Abstract
In 2006, two rotavirus vaccines were licensed. We summarize the impact of rotavirus vaccination on hospitalizations and deaths from rotavirus and all-cause acute gastroenteritis (AGE) during the first 10 years since vaccine licensure, including recent evidence from high child mortality countries. We used standardized guidelines (PRISMA) guidelines to identify observational evaluations of rotavirus vaccine impact among children <5 years of age that presented at least 12 months of pre- and post-vaccine introduction surveillance data. We identified 57 articles from 27 countries. Among children <5 years of age, the median percent reduction in AGE hospitalizations was 38% overall and 41%, 30%, 46% in low, medium, and high child mortality countries, respectively. Hospitalizations and ED visits due to rotavirus AGE were reduced by a median of 67% overall and 71%, 59%, and 60% in low, medium and high child mortality countries, respectively. Implementation of rotavirus vaccines has substantially decreased hospitalizations from rotavirus and all-cause AGE.
Keywords: Rotavirus vaccine, diarrhea, rotavirus
Introduction
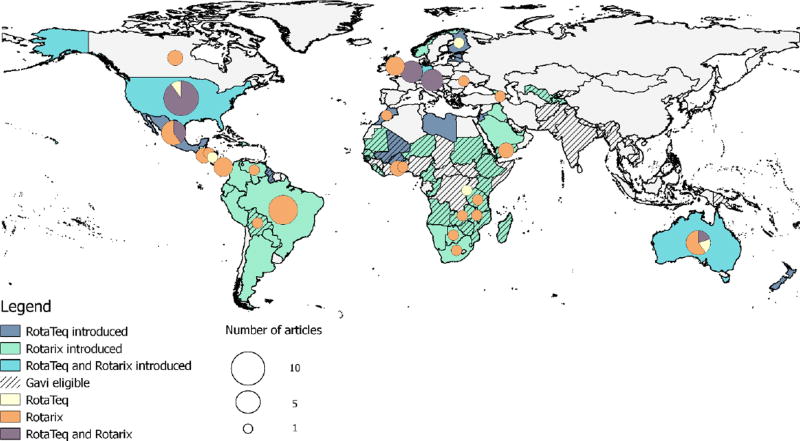
Rotavirus accounts for about 40% of acute gastroenteritis (AGE) hospitalizations in children <5 years of age worldwide [1]. Since two live, oral rotavirus vaccines for infants were licensed in 2006, more than 80 countries have introduced rotavirus vaccine into their national routine immunization programs (Figure 1). Rotarix (RV1, GSK Biologics, Rixensart, Belgium) is a two-dose monovalent human rotavirus vaccine and RotaTeq (RV5, Merck and Co., Westpoint, PA) is a three-dose pentavalent bovine-human reassortant rotavirus vaccine. These two rotavirus vaccines, as well as three nationally licensed rotavirus vaccines, are available subnationally and on the private market in additional countries.
Figure 1.
Map of rotavirus vaccine national introductions and estimates of rotavirus vaccine impact by individual countries.
Initially, in 2006, the World Health Organization (WHO) recommended rotavirus vaccine introductions only in high and middle income countries in regions with available efficacy data and with strong existing vaccination delivery infrastructure [2–4]. Early introducing countries in the Americas, Europe, and Australia reported large declines in rotavirus diarrhea and all-cause AGE hospitalizations and mortality shortly following national vaccine introduction [5–7]. With the availability of additional efficacy data from low income countries in Africa and Asia, WHO extended their routine rotavirus vaccine recommendation to all countries in 2009 [8, 9].
Previous reviews have summarized the impact of rotavirus vaccines on hospitalizations and deaths from diarrhea, but the data have been limited to a few years immediately following vaccine introduction primarily from the early introducing high and middle income countries [5–7]. We summarize the impact of rotavirus vaccination on hospitalizations and deaths from rotavirus and AGE during the first 10 years since vaccine licensure, including recently published evidence from low income countries with high child mortality that have introduced rotavirus vaccine.
Methods
Search strategy and selection criteria
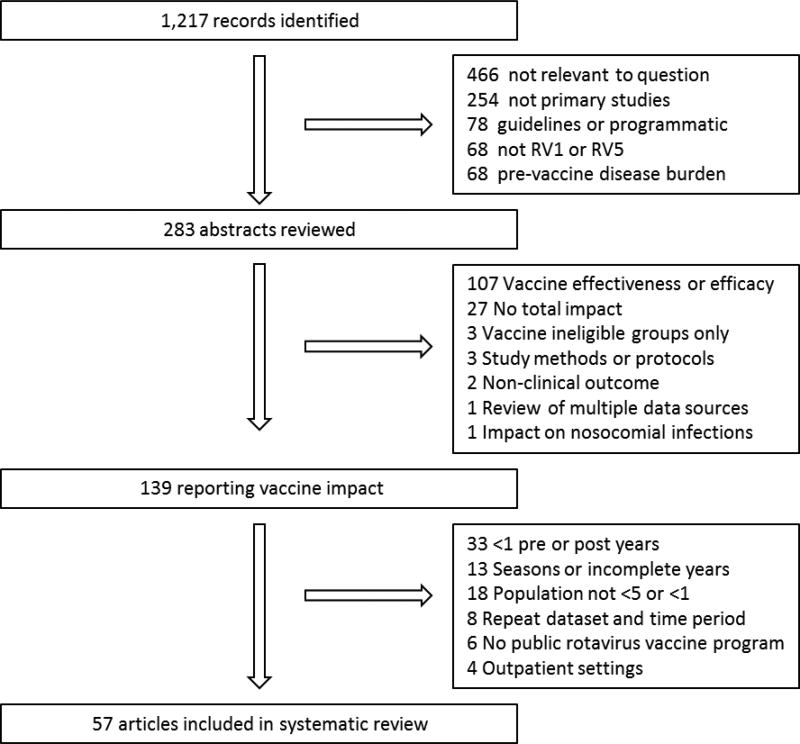
We identified articles published between January 1, 2006 and December 6, 2016 by searching for the terms “rotavirus” and either “vaccin*” or “immuni*” in the PubMed database per the PRISMA guidelines [10]. We included post-licensure, observational evaluations of rotavirus vaccine impact on rotavirus AGE or all-cause AGE among children <5 years of age in countries where RV1 or RV5 had been licensed (Figure 2). We excluded studies if they were not relevant to our question, such as those addressing safety, programmatic considerations, parent or healthcare provider knowledge attitudes and practices, laboratory methods, vaccine effectiveness, and economic impact. We excluded articles that did not present an overall impact estimates, including those that compared subnational populations or those that presented rotavirus strain-specific impact. We also excluded articles that included a pediatric population but did not specify the age group. We included studies that presented at least 12 months of pre- and post-vaccine introduction surveillance data. When updated analyses from the same dataset were available in separate publications, we included all peer-reviewed articles.
Figure 2.
Article review and selection process.
Data abstraction
We developed a standardized data abstraction form, and two reviewers (EB and CJ) independently abstracted information on vaccine licensure and national rotavirus vaccine introduction, the type and duration of surveillance, the number and age groups of participants, and reported outcomes; discrepancies were reconciled. We developed a database using Microsoft Excel 2013, which included information related to annual changes in the percentage of rotavirus-positive laboratory specimens as well as changes in rotavirus AGE and all-cause AGE hospitalizations and mortality. As this review focuses on the overall impact of rotavirus vaccines, we did not distinguish between the impact of RV1 and RV5. Using UNICEF 2014 <5 child mortality rates, we created three mortality strata where low mortality countries were defined as those with rates in the lowest quartile, medium mortality countries as those in the second lowest quartile, and high mortality countries as those in the highest two quartiles [11]. The mortality rate for children <5 years of age ranged from 1.9 to 7 deaths per 1,000 live births in the ‘low mortality’ strata, from 8 to 17 in the ‘medium mortality’ strata and from 18 to 157 in the ‘high mortality’ strata in 2015. Full course rotavirus vaccine coverage among infants was based on the median UNICEF estimate for the final year of the study [12].
Data Analysis
We performed these analyses using SAS 9.4 Figures were created using R v2.7, Quantum GIS v2.8, and Microsoft Excel. When individual years were presented, estimates were included starting with the first full year after introduction, referred to as ‘year 1’.
Results
We included 57 articles reporting on 59 data sources from 27 countries in this analysis; two articles reported data from multiple countries (Figure 1, Supplementary Table 1). Of these data sources, 37 were from countries using RV1 only, five from countries using RV5 only, and 17 from countries using both vaccines. Of these, 53 reported results from inpatient or ED settings, 26, 13, and 14 from low, medium, and high mortality countries, respectively; 12 reported mortality results, 11 and 5 from medium and high mortality countries, respectively.
Of the 53 reports from inpatient or ED settings in countries with national rotavirus vaccine introduction, the median number of years of follow-up after vaccine introduction was 3 (range: 1, 9); the median was 3, 4, and 3 post-vaccine introduction years in low, medium, and high child mortality strata countries, respectively. Overall, the median UNICEF estimate of the full course rotavirus vaccine coverage among infants for the final year of evaluation was 83% (range: 44%, 98%); the median coverage was 77%, 83%, and 85% in low, medium, and high child mortality strata countries, respectively.
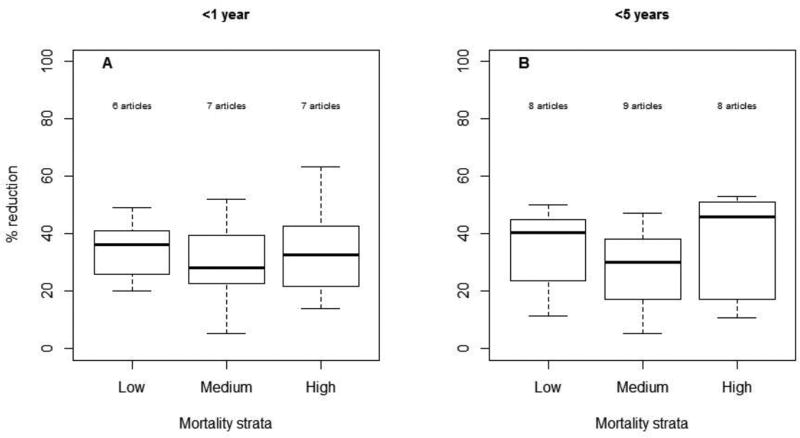
Among children <1 year of age, the median percent reduction in AGE hospitalizations and/or ED visits was 32% (range: 5, 63; n=20) overall and 36%, 28%, 33% in low (n=6), medium (n=7) and high (n=7) child mortality countries, respectively (Figure 3). Among children <5 years of age, the median percent reduction in AGE hospitalizations and/or ED visits was 38% (range: 5, 53; n=25) overall and 41%, 30%, 46% in low (n=8), medium (n=9) and high (n=8) child mortality countries, respectively.
Figure 3.
Reduction in AGE hospitalizations by age group and country's child mortality strata
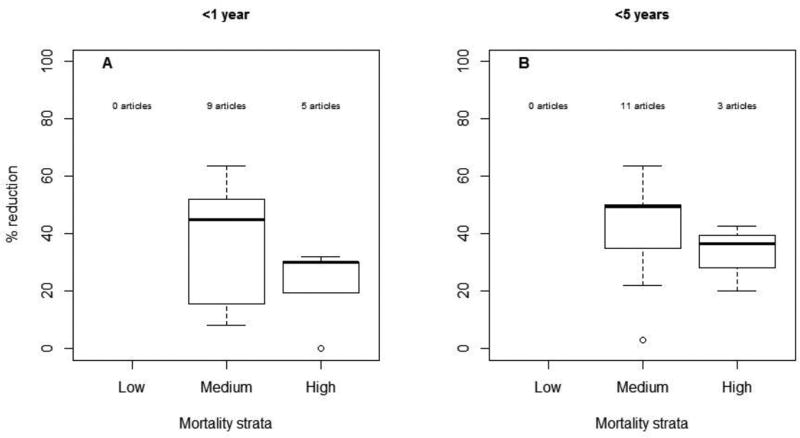
Reductions in AGE mortality were reported in medium and high child mortality countries; no estimates have been published from low child mortality countries. The median reduction in AGE mortality among children <1 year of age was 31% (range: 0, 64; n=14) overall; in medium (n=9) and high (n=5) child mortality strata countries, it was 45% and 30%, respectively (Figure 4). The median reduction in AGE mortality in children <5 years was 42% (range: 3, 64; n=14) overall; in medium (n=11) and high (n=3) child mortality strata countries, it was 50% and 36%, respectively. None of the articles examined reductions in mortality due to rotavirus specifically.
Figure 4.
Reduction in AGE deaths by age group and country's child mortality strata
Hospitalizations and ED visits due to laboratory-confirmed rotavirus AGE were reduced by a median of 80% (range: 25, 89; n=20) among children <1 year of age overall and by 80%, 78% and 46% in countries with low (n=15), medium (n=3), and high (n=2) child mortality, respectively. Among children <5 years of age, rotavirus AGE hospitalizations and ED visits were reduced by 67% (range: 18, 84; n=21) overall and by 71%, 59%, and 60% in low (n=14), medium (n=2), and high (n=5) child mortality, respectively.
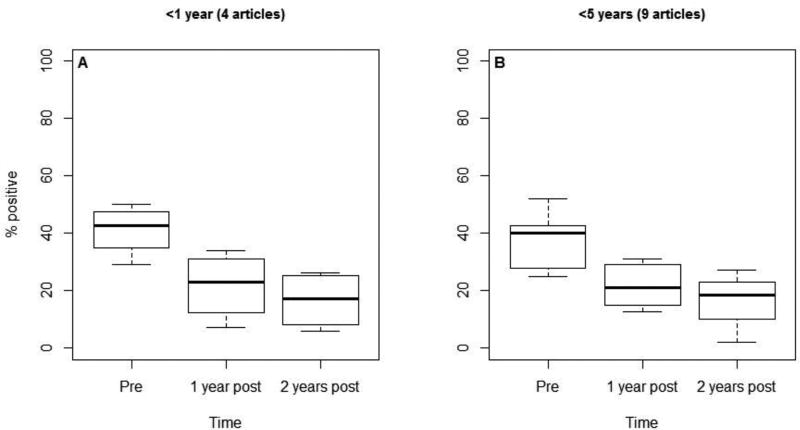
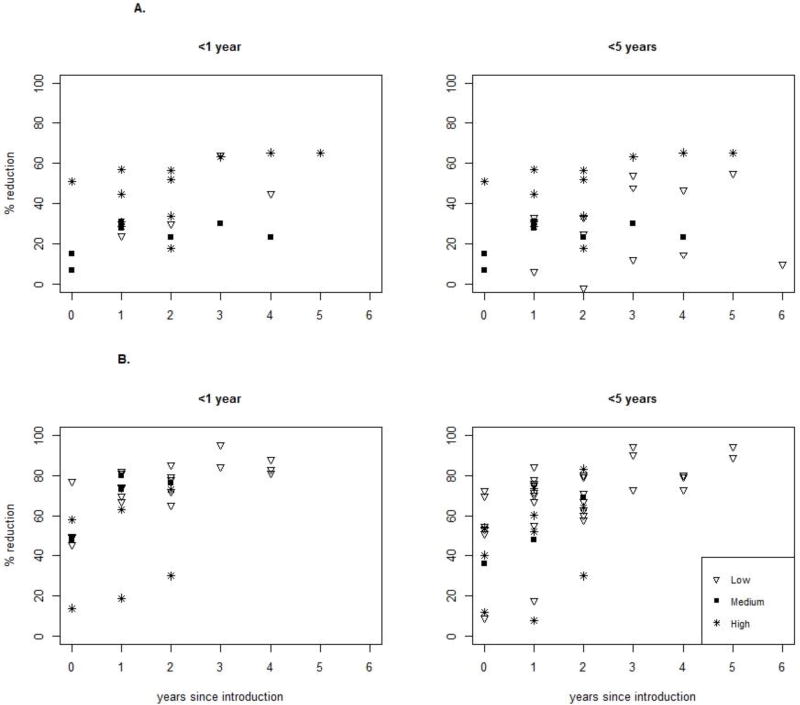
Four evaluations reported the percentage of rotavirus positive specimens among children <1 year of age over time, with 0, 1, and 3 estimates from low, medium, and high mortality strata countries, respectively. The median rotavirus positivity among children <1 year of age before vaccine introduction was 43%; it was 23% the first year and 17% the second year after introduction (Figure 5). This represents median reductions of 46% and 60%, respectively. Nine estimates of rotavirus positivity were reported among children <5 years of age over time, with 2, 2, and 5 estimates from low, medium, and high mortality strata countries, respectively. The median rotavirus positivity among children <5 years of age before vaccine introduction was 40%; it was 21% the first year and 19% the second year after introduction. This represents reductions of 48% and 53% in the median rotavirus positivity, respectively. After rotavirus vaccine introduction, we also noted an increase in the percent reduction in rotavirus hospitalizations and ED visits over time, though this was not evident in AGE hospitalizations (Figures 6A and 6B).
Figure 5.
Percentage of specimens positive for rotavirus by age group
Figure 6.
A. Reduction in AGE hospitalizations by age group and country's child mortality strata over time
B. Reduction in rotavirus hospitalizations by age group and country's child mortality strata over time
Indirect effects in older unvaccinated children
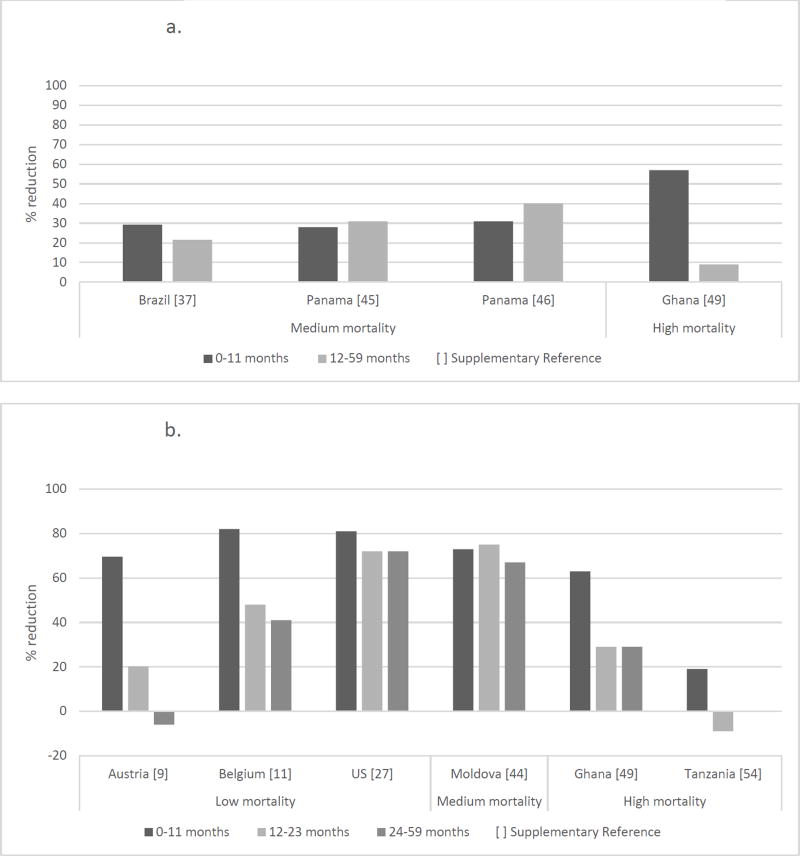
We identified four datasets that reported reductions in AGE hospitalizations in 0–11 months and 12–59 months of age subgroups in the year following rotavirus vaccine introduction (Figure 7a). In this time frame, all of the 0–11 month old children but only a very small proportion of 12–59 month old children (primarily in the 12–23 month age group) would have been eligible for vaccination. Reductions were observed in both age groups in all datasets, though these reductions were higher in the older age group than in the 0–11 month age group in two datasets from Panama. Seven articles reported reductions in rotavirus hospitalizations in the year following rotavirus vaccine introduction with 0–11 months, 12–23 months and 24–59 months of age subgroups (Figure 7b). In the US and Moldova, reductions were comparable for all three age groups. In Armenia, Belgium, and Ghana, reductions were modest in the 24–59 month age group. We saw an increase in rotavirus hospitalizations in Austria in the 24–59 month age group and in Tanzania in the 12–23 month age group.
Figure 7.
a. Reduction in AGE hospitalizations by age group in the first full year after vaccine introduction.
b. Reduction in rotavirus hospitalizations by age group in the first full year after vaccine introduction
Discussion
We found substantial reductions in rotavirus and all-cause AGE hospitalizations and AGE mortality among children <1 year of age and <5 years of age in publications from 27 countries that introduced rotavirus vaccine into their national routine immunization programs since 2006. Proportional reductions in rotavirus AGE hospitalizations were larger than reductions in all-cause AGE hospitalizations. Generally, there were minimal differences in impact between <1 year and <5 year age groups. This is likely because the highest disease burden is in the youngest children and estimates from this literature review included time periods after rotavirus vaccine introduction where most age groups among children <5 years would have been age eligible for vaccination. Similar reductions were seen across mortality strata. It is particularly encouraging to see the large impacts in low income settings given concerns about vaccine performance. Among children hospitalized for diarrhea, the percentage of specimens that tested positive for rotavirus decreased during the two years following rotavirus vaccine introduction in both age groups. In several but not all studies, we observed reductions in rotavirus and AGE hospitalizations in age groups explicitly not eligible for vaccination, indicating evidence of indirect protection. As the mortality impact and herd benefits had not been assessed in trials, so these post-licensure findings are noteworthy.
This literature review has several limitations. First, published data are limited on vaccination impact on mortality; no studies have been published from high burden settings and limited data are available from low and medium burden settings. Second, while comparable endpoints were reported, there was some variation in protocol and analysis methods in the papers. For example, some publications presented annual hospitalization reductions and others presented multi-year summaries. This variability in reporting of findings led to some sparseness in our data and reduced nuance in our results. Finally, many articles did not present specific age groups, making it difficult to assess indirect effects and potentially diluting the reported impact in our main analysis.
Our results show the marked reductions on rotavirus hospitalization and AGE hospitalizations and mortality in a range of child mortality settings since the licensure of rotavirus vaccines a decade ago. Future research is needed to address the impact of rotavirus vaccine on hospitalizations in countries in Asia, indirect impact on age groups not targeted for vaccination, particularly in high child mortality settings, and the percentage of stool specimens positive for rotavirus for additional years post-introduction to provide a more complete and nuanced description of rotavirus vaccine impact.
Supplementary Material
Acknowledgments
No funding was provided for this project;
Footnotes
Disclaimer: The authors have no funding or conflicts of interest to disclose.
The authors either do not have a commercial or other association that might pose a conflict of interest;
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
These results have not previously been presented;
References
- 1.Centers for Disease C, Prevention. Rotavirus surveillance--worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep. 2008;57(46):1255–7. [PubMed] [Google Scholar]
- 2.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.Organization WH. Rotavirus vaccines: WHO position paper- January 2013. Weekly epidemiological review. 2013;88(5):49–64. [Google Scholar]
- 5.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 Suppl):S56–60. doi: 10.1097/INF.0b013e3181fefdc0. [DOI] [PubMed] [Google Scholar]
- 6.Tate JE, Patel MM, Steele AD, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines. 2010;9(4):395–407. doi: 10.1586/erv.10.17. [DOI] [PubMed] [Google Scholar]
- 7.Desai R, Oliveira LH, Parashar UD, Lopman B, Tate JE, Patel MM. Reduction in morbidity and mortality from childhood diarrhoeal disease after species A rotavirus vaccine introduction in Latin America - a review. Mem Inst Oswaldo Cruz. 2011;106(8):907–11. doi: 10.1590/s0074-02762011000800002. [DOI] [PubMed] [Google Scholar]
- 8.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Reported estimates of rotavirus last dose coverage. [Accessed January 9, 2017]; [Google Scholar]
- 12.Prelog M, Gorth P, Zwazl I, et al. Universal mass vaccination against Rotavirus: indirect effects on Rotavirus infections in neonates and non-vaccinated small infants not eligible for vaccination. J Infect Dis. 2016 doi: 10.1093/infdis/jiw186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.