-L2 overexpression in CRC cells, under the regulation by IFNγ and glycosylation, associates with poor patient survival. These findings highlight PD-L2 as a promising therapeutic target in CRC, and suggest potential routes to control PD-L2 expression in CRC cells.
Materials and methods
Cell culture
The human CRC HCT116 cells were maintained in RPMI-1640 medium (Hyclone, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, USA, Australian origin), and cultured in a humidified incubator at 37°C under 5% CO2. Cells were plated at 40% confluence in six-well tissue plates for 24 h, followed by treatment of IFNγ at different concentrations (0, 50, and 100 μM) for 12 h. In the glycosylation blockade test, tunicamycin was used to treat cells at different concentrations (0, 10, and 20 μM) for 12 h.
Western blot
Cells treated by IFNγ or tunicamycin were harvested at indicated time point, and 30–50 μg of proteins were then used for electrophoresis. After transferred to nitrocellulose or PVDF membranes, proteins were blocked in 5% non-fat milk for 1 h at room temperature, and then incubated with rabbit anti-PD-L2 (Abcam, USA) at 1:800 dilution overnight at 4°C. Membranes were next washed five times with Tris-buffered saline with Triton (TBST) and bound with the horseradish peroxidase-conjugated anti-rabbit secondary antibodies at 1:5,000 dilution for 1 h at room temperature. An enhanced chemiluminescence system (Pierce Biotechnology, Rockford, IL, USA) was applied for detection of the bound secondary antibodies.
Sample preparation and immunohistochemistry
Tissue microarrays containing 180 CRC tissue specimens were purchased from BioChip (Shanghai, China), with informed consent obtained from patients. The study has been approved by the ethical committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University. IHC was performed as described previously.2 Briefly, the tissue microarray slides were deparaffinized in xylene and rehydrated using a graded series of ethanol. Heat-induced antigen retrieval was performed in high-pH antigen retrieval buffer (Dako Cytomation, Glostrup, Denmark). Endogenous peroxidase was blocked by incubation in 3% H2O2 for 5 min. After blocking with goat serum in 5% BSA, anti-PD-L2 (Abcam, USA) was incubated with the tissue slides overnight at 4°C. Following secondary antibody incubation, these sections were visualized by the HRP-labeled polymer method. Immunostained sections were counterstained with haematoxylin, dehydrated in ethanol and cleared in xylene. A total of 124 cases with complete clinicopathological data were successfully detected for PD-L2 expression by IHC (criteria: without loss of tissue during the process; both tumor cells and para-tumor sites could be observed). The staining intensity of PD-L2 in tumor cells was assigned with scores 0 (negative), 1(weak), 2 (moderate), and 3 (strong). The membranous distribution pattern of PD-L2 and the expression of PD-L2 in inflammatory cells were evaluated as weak or strong.
Statistical analysis
Comparisons between groups were performed using two-sided Student t-test (for sex and tumor volume), or chi-square test (for histological type and other factors). The survival curves of patients with high and low expression of PD-L2 were compared using the log rank (Kaplan–Meier) test, which is constructed by computing the observed and expected number of events at each observed event time and then adding these to obtain an overall summary across all time points. The multivariate COX regression model was used to analyze the effects of PD-L2 expression, AJCC stage, and tumor grade on patient survival. Both enter model and the forward stepwise conditional model were applied to evaluate the prognostic effects of above-mentioned factors. A p value of less than 0.05 was considered statistically significant, and all statistical tests were two-sided.
Validation data and pretreatment
The expression level of PD-L2 was extracted from the microarray data set (probe 220049_s_at) that is available from the GEO database (accession GSE17537), which was developed to evaluate the association between gene expression profile and prognosis of CRC.31 All patients were included in the survival analysis.
Gene set enrichment analysis
GSEA is a method of analyzing and interpreting microarray and such data using biologic knowledge.32 GSEA was performed using GSEA version 2.3 from the Broad Institute at MIT, based on TCGA data set including 246 CRC samples. GSEA first generated an ordered list of all genes according to their correlation with PD-L2 expression, and then a predefined gene set (signature of gene expression upon perturbation of certain cancer-related gene) receives an enrichment score (ES), which is a measure of statistical evidence rejecting the null hypothesis that its members are randomly distributed in the ordered list. Parameters used for the analysis were as follows. The indicated gene sets were used for running GSEA and 1,000 permutations were used to calculate p-value and permutation type was set to gene. The maximum and minimum sizes of gene sets were set in default. The “Metric for ranking genes” was set to Pearson Correlation. All other basic and advanced fields were set to default.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This project was supported by grants from the National Key Research&Development (R&D) Plan (2016YFC0906000, 2016YFC0906002); National Natural Science Foundation of China (81572326, 81322036, 81272383, 81602518, 81502015, 81572303, 81530072, 81421001, 81320108024); Top-Notch Young Talents Program of China (ZTZ2015–48); Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152514); “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (15SG16); and National Key Technology Support Program (2015BAI13B07).
References
- 1.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 2016; 13:691-706; PMID:27848961; https://doi.org/ 10.1038/nrgastro.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Qian J, Hu Y, Kong X, Chen H, Shi Q, Jiang L, Wu C, Zou W, Chen Y et al.. ArhGAP30 promotes p53 acetylation and function in colorectal cancer. Nat Commun 2014; 5:4735; PMID:25156493; https://doi.org/ 10.1038/ncomms5735 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3):177-93; PMID:28248415; https://doi.org/ 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 4.Bever KM, Le DT. An expanding role for immunotherapy in colorectal cancer. J Natl Compr Cancer Netw 2017; 15:401-10; PMID:28275038; https://doi.org/ 10.6004/jnccn.2017.0037 [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16:275-87; PMID:27079802; https://doi.org/ 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obenauf AC, Zou Y, Ji AL, Vanharanta S, Shu W, Shi H, Kong X, Bosenberg MC, Wiesner T, Rosen N et al.. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015; 520:368-72; PMID:25807485; https://doi.org/ 10.1038/nature14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450-61; PMID:25858804; https://doi.org/ 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, Moriceau G, Hong A, Dahlman KB, Johnson DB et al.. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 2015; 162:1271-85; PMID:26359985; https://doi.org/ 10.1016/j.cell.2015.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li G, Liang J, Hu H, Liu Y, Zhang W et al.. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology 2016; 5:e1196310; PMID:27999734; https://doi.org/ 10.1080/2162402X.2016.1196310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509-20; PMID:26028255; https://doi.org/ 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; https://doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan S, Zhang CWH, Gao GF. Seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Signal Transduct Targeted Ther 2016; 1:16029; https://doi.org/ 10.1038/sigtrans.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obeid JM, Erdag G, Smolkin ME, Deacon DH, Patterson JW, Chen L, Bullock TN, Slingluff CL. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology 2016; 5:e1235107; PMID:27999753; https://doi.org/ 10.1080/2162402X.2016.1235107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta HB, Clark CA, Yuan B, Sareddy G, Pandeswara S, Padron AS et al.. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct Targeted Ther 2016; 1:16030; https://doi.org/ 10.1038/sigtrans.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to Anti–PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; https://doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; https://doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, Wang X, Timmons CL, Hu J, Liu B et al.. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PloS One 2013; 8:e65821; PMID:23785454; https://doi.org/ 10.1371/journal.pone.0065821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Modern Pathol 2016; 29:1104-12; PMID:27198569; https://doi.org/ 10.1038/modpathol.2016.95 [DOI] [PubMed] [Google Scholar]
- 19.Inaguma S, Lasota J, Wang Z, Felisiak-Golabek A, Ikeda H, Miettinen M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Modern Pathol 2017; 30:278-85; PMID:27813511; https://doi.org/ 10.1038/modpathol.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi M, Roemer MG, Chapuy B, Liao X, Sun H, Pinkus GS, Shipp MA, Freeman GJ, Rodig SJ. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol 2014; 38:1715-23; PMID:25025450; https://doi.org/ 10.1097/PAS.0000000000000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, Sicinska E, Goldberg MS, Freeman GJ, Rodig SJ et al.. Epithelial PD-L2 expression marks Barrett's esophagus and esophageal adenocarcinoma. Cancer Immunol Res 2015; 3:1123-9; PMID:26081225; https://doi.org/ 10.1158/2326-6066.CIR-15-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE et al.. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 2010; 138:958-68; PMID:19914252; https://doi.org/ 10.1053/j.gastro.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T et al.. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun 2016; 7:12632; PMID:27572267; https://doi.org/ 10.1038/ncomms12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habicht A, Kewalaramani R, Vu MD, Demirci G, Blazar BR, Sayegh MH, Li XC. Striking dichotomy of PD-L1 and PD-L2 pathways in regulating alloreactive CD4(+) and CD8(+) T cells in vivo. Am J Transplant 2007; 7:2683-92; PMID:17924994; https://doi.org/ 10.1111/j.1600-6143.2007.01999.x [DOI] [PubMed] [Google Scholar]
- 25.Laurent C, Charmpi K, Gravelle P, Tosolini M, Franchet C, Ysebaert L, Brousset P, Bidaut A, Ycart B, Fournié JJ. Several immune escape patterns in non-Hodgkin's lymphomas. Oncoimmunology 2015; 4:e1026530; PMID:26405585; https://doi.org/ 10.1080/2162402X.2015.1026530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, Wei H, Wang X, Bai Y, Wang P, Wu J, Jiang X, Wang Y, Cai H, Xu T et al.. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov 2017; 3:17004; PMID:28280600; https://doi.org/ 10.1038/celldisc.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickmott L, De La Pena H, Turner H, Ahmed F, Protheroe A, Grossman A, Gupta A. Anti-PD-L1 atezolizumab-induced autoimmune diabetes: a case report and review of the literature. Targeted Oncol 2017; 12(2):235-41; PMID:28255845; https://doi.org/ 10.1007/s11523-017-0480-y [DOI] [PubMed] [Google Scholar]
- 28.Rao M, Valentini D, Dodoo E, Zumla A, Maeurer M. Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis 2017; 56:221-8; PMID:28163164; https://doi.org/ 10.1016/j.ijid.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 29.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH et al.. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med 2014; 211:943-59; PMID:24752301; https://doi.org/ 10.1084/jem.20130790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahalingam J, Lin YC, Chiang JM, Su PJ, Fang JH, Chu YY, Huang CT, Chiu CT, Lin CY. LAP+CD4+ T cells are suppressors accumulated in the tumor sites and associated with the progression of colorectal cancer. Clin Cancer Res 2012; 18:5224-33; PMID:22879386; https://doi.org/ 10.1158/1078-0432.CCR-12-0211 [DOI] [PubMed] [Google Scholar]
- 31.Freeman TJ, Smith JJ, Chen X, Washington MK, Roland JT, Means AL, Eschrich SA, Yeatman TJ, Deane NG, Beauchamp RD. Smad4-mediated signaling inhibits intestinal neoplasia by inhibiting expression of beta-catenin. Gastroenterology 2012; 142:562-71 e2; PMID:22115830; https://doi.org/ 10.1053/j.gastro.2011.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croken MM, Qiu W, White MW, Kim K. Gene set enrichment analysis (GSEA) of toxoplasma gondii expression datasets links cell cycle progression and the bradyzoite developmental program. BMC Genom 2014; 15:515; PMID:24962434; https://doi.org/ 10.1186/1471-2164-15-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.