Abstract
This review surveys the use of pluripotent and multipotent stem cells in skeletal tissue engineering. Specific emphasis is focused on evaluating the function and activities of these cells in the context of development in vivo, and how technologies and methods of stem cell-based tissue engineering for stem cells must draw inspiration from developmental biology. Information on the embryonic origin and in vivo differentiation of skeletal tissues is first reviewed, to shed light on the persistence and activities of adult stem cells that remain in skeletal tissues after embryogenesis. Next, the development and differentiation of pluripotent stem cells is discussed, and some of their advantages and disadvantages in the context of tissue engineering is presented. The final section highlights current use of multipotent adult mesenchymal stem cells, reviewing their origin, differentiation capacity, and potential applications to tissue engineering.
Keywords: biomaterials, bone, cartilage, cell differentiation, disease model, intervertebral disc, mesenchymal stem cells, pluripotent stem cells, scaffold, skeletal development
1. INTRODUCTION
In 1963, Till and McCulloch were the first to demonstrate the clonal colony forming capacity and multilineage differentiation potential of hematopoietic stem cells (1). Developmental biologists would go on to use these same attributes—capacity for clonal self-renewal and multilineage differentiation to define different stem cell populations in development. All vertebrates develop from a single zygote—a cell that is considered to be totipotent, meaning that it is capable of differentiation into all the tissues of an organism as well as the extra-embryonic tissues. In 1981, embryonic stem cells (ESCs) were first derived from an inner cell mass of mouse blastocysts at the University of Cambridge (2). These cells had the capacity for clonal expansion and self-renewal and retained their ability to differentiate into all cells of the organism, fitting the definition for a population of pluripotent stem cells (PSCs). Tissue engineers later appropriated the stem cell term to define cells isolated from the bone marrow of adult organisms with capacity for limited self-renewal and multi-lineage differentiation into mesenchymal tissues. These cells were termed mesenchymal stem cells (3). Multipotent progenitors have now been isolated from a number of tissues and in vivo niches, and this review refers to these cell populations together with mesenchymal stem cells as multipotent stem cells (MSCs).
For skeletal tissue engineering, PSCs and MSCs have become the cell types of choice due to their capacity for expansion and ability to differentiate into multiple skeletal tissues. The first part of this review discusses the markers and signaling pathways that influence the differentiation of skeletal tissues during development. The second part of the review discusses how the knowledge gained from these studies has been applied to tissue engineering to influence the differentiation of pluripotent stem cells and mesenchymal stem cells.
2. STEM CELLS IN DEVELOPMENT AND ADULT HOMEOSTASIS
In mammals, skeletal tissues retain some capacity for regeneration after the embryonic period with tissue specific stem cells that are capable of healing limited defects. The capacity for regeneration varies both by tissue type and with age. The healing and regeneration of bone after a fracture is a well-described phenomenon (4). Tendons possess a limited ability to repair (5). Humans are capable of imperfect repair of articular cartilage defects with fibrocartilage (6). There is evidence from other animals that regeneration of articular cartilage is possible. The MRL/MpJ mouse strain, for example, displays an intrinsic ability to regenerate articular cartilage defects (7). Unfortunately, this innate supply of stem cell renewal capacity decreases dramatically with aging (8). Neonatal mice and humans are capable of complete regeneration of amputated digit tips, an ability that is lost with aging. Lineage tracing study of newborn mouse digit tip regeneration demonstrates that stem cells necessary for this process are recruited from their native tissues (9). By understanding the developmental origins of skeletal tissues and adult progenitors, researchers gain insights into how to sustain and enhance the potential of these stem cell populations as well how to engineer functional tissues utilizing stem cells.
2.1. Mesenchymal precursors
All cells in the developing organism are descended from one of three germline lineages: endoderm, mesoderm, and ectoderm. The mesoderm is formed during gastrulation when cells migrate through the primitive streak under the influence of the fibroblast growth factor (FGF) family gene Snail and Sox3 (10). Another important signaling pathway in specification of mesoderm is transforming growth factor β (TGFβ) family ligand, Nodal. Nodal misexpression in frogs and zebrafish induces ectopic formation of mesoderm, while loss of Nodal in mice disrupts mesoderm formation. Nodal is responsible for inducing the T-box transcription factor and mesodermal marker Brachyury (T) (11).
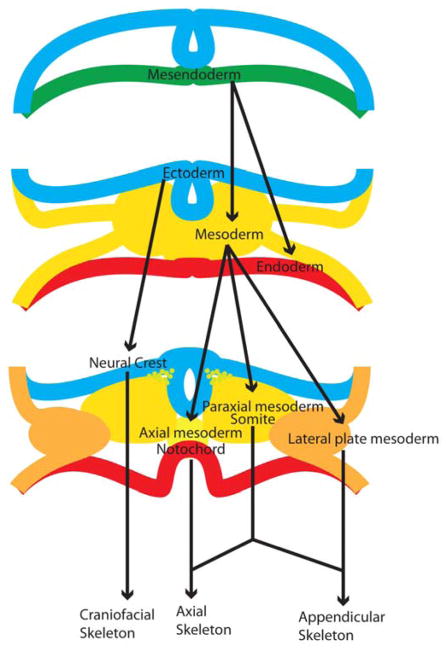
At this stage the mesoderm exists in multiple compartments (Figure 1). The lateral plate mesoderm, with contributions from the paraxial mesoderm derived somites for tendon and ligaments, eventually gives rise to the appendicular skeleton and pelvis (12). Mesenchymal precursor cells in the limb are marked by expression of Prx1 (13). The axial mesoderm gives rise to the notochord, which contributes towards the formation of the nucleus pulposus (NP) of the intervertebral discs (IVD). The paraxial mesoderm gives rise to the somites that will form the ribs, vertebral bodies, annulus fibrosus of the IVD (14), and part of the craniofacial skeletal tissues. The majority of the craniofacial skeletal tissues are derived from neural crest cells, a population of cells that derives from the ectoderm adjacent to the neural tube (15).
Figure 1. Developmental origins of skeletal tissues.
The axial and appendicular skeletons are derived from the mesoderm with the paraxial mesoderm somites making contributions to both the axial and appendicular skeleton. The craniofacial skeleton is derived primarily from neural crest, a subpopulation of ectoderm that has undergone epithelial-mesenchymal transition.
One of the key pathways inducing the expansion of mesenchymal precursors is the FGF signaling pathway. In the limb bud, Wnt3a signaling from the lateral plate mesoderm induces expression of FGF10 necessary for the development of an apical ectodermal ridge (AER). Once established, the AER secretes FGF4 and FGF8 to stimulate outgrowth of the limb bud and expansion of mesenchymal progenitors. FGF signaling from the AER also induces expression of the noncanonical Wnt5a in the immediate underlying mesenchyme to create a gradient towards which mesenchymal cells migrate to shape the developing limb (16). The AER FGFs help to establish the sonic hedgehog (Shh)-Grem1-AER/FGF feedback loop that patterns the limb bud and controls the onset of mesenchymal precursor condensation (17). Interestingly, Grem1 has recently been identified as a marker of multipotent skeletal stem cells in adult mice (18).
Mesenchymal precursor condensation is controlled primarily by the action of the bone morphogenetic protein (BMP) signaling pathway. The progression of proliferating mesenchyme to chondrogenesis in the limb bud is regulated by the termination of this Shh-Grem1-AER/FGF feedback loop due to outgrowth of the limb bud. Grem1 expressing cells in the mesenchyme escape the influence of Shh from the zone of polarizing activity (ZPA) in the posterior limb bud due to proliferation of the cells that separate them from the ZPA. As hedgehog signaling is silenced in the anterior limb by the repressive hedgehog transcription factor Gli3, Grem1 levels fall and BMP activation necessary for chondrogenic initiation can occur (17). BMP activation triggers the initiation of chondrogenic condensation via the upregulation of N-cadherin (Cdh2) and N-cell adhesion molecule-1 (Ncam1), allowing cell-cell communication to facilitate further condensation via Ncam1 and β-catenin signaling. Ncam1 and β-catenin signaling upregulate the expression of syndecans and fibronectin necessary for condensation (19). Wnt signaling is activated in areas between condensations and recent work has proposed that a Turing type reaction-diffusion mechanism controls the alternating pattern of BMP activation in digital condensations and Wnt activation in interdigital regions (20). Although TGFβ is a potent inducer of in vitro chondrogenesis of mesenchymal stem cells via its Type 2 receptor (Tgfbr2), deletion of Tgfbr2 from the limb mesenchymal precursors in mice does not affect chondrogenesis and results in fusion of phalanges and shortened skeletal elements, indicating that TGFβ signaling may function to limit chondrogenesis rather than promote chondrogenesis in vivo (21).
In adults, multipotent mesenchymal precursors have been demonstrated to exist in a number of niches. For example, cells with the characteristics of mesenchymal precursors including capacity for self-renewal and differentiation into multiple skeletal lineages have been shown to reside as pericytes in vivo (22). Stimulating the regenerative capacity of these perivascular MSCs in situ and/or isolating them for use in tissue engineering has been a major focus of research over the past few decades and will be discussed in the section on mesenchymal stem cells.
2.2 Cartilage
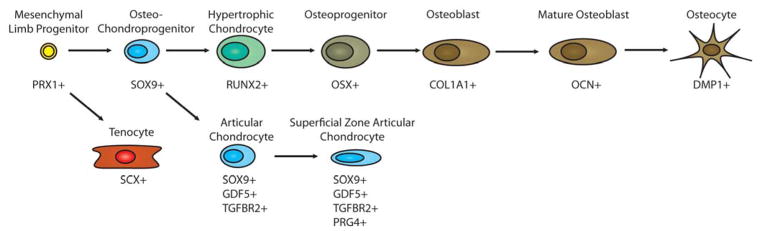
In the limb bud, the process of mesenchymal precursor condensation coincides with the initiation of chondrogenesis via the expression of the master chondrogenic transcription factors, Sox5, Sox6, and Sox9 (23). These transcription factors regulate the expression of a number of chondrocyte specific genes including collagen type II (Col2a1), collagen type XI (Col11a1), collagen IX (Col9a1), aggrecan (Acan), and cartilage oligomatrix protein (Comp) among others (24). These extracellular matrix (ECM) proteins assemble to generate a characteristic chondrocyte ECM that is known as hyaline cartilage specific matrix. Recent lineage tracing experiments with inducible reporters downstream of promoters for Col2a1, Acan, and Sox9 reveals that, in addition to articular cartilage, all osteoblast and marrow stroma cells are derived from precursors that express these earlier chondrogenic markers at one point (25). Although all chondrocytes express the same earlier marker genes, chondrocytes continue to differentiate into functionally dissimilar fates. In the case of growth plate chondrocytes, they are primarily destined for apoptosis/hypertrophy. In the case of articular chondrocytes, they achieve a homeostatic balance as a mature chondrocyte within a permanent hyaline matrix.
2.2.1 Growth plate chondrocytes
Chondrocytes at the center of the cartilage model are the first to hypertrophy and begin secreting signals that set up the structure of the growth plate, a transient developmental structure that eventually closes via endochondral ossification, that takes place during puberty in humans. The growth plate is a highly organized arrangement of chondrocytes that includes a resting zone, a proliferating zone, a pre-hypertrophic zone, and a hypertrophic zone. Resting and proliferating chondrocytes are marked by expression of parathyroid hormone related peptide (PTHrP), which is known to inhibit chondrocyte hypertrophy. Proliferating chondrocytes are organized into columns of cells oriented with the long axis towards the hypertrophic region, and this organization is essential for the control of bone shape. The stacking of proliferating chondrocytes is believed to involve elements of planar cell polarity, primary cilium orientation, matrix proteins, and diffusion gradients of growth factors such as BMPs, Indian hedgehog (Ihh), and Wnts and is an area of active investigation (26). Early pre-hypertrophic chondrocytes are marked by expression of Ihh. As the cells enlarge and enter into hypertrophy, they are marked by expression of the matrix protein, collagen type X (Col10a1). Additionally, they express a number of genes necessary for facilitating the remodeling of the ECM for blood vessel and osteoblast invasion, including matrix metalloproteinases (MMP9 and MMP13) and vasculoendothelial growth factor (VEGFA). Hypertrophic chondrocytes are also marked by expression of the early osteoblast transcription factors Runx2 and Osterix (Sp7). More mature hypertrophic chondrocytes also express apoptosis markers, such as Bcl2 (27).
The Ihh/PTHrP feedback loop is a major regulator of growth plate chondrocyte hypertrophy. Ihh secreted by the pre-hypertrophic chondrocytes stimulates hypertrophy of proliferating chondrocytes, but also stimulates expression of PTHrP, which inhibits chondrocyte hypertrophy. The interplay of these two signals sets up the orderly progression of chondrocytes through the growth plate. FGFs also play an important role in regulating chondrocyte hypertrophy. Constitutive activation of FGFR3 results in accelerated hypertrophy and premature growth plate closure, characteristic of diseases of growth plate disorders, such as achondroplasia (28, 29).
As indicated above, recent lineage tracing and cell population sorting studies have revealed that growth plate chondrocytes harbor a population of persistent skeletal stem cells with the capacity to differentiate into bone, cartilage, and marrow stroma that becomes progressively restricted (30). These cells represent a potent and defined source of tissue specific stem cells for regeneration of adult tissues.
2.2.2 Articular Chondrocytes
Articular chondrocytes represent a distinct population of chondrocytes, distinguished from growth plate chondrocytes by virtue of their ability to resist hypertrophy and calcification. Cavitation takes place within specified sites of the cartilage anlagen, and leads to the formation of an interzone consisting of flattened cells that gives rise to joints and articular chondrocytes. A recent review gives an excellent overview of the current state of what is known about the development of articular chondrocytes and related joint structures (31). Although there are many markers common to all chondrocytes, articular chondrocytes of the joint express a specific set unique from growth plate chondrocytes. Growth and differentiation factor 5 (Gdf5) is a BMP protein that has been shown with mouse models and in vitro experiments to be expressed specifically within articular chondrocyte precursors and to be essential for normal joint formation (32). PTHrP is known to inhibit chondrocyte hypertrophy and is expressed at high levels in both resting chondrocytes and articular chondrocytes during development. It has been proposed to function in the maintenance of articular chondrocyte phenotype (33). At the articular surface, synovial cells are marked by expression of lubricin (Prg4), a protein necessary for joint lubrication (34). Laser dissection and microarray analyses have identified a number of genes specific to articular chondrocytes and their precursors in development, and have also begun to explore expression specific to certain joint such as elbow versus knee (35, 36). The functional significance of the expression of these molecules is still being explored in developmental biology studies, but promises to yield new strategies for engineering adult articular cartilage.
Dysfunction of several signaling pathways has been described in the degenerative joint disease, osteoarthritis, including elevated BMP, hedgehog, and Wnt signaling and decreased TGFβ signaling. The effects of these changes, whether they are compensatory or pathologic, and their effects on articular cartilage repair are active areas of research. As an example, recent studies have demonstrated that Tgfbr2 is expressed in and specifically labels the joint versus growth plate. TGFβ signaling is known to support the development and homeostasis of articular cartilage by preventing chondrocyte hypertrophy (37). Interestingly, overexpression of TGFβ-1 in adjacent subchondral bone has been shown to induce cartilage degeneration, demonstrating the importance of restricting developmental signals to their proper tissues (38).
In the adult there is believed to be a population of cartilage-derived stem/progenitor cells (CPSCs) that migrate within the articular cartilage zone, in a manner responsive to degenerative changes. Although CPSCs have been isolated from articular cartilage, further work on their origin and derivation has been hampered by lack of a specific marker. It is hoped that stimulation of this population of cells may enhance cartilage regeneration (39).
2.3 Bone
Developmentally, bone is formed by two fundamental processes: endochondral ossification and intramembranous ossification. As described above, endochondral ossification involves the formation of the growth plate, a spatially organized structure within which chondrocytes mature through oriented proliferation, hypertrophy, and eventually either apoptosis or differentiation into osteoblasts; invading osteoblastic progenitor cells then populate the skeletal model, giving rise to a fully formed bone. In contrast, intramembranous ossification involves the direct conversion of mesenchymal progenitors to osteoblasts without the intervening chondrocyte maturation or growth plate structure, and involves the gradual fusion of clusters of osteoblasts known as spicules. The axial and craniofacial skeletons are formed primarily via intramembranous ossification (15).
Mature bone is composed of three types of cells: osteoblasts, osteocytes, and osteoclasts. Osteoclasts, responsible for bone resorption, are derived from hematopoietic stem cells. Their regulation and differentiation are tightly linked to osteoblasts and systemic regulators and are discussed in detail elsewhere (40). Osteoblasts, responsible for bone synthesis, are derived from Sox9+ mesenchymal progenitors that can either continue as chondrocytes or differentiate to preosteoblasts (41) under the influence of the transcription factors Runx2, and later, Osterix (Sp7). Runx2 and Sp7 are often used as markers of osteoblast differentiation. Mature osteoblasts express the transcription factor Atf4. Atf4 is a direct regulator of osteocalcin (Ocn) and stimulates expression of Ihh in chondrocytes to encourage maturation and osteoblast differentiation (42). Osteoblasts that become embedded within the bony matrix continue to differentiate into osteocytes. Osteocytes, marked by the expression of sclerostin (Sost) and dentin matrix protein 1 (Dmp1), compose more than 90% of all mature bone cells and play important roles in signaling to control calcium balance and bone remodeling in response to mechanical and hormonal cues via control of osteoblast and osteoclast differentiation (43).
The in vivo hallmark of osteoblastic cells is their ability to lay down a calcified bony matrix. This matrix is composed primarily of type I collagen (Col1a1) along with Ocn. Calcification requires the expression of alkaline phosphatase (Alpl) to provided the necessary phosphate for forming hydroxyapatite along with a host of matrix proteins that support the formation of calcified matrix including osteonectin (Sparc), integrin binding sialoprotein (Ibsp), and osteopontin (Spp1) (42).
A number of signaling pathways control the differentiation of osteoblasts. Ihh expression is essential for bone formation—loss of Ihh in mice results in complete failure of bony ossification (44). Mutations in the Notch signaling pathway demonstrate that Notch signaling suppresses osteoblast differentiation. Loss of function mutations in the Wnt co-receptor LRP5 result in severe osteoporosis, and the canonical Wnt transcription factor β-catenin is required for bone formation in the embryo. A critical threshold of BMP signaling is required for differentiation of mature osteoblasts, but the roles of different receptors and ligands is still an area of active research. FGF signaling plays a role in preosteoblast proliferation and mature osteoblast differentiation, but the precise temporal specificity remains to be investigated (42).
Mammals are capable of complete and perfect regeneration of bone following fracture. Fracture repair recapitulates the pathway of normal embryonic bone development, including activation of the Ihh, BMP, and Wnt pathways. The majority of fractures are healed by a combination of intramembranous and endochondral ossification, with endochondral ossification taking place in less stable fractures outside the periosteum and intramembranous ossification occurring within the periosteum at the edges of the callus. Following a fracture there is a brief inflammatory period after which periosteal and bone marrow mesenchymal precursors migrate to the site of fracture to initiate formation of a callus that is later remodeled to mature bone (4). Bmp2 is integral to bone healing—mice lacking the ability to produce Bmp2 in limb bones develop spontaneous fractures that do not resolve with time despite the presence of many osteogenic stimuli (45). Molecular understanding of bone development has already seen clinical translation in the form of recombinant BMP-2 for spinal fusions (46). As understanding of these processes grows, more adept manipulation of pathways and cells that control bone formation will help to reverse degenerative bone diseases such as osteoporosis as well as to expand reconstructive options and accelerate natural bone healing.
2.4 Intervertebral disc
The adult intervertebral disc (IVD) is formed from the interactions of notochord and neighboring somites during development. The mature IVD is composed of the central, largely acellular, proteoglycan rich nucleus pulposus surrounded by a dense ring of fibrous tissue known as the annulus fibrosus. The developmental formation of the IVD and vertebrae is driven largely by Shh secreted from the notochord, which is dependent on expression of transcription factors Foxa1 and Foxa2 (47). Shh from the notochord is responsible for inducing and maintaining the notochordal sheath. The notochordal sheath functions to restrict nucleus pulposus cells to the IVD and segregate the notochord between vertebrae (48). Shh exerts its function via induction of Pax1 and Pax9 to maintain the notochordal sheath and induces the surrounding somite to form the annulus fibrosus. Loss of Pax1 and Pax9 results in a single, unsegmented cartilaginous rod (49). The notochord and later annulus fibrosus secrete the BMP inhibitor, Noggin, which acts to counteract the BMPs secreted from developing vertebral bodies and prevents the ossification of the IVD (50). The formation and maintenance of the annulus fibrosus is also driven by TGFβ signaling through Tgfbr2 (51). Wnt and FGF signaling in the annulus fibrosus are thought to play a role in regulating interaction with the vertebral endplate (52).
Degeneration of the IVD is closely associated with aging, DNA damage, inflammation, and decreased stem cell function (53). In the nucleus pulposus, both cell density and proteoglycan content of the matrix decrease with age. The annulus fibrosus shows loss of structural integrity with aging, including tears and altered mechanical properties associated with matrix changes (14). Aging IVDs show decreased Shh and Wnt signaling, and pharmacological activation of these pathways has been shown to increase cell proliferation and matrix secretion in aged IVDs, potentially restoring a younger phenotype (54, 55). In the annulus fibrosus, stimulation of the TGFβ signaling pathway has been shown to increase the matrix retention and augment the repair of aging annulus fibrosus (56). Future studies to explore the interactions of these and other signaling pathways in development, aging, and repair will be essential to efforts to engineer functional IVDs and/or to regenerate aging IVDs.
2.5 Tendon and ligament
Tendons and ligaments are derived from the syndetome, a somitic compartment that arises during development between the sclerotome and myotome. The syndetome is marked by expression of scleraxis (Scx) (57). Scx expression is positively regulated by the transcription factors Pea3 and Erm, which are induced by FGF8 secreted from the myotome (58). Scx also suppresses expression of Pax1, which is specific to the neighboring sclerotome. Scx promotes expression of Col1a1 and the late tendon/ligament marker tenomodulin (Tnmd). Tnmd is a type II transmembrane glycoprotein protein whose deletion in mice results in poorly developed tendons with decreased tenocyte proliferation and reduced tenocyte density (59). TGFβ signaling also plays an important role in tendon development. Deletion of TGFβ2 and TGFβ3 or Tgfbr2 in mice resulted in loss of most tendons and ligaments in the limbs, trunk, tail, and head (60).
Mechanical stimulation is known to be essential to the development of tendon. During embryonic development, the composition of the ECM of developing tendons gradually changes in response to both mechanical signals and growth factors (61). The program of tendon differentiation and the different chemical and mechanical cues that control it is still an area of active research. Recently, another important transcription factor, Mohawk, was identified as necessary and specific for tendon/ligament development(62). Transcriptomic analyses of tendon progenitor cells from different stages in development will lend insight into the signaling pathways and transcription factors that regulate tenogenesis (63).
Some capacity for regeneration is retained in adult in the form of tendon stem cells. These cells are believed to reside in the sheath surrounding mature tendons known as the paratenon. There is evidence that they become activated and increase proliferation and differentiation in response to injury with some capacity to remodel and regenerate tendons (5). During this regenerative process, areas of tendon undergoing repair are enriched in expression of embryonic tendon markers such as Scx, Mohawk, and Tnmd. Deletion of Tnmd results in reduced self-renewal and augmented senescence in tendon stem cells, suggesting that the developmental program of tenogenesis is key to in vivo tendon repair (64).
3. STEM CELLS IN TISSUE ENGINEERING
The use of stem cells in tissue engineering opened a new era for the treatment of musculoskeletal diseases. First attempts to engineer skeletal tissues utilized fully differentiated somatic cells transplanted into the lesion area. These efforts have seen moderate success with the clinical implementation of therapies such as matrix assisted chondrocyte implantation (MACI) (65), but still have a number of drawbacks including limited expansion potential, invasive harvesting of autologous cells, limited capacity for remodeling and integration, uncontrolled hypertrophy, and overgrowth. Stem cells represent a more naïve population of cells similar to those involved in the embryogenesis and the original formation of tissues. Compared to somatic cells, stem cells have significantly greater capacity for expansion due to self-renewal. The multilineage differentiation capacity of stem cells suggests that they can be combined with scaffolds, mechanical stimulation, bioreactors, and growth factors to provide the stem cells with microenvironments for the development of complex tissues composed of multiple cell types (66, 67). Stem cells also avoid problems such as donor site morbidity and immunogenicity. Stem cells used in tissue engineering fall into two categories: pluripotent and multipotent stem cells. Pluripotent stems cells include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Multipotent stem cells (MSCs) used in clinical applications include mesenchymal stem cells and other tissue specific adult stem cells along with hematopoietic stem cells (HSCs). This section discusses the use of stem cells capable of skeletal tissue differentiation and, thus, HSCs will not be discussed in detail.
3.1 Pluripotent stem cells (PSCs)
In 1981, embryonic stem cells (ESCs) were first derived from mouse embryos at the University of Cambridge (2). In 1998, Thomson et al. at the University of Wisconsin-Madison first isolated and grew human ESCs in culture (68). They proposed that the essential characteristics of ESCs should include: (i) derivation from the preimplantation or peri-implantation embryo, (ii) undifferentiated proliferation for prolonged periods and (iii) the potential to differentiate into cells of all three germ layers. The ability to differentiate into all three germ layers is usually assayed via teratoma formation, and in mice, the incorporation of ESCs into germline. Their human ESCs expressed stage-specific embryonic antigen-3 (SSEA3), SSEA4, TRA-1-60, TRA-1-81, and alkaline phosphatase (Alpl) after isolation and culture in vitro. The cells maintained the developmental potential to form derivatives of all three embryonic germ layers (endoderm, mesoderm and ectoderm) after undifferentiated proliferation for months.
After the discovery that somatic cells could be reprogrammed by nuclear transfers (69) or by fusion with ESCs (70, 71), researchers at Kyoto University postulated that the factors which play important roles in the maintenance of ESC state would also induce of pluripotency in somatic cells (72). In 2006, they succeeded in generating pluripotent stem cells from mouse fibroblasts via retroviral transduction of four transcription factors: Oct3/4, Sox2, c-Myc, and Klf4. In the following year, two independent groups reported similar success in creating human induced-pluripotent stem cells (iPSC). Yamanaka and colleagues used the same four genes while Thompson and colleagues used Oct4, Sox2, Nanog and Lin28 (73, 74). iPSCs were shown to have the morphology and growth properties of ESCs and also to express ESC markers. Additionally, they demonstrated high telomerase activity and could proliferate indefinitely while maintaining their differentiation potential.
Although iPSCs share many similarities with ESCs, there are a number of practical differences. As transgenes and vectors are most commonly used to generate iPSCs and the integration is permanent, there is the possibility that the integrations will cause insertional mutations that could influence differentiation or cause tumor formation. To overcome this limitation, new methods of creating iPSCs have been developed, such as non-integrating or excisable vectors (75–77), single and multiple transient transfections (78, 79), protein transduction (80–82), mRNA-based transcription factor delivery (83, 84), RNA-based Sendai viruses (85–87), microRNA transfections (88), and the use of chemical compound to generate iPSCs (89, 90). In addition, iPSCs derived by factor-based reprogramming have a residual DNA methylation profile related to their tissue of origin, which is referred to as ”epigenetic memory”, which restrains to varying extent their differentiation potential. However, the methylation profile of iPSCs can also be reset by serial reprogramming and or by treatment with chromatin-modifying drugs (91). Human iPSCs have been derived from many cell types, such as fibroblast, peripheral blood cell, adipose stem cell, amniotic cell, mesenchymal stem cell, hepatocyte, and neural stem cell, among many others (92).
3.2 Pluripotent stem cell technology
Pluripotent stem cells have a number of features that make them attractive cell sources for skeletal tissue engineering. In their pluripotent state, PSCs can be expanded indefinitely and represent a nearly unlimited supply of cells. Additionally, the ability to expand PSCs from a single cell makes the implementation of multiple genetic modifications and alterations to cell lines considerably more feasible than in cells with limited expansion capacity. In the case of iPSCs, they represent a potentially unlimited source of autologous cells, making them ideal for engineering personalized tissues that avoid immune rejection. However, there is some controversy surrounding this concept due to reports that syngeneic and autologous iPSCs generated both by retroviral approach and episomal approach stimulate an immune response leading to rejection (93). Further studies have demonstrated this may be due to the unnatural pluripotent state, as terminally differentiated cells derived from iPSCs display negligible immunogenicity (94, 95). A number of advances have been made in expansion culture of PSCs that have expanded understanding of the pluripotent state and will eventually pave the way for safe clinical studies. Concomitant with these advances, methods and protocols for differentiation of PSCs into skeletal tissues have also been refined. These advances have opened possibilities for PSCs in various applications, including drug screening and basic science studies to pre-clinical applications.
3.2.1 Expansion culture of PSCs
PSCs have a number of properties that allows them to be expanded indefinitely. High telomerase activity maintains the length and integrity of telomeres, which contributes to PSCs’ resistance to senescence (96). Their pluripotent state is largely governed by transcription factors Oct4, Nanog, and Sox2. These three transcription factors activate the expression of protein-coding and micro RNA genes necessary to maintain the pluripotency state, while repressing expression of genes encoding lineage-specific proteins and miRNAs. When any one of the factors is not available or is below threshold level, the cells will begin to undergo differentiation (97). Some of the key regulators of the pluripotent state and continued proliferation include growth factors, mechanical stimulation, oxygen tension, and biomaterials.
3.2.1.1 Expansion culture growth factors
As in embryonic development, a number of growth factors, which play important roles in morphogenesis, have been shown to act on PSCs during in vitro expansion by stimulating proliferation, maintain stemness, and preparing them for subsequent differentiation.
While in development FGF signaling promotes differentiation of mesoderm, in culture it serves to maintain pluripotency. FGF2 is a foundational component of PSC expansion media. With a targeted phosphoproteomics approach, researchers show that upon FGF2 treatment, all four FGF receptors in PSCs are activated and about 40% of investigated proteins showed differential phosphorylation. These phosphorylated proteins include pluripotency factors, such as Sox2, Oct3/4, Nanog, and their target proteins. FGF2 activates MAPK, AKT and PI3K pathways, which mediate pluripotency, protect human ESCs (hESCs) from cell death, and promote ESC adhesion and cloning efficiency (98–100). Src kinase family activation by FGF2 and loss of pluripotent marker expression upon Src kinase inhibition indicate that regulation of cytoskeletal and actin dependent processes contribute to the maintenance of ESC in undifferentiated state.
Another growth factor family shown to regulate stem cell expansion is the Wnt family of secreted glycoproteins. While Wnt signaling has been demonstrated to maintain MSCs in an undifferentiated and proliferative state (101), the role of Wnt signaling in hESCs is controversial. Sato et al. found that activation of Wnt/β-catenin pathway with Wnt3a or GSK3 inhibitors maintained the self-renewal of hESCs (102). However, other groups have reported that Wnt3a or GSK3 inhibitors drive the differentiation of hESCs toward primitive streak and definitive endoderm (103, 104). One explanation for these results is that GSK3 inhibitors can maintain the expression of pluripotency marker for only a short time but are not sufficient to expand hESCs in a lon- term culture system (105). More recently, Davidson and colleagues used a sensitive reporter to prove that Wnt/β-catenin pathway is not activated during ESC self-renewal, whereas activation led to loss of self-renewal and induction of mesoderm markers (106). They also demonstrated that the canonical Wnt/β-catenin pathway is repressed by Oct4, and that knockdown of Oct4 activates β-catenin in hESCs. Supported by current data, Wnt/β-catenin pathway is more likely to induce differentiation rather than maintain the self-renewal of hESCs. The opposing role of Wnt/β-catenin pathway in human MSCs and ESCs shows a different response to the same growth factor in distinct stem cell types.
Although known to be a potent inducer of stem cell chondrogenic differentiation, TGFβ is also necessary for the self-renewal of both hMSC and hESC (107, 108). Using a TGF-β/activin/nodal signaling inhibitor, Tang and colleagues showed that TGF-β inhibits early chondrogenic induction of hESCs but is required for later stage of differentiation. TGFβ/activin/nodal signaling maintains the pluripotency markers of hESC through the signal transducer SMAD2/3, whose activation is required downstream of Wnt signaling (109). These results reveal an interconnection between TGF-β and Wnt signaling. Accordingly, exogenous activin A maintains hESCs in an undifferentiated state (109, 110). Xiao and colleagues identified Nodal/Activin, FGF, Wnt and Hedgehog as important pathways for maintaining self-renewal of hESCs with a microarray analysis. Activin A induces the expression of Oct4, Nanog, Wnt3 and FGF2, indicating a complex signaling network that retains the pluripotent state of hESCs.
3.2.1.2 Influence of oxygen tension in expansion culture
PSCs are typically cultured in a normoxic atmosphere (21% oxygen), but the mammalian reproductive tract contains only 1.5–5.3% oxygen. Some studies suggest that a hypoxic environment is more physiologically relevant for the growth of PSCs and that normoxic conditions may not be suitable for PSC culture (111). Several groups have investigated the effect of oxygen tension on culture of hESCs. Mild hypoxia (12% oxygen) increases the number of cells expressing Oct4/Nanog and reduces the frequency of apoptosis and chromosomal abnormalities compared to normoxia in long-term culture. In long-term culture, even lower tension of oxygen (5% oxygen) maintains the hESCs in a homogenous and flat colony in contrast to hESCs cultured in normoxia, where spontaneous differentiation occurs in the central zone of colony (112). Ezashi and colleagues found that in lower oxygen tension (3% and 5% oxygen), hESCs grow as well as in normoxia and are less prone to differentiation, with a reduction in proliferation seen only at 1% oxygen (113). Other groups have shown enhanced proliferation of ESCs cultured under 5% oxygen accompanied by increased expression of pluripotency markers SOX2, NANOG and OCT4 (114).
Upon exposure to low oxygen tension, hypoxia inducible factor (HIF) 1α is transiently expressed and both HIF2α and HIF3α are significantly upregulated. HIF2α regulates both hESC pluripotency and proliferation (114, 115). During prolonged hypoxia, binding of HIF1α to the hypoxia-response element (HRE) in the VEGF promoter significantly upregulates the expression level of VEGFA (116). VEGFA signaling is related to decreased apoptosis, as inhibition of VEGFA increases hESC apoptosis by about 10-fold and overexpression of Nrp-1, a VEGF receptor, decreased hypoxia-induced apoptosis by about 3-fold. Thus, VEGFA signaling plays a significant role in hESC survival during prolonged hypoxia. Besides these mechanisms, p53 and Notch signaling have also been shown to play a role in the maintenance of pluripotency by hypoxia (117, 118).
As factors like HIFs that mediate hypoxia responses in hESCs also exist in human iPSCs, hypoxia is hypothesized to regulate stemness and pluripotency in iPSCs. Sugimoto and colleagues found that mouse iPSCs cultured under 5% oxygen demonstrated rapid cell growth compared to that at 20% oxygen (119). How hypoxia regulates pluripotency in iPSCs is not fully understood, partly due to different approaches and cell sources in generating iPSCs. Besides expansion, hypoxia may exert additional effects on iPSCs. Yoshida and colleagues report that conducting reprogramming in hypoxia can also improve the efficiency for both mouse and human cells (120).
3.2.1.3 Feeder versus feeder-free culture systems
ESCs or iPSCs are not able to proliferate directly on tissue culture materials without feeder cells or coating. Mouse fibroblast feeder cells, such as SNL cells, combined with ESC culture medium containing FGF2 is the standard culture substrate for ESCs and iPSCs. The feeder-free culture on Matrigel with conditioned medium has become more popular due to decreased risk of animal/human pathogen to the stem cells (73). The feeder free culture system, which includes the critical factors for the maintenance of pluripotency, has greatly increased the possibility for clinical application of human ESCs and iPSCs. The TeSR family of feeder free media was introduced in 2006 (121). TeSR1 is a serum-free and animal product-free medium while mTeSR1 is a modification of TeSR1 medium that uses animal-source proteins but with less cost. More simplified medium such as E8 medium was introduced that contains less composition than TeSR medium (122). However, mTeSR1 remains the most published medium for the culture of PSCs due to their practical features and simple protocol. Besides Matrigel, multiple proteins such as leukemia inhibitory factor (LIF), laminin, vitronectin and fibronectin have been identified as supportive for pluripotent stem cell maintenance by approaches such as comparative proteomics analysis, (123, 124). Unfortunately, most of these are still too expensive for large-scale usage (122). ROCK inhibitor (HA100 or Y27632) has been reported to decrease the dissociation-induced apoptosis and promote colony formation (125). Actin-myosin contraction is a downstream target of ROCK pathway, and inhibitors of actin-myosin contraction such as blebbistatin have also been shown to increase cell survival and cloning efficiency (126).
3.2.1.4 Influence of biomaterial in expansion culture
Although conventional culture systems (with feeder cell or Matrigel coating) are currently used to maintain PSCs in defined growth factors, there are also developments in the use of biomaterial scaffolds to mimic the microenvironment of stem cells. Some scaffolds support human PSC growth in 2D culture and the others provide the stem cells with a well-defined three-dimensional (3D) microenvironment to simulate the in vivo stem cell niche. Major scaffolding approaches include pre-made porous scaffolds, decellularized ECM, cell sheets with secreted ECM, and cells encapsulated in self-assembled hydrogels (127).
Several cross-linked 3D hydrogels and scaffolds are used to provide hPSC spheroids and aggregates with fixed cues. Gerecht and colleagues developed a synthetic hyaluronic acid hydrogel that supports long-term self-renewal of hESCs and can direct cell differentiation (128). Nanofibrillar cellulose hydrogels and calcium alginate hydrogels have also been used to create tunable 3D environments for human PSCs (129, 130). Porous polymer scaffolds and 3D nanofibrous scaffolds have been shown to be capable of sustaining self-renewal of human PSCs in engineered 3D systems with conditioned medium (131, 132).
Biomaterials influence cell behavior of human PSCs by mechanisms distinct from growth factors and oxygen tension, such as control of cell morphology and cytoskeletal organization. Scaffold substrates that present cell adhesion elements such as heparin-binding peptides support PSCs by mimicking physiological cues (133, 134). Other properties such as surface roughness, stiffness, and hydrophilicity/hydropholicity all affect self-renewal of PSCs. Smooth and rigid substrates provide superior support to hESCs versus nano-rough and soft substrates in terms of adhesion, proliferation and self-renewal (135, 136). The enhanced self-renewal of PSCs in scaffolds is likely related to the activation of the small GTPase Rac, the PI3K pathway, and elevated expression of Nanog (132). Activation of Rac is accompanied by Rac-dependent changes like cytoskeletal reorganization, fibronectin deposition, and increased cell proliferation (137).
Although expansion culture of human PSCs can be improved by defined growth factors, proper oxygen tension and biomaterials, generating a large number of cells with high quality remains a challenge. Scalable expansion and differentiation of hPSCs is needed for biomedical applications, and two important technologies that have been developed to address this issue include microcarriers and thermoreversible hydrogels. Microcarriers have been modified by Matrigel or mouse embryonic fibroblasts and placed in suspension spinner flasks for hPSC culture (138–141). The recovery of hESCs adherent to microcarriers during cryopreservation is improved compared to the recovery of cells cryopreserved in freely-suspended colonies (139). Excellent reviews are available on the comparison of different microcarrier culture systems for the expansion of hPSCs (142). Thermoreversible hydrogels represent a novel, scalable system for expansion and differentiation of hPSCs (143). The hydrogel is liquid at low temperature and transfers into an elastic hydrogel when warmed, allowing cells to be mixed with the liquid polymeric solution at low temperature, suspended in solid gel at 37°C, and passaged by reliquifying the hydrogel at lower temperature. Optimized protocols enable long-term serial expansion of hPSCs with high proliferation rate.
3.2.2 Differentiation of pluripotent stem cells
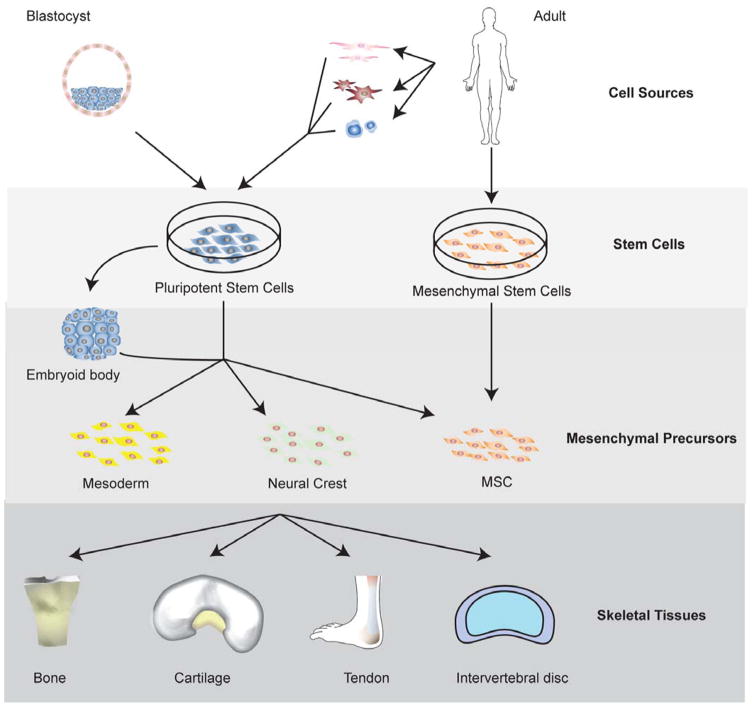
There are essentially four different methods for generating differentiated skeletal tissue cells from PSCs. The most commonly used method is to allow PSCs to form embryoid bodies. Embryoid bodies contain the three germ layers, and mesoderm can be manually separated from the endoderm and ectoderm. The second method is through the generation of MSCs either by outgrowth from embryoid bodies, direct plating of PSCs, or other methods. The third method is direct co-culture of PSCs with fully differentiated tissues. The fourth method involves stepwise differentiation through mesendoderm, mesoderm, precursor stages, and eventually to mature cells of specific lineage. Of these approaches, the step-wise differentiation method is the most tedious, but also yields the highest percentage of differentiated cells. It also eliminates steps that require manual dissection associated with embryoid body formation (144).
3.2.2.1 Differentiation of PSCs into MSCs, mesoderm, and neural crest
In order to achieve higher homogeneity necessary for the application of PSCs in musculoskeletal tissue engineering, efforts have been made to induce PSCs to a transient, mesenchymal stem cell-like cell type that can be differentiated with standard mesenchymal stem cell differentiation protocols. These mesenchymal stem cell-like cells derived from hPSCs are also called PMPs (PSC-derived mesenchymal progenitor cells). PMPs express cell surface markers of MSCs and are multipotent, which means they can differentiate into bone, cartilage, and adipose upon induction. Derivation of PMPs is of great interest because this approach combines the advantage of the infinite proliferative capacity of PSCs with the well-known properties of mesenchymal stem cells, and using iPSCs as the starting cell type also offers the possibility of developing autologous tissues. As the proliferative potential and differentiation capacity of mesenchymal stem cells are related to donor age, gender, and health condition, the use of PMPs opens the possibility to generate uniform batches of renewed mesenchymal stem cells.
There are several methods to derive PMPs from hPSCs. Outgrowth of embryoid body (EB) (145), spontaneous differentiation of PSC colonies (146), indirect co-culture (147), and treatment with mesenchymal stem cell growth medium (148) have all proved to be feasible approaches. Although morphology, surface markers and differentiation potential of these PMPs are similar to mesenchymal stem cells, they may still display a unique expression pattern of mesenchymal and pluripotency genes. Some mesenchymal progenitor cells derived from iPSCs are reported to be less responsive to traditional mesenchymal stem cell differentiation protocols compared with parental mesenchymal stem cells, suggesting that PMPs are a distinct population of cells with mesenchymal stem cell-like characteristics (149).
In contrast to methods that seek to go directly from PSCs to mesenchymal stem cells, Oldershaw et al. used a stepwise differentiation technique to generate first mesendoderm followed by mesodermal precursors (150). They begin with Wnt3a and Activin/nodal treatment. As mentioned above, activation of these signaling pathways is necessary for the formation of primitive streak and the differentiation of hESCs into the bi-potent mesendoderm (151–153). In development, the further differentiation of mesendoderm into mesoderm is regulated by members of the bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) families. Accordingly, treatment with FGF2 and BMP agonist is used to convert mesendoderm to mesoderm (154–157).
There are also a number of groups who have sought to differentiate neural crest from PSCs. Successful approaches thus far have sought to mimic developmental signaling that specifies neural crest including activation of canonical Wnt signaling combined with suppression of Nodal signaling (158). Another group has recently demonstrated that inhibition of the ROCK/Myosin II pathway can induce neural crest specification of ESCs (159). Because of the plasticity of in vivo neural crest cells and their contributions to multiple mature skeletal tissue types, these approaches hold a great deal of promise for the study of development and tissue engineering.
3.2.2.2 Differentiation of PSCs into chondrocytes
Chondrocytes have been differentiated from PSCs with a number of methods. The final stage of the Oldershaw et al. protocol is the induction of chondrocyte differentiation with GDF5 and NT4 from the mesodermal precursors. These derived chondrocytes express high level of SOX9, deposit a sulfated glycosaminoglycan and collagen type II matrix, and represent 80–99% differentiation of the PSC population (150). Yang and colleagues later simplified the protocol to a two-stage process using only six growth factors (160). Other methods have used direct co-culture of PSCs and chondrocytes, standard chondrogenic medium to induce differentiation of PMPs, and standard chondrogenic medium to induce mesoderm isolated from embryoid bodies and have been reviewed extensively (144). PSCs can also be transfected with virus-based promoter-reporter constructs to aide in selection of chondrogenic clones. In one study, chondrogenically differentiated PSCs were purified by green fluorescent protein expression driven by the chondrogenic Col2a1 gene promoter, allowing them to be identified and isolated (161).
Besides growth factors and matrix proteins, other modifications of in vitro culture systems can also result in improved chondrogenesis, such as oxygen tension, biomaterial scaffolds and mechanical stimulation. Although hypoxia has a critical role in maintaining the undifferentiated state of hPSCs, it also promotes chondrogenesis of MSCs and hPSCs. It has been shown that hESCs cultured in hypoxia during chondrogenic differentiation have enhanced ability to produce collagen type II, collagen type I, and glycosaminoglycans, resulting in better biomechanical functionality (162).
Biomechanical scaffolds have an important role in directing MSC chondrogenesis mainly through regulation of cell shape and actin cytoskeletal organization. Studies using scaffolds to promote hPSC chondrogenesis is being undertaken. An ideal scaffold for cartilage repair should provide the stem cells with proper porosity for nutrients diffusion, necessary mechanical strength, and promote differentiation. Many biomaterials have been used in musculoskeletal tissue engineering. Natural materials such as collagen, gelatin, fibrin and hyaluronan can mimic some aspects of native ECM and are relatively biodegradable, but require extensive purification and may have the risk of pathogen contamination. Synthetic biomaterial such as poly (α-hydroxy esters) and ceramics provide a higher stability and are more capable of forming macro-/microstructure (163).
In another approach, rigid scaffolds like PLA provide more mechanical strength but may not be readily degraded, thus affecting the properties of mature chondrocytes. Natural or synthetic hydrogels have the advantage that they are usually highly permeable and the cells can be distributed evenly in the gel before polymerization, but hydrogel lack sufficient mechanical strength (164). Scaffolds fabricated with electrospinning, which can produce continuous polymeric fibers ranging from nanometers to microns and serve as a 3D porous scaffold, represent another option. Other natural materials such as gelatin, hyaluronic acid and glycosaminoglycan can enhance the cell affinity of electrospun fibers (165–170). Cartilage defects implanted with cells in nanofibrous scaffold using PCL and gelatin have been shown to exhibit an improved appearance, higher cartilage-related gene expression and protein levels, and enhanced subchondral bone regeneration (171). Direct plating of undifferentiated iPSCs into high-density micromass cultures in the presence of BMP2 promoted chondrogenic differentiation, but with a mix of phenotypes including articular, growth plate, and fibrocartilage. However, the expression of articular markers was higher in micromass than in pellet cultures (172).
Li and colleagues demonstrated that a local cyclic stress applied through focal adhesion induced spreading and differentiation of mESCs, as evidenced by the down regulation of Oct3/4 gene expression (173). Fluid shear stress can also direct the differentiation of ESCs (174). Besides mechanical forces like cyclic stress and shear stress, matrix stiffness has been shown to influence differentiation of ESCs. Evans and colleagues assessed the effects of substrate stiffness on lineage specification and found that genes expressed in the primitive streak and during early mesendoderm differentiation are upregulated in cells cultured on stiffer substrate (175). Although mechanical stimulation influences PSC differentiation, how to combine it appropriately with other factors (growth factor, scaffolds, etc.) for the induction of PSC to cartilage still needs to be elucidated.
3.2.2.3 Differentiation of PSCs into osteoblasts
Compared to chondrogenesis of PSCs, osteogenesis is relatively successful, even without supplementation of recombinant growth factors. PSCs are typically differentiated into mesenchymal progenitors, and osteogenesis is induced with standard components of osteogenic mesenchymal stem cell medium, including dexamethasone, ascorbic acid, and β-glycerophosphate (148). Similar to their role and expression in development of cartilage anlagen that form the bones in the limb, BMP2, BMP4, and BMP7 have all been show to have an effect on osteogenic differentiation of PSCs. BMP2 has been used by several groups as a supplement in hESC osteogenic medium to promote osteogenesis (176). Another member of BMP family, BMP4 has also been shown to enhance osteogenesis of hPSCs (177). BMP7 has little effect alone, but has a synergistic effect in promoting osteogenesis of hESCs upon addition with dexamethasone (178). Most protocols induce osteogenesis of hPSCs via PSC-derived mesenchymal progenitors. Few studies have been performed to mimic various stages of development for differentiation from hPSC to osteoblast.
Unlike the positive effect of low oxygen tension on chondrogenesis, the effect of hypoxia on osteogenesis shows less consistency (179–181). Yang and colleagues found that hypoxia inhibits osteogenesis of human mesenchymal stem cells through down regulation of RUNX2 by TWIST, a downstream target of HIF1α (181). A general trend is that low oxygen tension reduces osteogenic differentiation. However, the relationship between oxygen tension and osteogenesis of hPSCs has not been demonstrated in published reports.
Given the mechanical strength of bone tissue, various scaffolds have been used to enhance bone regeneration. Scaffolds such as decellularized bone matrix (148), nanofibrous polylactic (PLLA) scaffold (178) and thermoreversible hydrogel (176) have all proved to be feasible for engineering bone from hPSCs.
3.2.2.4 Differentiation of PSCs into other skeletal lineages
PSCs are also a promising cell source for tendon repair. hESCs are capable of differentiating into tenocytes via ESC-derived MSCs and secret fetal tendon matrix and differentiating factors (182, 183). Mechanical load combined with tendon-specific transcription factor scleraxis synergistically promotes the commitment of hESC-derived MSCs to tenocytes (182). In a large animal tendinitis model, injection of ESCs significantly improved architecture, tendon size and tendon linear fiber pattern compared with controls (184).
3.2.3 Pluripotent stem cells application
Although patient-derived iPSCs may overcome the issue of immunological rejection and ethical problems in cell therapy, challenges still remain. Low efficiency of reprogramming is one of the hurdles, and significant genomic changes have been observed in human iPSCs despite the use of non-integrating reprogramming techniques (185). PSCs also tend to form teratomas because undifferentiated cells cannot be eliminated with current differentiation protocols (186). However, the expansion potential and flexibility of PSCs, combined with our increasing understanding of developmental programs necessary to generate homogenous populations of differentiated cells and next generation genome editing techniques, will likely make PSCs the ultimate cells of choice in skeletal tissue engineering applications. For now, the applications of PSCs are mostly limited to screening technologies, disease modeling, and furthering understanding of development with limited pre-clinical testing.
3.2.3.1 PSCs and molecular genetics technology
Combined with next generation genetic tools such as site specific CRISPR-Cas deletional and insertional mutagenesis (187) along with an ever increasing understanding of the genetic control of tissue morphogenesis, PSCs will play an integral part in the future of biological engineering. The expansion potential of these cells will allow researchers to build complex and highly regulated genetic structures with cell lines that can be rigorously tested and qualified before human trials. Fail-safe switches that trigger cell-apoptosis with the loss of cycle control, forced differentiation and maintenance of lineage commitment, cell-environment responsive expression switches, and custom metabolic pathways for cell specific activation of pharmacologic agents are all possibilities. There also exists with pluripotent stem cells the potential for whole organ engineering in an intact organism. It has been demonstrated that a different species is capable of developing and maintaining an organ of another species after blastocyst complementation with induced pluripotent stem cells (188). This raises the possibility for growing human skeletal tissues such as tendons, ligaments, menisci, articular cartilage, IVD, and bone within model organisms such as sheep, dogs, pigs, and/or cows that could be used for transplant or enhanced testing of drugs/interventions.
3.2.3.2 Cell therapy
As large quantities of cells are required for stem cell-based therapies, PSCs gain much attention because of their unlimited proliferative capacity and the ability to differentiate into any cell type. Use of iPSCs overcomes many of the ethical and legal controversies of ESCs, but challenges still remain as the generation of iPSCs is labor-intensive and costly, and genetic aberrations in iPSCs are common (185, 189). Moreover, the naivety of PSCs means that a series of defined intermediate cell types are required and generated with high fidelity in order to differentiate them into target cell types efficiently. MSCs are still the main source of current cellular therapies for musculoskeletal diseases due to their earlier discovery than PSCs. PSCs have shown some exciting results in treating neurodegenerative diseases (186, 190), diabetes (191) and some cardiovascular diseases (192). Although some promising results show in vivo differentiation of PSCs into skeletal lineages, a greater understanding of the identity and characteristics of PSCs is needed before the clinical application of hPSCs for skeletal tissue repair can progress to ensure safety and efficacy are maximized.
3.2.3.3 Disease modeling
The research of many human genetic diseases is hindered by the source of experimental material. The generation of human iPSCs provides the possibility of establishing disease-specific models as patient-derived iPSCs can be used to create cell lines reflecting certain defects. Some human disease models have been established in iPSCs, such as spinal muscular atrophy, Huntington’s disease, and Down syndrome (193, 194). Barruet and colleagues established an iPSC-derived model for fibrodysplasia ossificans progressiva (FOP), a congenital disease of increased pathologic ossification. They describe the derivation of iPSC from FOP patients and differentiate these iPSCs towards osteogenic lineage (195). Kim and colleagues generated human iPSCs from osteoarthritis patient-derived synovial cells and these iPSCs were able to differentiate into distinct mesenchymal cell lineages, deomnstrating that iPSCs can be developed into disease-relevant cell types for use in drug discovery and disease modeling (196). iPSCs derived from osteoarthritic chondrocytes are also reported, which provide a potential solution to osteoarthritic cell replacement problem (197). The genetic defect in iPSCs derived from an osteogenesis imperfecta patient was corrected with gene therapy to produce normal collagen and form bone in vivo, demonstrating that the combination of gene targeting and iPSCs could be used to cure many genetic disorders (198). Once the disease models have been established, novel drugs can be tested on these iPSC based models. Such models would be directly relevant to human disease and potentially cost saving.
3.3 Multipotent Stem Cells (MSCs)
Multipotent skeletal stem cells represent a wide range of cell populations that have application potential for skeletal tissue engineering. Several excellent reviews have been published on the history of multipotent mesenchymal stem cells (MSCs) e.g. (3). When the first multipotent stem cells isolated were from bone marrow stroma along with HSCs, hematologists quickly realized that tissue culture plastic adherent stromal cells could support HSC expansion. It was soon learned that these stromal cells were also capable of differentiation into multiple skeletal tissue lineages, including bone, cartilage, and fat and had capacity for self-renewal.(199, 200) With the emergence of tissue engineering in the 1990s, the accessibility, multipotential differentiation capacity, and expansion potential of MSCs made them ideal cells for skeletal tissue engineering. Bone marrow derived MSCs were applied to a number of musculoskeletal clinical challenges with great hope, but often with limited success. It has since been recognized that this population of multipotent progenitors defined by post hoc characteristics of multilineage differentiation capacity and an assortment of non-specific cell surface markers represents a heterogenous population of cells with varying capacities for self-renewal, differentiation, and engraftment (201). This section focuses on describing the current state of defining skeletal and skeletal tissue specific MSCs, and the knowledge of their skeletogenic differentiation potential and its relationship to embryonic development. Other important characteristics of MSCs, including their trophic effects and immunomodulatory properties are not covered, as they have been reviewed extensively elsewhere (202, 203).
3.3.1 MSC definition and source
For the purpose of this review, multipotent stem cells (MSCs) of the skeletal tissues are defined to include mesenchymal stem cells as well as tissue specific stem cells, such as those that reside in the periosteum and are recruited for fracture healing (204), and skeletal stem cells that continue to give rise to and renew adult skeletal tissues (30). The subset of multipotent stem cells collectively referred to as mesenchymal stem cells are defined by a number of cell surface marker expression and by multipotential differentiation capacity into osteoblasts, chondrocytes, and adipocytes. The minimum criteria for a mesenchymal stem cell includes (i) adherence to tissue culture plastic under standard tissue culture conditions, (ii) negative for expression of CD45, CD34, CD14 or CD11b, CD79a or C19, and HLA-DR (iii) positive for expression of CD105, CD73, and CD90, (iv) the ability to differentiate into osteoblasts, chondrocytes, and adipocytes in vitro (205). Other surface antigens commonly expressed by MSCs include CD13, CD29, CD44, and CD10 (201, 206). It is important to note that the 3 positive markers CD70/CD90/CD105 are co-expressed in a wide variety of cells and thus incapable of identifying an MSC in vivo. Stro-1 is another commonly used identifier, but has been shown to be an endothelial antigen and its ability as a definitive marker to identify MSCs in vivo is unproven. There is also some evidence that the proposed negative marker CD34 is expressed in native MSCs, especially in those derived from adipose (207). MSCs are by nature a heterogeneous population—this makes them difficult to study because their definition is functional. Some MSCs are more lineage committed. Other MSCs express pluripotency markers like Oct-4 and Sox-2. Comparison of human MSCs derived from periodontal ligament, dental pulp, bone marrow, adipose, and umbilical cord tissue shows that even from topographically closely related tissues such as dental pulp and periodontal ligament, there are significant functional differences including multipotency and immunomodulatory properties and proliferation potential (208).
More recent studies have utilized lineage-tracing approaches to identify skeletal tissue specific stem cells within their in vivo niche. By labeling cells at specific time points using a rainbow reporter mouse to track areas of tissue with common origin and following their progeny to determine to which tissues these cells contribute, different populations of stem cells have been isolated. Furthermore, these studies identified several cell types with increasingly restricted differentiation potential down the differentiation lineage. They also showed that these cells responded appropriately to BMP2 with osteogenesis, and to BMP2 with VEGF inhibition with chondrogenesis (30). Although there is no single accepted marker of MSCs in humans, mouse experiments have commonly used Stro-1 and Nestin as markers of MSCs. Nestin is an intermediate filament protein that was found to be highly expressed in bone marrow stromal cells that possess multi-lineage differentiation potential and self-renewal. Recent studies have identified Gremlin-1 (Grem1) as a highly specific marker of multipotential progenitors that is significantly more enriched for multipotent progenitors than mesenchymal stem cells isolated via standard protocols (18).
3.3.2 MSC differentiation
MSCs have been shown to differentiate under the influence of a number of stimuli including growth factor treatment, mechanical stimulation, hypoxia, and substrate stiffness (209). The differentiation capacity and robustness of a population of MSCs depend heavily on their origin and purity. Tissue engineers have designed a number of scaffolds, bioreactors, growth factor delivery systems, treatment protocols, and drugs designed to optimize differentiation. These topics have each been extensively reviewed in numerous publications (202, 210–212) and will not be discussed in detailed here. This section focuses on the methods used to differentiate MSCs into skeletal tissues and how these methods draw their inspiration from and also differ from the developmental programs described earlier in this review. Despite these differences, ultimate measure of an MSC remains the same—the ability to differentiate into a cell that expresses the genes characteristic of cells of multiple skeletal tissues.
Some studies have shown that canonical Wnt signaling can promote MSC expansion without reducing multipotency (101, 213). This is supported by findings that mesenchymal lineage stem cell populations are reduced in Sirt1 knock out mice that have loss of β-catenin activating functions (214). Wnt3a treatment, which stimulates the canonical Wnt pathway, has also been shown to promote MSC expansion while inhibiting osteogenic (101) and chondrogenic differentiation (215). This finding is in line with the observation that chondrogenic condensations in the developing limb have quiescent β-catenin signaling, while mesenchymal precursors excluded from condensations have activated β-catenin signaling (20).
3.3.2.1 Differentiation of MSCs into chondrocytes
One of the first differentiation commitments of MSCs is chondrogenesis, leading to cartilage formation. Using micromass cultures of adherent cells that were isolated from bone marrow, many groups have investigated the mechanisms by which these precursors condensed, underwent chondrogenesis, and subsequent maturation into hypertrophic and calcifying chondrocytes (216). Markers used to identify cartilage differentiation largely include early chondrocyte markers such as Col2a1, Col11a1, Acan, and Sox9. These genes are essential for the synthesis of the characteristic hyaline cartilage matrix, and Sox9 is a transcription factor known to be essential for their expression.
Mesenchymal stem cells can be induced to differentiate into chondrocytes with application of TGFβ. The standard protocol for chondrocyte differentiation of MSCs calls for treatment with TGFβ1 or TGFβ3 in Dulbecco’s Modified Eagle Medium (DMEM) supplemented only with insulin/selenium/transferrin (ITS), ascorbic acid (to enhance collagen production), and dexamethasone. TGFβ stimulation of isolated perivascular cells in vitro has been demonstrated to induce expression of chondrogenic markers via Tgfbr2 (217). Interestingly, deletion of Tgfbr2 from the limb mesenchymal precursors in mice does not affect chondrogenesis, but results in fusion of phalanges and shortened skeletal elements, indicating that TGFβ signaling may function to limit chondrogenesis rather than promote chondrogenesis in vivo (21). This shows that despite similarities in terms of self-renewal and differentiation capacity, there are differences between adult perivascular cells, such as MSCs, and embryonic mesenchymal precursors in vivo. There is indication that within heterogenous MSC populations there are variations in expression of tissue type and donor type differentiation genes. Single cells within an MSC population may use distinct molecular mechanisms to reach a common cell fate (218). However, by and large, the differentiation mechanisms and regulatory genes used by adult MSCs are very similar to those in development, specifically, the master chondro-regulatory gene, Sox9, is induced in both cases.
Tissue engineering applications generally require a significant number of tissue progenitor cells, which represents a challenge for MSCs, as they generally have a long doubling time and show compromised differentiation potency with extensive culture expansion. FGF2 has been found to enhance hMSC proliferative activity and support chondrogenesis (219). A number of studies in the last decade have reported that MSCs have enhanced growth kinetics upon culture under hypoxic conditions, reaching higher population doubling number and express a less spread morphology with higher expression of MSC-associated markers than cultures maintained under normoxia, although there is also evidence that there is impairment of subsequent differentiation ability (220, 221).
A number of signaling pathways, growth factors, microRNAs, and treatment regimes have been identified that play a role in MSC chondrogenesis. For example, overexpression of Wnt11 has been shown to inhibit MSC proliferation and induce expression of Acan and Col2a1 along with Sox9, Runx2 through syngerism with TGFβ (222). mIR-29a is one of most downregulated miRNAs during chondrogenesis and is directly regulated by Sox9. Its expression directly inhibits FOXO3A, a necessary transcription factor for chondrogenesis (223). Hypoxic pre-conditioning impairs differentiation but enhances clonogenicity due to enhanced VEGFA and proliferation, possibly associated with enhanced mineral deposition and increased osteogenic differentiation at the expense of adipogenic and chondrogenic differentiation (221). Co-cultures of articular chondrocytes with MSCs in porous scaffolds show enhanced chondrogenesis and matrix synthesis with lowered oxygen tension, but MSCs alone showed increased hypertrophy and calcification with hypoxia (224). Under standard chondrogenic culture conditions as used for bone marrow MSCs, adipose derived MSCs (ASCs) are able to synthesize cartilage matrix markers including Comp, Acan, Col2a1 and also express BMP receptors; however, the level of chondrogenesis is generally lower (225). It has been shown that the inclusion of BMP6 as a culture supplement enhances chondrogenic differentiation of ASCs, and also. prevents expression of the chondrocyte hypertrophy marker, collagen type X (226).
A major challenge in the application of MSCs for cartilage tissue engineering, which generally aims to repair the hyaline articular cartilage, is the tendency of chondrifying MSCs to under hypertrophy, similar to the events in the cartilage growth plate during developmental endochondral ossification. In vitro, pellet cultures of MSCs may be further induced to undergo robust hypertrophy upon treatment with the thyroid hormone, triiodothryonin (T3) (216). Recent studies have demonstrated that PTHrP (1–40) treatment of MSC pellets reduces ALP expression, a marker of hypertrophy, but does not significantly diminish T3 induced hypertrophy (227). Interestingly, Luyten et al. have reported expression of a specific gene set as a predictive indicator of the pro-hypertrophy characteristic of specific MSC clones (228). There is some suggestion that ALP activity maybe a good indicator of MSC osteogenic/chondrogenic potential. Thus, MSCs with high levels of ALP activity appeared to be more osteogenic and less chondrogenic, were less responsive in terms of induced expression of Col2a1, and showed increased expression of chondrocyte hypertrophy markers (229). Precise knowledge on the regulation and control of hypertrophy is critical to successful application of MSCs for cartilage tissue engineering.
In addition to biologics, biomaterials such as bioactive glasses and glass-ceramics with specific nanoscale characteristics and topology have been shown to promote chondrogenesis of MSCs (230, 231).(230–232)(230) In addition, 3D porous aqueous-derived silk, agarose, alginate, and gelatin scaffolds have all been shown to support the chondrogenic differentiation of ASCs (231, 233). Functionalizing these scaffolds with biologic cues also represents a method to enhance chondrogenesis. One group has used polyacrylate substrates modified to include functional groups that mimicked the RGD integrin binding site induced chondrogenesis in MSCs in the absence of TGFB-3 (234).
Finally, recent efforts have focused on the engineering of specific zones of cartilage, such as the superficial zone. For example, one such study showed that co-culture of MSCs with the superficial zone cartilage explants enhanced Sox9 and Col2a1 expression along with expression of the superficial zone marker lubricin (Prg4) (235). Interestingly, building on knowledge gained from analysis of gene expression events during mesenchymal condensation and chondrogenesis during embryonic development, a recent study reported the construction of large, mechanically functional cartilage constructs from fusion of condensed mesenchymal cell bodies (236).
3.3.2.2 Differentiation of MSCs into osteoblasts
The differentiation of MSCs into osteoblastic cells, with the ability to produce a mineralized matrix, was one of the earliest observed functional phenotype of MSCs (199). The major criterion used to assess osteoblastic differentiation is the ability of the cultured cells to form a calcified matrix. Histologically, this includes positive staining with alizarin red S and von Koss staining, corresponding to the presence of calcium phosphate. [Note: Caution is required to distinguish between pathologic calcification versus genuine hydroxyapatite mineral, the latter denoting osteoblast-mediated matrix mineralization.] Other characteristic markers include expression of known osteoblastic genes, including Ocn, Col1a1, Alpl, Bmp2, Ibsp, and others (237).
Mesenchymal osteogenesis during development, e.g., during intramembranous ossification, is characterized by a distinct sequence of cell phenotype and gene expression changes, which may be reproduced in in vitro cultured mesenchymal cells, such as MC3T3 and ROS cells and MSCs (237). These studies have revealed the importance of signaling pathways from development in adult formation of bone. For example, the BMP family proteins, BMP2, BMP4, and BMP7, have been shown to induce the formation of bone in vivo and the differentiation of osteoblasts. Under the influence of BMP2, expression of Ocn, Alpl, Col1a1, Bsp2, and other bone markers is induced in MSCs. Interestingly, BMP7 was reported to transduce human fibroblasts to form bone in vivo, suggesting that activation of the BMP pathway is sufficient for in vivo bone formation (238). The importance of BMP2 for adult regenerative bone response is underscored by the observation that it is necessary for fracture healing (45).
Many studies indicate that exposure to bony matrix components, including growth factors such as IGF-1 as well as hydroxyapatite, has the ability to induce MSCs to differentiate into bone (239). This has been exploited to use MSC homing to bone to improve bone formation and healing. An approach was recently reported that involved tagging of MSCs with the bone-specific drug alendronate via an α4β1 integrin binding peptide, thus allowing the sequestration of MSCs to bone, and resulting in increased bone mass in vivo (240).
As is the case for cartilage, the use of biomimetic scaffolds is critical for optimal bone tissue engineering. Given the mineralized nature of the bone matrix, most biomaterials used for bone tissue engineering contain hydroxyapatite, e.g., nano-hydroxyapatite/polyamide composite scaffolds (241) and photocrosslinked collagen/hyaluronan hydrogel with direct inclusion of hydroxyapatite (242).
3.3.2.3 Differentiation of MSCs into other skeletal lineages
Tendon
MSCs have been induced to differentiate into tenocytes by application of mechanical stimulation (243), treatment with extracted decellularized tendon extracellular matrix (244), TGFβ induction, and forced overexpression of Scx (245). However, current approaches have not been able to meet the critical requirements for functional engineered tendon/ligament, including mechanical strength, scalable construct, and vascularized structures [see (246) for an in-depth discussion of the current challenges in tendon/ligament tissue engineering].
Intervertebral disc
Recent studies have shown that MCSs can be differentiated into nucleus pulposus cells of the IVD with hypoxia and TGFB1 treatment (247, 248), as well as exposure to factors secreted by notochondal cells (249). The use of an MSC-seeded composite biomaterial scaffold consisting of a nanofibrous cortex and a hyaluronan-based interior has also been shown to support the formation of an IVD-like tissue construct, with composition resembling an exterior annulus fibrosus and an internal nucleus pulposus (250). Recently, an MSC-based collagenous construct has been reported to produce a multicomponent spinal motion segment (251). The construct consists of two osteochondral subunits, a nucleus pulposus-like core and a multi-lamellae annulus fibrosus-like component, maintained in a chondrogenic medium and conditioned with cyclic compressive and torsional loading. Clearly, IVD tissue engineering is at an early stage of development. Lessons gained from embryonic axial skeletal development, including differential gene expression and signaling pathway activation, must be exploited and tested for successful IVD tissue engineering (252).
3.3.3 Mesenchymal stem cell applications
Tissue sourcing and optimization
MSCs are the current cell type of choice for clinical stem cell therapy due primarily to their relatively ready accessibility. Autologous bone marrow aspirates from iliac crest have been used to augment fusions since the 1980’s. With the recent insight that MSCs can be isolated in large quantities from a number of vascularized tissues, such as adipose and muscle, allows the isolation of large enough quantities of MSCs to potentially avoid ex vivo expansion steps (211).
At present, multipotent stem cells from a variety of sources have been analyzed and characterized based on cell surface marker expression, colony formation capacity, immunogenicity, in vitro differentiation capacity, and in vivo differentiation capacity (201). However, the reported failure of a number of recent MSC clinical trials to reach primary endpoints indicates that there remains a poor understanding of the role and function of these cells in vivo. Studies of the behavior of MSCs are complicated by the fact that MSC populations are inherently heterogeneous, with large inter-subject variability, and have phenotypes that are altered dramatically by cell culture conditions (253).
One potential solution to the issue of heterogeneity may be the derivation of a homogeneous population of MSCs from a well-characterized cell line. Homogenization of MSCs for commercial purposes may be achieved through the derivation of multipotent stem cells from pluriopotent stem cells, such as ESCs or iPSCs. Recent work has shown that MSCs differentiated from induced pluripotent stem cells, while phennotypically similar to parental MSCs from the same patient, are functionally different in a number of ways (149). This observation suggests that further optimization of such iPSC-derived MSCs, both in terms of culture expansion conditions as well as requirements for lineage-specific differentiation, is clearly needed.
Modeling tissue physiology, development, and disease pathogenesis
In addition to direct clinical application, considerable attention has been paid to the use of MSCs to model human diseases. Several recent studies have addressed the importance of reconstructing multiple components of complex skeletal tissues at once. This more faithfully recapitulates the process of development where the differentiation of tissue and cell type is often encouraged and reinforced by those of neighboring tissue types. A recent study demonstrated improved subchondral bone and cartilage integration of a composite implant constructed of scaffold free synovial membrane derived MSCs juxtaposed with a hydroxyapatite artificial bone versus single implant alone (254). One of the difficulties of culturing tissues with different cells types is that cues for one lineage differentiation may differ or be at odds with those for another lineage. For example, the standard medium for osteogenic differentiation of MSCs contains serum, while that for chondrogenic differentiation does not. One way around this limitation is a temporary early treatment with serum containing medium to jump start osteogenic differentiation, followed by serum free chondrogenic medium (255). Another advance that is aiding the construction of complex skeletal tissues is the development of microphysiological tissue systems (256, 257). To simulate the osteochondral unit of the articular joint, 3D microfabrication techniques such as projection stereolithography, have been used to produce microbioreactors that maintain MSC-derived osteochondral constructs consisting of contiguous chondral and osseous components, fed by independent culture medium streams. Such biphasic microphysiological systems mimic physiologically the osteochondral junction in vivo and are being used to model the pathogenesis of osteoarthritis and tissue responses to pro-inflammatory cytokines, such as IL-1β (258–260). Similar systems to mimic complex musculoskeletal tissues such as the muscle tendon enthesis are also being developed (261). Innovative culture techniques and bioreactors will allow for the construction of these more complex skeletal tissue engineered constructs. Finally, combinations of different biomaterials have also been used to induce zonal organization with specific chondrocyte phenotypes corresponding to the different layers of articular cartilage in a single construct (262).
4. CONCLUSIONS
The overview of stem cells in skeletal tissue engineering clearly indicates that a greater and more in-depth understanding of the developmental biology of stem cells is critical for formulating experimental approaches that more faithfully replicate natural tissues, and enhancing probability of success. A great deal of progress has been made in the past 20 years in elucidating the identity and mechanism of action of genes that contribute to these developmental programs, using tools such as inducible gene expression, and the Cre-lox system for conditional gene deletion/addition. As we apply our understanding of development to generate more sophisticated and faithful recapitulations of native tissue, we also generate more sophisticated models to investigate the underlying pathways and mechanisms of developmental biology further. By incorporating contemporary molecular biology based research technologies, such as RNA-seq, genomic editing, and analytical non-invasive imaging, as well as sophisticated biomaterial engineering approaches into stem cell-based tissue engineering studies, it should be possible to construct truly informative and biomimetic experimental models for diagnosing disease, understanding pathogenesis, and engineering personalized regenerative approaches.
Figure 2. Developmental markers of appendicular skeletal tissue differentiation.
Lineage tracing experiments in mice have revealed that expression of specific genes is limited to certain skeletal lineages. The above depicts the currently understood hierarchy of markers that label specific mesenchymal lineages in vivo.
Figure 3. Schema for isolation, differentiation, and application of stem cells in musculoskeletal therapies.
In general, stem cells derive either from the embryonic blastocyst or adult organisms. Pluripotent stem cells can be derived from the inner cell mass of the blastocyst, or from reprogramming of adult somatic cells. Mesenchymal stem cell populations can be isolated from many adult tissues. Differentiation of pluripotent stem cells to skeletal lineages can proceed through an embryoid body from which mesoderm must be manually isolated; through direct conversion to a mesenchymal precursor cell type such as neural crest, mesoderm, or MSC followed by skeletal differentiation; or by direct conversion to skeletal lineage. Multipotent stem cell isolated from adults can be directly converted to skeletal lineages.
Table 1.
Differentiation of Chondrocytes from PSCs
| Method to derive mesenchymal progenitors | Growth factors for induction of chondrogenesis | Biomaterial | Reference |
|---|---|---|---|
| EB outgrowth | BMP2,TGFβ3 | (263) | |
| “ | TGFβ1,BMP2,FGF2,PDGF-bb | Gelatin/type II collagen coating | (264) |
| “ | TGFβ1 | Hydrogel | (265) |
| “ | TGFβ1 | PCL/gelatin nanofibrous scaffolds | (171) |
| Coculture with chondrocytes | TGFβ1 or TGFβ3 | (266, 267) | |
| Single cell dissociation, switch to BMSC media | TGFβ1,BMP7 | (268) |
Table 2.
Differentiation of Osteoblasts from PSCs
| Method to derive mesenchymal progenitors | Growth factors for induction of osteogenesis | Biomaterial | Reference |
|---|---|---|---|
| N/A | BMP2 | HA-PLGA scaffold | (269) |
| Switch to MSC medium | Dexamethasone, ascorbic acid, β-Glycerophosphate | 3D osteoconductive scaffolds in bioreactors | (148) |
| N/A | Disassociate into single cell, Dexamethasone, ascorbic acid, β-Glycerophosphate | fibronectin-coating | (270) |
| EB outgrowth | Dexamethasone, ascorbic acid, β-Glycerophosphate | (271) | |
| EB outgrowth | Dexamethasone, ascorbic acid, β-Glycerophosphate | HA-PLGA scaffold | (272) |
| EB outgrowth (with or without BMP2 gene transduction) | Dexamethasone, ascorbic acid, β-Glycerophosphate | Calcium Phosphate Cement Scaffold | (273) |
| Switch to MSC medium | Dexamethasone, ascorbic acid, β-Glycerophosphate | Decellularized bone matrix | (276) |
| Switch to MSC medium | Dexamethasone, ascorbic acid, β-Glycerophosphate | PCL scaffold | (67) |
Table 3.
Differentiation of MSCs from PSCs
Acknowledgments
The authors would like to acknowledge members of the Center for Cellular and Molecular engineering for helpful discussions on the related subject matter of this review. This work was supported by grants from the NIH to MTL (5T32HL076124-08) and by grants from the Commonwealth of PA Department of Health and the Pittsburgh Foundation to RST.
References
- 1.Till JE, Mc CE. Early repair processes in marrow cells irradiated and proliferating in vivo. Radiation research. 1963;18:96–105. [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue engineering. 2005;11(7–8):1198–211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 4.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. Journal of dental research. 2008;87(2):107–18. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyment NA, Liu CF, Kazemi N, Aschbacher-Smith LE, Kenter K, Breidenbach AP, et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PloS one. 2013;8(3):e59944. doi: 10.1371/journal.pone.0059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. The Journal of the American Academy of Orthopaedic Surgeons. 2013;21(5):303–11. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(11):1319–26. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Boyette LB, Tuan RS. Adult Stem Cells and Diseases of Aging. Journal of clinical medicine. 2014;3(1):88–134. doi: 10.3390/jcm3010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(51):20609–14. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acloque H, Ocana OH, Matheu A, et al. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev Cell. 2011;21(3):546–58. doi: 10.1016/j.devcel.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman B. Taking the Middle Road: Vertebrate Mesoderm Formation and the Blastula-Gastrula Transition. In: Moody SA, editor. Principles of Developmental Genetics. 2. Washington, DC: Elsevier; 2014. pp. 203–36. [Google Scholar]
- 12.Malashichev Y, Christ B, Prols F. Avian pelvis originates from lateral plate mesoderm and its development requires signals from both ectoderm and paraxial mesoderm. Cell and tissue research. 2008;331(3):595–604. doi: 10.1007/s00441-007-0556-6. [DOI] [PubMed] [Google Scholar]
- 13.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 14.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Disease models & mechanisms. 2011;4(1):31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134(17):3133–44. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- 16.Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Current biology: CB. 2010;20(22):1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pignatti E, Zeller R, Zuniga A. To BMP or not to BMP during vertebrate limb bud development. Seminars in cell & developmental biology. 2014;32:119–27. doi: 10.1016/j.semcdb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269–84. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuan RS. Cellular signaling in developmental chondrogenesis: N-cadherin, Wnts, and BMP-2. The Journal of bone and joint surgery American volume. 2003;85-A(Suppl 2):137–41. doi: 10.2106/00004623-200300002-00019. [DOI] [PubMed] [Google Scholar]
- 20.Raspopovic J, Marcon L, Russo L, Sharpe J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science. 2014;345(6196):566–70. doi: 10.1126/science.1252960. [DOI] [PubMed] [Google Scholar]
- 21.Seo HS, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Developmental biology. 2007;310(2):304–16. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2001;9(Suppl A):S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Molecular and cellular biology. 1997;17(4):2336–46. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Tsang KY, Tang HC, Chan D, Cheah KSE. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12097–102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuss P, Kraft K, Stumm J, Ibrahim D, Vallecillo-Garcia P, Mundlos S, et al. Regulation of cell polarity in the cartilage growth plate and perichondrium of metacarpal elements by HOXD13 and WNT5A. Developmental biology. 2014;385(1):83–93. doi: 10.1016/j.ydbio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 28.Vortkamp A. Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2001;9(Suppl A):S109–17. [PubMed] [Google Scholar]
- 29.Yeung Tsang K, Wa Tsang S, Chan D, Cheah KS. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth defects research Part C, Embryo today: reviews. 2014;102(1):52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- 30.Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1–2):285–98. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix biology: journal of the International Society for Matrix Biology. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Developmental biology. 2008;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macica C, Liang G, Nasiri A, Broadus AE. Genetic evidence of the regulatory role of parathyroid hormone-related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis and rheumatism. 2011;63(11):3333–43. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ, Lassar AB. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes & development. 2014;28(2):127–39. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pazin DE, Gamer LW, Cox KA, Rosen V. Molecular profiling of synovial joints: use of microarray analysis to identify factors that direct the development of the knee and elbow. Developmental dynamics: an official publication of the American Association of Anatomists. 2012;241(11):1816–26. doi: 10.1002/dvdy.23861. [DOI] [PubMed] [Google Scholar]
- 36.Ochi K, Derfoul A, Tuan RS. A predominantly articular cartilage-associated gene, SCRG1, is induced by glucocorticoid and stimulates chondrogenesis in vitro. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2006;14(1):30–8. doi: 10.1016/j.joca.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. The Journal of cell biology. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nature medicine. 2013;19(6):704–12. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nature reviews Rheumatology. 2014 doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14665–70. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nature reviews Molecular cell biology. 2012;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 43.Bonewald LF. The amazing osteocyte. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes & development. 1999;13(16):2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nature genetics. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 46.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ journal of surgery. 2007;77(8):626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 47.Maier JA, Lo Y, Harfe BD. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PloS one. 2013;8(1):e55528. doi: 10.1371/journal.pone.0055528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9484–9. doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126(23):5399–408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 50.DiPaola CP, Farmer JC, Manova K, Niswander LA. Molecular signaling in intervertebral disk development. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2005;23(5):1112–9. doi: 10.1016/j.orthres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Sohn P, Cox M, Chen D, Serra R. Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc. BMC developmental biology. 2010;10:29. doi: 10.1186/1471-213X-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine. 2009;34(5):447–55. doi: 10.1097/BRS.0b013e3181990c64. [DOI] [PubMed] [Google Scholar]
- 53.Kadow T, Sowa G, Vo N, Kang JD. Molecular Basis of Intervertebral Disc Degeneration and Herniations: What Are the Important Translational Questions? Clinical orthopaedics and related research. 2014 doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PloS one. 2012;7(4):e35944. doi: 10.1371/journal.pone.0035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winkler T, Mahoney EJ, Sinner D, Wylie CC, Dahia CL. Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PloS one. 2014;9(6):e98444. doi: 10.1371/journal.pone.0098444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS letters. 2011;585(8):1209–15. doi: 10.1016/j.febslet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113(2):235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 58.Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131(16):3885–96. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- 59.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth defects research Part C, Embryo today: reviews. 2013;99(3):203–22. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136(8):1351–61. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown JP, Finley VG, Kuo CK. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. Journal of biomechanics. 2014;47(1):214–22. doi: 10.1016/j.jbiomech.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10538–42. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, et al. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141(19):3683–96. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 64.Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D. Loss of Tenomodulin Results in Reduced Self-Renewal and Augmented Senescence of Tendon Stem/Progenitor Cells. Stem cells and development. 2014 doi: 10.1089/scd.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England journal of medicine. 1994;331(14):889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 66.Brown PT, Handorf AM, Jeon WB, Li WJ. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Current pharmaceutical design. 2013;19(19):3429–45. doi: 10.2174/13816128113199990350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC, et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Scientific reports. 2013;3:2243. doi: 10.1038/srep02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 69.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 70.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309(5739):1369–73. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 71.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Current biology: CB. 2001;11(19):1553–8. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–12. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 76.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Si-Tayeb K, Noto FK, Sepac A, Sedlic F, Bosnjak ZJ, Lough JW, et al. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC developmental biology. 2010;10:81. doi: 10.1186/1471-213X-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 80.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell stem cell. 2009;4(5):381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116(3):386–95. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- 83.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochemical and biophysical research communications. 2010;394(1):189–93. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- 85.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura K, Sano M, Ohtaka M, Furuta B, Umemura Y, Nakajima Y, et al. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. The Journal of biological chemistry. 2011;286(6):4760–71. doi: 10.1074/jbc.M110.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell stem cell. 2010;7(1):11–4. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15768–73. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desponts C, Ding S. Using small molecules to improve generation of induced pluripotent stem cells from somatic cells. Methods in molecular biology. 2010;636:207–18. doi: 10.1007/978-1-60761-691-7_13. [DOI] [PubMed] [Google Scholar]
- 90.Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31(1):36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao J, Jiang WJ, Sun C, Hou CZ, Yang XM, Gao JG. Induced pluripotent stem cells: origins, applications, and future perspectives. J Zhejiang Univ Sci B. 2013;14(12):1059–69. doi: 10.1631/jzus.B1300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 94.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–4. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 95.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell stem cell. 2013;12(4):407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 96.Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013;45(5):289–304. doi: 10.1111/and.12008. [DOI] [PubMed] [Google Scholar]
- 97.Young RA. Control of the embryonic stem cell state. Cell. 2011;144(6):940–54. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding VM, Boersema PJ, Foong LY, Preisinger C, Koh G, Natarajan S, et al. Tyrosine phosphorylation profiling in FGF-2 stimulated human embryonic stem cells. PloS one. 2011;6(3):e17538. doi: 10.1371/journal.pone.0017538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zoumaro-Djayoon AD, Ding V, Foong LY, Choo A, Heck AJ, Munoz J. Investigating the role of FGF-2 in stem cell maintenance by global phosphoproteomics profiling. Proteomics. 2011;11(20):3962–71. doi: 10.1002/pmic.201100048. [DOI] [PubMed] [Google Scholar]
- 100.Eiselleova L, Matulka K, Kriz V, Kunova M, Schmidtova Z, Neradil J, et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem cells. 2009;27(8):1847–57. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–30. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 102.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 103.Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124(Pt 12):1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakanishi M, Kurisaki A, Hayashi Y, Warashina M, Ishiura S, Kusuda-Furue M, et al. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23(1):114–22. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 105.Ullmann U, Gilles C, De Rycke M, Van de Velde H, Sermon K, Liebaers I. GSK-3-specific inhibitor-supplemented hESC medium prevents the epithelial-mesenchymal transition process and the up-regulation of matrix metalloproteinases in hESCs cultured in feeder-free conditions. Mol Hum Reprod. 2008;14(3):169–79. doi: 10.1093/molehr/gan001. [DOI] [PubMed] [Google Scholar]
- 106.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, et al. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(12):4485–90. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mimura S, Kimura N, Hirata M, Tateyama D, Hayashida M, Umezawa A, et al. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol. 2011;55(2):181–7. doi: 10.1387/ijdb.103232sm. [DOI] [PubMed] [Google Scholar]
- 108.Yang Z, Sui L, Toh WS, Lee EH, Cao T. Stage-dependent effect of TGF-beta1 on chondrogenic differentiation of human embryonic stem cells. Stem cells and development. 2009;18(6):929–40. doi: 10.1089/scd.2008.0219. [DOI] [PubMed] [Google Scholar]
- 109.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132(6):1273–82. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 110.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem cells. 2006;24(6):1476–86. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 111.Lim HJ, Han J, Woo DH, Kim SE, Kim SK, Kang HG, et al. Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long-term culture and differentiating embryoid bodies. Mol Cells. 2011;31(2):123–32. doi: 10.1007/s10059-011-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zachar V, Prasad SM, Weli SC, Gabrielsen A, Petersen K, Petersen MB, et al. The effect of human embryonic stem cells (hESCs) long-term normoxic and hypoxic cultures on the maintenance of pluripotency. In vitro cellular & developmental biology. Animal. 2010;46(3–4):276–83. doi: 10.1007/s11626-010-9305-3. [DOI] [PubMed] [Google Scholar]
- 113.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(13):4783–8. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139(1):85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cameron CM, Harding F, Hu WS, Kaufman DS. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Experimental biology and medicine. 2008;233(8):1044–57. doi: 10.3181/0709-RM-263. [DOI] [PubMed] [Google Scholar]
- 116.Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. The Journal of biological chemistry. 2005;280(5):3493–9. doi: 10.1074/jbc.M406613200. [DOI] [PubMed] [Google Scholar]
- 117.Das B, Bayat-Mokhtari R, Tsui M, Lotfi S, Tsuchida R, Felsher DW, et al. HIF-2alpha suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells. Stem cells. 2012;30(8):1685–95. doi: 10.1002/stem.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prasad SM, Czepiel M, Cetinkaya C, Smigielska K, Weli SC, Lysdahl H, et al. Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif. 2009;42(1):63–74. doi: 10.1111/j.1365-2184.2008.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sugimoto K, Yoshizawa Y, Yamada S, Igawa K, Hayashi Y, Ishizaki H. Effects of hypoxia on pluripotency in murine iPS cells. Microsc Res Tech. 2013;76(10):1084–92. doi: 10.1002/jemt.22269. [DOI] [PubMed] [Google Scholar]
- 120.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell stem cell. 2009;5(3):237–41. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 121.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3(8):637–46. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 122.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soteriou D, Iskender B, Byron A, Humphries JD, Borg-Bartolo S, Haddock MC, et al. Comparative proteomic analysis of supportive and unsupportive extracellular matrix substrates for human embryonic stem cell maintenance. The Journal of biological chemistry. 2013;288(26):18716–31. doi: 10.1074/jbc.M113.463372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hughes C, Radan L, Chang WY, Stanford WL, Betts DH, Postovit LM, et al. Mass spectrometry-based proteomic analysis of the matrix microenvironment in pluripotent stem cell culture. Molecular & cellular proteomics: MCP. 2012;11(12):1924–36. doi: 10.1074/mcp.M112.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature biotechnology. 2007;25(6):681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 126.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell stem cell. 2010;7(2):240–8. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(Suppl 4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lou YR, Kanninen L, Kuisma T, Niklander J, Noon LA, Burks D, et al. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem cells and development. 2014;23(4):380–92. doi: 10.1089/scd.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siti-Ismail N, Bishop AE, Polak JM, Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29(29):3946–52. doi: 10.1016/j.biomaterials.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 131.Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 2010;31(3):404–12. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 132.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem cells. 2006;24(2):426–33. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 133.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7(12):989–94. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villa-Diaz LG, Ross AM, Lahann J, Krebsbach PH. Concise review: The evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem cells. 2013;31(1):1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen W, Villa-Diaz LG, Sun Y, Weng S, Kim JK, Lam RH, et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS nano. 2012;6(5):4094–103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y, Villa-Diaz LG, Lam RH, Chen W, Krebsbach PH, Fu J. Mechanics regulates fate decisions of human embryonic stem cells. PloS one. 2012;7(5):e37178. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three dimensional nanofibrillar surfaces induce activation of Rac. Biochemical and biophysical research communications. 2005;331(2):428–34. doi: 10.1016/j.bbrc.2005.03.195. [DOI] [PubMed] [Google Scholar]
- 138.Oh SK, Chen AK, Mok Y, Chen X, Lim UM, Chin A, et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2(3):219–30. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25(1):20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue engineering Part A. 2009;15(8):2051–63. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138(1–2):24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 142.Storm MP, Orchard CB, Bone HK, Chaudhuri JB, Welham MJ. Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnology and bioengineering. 2010;107(4):683–95. doi: 10.1002/bit.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(52):E5039–48. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsumaki N, Okada M, Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48–54. doi: 10.1016/j.bone.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 145.Teramura T, Onodera Y, Mihara T, Hosoi Y, Hamanishi C, Fukuda K. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cellular reprogramming. 2010;12(3):249–61. doi: 10.1089/cell.2009.0086. [DOI] [PubMed] [Google Scholar]
- 146.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental hematology. 2008;36(3):350–9. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS medicine. 2005;2(6):e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, et al. Engineering bone tissue from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8705–9. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem cells and development. 2014;23(14):1594–610. doi: 10.1089/scd.2013.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nature biotechnology. 2010;28(11):1187–94. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 151.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16806–11. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135(17):2969–79. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 153.Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132(19):4363–74. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- 154.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107(1):111–7. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 155.Era T, Izumi N, Hayashi M, Tada S, Nishikawa S, Nishikawa S. Multiple mesoderm subsets give rise to endothelial cells, whereas hematopoietic cells are differentiated only from a restricted subset in embryonic stem cell differentiation culture. Stem cells. 2008;26(2):401–11. doi: 10.1634/stemcells.2006-0809. [DOI] [PubMed] [Google Scholar]
- 156.Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, et al. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127(9):1931–41. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- 157.Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111(4):1933–41. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 158.Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(48):19240–5. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim K, Ossipova O, Sokol SY. Neural Crest Specification by Inhibition of the ROCK/Myosin II Pathway. Stem cells. 2015;33(3):674–85. doi: 10.1002/stem.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang SL, Harnish E, Leeuw T, Dietz U, Batchelder E, Wright PS, et al. Compound screening platform using human induced pluripotent stem cells to identify small molecules that promote chondrogenesis. Protein & cell. 2012;3(12):934–42. doi: 10.1007/s13238-012-2107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saito T, Yano F, Mori D, Ohba S, Hojo H, Otsu M, et al. Generation of Col2a1-EGFP iPS cells for monitoring chondrogenic differentiation. PloS one. 2013;8(9):e74137. doi: 10.1371/journal.pone.0074137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(12):1450–6. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 163.Noth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Advanced drug delivery reviews. 2010;62(7–8):765–83. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 164.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(47):19172–7. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim MS, Jun I, Shin YM, Jang W, Kim SI, Shin H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol Biosci. 2010;10(1):91–100. doi: 10.1002/mabi.200900168. [DOI] [PubMed] [Google Scholar]
- 166.Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2010;93(1):247–57. doi: 10.1002/jbm.a.32535. [DOI] [PubMed] [Google Scholar]
- 167.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 168.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26(25):5158–66. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 169.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of biomedical materials research. 2002;60(4):613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 170.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta biomaterialia. 2006;2(4):377–85. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 171.Liu J, Nie H, Xu Z, Niu X, Guo S, Yin J, et al. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PloS one. 2014;9(11):e111566. doi: 10.1371/journal.pone.0111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. Journal of cellular biochemistry. 2013;114(2):480–90. doi: 10.1002/jcb.24388. [DOI] [PubMed] [Google Scholar]
- 173.Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9(1):82–8. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Garin G, Berk BC. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium. 2006;13(6):375–84. doi: 10.1080/10623320601061599. [DOI] [PubMed] [Google Scholar]
- 175.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. European cells & materials. 2009;18:1–13. doi: 10.22203/ecm.v018a01. discussion -4. [DOI] [PubMed] [Google Scholar]
- 176.Kim MJ, Park JS, Kim S, Moon SH, Yang HN, Park KH, et al. Encapsulation of bone morphogenic protein-2 with Cbfa1-overexpressing osteogenic cells derived from human embryonic stem cells in hydrogel accelerates bone tissue regeneration. Stem cells and development. 2011;20(8):1349–58. doi: 10.1089/scd.2010.0311. [DOI] [PubMed] [Google Scholar]
- 177.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36(5):758–69. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 178.Hu J, Smith LA, Feng K, Liu X, Sun H, Ma PX. Response of human embryonic stem cell-derived mesenchymal stem cells to osteogenic factors and architectures of materials during in vitro osteogenesis. Tissue engineering Part A. 2010;16(11):3507–14. doi: 10.1089/ten.tea.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sengupta S, Park SH, Patel A, Carn J, Lee K, Kaplan DL. Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue engineering Part A. 2010;16(12):3623–34. doi: 10.1089/ten.tea.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290(4):C1139–46. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 181.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PloS one. 2011;6(9):e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen X, Yin Z, Chen JL, Shen WL, Liu HH, Tang QM, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Scientific reports. 2012;2:977. doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem cells. 2009;27(6):1276–87. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 184.Watts AE, Yeager AE, Kopyov OV, Nixon AJ. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem cell research & therapy. 2011;2(1):4. doi: 10.1186/scrt45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5856–61. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rashid T, Kobayashi T, Nakauchi H. Revisiting the flight of Icarus: making human organs from PSCs with large animal chimeras. Cell stem cell. 2014;15(4):406–9. doi: 10.1016/j.stem.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 189.Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem cells. 2012;30(6):1120–33. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 191.Jeon K, Lim H, Kim JH, Thuan NV, Park SH, Lim YM, et al. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem cells and development. 2012;21(14):2642–55. doi: 10.1089/scd.2011.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120(5):408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Barruet E, Hsiao EC. Using Human Induced Pluripotent Stem Cells to Model Skeletal Diseases. Methods in molecular biology. 2014 doi: 10.1007/7651_2014_171. [DOI] [PubMed] [Google Scholar]
- 196.Kim MJ, Son MJ, Son MY, Seol B, Kim J, Park J, et al. Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells. Arthritis and rheumatism. 2011;63(10):3010–21. doi: 10.1002/art.30488. [DOI] [PubMed] [Google Scholar]
- 197.Wei Y, Zeng W, Wan R, Wang J, Zhou Q, Qiu S, et al. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. European cells & materials. 2012;23:1–12. doi: 10.22203/ecm.v023a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Deyle DR, Khan IF, Ren G, Wang PR, Kho J, Schwarze U, et al. Normal collagen and bone production by gene-targeted human osteogenesis imperfecta iPSCs. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(1):204–13. doi: 10.1038/mt.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 200.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 201.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem cells. 2014;32(6):1408–19. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 202.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology. 2014;32(3):252–60. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo. Journal of inflammation. 2010;7:51. doi: 10.1186/1476-9255-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10–8. doi: 10.1016/j.bone.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 205.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 206.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis research & therapy. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lin CS, Xin ZC, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histology and histopathology. 2013;28(9):1109–16. doi: 10.14670/hh-28.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vasandan AB, Shankar SR, Prasad P, Sowmya Jahnavi V, Bhonde RR, Jyothi Prasanna S. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. Journal of cellular and molecular medicine. 2014;18(2):344–54. doi: 10.1111/jcmm.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 210.Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem cells translational medicine. 2012;1(3):237–47. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Murray IR, Corselli M, Petrigliano FA, Soo C, Peault B. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. The bone & joint journal. 2014;96-B(3):291–8. doi: 10.1302/0301-620X.96B3.32789. [DOI] [PubMed] [Google Scholar]
- 212.Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39(4):678–83. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 213.Hoffman MD, Benoit DS. Agonism of Wnt-beta-catenin signalling promotes mesenchymal stem cell (MSC) expansion. Journal of tissue engineering and regenerative medicine. 2013 doi: 10.1002/term.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO molecular medicine. 2013;5(3):430–40. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Qu F, Wang J, Xu N, Liu C, Li S, Wang N, et al. WNT3A modulates chondrogenesis via canonical and non-canonical Wnt pathways in MSCs. Frontiers in bioscience. 2013;18:493–503. doi: 10.2741/4116. [DOI] [PubMed] [Google Scholar]
- 216.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis and rheumatism. 2008;58(5):1377–88. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental cell research. 1998;238(1):265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 218.Jaager K, Fatkina A, Velts A, Orav E, Neuman T. Variable expression of lineage regulators in differentiated stromal cells indicates distinct mechanisms of differentiation towards common cell fate. Gene. 2014;533(1):173–9. doi: 10.1016/j.gene.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 219.Handorf AM, Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PloS one. 2011;6(7):e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Palumbo S, Tsai TL, Li WJ. Macrophage migration inhibitory factor regulates AKT signaling in hypoxic culture to modulate senescence of human mesenchymal stem cells. Stem cells and development. 2014;23(8):852–65. doi: 10.1089/scd.2013.0294. [DOI] [PubMed] [Google Scholar]
- 221.Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem cells translational medicine. 2014;3(2):241–54. doi: 10.5966/sctm.2013-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu S, Zhang E, Yang M, Lu L. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-beta. Molecular and cellular biochemistry. 2014;390(1–2):123–31. doi: 10.1007/s11010-014-1963-0. [DOI] [PubMed] [Google Scholar]
- 223.Guerit D, Brondello JM, Chuchana P, Philipot D, Toupet K, Bony C, et al. FOXO3A regulation by miRNA-29a Controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem cells and development. 2014;23(11):1195–205. doi: 10.1089/scd.2013.0463. [DOI] [PubMed] [Google Scholar]
- 224.Meretoja VV, Dahlin RL, Wright S, Kasper FK, Mikos AG. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials. 2013;34(17):4266–73. doi: 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochemical and biophysical research communications. 2002;290(2):763–9. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 226.Roux C, Pisani DF, Yahia HB, Djedaini M, Beranger GE, Chambard JC, et al. Chondrogenic potential of stem cells derived from adipose tissue: a powerful pharmacological tool. Biochemical and biophysical research communications. 2013;440(4):786–91. doi: 10.1016/j.bbrc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 227.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Kujat R, et al. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. International orthopaedics. 2013;37(5):945–51. doi: 10.1007/s00264-013-1800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Eyckmans J, Roberts SJ, Bolander J, Schrooten J, Chen CS, Luyten FP. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials. 2013;34(19):4612–21. doi: 10.1016/j.biomaterials.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim YH, Yoon DS, Kim HO, Lee JW. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem cells and development. 2012;21(16):2958–68. doi: 10.1089/scd.2011.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 231.Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26(34):7082–94. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 232.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–74. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 233.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 234.Glennon-Alty L, Williams R, Dixon S, Murray P. Induction of mesenchymal stem cell chondrogenesis by polyacrylate substrates. Acta biomaterialia. 2013;9(4):6041–51. doi: 10.1016/j.actbio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Coates EE, Fisher JP. Engineering superficial zone chondrocytes from mesenchymal stem cells. Tissue engineering Part C, Methods. 2014;20(8):630–40. doi: 10.1089/ten.tec.2013.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(19):6940–5. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrine reviews. 1993;14(4):424–42. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 238.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Human gene therapy. 2000;11(8):1201–10. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 239.Lu Z, Roohani-Esfahani SI, Wang G, Zreiqat H. Bone biomimetic microenvironment induces osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. Nanomedicine: nanotechnology, biology, and medicine. 2012;8(4):507–15. doi: 10.1016/j.nano.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 240.Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nature medicine. 2012;18(3):456–62. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28(22):3338–48. doi: 10.1016/j.biomaterials.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 242.Lin H, Zhang D, Alexander PG, Yang G, Tan J, Cheng AW, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 2013;34(2):331–9. doi: 10.1016/j.biomaterials.2012.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue engineering Part A. 2008;14(10):1615–27. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 244.Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34(37):9295–306. doi: 10.1016/j.biomaterials.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Alberton P, Popov C, Pragert M, Kohler J, Shukunami C, Schieker M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem cells and development. 2012;21(6):846–58. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports medicine, arthroscopy, rehabilitation, therapy & technology: SMARTT. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, et al. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2014 doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29(23):2627–32. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 249.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem cell research & therapy. 2010;1(2):18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nesti LJ, Li WJ, Shanti RM, Jiang YJ, Jackson W, Freedman BA, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue engineering Part A. 2008;14(9):1527–37. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chik TK, Chooi WH, Li YY, Ho FC, Cheng HW, Choy TH, et al. Bioengineering a Multicomponent Spinal Motion Segment Construct-A 3D Model for Complex Tissue Engineering. Advanced healthcare materials. 2015;4(1):99–112. doi: 10.1002/adhm.201400192. [DOI] [PubMed] [Google Scholar]
- 252.Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. Journal of cellular physiology. 2010;222(1):23–32. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 253.Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Molecular biology of the cell. 2005;16(3):1131–41. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, et al. Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue engineering Part A. 2014;20(17–18):2291–304. doi: 10.1089/ten.tea.2013.0414. [DOI] [PubMed] [Google Scholar]
- 255.France LA, Scotchford CA, Grant DM, Rashidi H, Popov AA, Sottile V. Transient serum exposure regimes to support dual differentiation of human mesenchymal stem cells. Journal of tissue engineering and regenerative medicine. 2014;8(8):652–63. doi: 10.1002/term.1567. [DOI] [PubMed] [Google Scholar]
- 256.Sutherland ML, Fabre KM, Tagle DA. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem cell research & therapy. 2013;4(Suppl 1):I1. doi: 10.1186/scrt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fabre KM, Livingston C, Tagle DA. Organs-on-chips (microphysiological systems): tools to expedite efficacy and toxicity testing in human tissue. Experimental biology and medicine. 2014;239(9):1073–7. doi: 10.1177/1535370214538916. [DOI] [PubMed] [Google Scholar]
- 258.Alexander PG, Gottardi R, Lin H, Lozito TP, Tuan RS. Three-dimensional osteogenic and chondrogenic systems to model osteochondral physiology and degenerative joint diseases. Experimental biology and medicine. 2014;239(9):1080–95. doi: 10.1177/1535370214539232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lin H, Lozito TP, Alexander PG, Gottardi R, Tuan RS. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1beta. Mol Pharm. 2014;11(7):2203–12. doi: 10.1021/mp500136b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lozito TP, Alexander PG, Lin H, Gottardi R, Cheng AW, Tuan RS. Three-dimensional osteochondral microtissue to model pathogenesis of osteoarthritis. Stem cell research & therapy. 2013;4(Suppl 1):S6. doi: 10.1186/scrt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rothrauff BB, Tuan RS. Cellular therapy in bone-tendon interface regeneration. Organogenesis. 2014;10(1):13–28. doi: 10.4161/org.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32(29):6946–52. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 263.Kim MS, Hwang NS, Lee J, Kim TK, Leong K, Shamblott MJ, et al. Musculoskeletal differentiation of cells derived from human embryonic germ cells. Stem cells. 2005;23(1):113–23. doi: 10.1634/stemcells.2004-0110. [DOI] [PubMed] [Google Scholar]
- 264.Toh WS, Guo XM, Choo AB, Lu K, Lee EH, Cao T. Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. Journal of cellular and molecular medicine. 2009;13(9B):3570–90. doi: 10.1111/j.1582-4934.2009.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem cells. 2007;25(11):2730–8. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 266.Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PloS one. 2008;3(6):e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bigdeli N, Karlsson C, Strehl R, Concaro S, Hyllner J, Lindahl A. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem cells. 2009;27(8):1812–21. doi: 10.1002/stem.114. [DOI] [PubMed] [Google Scholar]
- 268.Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis and rheumatism. 2009;60(12):3686–92. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- 269.Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20379–84. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Arpornmaeklong P, Wang Z, Pressler MJ, Brown SE, Krebsbach PH. Expansion and characterization of human embryonic stem cell-derived osteoblast-like cells. Cellular reprogramming. 2010;12(4):377–89. doi: 10.1089/cell.2009.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bilousova G, Jun du H, King KB, De Langhe S, Chick WS, Torchia EC, et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem cells. 2011;29(2):206–16. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hwang NS, Varghese S, Lee HJ, Zhang Z, Elisseeff J. Biomaterials directed in vivo osteogenic differentiation of mesenchymal cells derived from human embryonic stem cells. Tissue engineering Part A. 2013;19(15–16):1723–32. doi: 10.1089/ten.tea.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen W, Zhou H, Weir MD, Tang M, Bao C, Xu HH. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair. Tissue engineering Part A. 2013;19(7–8):915–27. doi: 10.1089/ten.tea.2012.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue engineering Part A. 2014;20(7–8):1295–305. doi: 10.1089/ten.tea.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu J, Chen W, Zhao Z, Xu HH. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials. 2013;34(32):7862–72. doi: 10.1016/j.biomaterials.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012;33(33):8329–42. doi: 10.1016/j.biomaterials.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20641–6. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem cells. 2006;24(8):1914–22. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 279.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem cells. 2007;25(2):425–36. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 280.Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem cells translational medicine. 2012;1(2):83–95. doi: 10.5966/sctm.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PloS one. 2012;7(3):e33225. doi: 10.1371/journal.pone.0033225. [DOI] [PMC free article] [PubMed] [Google Scholar]