Lignin polymers in the tough outer skin (endocarp) of palm fruits are produced, in part, from a new class of monomers, hydroxystilbenes, including the valuable resveratrol and piceatannol.
Abstract
Lignin, the plant cell wall polymer that binds fibers together but makes processing difficult, is traditionally formed from three monomers, the so-called monolignols (p-coumaryl, coniferyl, and sinapyl alcohols). Recently, we discovered, in grass lignins, a phenolic monomer that falls outside the canonical lignin biosynthetic pathway, the flavone tricin. As we show here, palm fruit (macaúba [Acrocomia aculeata], carnauba [Copernicia prunifera], and coconut [Cocos nucifera]) endocarps contain lignin polymers derived in part from a previously unconsidered class of lignin monomers, the hydroxystilbenes, including the valuable compounds piceatannol and resveratrol. Piceatannol could be released from these lignins upon derivatization followed by reductive cleavage, a degradative method that cleaves β-ether bonds, indicating that at least a fraction is incorporated through labile ether bonds. Nuclear magnetic resonance spectroscopy of products from the copolymerization of piceatannol and monolignols confirms the structures in the natural polymer and demonstrates that piceatannol acts as an authentic monomer participating in coupling and cross-coupling reactions during lignification. Therefore, palm fruit endocarps contain a new class of stilbenolignin polymers, further expanding the definition of lignin and implying that compounds such as piceatannol and resveratrol are potentially available in what is now essentially a waste product.
Lignin has long been considered to be a complex phenylpropanoid polymer derived essentially from the oxidative radical coupling of three p-hydroxycinnamyl alcohols (monolignols) differing in their degree of methoxylation, p-coumaryl, coniferyl, and sinapyl alcohols, that form the p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, respectively, when incorporated into the lignin polymer (Boerjan et al., 2003; Ralph et al., 2004; Morreel et al., 2010). Once synthesized in the cytoplasm, the monolignols are transported to the cell wall, where they are oxidized and polymerized. The oxidation/dehydrogenation reaction is initiated by one-electron oxidation of a phenolic monolignol to its phenoxy radical by plant peroxidases and laccases. Lignification then proceeds by radical coupling of two phenoxy radicals between positions dictated by resonance delocalization of the single-electron density and usually in an end-wise manner between a monomer and the growing lignin chain, giving rise to variously interconnected monomer-derived units characterized by their interunit ether and carbon-carbon linkages.
It is now increasingly appreciated that lignins also derive from monomeric units beyond the traditional monolignols. As has been reviewed (Ralph, 2010; Vanholme et al., 2012; Mottiar et al., 2016), several other phenolic compounds, all deriving from the shikimate-derived monolignol biosynthetic pathway, have been found to behave as lignin monomers in many plants, participating in radical coupling reactions during lignification and resulting in cross-coupled structures. Several monomers derived from truncated monolignol biosynthesis, such as the hydroxycinnamaldehydes that are the immediate precursors of monolignols, also are especially prevalent in various mutant and transgenic plants. Incompletely methylated monomers, caffeyl and 5-hydroxyconiferyl alcohols, are found in O-methyltransferase-deficient mutants and transgenics and can be found as the sole lignin monomers involved in seed coat lignification in several plants (Chen et al., 2012, 2013). Monolignol ester conjugates (with acetate, p-coumarate, and p-hydroxybenzoate) also are used as lignin monomers in a variety of natural plants (Ralph, 2010). We recently exploited the clear metabolic malleability of lignification by extending the monolignol conjugates to include monolignol ferulates. Introducing an exotic feruloyl-CoA monolignol transferase gene/protein into poplar (Populus spp.) and Arabidopsis (Arabidopsis thaliana), in a nature-inspired manner, was recently shown to successfully introduce readily chemically cleavable ester linkages into the lignin backbone, facilitating its depolymerization during pretreatments or pulping (Wilkerson et al., 2014). It was shown subsequently, via more sensitive analytical methods, that nature has, in fact, been biosynthesizing lignins with low levels of these conjugates all along in a variety of plant species but not universally (Karlen et al., 2016).
In all of the cases above, however, the monomers have been derived from the monolignol biosynthetic pathway. The recent discovery of the flavone tricin in the lignins from grasses and other monocots, therefore, was unanticipated, but is now well established (del Río et al., 2012b; Rencoret et al., 2013; Lan et al., 2015, 2016a, 2016b; Eloy et al., 2017). Tricin, unlike monolignols that originate from the shikimate biosynthetic pathway, is biosynthesized from a combination of the shikimate and acetate/malonate-derived polyketide pathways. Tricin’s only mode of incorporation is via 4′-O-β-coupling with a monolignol; therefore, it can only appear at the initiating end of the lignin chain.
All these discoveries indicate that lignification is a flexible mechanism and that the plant is capable of using a variety of phenolic compounds for the formation of the lignin polymers. The discovery of nonconventional phenolic precursors, different from the three canonical monolignols, illustrates the high metabolic plasticity of lignification and reveals that any phenolic compound that is transported to the cell wall may be oxidized and incorporated into the lignin polymer during lignification via radical coupling reactions, subject exclusively to simple chemical compatibility (Boerjan et al., 2003; Ralph et al., 2004, 2008; Ralph, 2006, 2010; Vanholme et al., 2008, 2012; Morreel et al., 2010; Mottiar et al., 2016). In this study, we report the occurrence of a second class of polyphenolic compounds, hydroxystilbenes, also arising from outside the monolignol biosynthetic pathway, in the lignins of palm fruit endocarps.
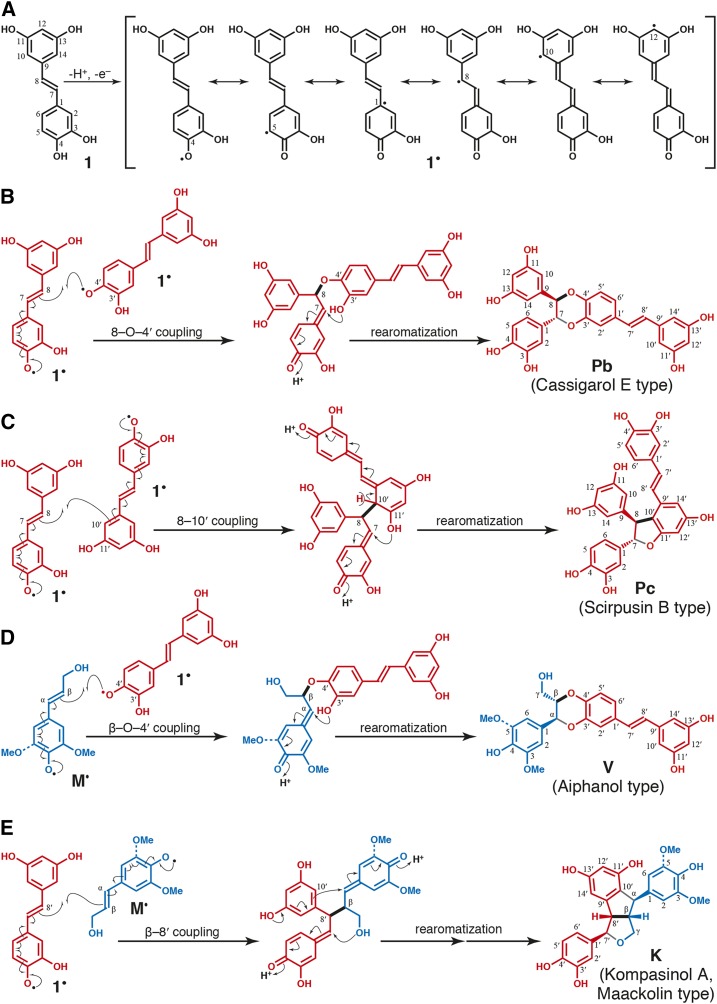
Hydroxystilbenes are a class of nonflavonoid polyphenolics that, like the flavonoids, are metabolic hybrids resulting from a combination of the shikimate-derived phenylpropanoid and the acetate/malonate-derived polyketide pathways. Hydroxystilbenes, as with monolignols, can be oxidized to form radicals that are resonance stabilized, as shown for piceatannol 1 in Figure 1A. Hydroxystilbene (dehydro)dimerization (Fig. 1, B and C), or the cross-coupling of different hydroxystilbenes, produces a wide variety of dimers and higher oligomers. Illustrating their latent chemical compatibility with lignification, hydroxystilbenes can cross-couple with monolignols M (Fig. 1, D and E); several stilbenolignans have been identified in a variety of plants from different families (Kobayashi et al., 1996; Lee et al., 2001; Yao et al., 2006; Begum et al., 2010). Therefore, it is reasonable to speculate that phenolic stilbenoids present in the cell wall also could cross-couple with monolignols and the growing lignin polymer to become integrally incorporated into the lignin structure, as occurs with other nontraditional monomers. We propose that such polymers be classed as stilbenolignins and the low-molecular-mass oligomers as stilbenolignols, as for the recently coined terms flavonolignins and flavonolignols (Lan et al., 2015, 2016a, 2016b).
Figure 1.
Piceatannol radical, and radical coupling reactions. A, The most stable phenolic radical is that from dehydrogenation of the 4-OH. Resonance forms show how coupling can occur at the 4-O-, 5-, 8-, and 10-positions, among others. B, Piceatannol dehydrodimerization by 8-O-4′ coupling to give Pb, cassigarol E (Li et al., 2005). C, Piceatannol dehydrodimerization by 8-10′ coupling to give Pc, scirpusin B (Nakajima et al., 1978). D, Cross-coupling of monolignols M, sinapyl and coniferyl alcohol, with piceatannol 1 via β-O-4′ coupling to give the stilbenolignols V, aiphanol (Lee et al., 2001; Begum et al., 2010) and its G analog. E, Cross-coupling of monolignols M with piceatannol 1 via β-8′ coupling to give the stilbenolignols K, kompasinol A or maackolin (Kobayashi et al., 1996; Lee et al., 2001; Yao et al., 2006; Begum et al., 2010) and their G analogs. Note that the names are often given for dimeric hydroxystilbenes or stilbenolignans that may be optically active; here, we are referring to the racemic compounds produced during lignification. Stereochemical rendering on Pb, Pc, V, and K is to show the trans-nature of the rings and does not imply optical activity: the other enantiomer is equally present in the racemates. These structures, other than K (for which no indication can be found), are evidenced in the lignins from macaúba, carnauba, and coconut palm endocarps.
RESULTS AND DISCUSSION
Release of Hydroxystilbenes by Derivatization Followed by Reductive Cleavage
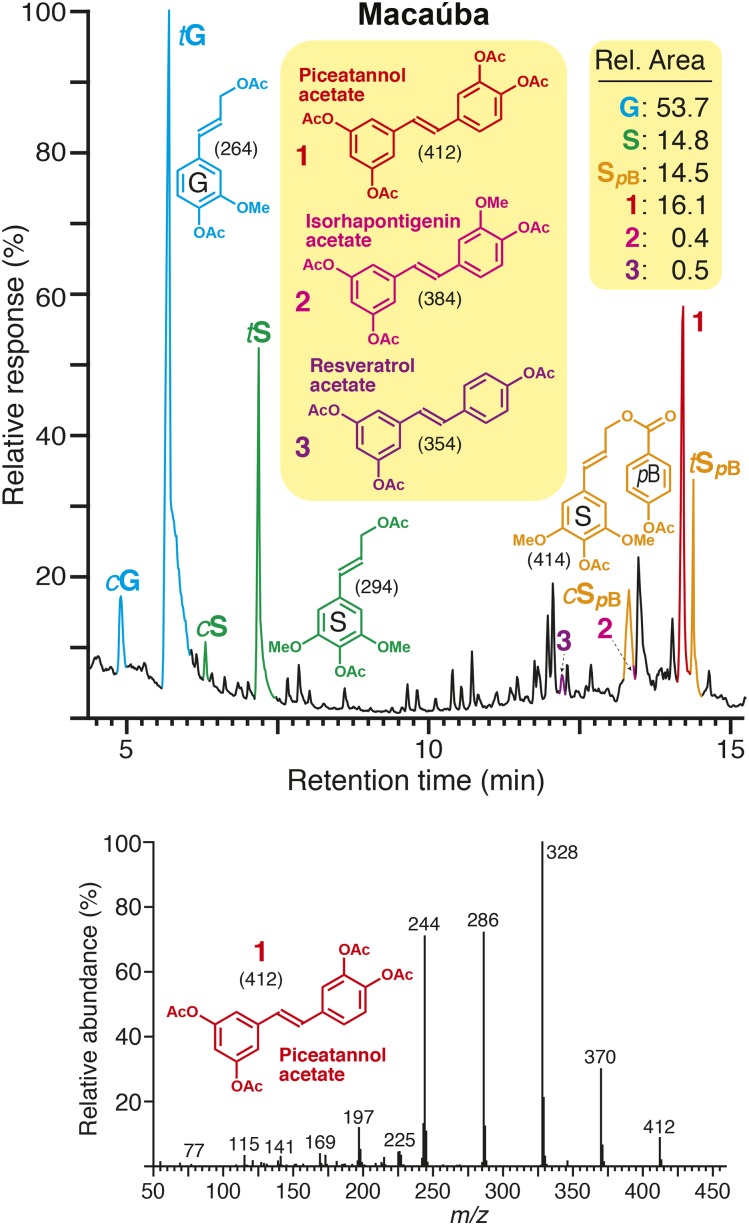
Lignins isolated from macaúba (Acrocomia aculeata), carnauba (Copernicia prunifera), and coconut (Cocos nucifera) palm fruit endocarps were analyzed by derivatization followed by reductive cleavage (DFRC), the degradation method that cleaves β-ether bonds in the lignin polymer but leaves γ-esters intact (Lu and Ralph, 1997a; Wilkerson et al., 2014; Lu et al., 2015; Karlen et al., 2016). The chromatograms of the DFRC degradation products from macaúba (Fig. 2) and from all three palms (Supplemental Fig. S1) show the released cis- and trans-isomers of G (cG and tG) and S (cS and tS) lignin monomers (as their acetylated derivatives) arising from normal units in lignin as well as peaks corresponding to γ-p-hydroxybenzoylated S (cSpB and tSpB) lignin units as usually noted from the lignins from palms (Ralph and Landucci, 2010; Rencoret et al., 2013; Lu et al., 2015). More interesting was a strong peak, released from both macaúba and carnauba lignins and at low levels from coconut lignin, that we had not observed previously and was identified as the hydroxystilbene piceatannol 1 (Fig. 2; Supplemental Fig. S1). Its identity was confirmed by comparison with an authentic piceatannol standard that presented exactly the same retention time and mass spectrum as the released compound. Two other related hydroxystilbenes, isorhapontigenin 2 and resveratrol 3, also were released from these lignins upon DFRC, although in lower amounts. The relative areas from the gas chromatography-mass spectrometry-total ion chromatogram peaks are given on the figures. For reasons that will become evident below, we note that neither of the products from other catechols, caffeyl or 5-hydroxyconiferyl alcohol, that have been observed in lignins from various O-methyltransferase-deficient plants, was evident beyond the trace levels that are always seen from the demethylation of G and S units via DFRC (and are always in proportion to the normal G and S monomer levels; Lu and Ralph, 1998).
Figure 2.
Hydroxystilbenes released from lignins by reductive cleavage. Top, Total ion chromatogram of the DFRC degradation products released from the lignin from macaúba fruit endocarps, showing the presence of stilbenoid compounds (1, piceatannol; 2, isorhapontigenin; 3, resveratrol, as their acetate derivatives). cG, tG, cS, and tS are the normal cis- and trans-coniferyl (G) and sinapyl (S) alcohol monomers (as their acetate derivatives). cSpB and tSpB are the cis- and trans-sinapyl p-hydroxybenzoates (as their acetate derivatives). Bottom, Electron-impact mass spectrum from peak 1 matches that of an authentic standard of piceatannol 1 (acetylated). The chromatograms from all three palm endocarps examined here (macaúba, carnauba, and coconut) are shown in Supplemental Figure S1. Relative peak areas are given, with all identified lignin-derived aromatic components, including those from the released hydroxystilbenes, totaling 100%. (For the traces from all three palm samples, see Supplemental Fig. S1).
Piceatannol, isorhapontigenin, and resveratrol have not been detected previously in native lignins. Such hydroxystilbenes, like the lignin monomers themselves, can only be released via DFRC from polymer units that are present in β-ether-linked structures. Unlike tricin, which has only one possible mode of incorporation, piceatannol would be expected to couple and cross-couple with other piceatannol molecules through different types of linkages, forming a variety of dimeric (e.g., Pb [Fig. 1B] and Pc [Fig. 1C]) and oligomeric structures. Indeed, such compounds, often optically active, are known from various plant extractives (Baba et al., 1994; Iliya et al., 2002; Li et al., 2005; Xiang et al., 2005; Morikawa et al., 2010; Quideau et al., 2011). Moreover, piceatannol also can cross-couple with monolignols (and oligolignols) in a variety of ways, two of which are shown in Figure 1, D and E. That such cross-coupling reactions can occur is evidenced by the array of stilbenolignans, including aiphanol V and kompasinol A (or maackolin) K, which are the radical coupling products of piceatannol and sinapyl alcohol (Kobayashi et al., 1996; Lee et al., 2001; Banwell et al., 2005; Begum et al., 2010). Although lignification and lignan formation are distinct processes separated in time and space (Ralph et al., 1999; Umezawa, 2003), the presence of such stilbenolignans reveals that piceatannol also is compatible with the radical coupling reactions that typify lignification. The actual amounts of piceatannol monomers being incorporated into these lignins is logically, due to the coupling modes available to them, higher than the level released by DFRC. An assessment of the extent to which the polymer derives from piceatannol (and the other hydroxystilbenes), and the types of structures produced, is most readily gained from NMR studies.
NMR Examination of the Lignins for Coupling and Cross-Coupling Products of Piceatannol
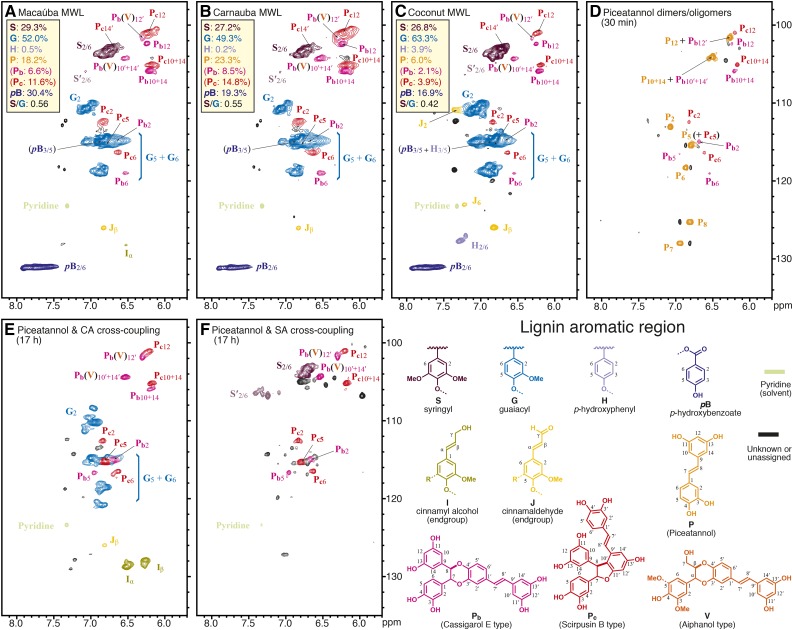
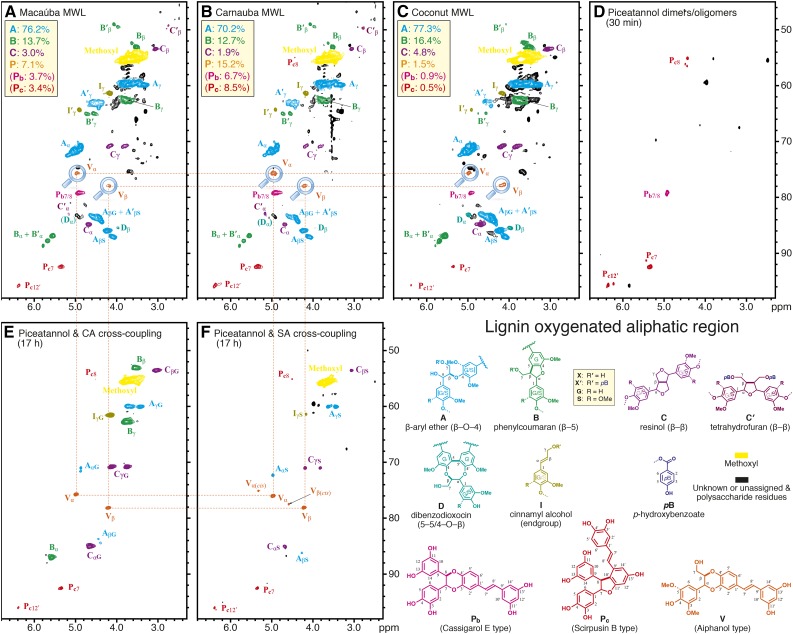
The lignins isolated from macaúba, carnauba, and coconut palm fruit endocarps were analyzed by 2D HSQC (heteronuclear single-quantum coherence) NMR in DMSO-d6:pyridine-d5 (4:1; Figs. 3 and 4); in order to assess the product levels in the entire material, the unfractionated cell wall material also was analyzed via previously described whole cell wall NMR methods (Mansfield et al., 2012; Supplemental Fig. S2). Large differences were observed in the spectra of these lignins with respect to the spectra of other typical lignins, particularly in the signals observed in the aromatic region (Fig. 3). The main aromatic correlation peaks corresponded to the different lignin (G and S) units as well as the pendant p-hydroxybenzoates (pB). The most striking feature was the presence of a previously unreported group of strong signals (labeled Pb and Pc) appearing at 100 to 107/5.8 to 6.8 ppm (δC/δH) that we assign here to piceatannol-derived units. Likewise, the oxygenated-aliphatic region of the spectra (Fig. 4) also showed signals other than those commonly observed from conventional lignin structures (β-aryl ethers A, phenylcoumarans B, resinols C, or cinnamyl alcohol end groups I). These correlation peaks, labeled Pb and Pc, are assigned here to structures involving piceatannol units. Definitive assignments of these signals were achieved by HSQC-TOCSY (Supplemental Fig. S3) and HMBC (Supplemental Fig. S4) experiments and by comparing with piceatannol polymerization products and in vitro biomimetic cross-coupling reaction results (Figs. 3 and 4, D–F). Thus, Pb was identified as a benzodioxane structure formed via 8-O-4′-type radical coupling of a piceatannol unit at its 8-position with another piceatannol unit (at its O-4′ position), followed by internal trapping of the quinone methide intermediate by the 3′-hydroxyl group (Fig. 1B); Pc was identified as a phenylcoumaran structure formed by the radical coupling of a piceatannol unit (at its 8-position) with another piceatannol unit (at its 10′-position) followed by a subsequent 11′-O-7 bonding during rearomatization of the quinone methide intermediate (Fig. 1C). The structure of Pc was confirmed by comparing with the HSQC spectrum of ε-viniferin, a related dehydrodimer of resveratrol (Ngoc et al., 2008; Wang et al., 2011). Assignment details are described in Supplemental Data S1.
Figure 3.
Aromatic regions of the 2D HSQC NMR spectra. A, Macaúba fruit endocarp milled wood lignin (MWL). B, Carnauba fruit endocarp MWL. C, Coconut endocarp MWL. D, Dimers and oligomers from biomimetic coupling of piceatannol. E, Piceatannol and coniferyl alcohol cross-coupled polymers from biomimetic coupling. F, Piceatannol and sinapyl alcohol cross-coupled polymers from biomimetic coupling. The piceatannol-derived peaks in the palm endocarp lignins are well matched with those from the biomimetic in vitro polymerization and dimerization products. Note that, as neither the piceatannol stilbene double-bond P7 and P8 peaks nor the corresponding P7′ and P8′ peaks from the piceatannol dimers Pb and Pc or the crossed-dimer V are evident in the lignins (A–C) or the cross-coupled synthetic lignins (E and F), the initial coupling of piceatannol at its 8-position must be highly dominant; the piceatannol-containing structures shown are to label the moieties from the various homo-coupled and cross-coupled entities but do not imply that such intact units are in the polymer (i.e. they are clearly further coupled in the polymer). All spectra were run in DMSO-d6:pyridine-d5 (4:1). Analogous spectra from unfractionated whole cell wall samples and the coniferyl alcohol + piceatannol biomimetic cross-coupling, showing most of the same spectral features, are shown in Supplemental Figure S2. Compositional percentages are from volume integration and are on an S + G + H + piceatannol = 100% basis; the p-hydroxybenzoate, because it is pendant on the lignin and because, as a more slowly relaxing unit, it is significantly overrepresented, is not included in this total but expressed as a percentage of that total.
Figure 4.
Oxygenated-aliphatic regions of the 2D HSQC NMR spectra. A, Macaúba fruit endocarp MWL. B, Carnauba fruit endocarp MWL. C, Coconut endocarp MWL. D, Piceatannol dimers and oligomers showing both Pb and Pc structures with correlations that are well matched with peaks in the three lignins. E, Piceatannol and coniferyl alcohol cross-coupled polymers. F, Piceatannol and sinapyl alcohol cross-coupled polymers. The piceatannol-monolignol polymers provided evidence of the cross-coupling reactions to produce benzodioxane structures V. Both trans- and cis-configurations are evidenced in the in vitro polymerization products, but only the trans-form can be found in the lignins. Again, analogous whole cell wall spectra are shown in Supplemental Figure S2. Structures are with R = H (G unit) or R = OMe (S unit) and are labeled as X (R′ = H) or X′ (R′ = pB), where X is generic for A, B, D, and I; C′ is a special case that gets its own structure. Aromatic rings also are designated as G or S (or G/S in the case of either being allowed), with a label’s color intensity signifying the main types; for example, structures C and C′ are largely S based, with such G units being minor. Percentages for the various units are from volume integration and total 100%.
In addition to the radical dehydrodimerization structures from two piceatannol monomers, an important structure V, arising from the cross-coupling of piceatannol and monolignols, also was identified in the lignins of all three palm fruit endocarps. As anticipated for cross-coupling of a monomer with a catechol unit, characteristic benzodioxane structures are clearly observed in the NMR spectra of Figure 4. The Cα/Hα (Vα) and Cβ/Hβ (Vβ) correlations were observed at δC/δH 75.8/4.97 and 78/4.2 in DMSO-d6:pyridine-d5 (4:1), whereas the Cγ/Hγ correlations appear at around δC/δH 60.1/3.44 and 3.63, superimposed on other signals. All these signals are in the same coupling network, as seen in the HSQC-TOCSY spectrum (Supplemental Fig. S3). These correlation signals match those for benzodioxane structures found in other lignins from the catechol monomers caffeyl alcohol and 5-hydroxyconiferyl alcohol, particularly with the trans-isomer (Chen et al., 2012, 2013; Tobimatsu et al., 2013), and are clearly different from the benzodioxane structure of homo-coupled piceatannols Pb. The benzodioxane structures do not result from the incorporation of caffeyl alcohol or from the related 5-hydroxyconiferyl alcohol, both of which may be evidenced in the spectra of lignins from O-methyltransferase-deficient plants (Ralph et al., 2001; Wagner et al., 2011), as the expected monomers from such involvement in lignins were not observed here by DFRC and the characteristic aromatic signals from such catechols also were not evident; all of the peaks in the HSQC spectra (Fig. 3, A–C) are fully consistent with those from the incorporation of piceatannol and with the piceatannol homo-coupled dimers/oligomers (Fig. 3D) and, for structures V in particular, its cross-coupling products with monolignols (Fig. 3, E and F). The cross-coupled benzodioxane structures found in the lignins from palm fruit endocarps, therefore, are uniquely formed via radical coupling of a monolignol (at its β-position) and the catechol moiety of piceatannol (at its O-4′-position), followed by internal trapping of the quinone methide intermediate by the 3′-hydroxyl group in the piceatannol unit to form the benzodioxane structure V (Fig. 1D). A similar cross-coupling product of piceatannol and sinapyl alcohol, the stilbenolignan aiphanol, having a benzodioxane bridge, has been found in the seeds of Aiphanes aculeata from the Arecaceae family (Lee et al., 2001). Biomimetic cross-coupling reactions between piceatannol and p-hydroxycinnamyl alcohols successfully proved that benzodioxane structures V could be easily formed during the radical reaction; both coniferyl alcohol (Fig. 4E) and sinapyl alcohol (Fig. 4F) produced respectable levels of benzodioxane structures V, in which the chemical shifts of the unique peaks are well matched with the correlations appearing in the lignins here (Fig. 4). The occurrence of these benzodioxane structures in the lignins from macaúba, carnauba, and coconut palm fruit endocarps compellingly demonstrates that the stilbene piceatannol acts as an authentic monomer participating in coupling and cross-coupling reactions during the lignification of these tissues.
Further strong evidence for the cross-coupling of piceatannol to monolignol or lignin components is the complete absence of P7 and P8 peaks from the piceatannol end group in cross-coupling reactions and in the lignin samples. Such P7 and P8 peaks of the piceatannol end group were detected and assigned from in vitro piceatannol homo-coupling dimerization/oligomerization reactions (Fig. 3D). Normally, monolignol end groups, including the double bonds in cinnamyl alcohol side chains (Fig. 3E, peaks for structure I), can be easily found in the spectra from synthetic lignins and also at lower levels in isolated lignin polymers. However, we could not detect the piceatannol end-group peaks (P7 and P8) in the spectra from any of the lignin samples (Fig. 3, A–C) or from the products of the in vitro cross-coupling experiments (Fig. 3, E and F), yet the piceatannol had clearly integrated into these polymers. It is apparent that most of the piceatannol must radically couple to monolignols or the phenolic end of the growing lignin polymer by coupling at P8 before further polymerization at its catechol and/or resorcinol ends. As neither the piceatannol stilbene double-bond P7 and P8 peaks nor the corresponding P7′ and P8′ peaks from the piceatannol dimers Pb and Pc or the crossed dimer V are evident in the lignins (Fig. 3, A–C) or the cross-coupled synthetic lignins (Fig. 3, E and F), the initial coupling of piceatannol at its 8-position must be highly dominant. Elucidating piceatannol’s mode of incorporation into the lignin polymer will require significantly more sophisticated studies.
Mechanisms for Dehydrodimerization of Piceatannol and Its Cross-Coupling with Monolignols and the Growing Lignin Polymer
As occurs with monolignols, piceatannol (as for other hydroxystilbenes) also is oxidized by peroxidases and/or laccases to form a radical that is stabilized by resonance (Fig. 1A). These radicals can couple and cross-couple with other stilbenoids, forming a variety of dimers and higher oligostilbenes (Fig. 1, B and C; Quideau et al., 2011; Keylor et al., 2015). In addition, piceatannol can cross-couple with monolignols via radical coupling reactions, generating a variety of stilbenolignans (Fig. 1, D and E); chiral compounds of this type are presumably used in plant defense (Lee et al., 2001; Begum et al., 2010). It is obvious from the polymers analyzed here that piceatannol (and also its dimers and higher oligomers) also can cross-couple with monolignols and the growing lignin polymer, in the typically chemically controlled fashion of lignification, to be integrally incorporated into the racemic lignin polymer. As we have concluded previously, any phenolic component present in the cell wall during lignification can be incorporated into the polymer, simply subject to its chemical compatibility with the radical coupling reactions involving the components in that zone (Ralph et al., 2008). The implication here is that, for whatever reason, these palms are both synthesizing and, possibly, transporting hydroxystilbenes to the cell wall for polymerization to produce these previously unknown polymers.
The mechanisms for the formation of the different structures involving piceatannol units identified in the lignins from palm fruit endocarps are detailed in Figure 1. Piceatannol dimerization can produce various structures characterized by their different interunit linkages, including the 8-O-4′ and 8-10′ structures found in the lignins from palm fruit endocarps. The 8-O-4′ (cassigarol E type) benzodioxane structure is formed via 8-O-4′-type radical coupling of a piceatannol unit at its 8-position (a position equivalent to the β-position of a monolignol) with another piceatannol unit (at its O-4′ position) followed by internal trapping of the quinone methide intermediate by the 3′-hydroxyl group, forming a benzodioxane structure (Fig. 1B), as in structures Pb in the lignins. The mechanism for the formation of the 8-10′ (scirpusin A type) phenylcoumaran structure (Pc in the lignin) involves the radical coupling of a piceatannol unit (at its 8-position) with another piceatannol unit (at its 10′-position) followed by a subsequent 11′-O-7 bonding during rearomatization of the quinone methide intermediate, producing the phenylcoumaran structure, as shown in Figure 1C. Such a coupling reaction is possible due to the highly extended conjugation in these stilbene systems, in which single-electron density from the radical produced by abstraction of the H from the 4-OH of piceatannol extends all the way out to C10 (and, in fact, C12; Fig. 1A). The mechanism for β-O-4′ cross-coupling of a p-hydroxycinnamyl alcohol (at its β-position) and a piceatannol (as its 4′-O-position) is similar to that for the formation of the benzodioxane structure from two piceatannols shown above and also involves the subsequent 3′-O-α′ bonding during quinone methide rearomatization, producing the benzodioxane structure V found in the lignins of the selected palm fruit endocarps (Fig. 1D).
In addition to the structures identified in the lignins from palm fruit endocarps, and due to the large variety of radical coupling products that can potentially form from the highly conjugated piceatannol and p-hydroxycinnamyl alcohols, several other structures coupled at different positions also could be formed by homo- and cross-coupling. Among these structures, we can refer to the stilbenolignans kompasinol A (isolated from Koompassia malaccensis, from the Fabaceae) and maackolin (isolated from Maackia amurensis, from the Fabaceae; Kobayashi et al., 1996; Begum et al., 2010). The key ring structure in kompasinol A is formed by radical coupling of sinapyl alcohol at its β-position with the 8′-position of piceatannol followed by internal trapping of the quinone methide on the piceatannol moiety by the γ-OH and rearomatization of the sinapyl alcohol-derived moiety’s quinone methide by nucleophilic attack at its α-position by the electron-rich 10′-position of the piceatannol (Fig. 1E). Although these β-8′ structures were not detected in the lignins here, they and other linkage types may exist, and the unambiguous identification of any of them would provide additional evidence for piceatannol’s being incorporated into the lignin polymer.
Mr Distributions and the Role of Stilbenolignins
The three lignins exhibited similar Mr distributions, around 5,500 to 6,500 g mol−1 with relatively narrow polydispersity, with weight-average (Mw)/number-average (Mn) Mr of ∼1.61 to 1.84 (Supplemental Fig. S5; Supplemental Data S2), which appears to indicate that the lignin polymer in the selected palm fruit endocarps is quite homogenous and, therefore, does not include simple stilbenes, dimers, or higher oligostilbenes mixed with the lignin. Therefore, these data support our contention that the lignin polymer in palm fruit endocarps includes hydroxystilbenes, mostly piceatannol, fully integrated into the polymeric structure.
We can only speculate on the role of these stilbenolignin polymers. Palm fruits are drupes that contain an extremely hard lignified endocarp surrounding the seed. Endocarp lignification, therefore, plays an important role in seed protection. The incorporation of hydroxystilbenes into the lignin polymer may allow the production of higher amounts of lignin (using other phenolic compounds present in the lignification zone) and appears to contribute to endocarp hardening. The piceatannol-derived components also could provide additional antioxidant properties to the endocarp, contributing to seed protection. Although piceatannol has been identified in the lignins of the fruit endocarps from the three palm species selected for this study, the analysis of a broader collection of palm species (and beyond) is required in order to establish the phylogenic range of the occurrence of such stilbenolignins.
CONCLUSION
It is evident from the three palm endocarp lignin polymers analyzed here that piceatannol can cross-couple with monolignols and the growing lignin polymer, in the typically chemically controlled fashion of lignification, to be integrally incorporated into the racemic lignin polymer. As we have concluded previously, any phenolic component present in the cell wall during lignification can be incorporated into the polymer, simply subject to its chemical compatibility with the radical coupling reactions involving the components in that zone (Ralph et al., 2008). The implication here is that, for whatever reason, these palms are synthesizing and, possibly, transporting hydroxystilbenes to their fruit endocarp cell walls for polymerization to produce these previously unknown copolymers; we cannot rule out the possibility that small stilbenolignols are produced in the cytoplasm in the same way that oligolignols also are implicated (Dima et al., 2015) and that it is these that are transported to the wall. The incorporation of nonconventional monomers, not usually present in the lignins of other plants, as is the case for the piceatannol described here, can open up new ways to design and engineer the lignin structure to produce polymers and plant-based biomaterials with altered properties. A whole new generation of modified lignin polymers can be envisioned via introducing hydroxystilbenes into plant lignification pathways, as already anticipated with other phenolic compounds (Grabber et al., 2010, 2012, 2015; Elumalai et al., 2012; Tobimatsu et al., 2012; Vanholme et al., 2012) and as already achieved with the introduction of monolignol ferulates and p-coumarates into plants that do not normally possess them (Wilkerson et al., 2014; Smith et al., 2015; Sibout et al., 2016). Additionally, hydroxystilbenes such as piceatannol and, in particular, resveratrol are quite valuable (with the cheapest prices being approximately $US80 and $280 per kg on alibaba.com), and their potential availability in bulk quantities from lignins will spur additional interest into deriving value from lignins (Rinaldi et al., 2016).
MATERIALS AND METHODS
Samples
Macaúba (Acrocomia aculeata) and carnauba (Copernicia prunifera) palm fruits were collected from native populations located in the municipality of Mirabela, Minas Gerais, Brazil. The coconut (Cocos nucifera) sample originated from India and was supplied from Bonnysa Agroalimentaria. The endocarps of the fruits were separated manually using a knife and subsequently dried in a forced-air oven at 40°C until reaching constant mass. The dried samples were milled using a knife mill (1-mm screen) and extracted successively for 8 h with acetone (200 mL) and hot water (100 mL; 3 h at 100°C) in a Soxhlet apparatus to purify the cell walls. Klason lignin content was estimated as the residue after sulfuric acid hydrolysis of the preextracted material according to the TAPPI method T222 om-88 (http://www.tappi.org/content/SARG/T222.pdf) and further corrected for ash and protein content. Three replicates were used for each sample. The data indicated that the selected palm fruit endocarps contained extremely high lignin contents, accounting for 39.8%, 38.8%, and 33.2% in macaúba, carnauba, and coconut, respectively.
Lignin Isolation and Purification
The lignin preparations were obtained from extractive-free samples according to the classical MWL procedure (Björkman, 1956). Around 40 g of extractive-free material was finely ball milled in a Retsch PM100 planetary ball mill (Retsch) for 25 h at 400 rpm using a 500-mL agate jar and agate ball bearings (20 × 20 mm). The ball-milled material was then extracted with dioxane:water, 96:4 (v/v; 20 mL of solvent g−1 milled fiber), and the isolated lignin was purified subsequently as described previously (del Río et al., 2012a). The final yields were ∼15% of the original Klason lignin content.
Analyses
DFRC
DFRC degradation was performed according to the developed protocol (Lu and Ralph, 1997a, 1997b) using the detailed procedure described previously (del Río et al., 2012a). The acetylated lignin degradation products were then analyzed by gas chromatography-mass spectrometry on a Saturn 4000 (Varian) instrument fitted with a medium-length high-temperature capillary column (DB5-HT; 15 m × 0.25 mm i.d., 0.1-μm film thickness; J&W Scientific). Helium was used as the carrier gas at a rate of 2 mL min−1. The samples were injected with an autoinjector (Varian 8200) directly onto the column using a septum-equipped programmable injector system that was programmed from 120°C (0.1 min) to 340°C at a rate of 200°C min−1 and held at the maximum temperature until the end of the analysis. The oven temperature was programmed from 120°C (1 min) to 340°C (10 min) at a rate of 10°C min−1. The temperature of the transfer line was set at 300°C during the analysis.
Chromatograms of the DFRC products from each of the palm endocarp samples are shown in Figure 2 and Supplemental Figure S1.
NMR Spectroscopy
Multidimensional NMR spectra (2D HSQC, 2D HMBC, 2D HSQC-TOCSY, and three-dimensional TOCSY-HSQC) experiments from lignin samples (∼40 mg) were acquired, in parallel, in DMSO-d6 (0.75 mL) on an AVANCE III 500-MHz instrument (Bruker) and in DMSO-d6:pyridine-d5 (4:1) on a Bruker Biospin (Billerica) AVANCE 700-MHz spectrometer, both fitted with cryogenically cooled 5-mm gradient probes with inverse geometry (proton coils closest to the sample). NMR of polymerization/dimerization products of piceatannols and p-hydroxycinnamyl alcohols also was analyzed in DMSO-d6:pyridine-d5 (4:1) on the 700-MHz NMR instrument. Whole cell wall samples were analyzed in DMSO-d6:pyridine-d5 (4:1) on the 700-MHz NMR instrument based on the gel-NMR method described previously (Kim and Ralph, 2010). The central residual DMSO peak was used as an internal reference (δC/δH 39.5/2.49). All NMR experiments used Bruker’s standard pulse programs: HSQC experiments used hsqcetgpsisp2.2 (adiabatic-pulsed version), the HMBC experiments used hmbcgplpndqf with long-range J-coupling evolution times of 62.5 ms (and/or 80 ms when required), the HSQC-TOCSY experiments used hsqcdietgpsisp.2, and the three-dimensional TOCSY-HSQC experiments (data not shown) used mlevhsqcetgp3d. The detailed NMR experimental conditions have been described elsewhere (del Río et al., 2012a). Integrals are from volume integration of contours from C/H pairs that are in similar coupling environments. Thus, for the aromatics (Fig. 3), the peaks used are S2/6, G2, H2/6, and Pc2; Pb2 was not resolved, so its expected integral was calculated from Pc2 via the ratio of the resolved Pb6 to Pc6 peaks; the pB2/6 peak was used for the p-hydroxybenzoates that are not included in the lignin background total and are expressed simply as a percentage of that total. In Figure 4, the various units were relatively quantified via the volume integrals of the Aα, Bα, Cα, C′α, Pb7, Pc7, and Vα correlation peaks.
The NMR spectra had the following parameters for the lignins: spectra were acquired from 11.5 to −0.5 ppm in F2 (1H) using 3,366 data points for an acquisition time of 200 ms, an interscan delay of 1 s, 215 to −5 ppm in F1 (13C) using 620 increments (F1 acquisition time of 8 ms) of 32 scans, with a total acquisition time of 7 h. For the in vitro polymerization products, 16 scans per increment were performed with a total acquisition time of 3.5 h. Processing used typical matched Gaussian apodization (GB = 0.001, LB = −0.5) in F2 and squared cosine-bell in F1. Interactive integrations of contours in 2D HSQC plots were carried out using Bruker’s TopSpin 3.5 (Mac) software, as was all data processing. Spectra from whole cell wall samples (Supplemental Fig. S2) were run under similar conditions but acquired from 11.5 to −0.5 ppm in F2 (1H) with 1,682 data points (acquisition time of 100 ms), 215 to −5 ppm in F1 (13C) with 620 increments (F1 acquisition time of 8 ms) of 56 scans with a 500-ms interscan delay; the d24 delay was set to 0.86 ms (1/8J, where J = 145 Hz). The total acquisition time for each was 6 h.
Gel-Permeation Chromatography
Gel-permeation chromatography was performed on the Shimadzu Prominence-i LC-2030 3D GPC system equipped with a photodiode array detector using the following conditions: column, PLgel 5-µm MIXED-D, 7.5 × 300 mm (Agilent Technologies); eluent, tetrahydrofuran; flow rate, 0.5 mL min−1; temperature, 40°C; sample detection, photodiode array response at 280 nm. The data acquisition and computation used LabSolution GPC software version 5.82 (Shimadzu). The Mr calibration was via polystyrene standards (Mw range from 5.8 × 102 to 3.24 × 106; Agilent Technologies).
Dimerization of Piceatannol and Polymerization with p-Hydroxycinnamyl Alcohols
Piceatannol 1 (20 mg, 0.082 mmol) was dissolved in acetone:water (1:10 [v/v], 11 mL). Horseradish peroxidase (5 mg; EC 1.11.1.7; 173 purpurogallin units per mg of solid; type II) was added to the reaction solution directly and stirred. Excess hydrogen peroxide (30%, 0.5 mL) was added at once into the reaction solution while the solution was stirred. The color changed to dark red immediately. The solution was stirred for 15 min at room temperature for the short-time dimerization reaction or for 1.5 h for the extended polymerization reaction. The crude products were extracted with ethyl acetate and washed with saturated aqueous solution and water. The ethyl acetate fraction was dried over anhydrous MgSO4 and evaporated.
Cross-coupling polymerization reactions between piceatannol (20 mg, 0.082 mmol) and coniferyl alcohol (14.8 mg, 0.082 mmol) or between piceatannol 1 (20 mg, 0.082 mmol) and sinapyl alcohol (17.2 mg, 0.082 mmol) were performed as above but stirred for 17 h.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Hydroxystilbenes released from lignins by reductive cleavage.
Supplemental Figure S2. 2D HSQC NMR spectra of whole cell walls in DMSO-d6:pyridine-d5.
Supplemental Figure S3. 2D HSQC-TOCSY and HMBC NMR spectra of isolated lignins in DMSO-d6 showing diagnostic Pb, Pc, and V correlations.
Supplemental Figure S4. 2D HMBC NMR spectra of isolated lignins in DMSO-d6 showing main correlations for piceatannol-derived units.
Supplemental Figure S5. Mr distribution of the lignins from the fruit endocarps of macaúba, carnauba, and coconut palm.
Supplemental Data S1. Assignments of the 1H/13C correlation signals of structures involving piceatannol units in the HSQC spectra (in DMSO-d6:pyridine-d5) of the lignins from palm fruit endocarps.
Supplemental Data S2. Mw and Mn Mr values and polydispersity of the MWL isolated from macaúba, carnauba, and coconut fruit endocarps.
Acknowledgments
We thank Anderson B. Evaristo (University of Viçosa) for providing the carnauba and macaúba palm fruits; Dr. Manuel Angulo for performing the preliminary NMR analyses, which were acquired on a Bruker AVANCE III 500-MHz instrument from the NMR facilities of the General Research Services of the University of Seville; as well as Gautham Ramapriya and Christos Maravelias (University of Wisconsin) for helping us find bulk prices for the hydroxystilbenes.
Glossary
- DFRC
derivatization followed by reductive cleavage
- MWL
milled wood lignin
- H
p-hydroxyphenyl
- G
guaiacyl
- S
syringyl
Footnotes
This work was supported by Spanish projects AGL2014-53730-R and CTQ2014-60764-JIN to J.C.d.R., J.Re., and A.G. (cofinanced by FEDER funds), the CSIC Project 2014-40E-097 to J.C.d.R., J.Re., and A.G., and the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494 to J.Ra. and H.K.).
Articles can be viewed without a subscription.
References
- Baba K, Kido T, Taniguchi M, Kozawaqa M (1994) Stilbenoids from Cassia garrettiana. Phytochemistry 36: 1509–1513 [Google Scholar]
- Banwell MG, Chand S, Savage GP (2005) An enantioselective total synthesis of the stilbenolignan (−)-aiphanol and the determination of its absolute stereochemistry. Tetrahedron Asymmetry 16: 1645–1654 [Google Scholar]
- Begum SA, Sahai M, Ray AB (2010) Non-conventional lignans: coumarinolignans, flavonolignans, and stilbenolignans. Fortschr Chem Org Naturst 93: 1–70 [DOI] [PubMed] [Google Scholar]
- Björkman A. (1956) Studies on finely divided wood. Part I. Extraction of lignin with neutral solvents. Sven Papperstidn 59: 477–485 [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Havkin-Frenkel D, Dixon RA, Ralph J (2012) A polymer of caffeyl alcohol in plant seeds. Proc Natl Acad Sci USA 109: 1772–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Jackson L, Nakashima J, Ralph J, Dixon RA (2013) Novel seed coat lignins in the Cactaceae: structure, distribution and implications for the evolution of lignin diversity. Plant J 73: 201–211 [DOI] [PubMed] [Google Scholar]
- del Río JC, Prinsen P, Rencoret J, Nieto L, Jiménez-Barbero J, Ralph J, Martínez ÁT, Gutiérrez A (2012a) Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J Agric Food Chem 60: 3619–3634 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Prinsen P, Martínez ÁT, Ralph J, Gutiérrez A (2012b) Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem 60: 5922–5935 [DOI] [PubMed] [Google Scholar]
- Dima O, Morreel K, Vanholme B, Kim H, Ralph J, Boerjan W (2015) Small glycosylated lignin oligomers are stored in Arabidopsis leaf vacuoles. Plant Cell 27: 695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloy NB, Voorend W, Lan W, Saleme ML, Cesarino I, Vanholme R, Smith RA, Goeminne G, Pallidis A, Morreel K, et al. (2017) Silencing CHALCONE SYNTHASE in maize impedes the incorporation of tricin into lignin and increases lignin content. Plant Physiol 173: 998–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elumalai S, Tobimatsu Y, Grabber JH, Pan X, Ralph J (2012) Epigallocatechin gallate incorporation into lignin enhances the alkaline delignification and enzymatic saccharification of cell walls. Biotechnol Biofuels 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber JH, Ress D, Ralph J (2012) Identifying new lignin bioengineering targets: impact of epicatechin, quercetin glycoside, and gallate derivatives on the lignification and fermentation of maize cell walls. J Agric Food Chem 60: 5152–5160 [DOI] [PubMed] [Google Scholar]
- Grabber JH, Santoro N, Foster CE, Elumalai S, Ralph J, Pan X (2015) Incorporation of flavonoid derivatives or pentagalloyl glucose into lignin enhances cell wall saccharification following mild alkaline or acidic pretreatments. BioEnergy Res 8: 1391–1400 [Google Scholar]
- Grabber JH, Schatz PF, Kim H, Lu F, Ralph J (2010) Identifying new lignin bioengineering targets. 1. Monolignol-substitute impacts on lignin formation and cell wall fermentability. BMC Plant Biol 10: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliya I, Tanaka T, Iinuma M, Ali Z, Furusawa M, Nakaya K (2002) Dimeric stilbenes from stem lianas of Gnetum africanum. Heterocycles 57: 1057–1062 [Google Scholar]
- Karlen SD, Zhang C, Peck ML, Smith RA, Padmakshan D, Helmich KE, Free HCA, Lee S, Smith BG, Lu F, et al. (2016) Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci Adv 2: e1600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keylor MH, Matsuura BS, Stephenson CR (2015) Chemistry and biology of resveratrol-derived natural products. Chem Rev 115: 8976–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ralph J (2010) Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org Biomol Chem 8: 576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Mahmud T, Yoshioka N, Hori K, Shiguya H, Kitagawa I (1996) Indonesian medicinal plants. XVIII. Kompasinol A, a new stilbeno-phenylpropanoid from the bark of Koompassia malaccensis (Fabaceae). Chem Pharm Bull (Tokyo) 44: 2249–2253 [Google Scholar]
- Lan W, Lu F, Regner M, Zhu Y, Rencoret J, Ralph SA, Zakai UI, Morreel K, Boerjan W, Ralph J (2015) Tricin, a flavonoid monomer in monocot lignification. Plant Physiol 167: 1284–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Morreel K, Lu F, Rencoret J, Del Río JC, Voorend W, Vermerris W, Boerjan W, Ralph J (2016a) Maize tricin-oligolignol metabolites and their implications for monocot lignification. Plant Physiol 171: 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Rencoret J, Lu F, Karlen SD, Smith BG, Harris PJ, Del Río JC, Ralph J (2016b) Tricin-lignins: occurrence and quantitation of tricin in relation to phylogeny. Plant J 88: 1046–1057 [DOI] [PubMed] [Google Scholar]
- Lee D, Cuendet M, Vigo JS, Graham JG, Cabieses F, Fong HH, Pezzuto JM, Kinghorn AD (2001) A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata. Org Lett 3: 2169–2171 [DOI] [PubMed] [Google Scholar]
- Li WL, He KK, Li Y, Hou ZJ (2005) Total synthesis of (±)-shegansu B, gnetuhainin F, (±)-maackin A and (±)-cassigarol E. Acta Chim Sin 63: 1607–1612 [Google Scholar]
- Lu F, Karlen SD, Regner M, Kim H, Ralph SA, Sun R, Kuroda K, Augustin MA, Mawson R, Sabarez H, et al. (2015) Naturally p-hydroxybenzoylated lignins in palms. BioEnergy Res 8: 934–952 [Google Scholar]
- Lu F, Ralph J (1997a) Derivatization followed by reductive cleavage (DFRC method), a new method for lignin analysis: protocol for analysis of DFRC monomers. J Agric Food Chem 45: 2590–2592 [Google Scholar]
- Lu F, Ralph J (1997b) The DFRC method for lignin analysis. 1. A new method for β-aryl ether cleavage: lignin model studies. J Agric Food Chem 45: 4655–4660 [Google Scholar]
- Lu F, Ralph J (1998) The DFRC method for lignin analysis. 2. Monomers from isolated lignins. J Agric Food Chem 46: 547–552 [DOI] [PubMed] [Google Scholar]
- Mansfield SD, Kim H, Lu F, Ralph J (2012) Whole plant cell wall characterization using solution-state 2D NMR. Nat Protoc 7: 1579–1589 [DOI] [PubMed] [Google Scholar]
- Morikawa T, Xu F, Matsuda H, Yoshikawa M (2010) Structures of novel norstilbene dimer, longusone A, and three new stilbene dimers, longusols A, B, and C, with antiallergic and radical scavenging activities from Egyptian natural medicine Cyperus longus. Chem Pharm Bull (Tokyo) 58: 1379–1385 [DOI] [PubMed] [Google Scholar]
- Morreel K, Kim H, Lu F, Dima O, Akiyama T, Vanholme R, Niculaes C, Goeminne G, Inzé D, Messens E, et al. (2010) Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal Chem 82: 8095–8105 [DOI] [PubMed] [Google Scholar]
- Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD (2016) Designer lignins: harnessing the plasticity of lignification. Curr Opin Biotechnol 37: 190–200 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Taguchi H, Endo T, Yosioka I (1978) The constituents of Scirpus fluviatilis (Torr.) A. Gray. I. The structures of two new hydroxystilbene dimers, scirpusin A and B. Chem Pharm Bull (Tokyo) 26: 3050–3057 [Google Scholar]
- Ngoc TM, Hung TM, Thuong PT, Na M, Kim H, Ha do T, Min BS, Minh PT, Bae K (2008) Inhibition of human low density lipoprotein and high density lipoprotein oxidation by oligostilbenes from rhubarb. Biol Pharm Bull 31: 1809–1812 [DOI] [PubMed] [Google Scholar]
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 50: 586–621 [DOI] [PubMed] [Google Scholar]
- Ralph J. (2006) What makes a good monolignol substitute? In Hayashi T, ed, The Science and Lore of the Plant Cell Wall: Biosynthesis, Structure and Function. Universal Publishers, Boca Raton, FL, pp 285–293 [Google Scholar]
- Ralph J. (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Brunow G, Harris PJ, Dixon RA, Schatz PF, Boerjan W (2008) Lignification: are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? In Daayf F, El Hadrami A, Adam L, Ballance GM, eds, Recent Advances in Polyphenol Research, Vol 1 Wiley-Blackwell Publishing, Oxford, pp 36–66 [Google Scholar]
- Ralph J, Landucci LL (2010) NMR of lignins. In Heitner C, Dimmel DR, Schmidt JA, eds, Lignin and Lignans: Advances in Chemistry. CRC Press, Boca Raton, FL, pp 137–234 [Google Scholar]
- Ralph J, Lapierre C, Marita JM, Kim H, Lu F, Hatfield RD, Ralph S, Chapple C, Franke R, Hemm MR, et al. (2001) Elucidation of new structures in lignins of CAD- and COMT-deficient plants by NMR. Phytochemistry 57: 993–1003 [DOI] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Ralph J, Peng J, Lu F, Hatfield RD, Helm RF (1999) Are lignins optically active? J Agric Food Chem 47: 2991–2996 [DOI] [PubMed] [Google Scholar]
- Rencoret J, Ralph J, Marques G, Gutiérrez A, Martínez Á, del Río JC (2013) Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J Agric Food Chem 61: 2434–2445 [DOI] [PubMed] [Google Scholar]
- Rinaldi R, Jastrzebski R, Clough MT, Ralph J, Kennema M, Bruijnincx PCA, Weckhuysen BM (2016) Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew Chem Int Ed Engl 55: 8164–8215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Le Bris P, Legée F, Cézard L, Renault H, Lapierre C (2016) Structural redesigning Arabidopsis lignins into alkali-soluble lignins through the expression of p-coumaroyl-CoA:monolignol transferase PMT. Plant Physiol 170: 1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Gonzales-Vigil E, Karlen SD, Park JY, Lu F, Wilkerson CG, Samuels L, Ralph J, Mansfield SD (2015) Engineering monolignol p-coumarate conjugates into poplar and Arabidopsis lignins. Plant Physiol 169: 2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu Y, Elumalai S, Grabber JH, Davidson CL, Pan X, Ralph J (2012) Hydroxycinnamate conjugates as potential monolignol replacements: in vitro lignification and cell wall studies with rosmarinic acid. ChemSusChem 5: 676–686 [DOI] [PubMed] [Google Scholar]
- Tobimatsu Y, Wagner A, Donaldson L, Mitra P, Niculaes C, Dima O, Kim JI, Anderson N, Loque D, Boerjan W, et al. (2013) Visualization of plant cell wall lignification using fluorescence-tagged monolignols. Plant J 76: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T. (2003) Diversity in lignan biosynthesis. Phytochem Rev 2: 371–390 [Google Scholar]
- Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196: 978–1000 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Ralph J, Boerjan W (2008) Lignin engineering. Curr Opin Plant Biol 11: 278–285 [DOI] [PubMed] [Google Scholar]
- Wagner A, Tobimatsu Y, Phillips L, Flint H, Torr K, Donaldson L, Pears L, Ralph J (2011) CCoAOMT suppression modifies lignin composition in Pinus radiata. Plant J 67: 119–129 [DOI] [PubMed] [Google Scholar]
- Wang CY, Lam SH, Tseng LH, Lee SS (2011) Rapid screening of lignans from Phyllanthus myrtifolius and stilbenoids from Syagrus romanzoffiana by HPLC-SPE-NMR. Phytochem Anal 22: 352–360 [DOI] [PubMed] [Google Scholar]
- Wilkerson CG, Mansfield SD, Lu F, Withers S, Park JY, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Rencoret J, et al. (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344: 90–93 [DOI] [PubMed] [Google Scholar]
- Xiang T, Uno T, Ogino F, Ai C, Duo J, Sankawa U (2005) Antioxidant constituents of Caragana tibetica. Chem Pharm Bull (Tokyo) 53: 1204–1206 [DOI] [PubMed] [Google Scholar]
- Yao CS, Lin M, Wang L (2006) Isolation and biomimetic synthesis of anti-inflammatory stilbenolignans from Gnetum cleistostachyum. Chem Pharm Bull (Tokyo) 54: 1053–1057 [DOI] [PubMed] [Google Scholar]