The Arabidopsis L-type lectin receptor-like kinase LecRK-IX.2 recruits calcium dependent protein kinases (CPKs) to phosphorylate NADPH oxidase RbohD to activate H2O2-SA circuit.
Abstract
Plant surface-localized pathogen recognition receptors (PRRs) perceive conserved microbial features, termed pathogen-associated molecular patterns (PAMPs), resulting in disease resistance. PAMP perception leads to calcium influx, MAPK activation, a burst of reactive oxygen species (ROS) mediated by RbohD, accumulation of the defense hormone salicylic acid (SA), and callose deposition. Lectin receptor-like kinases (LecRKs) belong to a specific PRR family and are important players in plant innate immunity. Here, we report that LecRK-IX.2 is a positive regulator of PRR-triggered immunity. Pathogen infection activated the transcription of Arabidopsis (Arabidopsis thaliana) LecRK-IX.2, and the LecRK-IX.2 knockout lines exhibited enhanced susceptibility to virulent Pseudomonas syringae pv tomato DC3000. In addition, LecRK-IX.2 is capable of inducing RbohD phosphorylation, likely by recruiting calcium-dependent protein kinases to trigger ROS production in Arabidopsis. Overexpression of LecRK-IX.2 resulted in elevated ROS and SA and enhanced systemic acquired resistance to P. syringae pv tomato DC3000. Our data highlight the importance of LecRKs in plant immune signaling and SA accumulation.
Plants do not have a circulating immune system but instead have evolved an efficient innate immune system to defend against invading pathogens. Cell surface-located pattern recognition receptors (PRRs) and cytosolic immune receptors are two classical types of plant immune receptors (Couto and Zipfel, 2016). PRRs perceive pathogen-associated molecular patterns (PAMPs) or host-derived damage-associated molecular patterns, leading to PAMP-triggered immunity (PTI; Zipfel, 2014). Typical PTI responses include Ca2+ influx, MAPK activation, reactive oxygen species (ROS) burst, accumulation of the defense hormone salicylic acid (SA), and callose deposition (Kim et al., 2005; Tsuda et al., 2013; Couto and Zipfel, 2016).
Known plant PRRs are either receptor-like kinases (RLKs) or receptor-like proteins (Couto and Zipfel, 2016). PRRs contain a variable ligand-binding ectodomain featuring Leu-rich repeats (LRRs), LysM, or lectin-type motifs that perceive pathogen-derived proteins, peptides, carbohydrate-based ligands, or other ligands. Several PRRs have been well studied in Arabidopsis (Arabidopsis thaliana), such as FLS2, EFR, and CERK1. The LRR-RLKs FLS2 and EFR bind bacterial flagellin (or the epitope flg22; Gómez-Gómez et al., 2000; Zipfel et al., 2004; Chinchilla et al., 2007) and elongation factor Tu (or the minimal epitope elf18; Kunze et al., 2004; Zipfel et al., 2006), respectively, and the LysM-RLK CERK1 coordinates with LYK5 to perceive chitin in Arabidopsis (Miya et al., 2007; Wan et al., 2008; Cao et al., 2014). Upon ligand perception, receptor-like cytoplasmic kinases are activated and can transphosphorylate members of the primary receptor complex, which further transduces signals to downstream components. For example, when FLS2 perceives flg22, it recruits the coreceptor BRI1-ASSOCIATED RECEPTOR KINASE1 to the complex (Lu et al., 2010). FLS2 activation also results in the phosphorylation of intracellular kinases, such as BOTRYTIS-INDUCED KINASE1 (BIK1), and multiple transphosphorylation events (Lu et al., 2010). Activated BIK1 phosphorylates and activates RbohD, the key NADPH oxidase responsible for producing the majority of extracellular ROS upon pathogen perception. Accumulating evidence shows that RbohD is one of the key components of PTI. rbohd mutants are compromised in multiple PTI responses and have an impaired ROS burst, decreased callose deposition, and SA-mediated disease resistance (Kadota et al., 2014; Li et al., 2014). SA also is required for defense priming, leading to heightened resistance against subsequent pathogen attack after initial pathogen perception (Fu and Dong, 2013).
In addition to the well-characterized PRRs that can directly recognize pathogen PAMPs, such as FLS2, EFR, and CERK1, accumulating evidence demonstrates that plant lectin receptor-like kinases (LecRKs) also are important immune regulators. For example, LecRK-VI.2 enhances PTI in Arabidopsis, and transgenic plants overexpressing LecRK-VI.2 display constitutively activated defense responses (Singh et al., 2012). LecRKI.9/DORN1 acts as a PRR for recognition of the damage-associated molecular pattern eATP, and Arabidopsis lecrk-I.9/dorn1 mutants exhibit reduced ATP-triggered defense responses, including impaired Ca2+ influx, MAPK activation, and defense gene expression (Choi et al., 2014). Similarly, the lecrk-IX.1 and lecrk-IX.2 mutants show compromised resistance to Phytophthora spp., and overexpression of these genes led to spontaneous cell death and enhanced disease resistance in Arabidopsis (Wang et al., 2015). There are 75 LecRK family members in Arabidopsis and 173 members in rice (Oryza sativa), which can be divided into three subclasses, the L-, G-, and C-type LecRLKs (Vaid et al., 2013). However, most LecRKs have not been characterized yet, and additional members are likely to be involved in mediating plant defense responses.
During infection, pathogens secrete diverse virulence proteins, called effectors, into host cells to interfere with PTI (Macho and Zipfel, 2015). The bacterial pathogen Pseudomonas syringae pv tomato (Pst) harbors over 30 effectors (Liu et al., 2011), some of which can directly target PRRs. For example, the AvrPtoB effector is an E3 ligase and has been shown to target FLS2, CERK1, and Fen kinases for proteasome-mediated degradation (Abramovitch et al., 2006; Rosebrock et al., 2007; Göhre et al., 2008; Gimenez-Ibanez et al., 2009). Another Pst effector protein, AvrPto, binds FLS2 and EFR to inhibit FLS2 and EFR kinase activity during PTI, thereby inhibiting the plant immune response (Xiang et al., 2008). However, plants have evolved intracellular NB-LRR immune receptors that recognize pathogen-secreted effectors to activate effector-triggered immunity. In resistant tomato (Solanum lycopersicum) cultivars, the Pto kinase and the associated NB-LRR protein Prf recognize AvrPto and AvrPtoB, leading to the hypersensitive response (Pedley and Martin, 2003), a hallmark of effector-triggered immunity.
SA is a signaling molecule that plays a major role in plant defense against biotrophic and hemibiotrophic pathogens (Tsuda et al., 2013). SA INDUCTION-DEFICIENT2 (SID2) encodes an isochorismate synthase, a key enzyme that controls SA biosynthesis (Nawrath and Métraux, 1999; Wildermuth et al., 2001; Tsuda et al., 2013). In Arabidopsis, SA biosynthesis is induced by pathogen infection and is required for PTI (Tsuda et al., 2008). However, it is not fully understood how PTI induces SA accumulation in Arabidopsis. Here, we report the characterization of the pathogen-inducible Arabidopsis LecRK-IX.2. LecRK-IX.2 positively contributes to disease resistance and is involved in flg22-induced SA signaling. Interestingly, LecRK-IX.2 recruits calcium-dependent protein kinases (CPKs) to phosphorylate and activate RbohD to generate ROS and enhances ROS-triggered SA biosynthesis.
RESULTS
LecRK-IX.2 Positively Regulates Disease Resistance to Pst DC3000
In Arabidopsis, there are 45 L-type LecRKs (Bouwmeester and Govers, 2009). In an attempt to discover LecRKs that are responsive to bacterial pathogen invasion, we analyzed the transcription of all 45 L-type LecRKs by surveying the Genevestigator database (https://genevestigator.com/gv). We found that close to 42% (19 out of 45 genes) of LecRK genes were induced by Pst infection. Four members, At5g65600 (LecRK-IX.2), At3g53810 (LecRK-IV.2), At3g45330 (LecRK-I.1), and At3g59740 (LecRK-V.7), were induced significantly by the bacterial pathogen Pst DC3000 ΔhrcC (a type III secretion system-deficient mutant) but suppressed by virulent Pst DC3000 (Supplemental Fig. S1A), suggesting that induction of these LecRKs may be inhibited by pathogen effector(s). Previous studies showed that lecrk-IX.1 and lecrk-IX.2 mutants exhibited compromised resistance to Phytophthora spp., and overexpression of these genes led to enhanced disease resistance (Wang et al., 2015). However, their roles in response to bacterial infection have not been characterized. We focused on LecRK-IX.2, whose expression was induced dramatically by Pst DC3000 ΔhrcC but was suppressed by Pst DC3000 (Fig. 1A). LecRK-IX.2 possesses a predicted transmembrane domain. There is a close homolog of LecRK-IX.2, LecRK-IX.1, whose expression was not induced by Pst infection according to the Genevestigator database (Supplemental Fig. S1A). We also included LecRK-IX.1 in pathogen inoculation assays, which was confirmed as a knockout line by RT-PCR (Supplemental Fig. S1B). The single and double LecRK-IX.1 and LecRK-IX.2 mutants displayed enhanced disease susceptibility to Pst DC3000, indicating that they positively regulate disease resistance against Pst DC3000 (Fig. 1B).
Figure 1.
LecRK-IX.2 positively regulates disease resistance to Pst DC3000. A, LecRK-IX.2 transcription is induced by Pst DC3000 and Pst DC3000 ΔhrcC. Four-week-old plants were infiltrated with 5 × 107 colony-forming units (cfu) mL−1 Pst DC3000 and Pst DC3000 ΔhrcC, then they were sampled at the indicated times. Mock is 10 mm MgCl2. qRT-PCR was used to determine gene expression. Values are means ± sd (n = 3 biological replicates). B, Growth curve analysis of Pst DC3000 in Columbia-0 (Col-0), lecrk-IX.2, lecrk-IX.1, and lecrk-IX.2/lecrk-IX.1 double mutants. Four-week-old plants were infiltrated with 5 × 104 cfu mL−1 Pst DC3000. Plants were subjected to bacterial growth curve analysis at 1 and 3 d postinoculation (dpi). Values are means ± sd (n = 6; ANOVA, P < 0.01). C, Overexpression of LecRK-IX.2 enhances disease resistance to Pst DC3000. Four-week-old homozygous transgenic plants were subjected to syringe inoculation as described in B. OE6-6 and OE14-1 are two independent 35S::LecRK-IX.2 lines. Values are means ± sd (n = 6; ANOVA, P < 0.01).
In order to ascertain the roles of LecRK-IX.2 in immune responses, we also generated the LecRK-IX.2 Arabidopsis overexpression lines. Similar to the observation of Wang et al. (2015), few 35S promoter-driven LecRK-IX.2 lines are phenotypically normal and can be used for pathogen inoculation assays (Wang et al., 2015). Using these lines, we found that the 35S::LecRK-IX.2 transgenic Arabidopsis exhibited enhanced resistance to Pst DC3000 (Fig. 1C). Taken together, these data demonstrate that LecRK-IX.2 is a positive regulator of plant disease resistance against Pst DC3000.
LecRK-IX.2 Is Involved in flg22-Induced PTI Responses
To investigate the role of LecRK-IX.2 in PTI, we first examined the expression of LecRK-IX.2 after flg22 treatment and found that it is largely dependent on FLS2 (Fig. 2A), indicating that it may have a role in bacterial pathogen infection. We then examined MAPK activation, which occurs early after the initial perception of flg22 (Frei dit Frey et al., 2014). Figure 2B shows that MAPK activation is reduced in the lecrk-IX.2 mutant when compared with Col-0. Two PTI-responsive genes, FRK1 and WRKY22, are reduced in lecrk-IX.2 mutants after flg22 treatment (Fig. 2C). A later PTI response is the deposition of callose, a β-1,3-glucan that can serve as a physical barrier (Kim et al., 2005). The result showed that the lecrk-IX.2 mutant accumulated significantly less callose than Col-0 (Fig. 2D). The above results indicated that PTI responses were compromised in the lecrk-IX.2 mutant.
Figure 2.
lecrk-IX.2 is compromised in flg22-induced PTI responses. A, Induction of LecRK-IX.2 by flg22 treatment requires FLS2. Wild-type (WT) Col-0 and fls2 were treated with 0.1 µm flg22. Samples were collected for qRT-PCR after 6 h. Mock treatment served as a negative control. Values are means ± sd (n = 3 biological replicates; Student’s t test, **, P < 0.01). B, MAPK signaling is impaired in lecrk-IX.2 mutants upon flg22 treatment. Three-week-old Col-0 and lecrk-IX.2 seedlings were sprayed with 1 μm flg22 and sampled at 0, 5, 10, and 15 min for immunoblotting. Activated MAPKs were detected by immunoblotting with phospho-p44/42 MAPK antibody. The corresponding bands are indicated for MPK3 and MPK6. Ponceau staining of Rubisco was used to estimate equal loading in each lane. The experiment was repeated three times with similar results. C, The expression of FRK1 and WRKY22 was reduced in lecrk-IX.2 mutants after flg22 treatment. Four-week-old Col-0 and lecrk-IX.2 mutants were infiltrated with 0.1 μm flg22. Relative expression levels of FRK1 and WRKY22 were analyzed at 3 h postinoculation (hpi). Values are means ± sd (n = 3 biological replicates; Student’s t test, **, P < 0.01). D, The lecrk-IX.2 mutant is compromised in callose deposition upon flg22 treatment. Four-week-old Col-0 and lecrk-IX.2 plants were infiltrated with 0.1 μm flg22. Leaves were stained with Aniline Blue at 12 h post treatment.
It has been reported that robust overexpression of LecRK-IX.2 results in spontaneous cell death and a dwarf phenotype (Supplemental Fig. S2A; Wang et al., 2015). Therefore, we conditionally expressed LecRK-IX.2 in Col-0 plants using a dexamethasone (Dex)-inducible promoter according to Kim et al. (2005). By using a low concentration of Dex (0.3 μm) to treat plants, the Dex::LecRK-IX.2 plants exhibited a similar phenotype to 35S::LecRK-IX.2 after 10 d (Supplemental Fig. S2A). However, 3 μm Dex treatment caused leaf cell death rapidly (Supplemental Fig. S2B). Interestingly, MPK3/MPK6 signaling and the expression of PTI marker genes FRK1 and WRKY22 also were activated in Dex-treated Dex::LecRK-IX.2 transgenic plants in the absence of flg22 (Supplemental Fig. S3, A–C), reinforcing the conclusion that LecRK-IX.2 is involved in PTI signaling downstream of the initial pathogen perception. Moreover, pretreatment with a low concentration of Dex in Dex::LecRK-IX.2 plants induced enhanced disease resistance to Pst DC3000, further confirming the disease resistance phenotype of 35S::LecRK-IX.2 plants (Fig. 1C; Supplemental Fig. S3D).
lecrk-IX.2 Mutants Showed Impaired flg22-Induced Activation of SA Signaling
Next, we examined the induction of marker genes in lecrk-IX.2 mutants upon flg22 treatment. The expression of PR1, a marker gene induced by SA (Tsuda et al., 2013), was reduced significantly when compared with wild-type plants after flg22 treatment (Fig. 3A). In contrast, expression of the jasmonic acid-responsive genes PDF1.2 and VSP1 was constitutively up-regulated in the lecrk-IX.2 mutant (Fig. 3, B and C). Furthermore, flg22-induced priming in systemic leaves was compromised in lecrk-IX.2 mutant plants (Fig. 3D). These data suggest that LecRK-IX.2 participates in flg22-activated SA signaling.
Figure 3.
The lecrk-IX.2 mutant showed reduced activation of flg22-induced gene expression and SA signaling. A, The induction of PR1 upon flg22 treatment is impaired in lecrk-IX.2 mutant plants. Four-week-old Col-0 and lecrk-IX.2 mutants were infiltrated with 0.1 μm flg22. qRT-PCR was performed at 0, 12, 24, and 48 hpi. Values are means ± sd (n = 3 biological replicates). B and C, The jasmonic acid-responsive genes VSP1 and PDF1.2 constitutively expressed in lecrk-IX.2 mutant plants. Four-week-old Col-0 and lecrk-IX.2 mutants were harvested for qRT-PCR. Values are means ± sd (n = 3 biological replicates; Student’s t test, **, P < 0.01). D, lecrk-IX.2 is impaired in flg22-induced systemic acquired resistance (SAR). Local leaves of 4-week-old Col-0 and lecrk-IX.2 plants were infiltrated with 0.1 μm flg22. Systemic leaves were infiltrated with 5 × 104 cfu mL−1 Pst DC3000 24 h later after flg22 treatment. Plants were subjected to bacterial growth curve analysis at 3 dpi. Mock is water. Values are means ± sd (n = 6; ANOVA, P < 0.01).
Interestingly, we found that when the local leaves of Dex::LecRK-IX.2 plants were treated with 3 μm Dex, both the local and systemic leaves displayed necrosis (Supplemental Fig. S2B), indicating that LecRK-IX.2 overexpression leads to a systemic response. We monitored cell death and hydrogen peroxide (H2O2) by Trypan Blue and 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining, respectively, in both Dex-treated and distal leaves (Supplemental Fig. S4A). We also used qRT-PCR to detect the expression of LecRK-IX.2 in local and systemic leaves after 0.3 or 3 μm Dex treatment. We found that the induction of LecRK-IX.2 in systemic leaves was much lower than that in local leaves after 0.3 μm Dex treatment (Supplemental Fig. S4B). Therefore, we hypothesize that the necrosis phenotype is correlated with increased accumulation of H2O2. Because 0.3 μm Dex treatment cannot induce cell death efficiently (Supplemental Fig. S4C) and the PR1 and PMR4 transcripts are highly up-regulated (Supplemental Fig. S4D), these data demonstrate that 0.3 μm Dex can be used for Dex::LecRK-IX.2 plants to mount appropriate defense.
Overexpression of LecRK-IX.2 Leads to SA Accumulation and Cell Death
SID2 encodes an isochorismate synthase, a key enzyme that controls SA biosynthesis (Tsuda et al., 2013). To determine whether the Dex::LecRK-IX.2-mediated cell death was dependent on SA, we first examined the expression of SID2. Mild expression of LecRK-IX.2 activated SID2 transcription in Dex::LecRK-IX.2 plants but not in Col-0 plants that were also treated with Dex (Supplemental Fig. S4E). Dex::LecRK-IX.2 plants pretreated with 0.3 μm Dex exhibited enhanced disease resistance in both local and systemic leaves (Fig. 4A). SA accumulation also is required for SAR. Therefore, we measured endogenous SA levels and observed a significant increase in SA accumulation in Dex::LecRK-IX.2 plants after Dex treatment (Fig. 4B).
Figure 4.
Overexpression of LecRK-IX.2 leads to SA accumulation and SAR. A, Mild expression of LecRK-IX.2 induces SAR. Three local leaves of Col-0 and Dex12-2 were pretreated with 0.3 μm Dex, and the leaves were inoculated with Pst DC3000 (5 × 104 cfu mL−1) 1 d later. The bacterial growth assay for local and systemic leaves was performed 3 d later. Values are means ± sd (n = 6; ANOVA, P < 0.01). B, Mild expression of LecRK-IX.2 induces SA accumulation. Four-week-old plant leaves were infiltrated with 0.3 μm Dex. Samples were harvested at the indicated times. The SA contents of local leaves were determined by HPLC-tandem mass spectrometry. Values are means ± sd (n = 3; Student’s t test, **, P < 0.01). C, LecRK-IX.2-induced cell death requires SID2. The Dex12-2 line was crossed with sid2 mutant plants, and the F2 homozygous lines were used for Dex treatment. The Dex12-2/sid2 plants were infiltrated with 3 μm Dex, and the photographs were taken 24 h later. The gels at bottom show equal expression of the proteins. D, LecRK-IX.2-induced cell death is mediated by SA. Wild-type (WT) and the NahG transgenic N. benthamiana plant leaves transiently expressed 35S::LecRK-IX.2 or 35S::GFP (negative control), and the plant leaves were stained by Trypan Blue at 48 hpi.
In order to determine if the LecRK-IX.2 phenotypes require SA, we crossed Dex::LecRK-IX.2 to the sid2 mutant. Dex-inducible expression of LecRK-IX.2 was unable to elicit cell death in a sid2 mutant background, demonstrating that LecRK-IX.2 robust expression-induced cell death requires SID2 (Fig. 4C). To further confirm that SA was responsible for LecRK-IX.2-mediated cell death, we transiently expressed LecRK-IX.2 in Nicotiana benthamiana leaves of either wild-type or NahG transgenic plants that are unable to accumulate SA. Cell death was then investigated using Trypan Blue staining. LecRK-IX.2-mediated cell death was detectable in wild-type N. benthamiana at 24 hpi, but cell death was almost completely abolished in NahG transgenic plants even at 48 hpi (Fig. 4D). Taken together, these results demonstrate that LecRK-IX.2 expression induces SA biosynthesis and that SA accumulation is required for the induction of LecRK-IX.2-mediated cell death.
LecRK-IX.2 Is an Active Kinase
L-type lectin receptor kinases contain an extracellular legume lectin-like domain, a transmembrane domain, and an intracellular kinase domain (Vaid et al., 2013). Interestingly, flg22 treatment strongly induced LecRK-IX.2 phosphorylation (Fig. 5A). Previous work by Wang et al. (2016) showed that LecRK-IX.2 is an RD kinase, and mutation of these two sites abolished LecRK-mediated cell death. However, it is unclear if LecRK-IX.2 is an active kinase. To test if LecRK-IX.2 has kinase activity, we purified recombinant LecRK-IX.2-CD (cytosolic domain) and three kinase-dead mutant variants, K379R, K477E, and D532N, which are conserved residues in RLKs and required for kinase activity, from Escherichia coli. Kinase activity assays demonstrated that LecRK-IX.2-CD phosphorylated the universal kinase substrate myelin basic protein, whereas the three kinase-dead variants did not (Fig. 5B). Furthermore, the wild type, but not the kinase-dead recombinant LecRK-IX.2-CD, was able to autophosphorylate (Fig. 5B).
Figure 5.
LecRK-IX.2 kinase activity is required for the cell death phenotype in N. benthamiana. A, Flg22 treatment activates the phosphorylation of LecRK-IX.2. Dex::LecRK-IX.2-FLAG transgenic plants were treated with 3 μm Dex and then infiltrated with 0.1 μm flg22 5 h later. Fifteen minutes after flg22 treatment, the plant leaves were sampled for immunoprecipitation with anti-Flag agarose beads. The precipitated proteins were subjected to immunoblotting. The phosphorylation of LecRK-IX.2 was probed with anti-pThr antibody. B, LecRK-IX.2 is an active kinase. The recombinant MBP-tagged cytoplasmic domain of LecRK-IX.2 and its kinase site-mutated variants were subjected to radioactive kinase assays. Myelin basic protein (MyBP) was used as a universal substrate. Kinase activity was detected by autoradiography. The bottom gel shows the protein abundance stained by Coomassie Brilliant Blue (CBB) in SDS-PAGE. The asterisk indicates a LecRK cleavage product. C, Transient expression of LecRK-IX.2 and its variants in N. benthamiana. 35S::GFP-FLAG (negative control), 35S::LecRK-IX.2-T7, and its kinase site mutant variants were expressed transiently in N. benthamiana leaves. Plant leaves were subjected to Trypan Blue staining and photographed at 48 hpi. Similar results were obtained from three biological replicates. The gels at right show equal expression of respective proteins.
To determine if kinase activity was required for LecRK-IX.2-mediated cell death, we transiently expressed the kinase-dead mutant variants K379R, K477E, and D532N in N. benthamiana. None of the kinase-dead mutant variants were able to elicit cell death in N. benthamiana (Fig. 5C). Thus, kinase activity is required for LecRK-IX.2-mediated cell death in N. benthamiana.
The LecRK-IX.2-Mediated Immune Response Requires RbohD
In order to identify members of the LecRK-IX.2 immune complex, we performed coimmunoprecipitation/mass spectrometry using Dex::LecRK-IX.2 transgenic Arabidopsis. Mass spectrometry results identified several proteins that were specifically identified after coimmunoprecipitation with LecRK-IX.2 (Supplemental Table S1). One of the identified proteins was the NADPH oxidase RbohD. RbohD is the main producer of extracellular ROS burst in Arabidopsis when plants are subjected to pathogen infection. Because H2O2 is able to induce SA accumulation and SAR (Chen et al., 1993; Dubiella et al., 2013), we hypothesize that RbohD may be responsible for the LecRK-IX.2-mediated systemic resistance phenotype.
To confirm the coimmunoprecipitation/mass spectrometry result, we tested if LecRK-IX.2 could associate with RbohD. We used Agrobacterium tumefaciens-mediated transient expression in N. benthamiana to coexpress RbohD-T7 and LecRK-IX.2-FLAG. Coimmunoprecipitation with anti-FLAG revealed that RbohD and LecRK-IX.2 can associate in planta (Fig. 6A). Next, we purified the N terminus of RbohD fused with MBP and the cytosolic domain of LecRK-IX.2 fused with GST from E. coli. In vitro pull-down assays revealed that LecRK-IX.2 can associate directly with the N terminus of RbohD (Fig. 6B). These data indicate that LecRK-IX.2 may recruit RbohD to activate cell death. Therefore, we crossed Dex::LecRK-IX.2 with the rbohd mutant to generate Dex::LecRK-IX.2/rbohd plants and examined if RbohD was genetically required for the LecRK-IX.2-mediated cell death. The results showed that, after Dex treatment, Dex::LecRK-IX.2 transgenic plants exhibited a cell death phenotype while Dex::LecRK-IX.2/rbohd plants did not (Fig. 6C). Thus, LecRK-IX.2-induced cell death requires RbohD.
Figure 6.
LecRK-IX.2 interacts physically and genetically with RbohD but cannot directly phosphorylate RbohD. A, LecRK-IX.2 interacts with RbohD in vivo by coimmunoprecipitation assay. Dex::LecRK-IX.2-Flag and 35S::RbohD-T7 were expressed transiently in N. benthamiana leaves. Plants were infiltrated with 3 μm Dex 48 h later to induce the expression of LecRK-IX.2-Flag. The immunoprecipitation was performed 3 h after Dex treatment. The experiment was repeated three times with similar results. B, LecRK-IX.2-CD interacts with the RbohD N terminus in vitro by GST pull-down assays. The recombinant GST-LecRK-IX.2 cytosolic domain and the MBP-RbohD N terminus were subjected to GST pull-down analysis. The interacting proteins were revealed by immunoblotting. The experiment was repeated three times with similar results. C, LecRK-IX.2-induced cell death requires RbohD. A Dex12-2 plant was crossed with rbohd mutant plants. Homozygous lines of Dex12-2/rbohd were treated with 3 μm Dex. The photographs were taken 24 h later. The gels at bottom show equal expression of the proteins before leaf collapse. D, LecRK-IX.2 cannot phosphorylate RbohD. The recombinant MBP-tagged cytoplasmic domain of LecRK-IX.2 was subjected to kinase activity assays with myelin basic protein (MyBP) or the RbohD N terminus as substrates. GST-BIK1 served as a positive control. Coomassie Brilliant Blue (CBB) shows the protein abundance.
Because LecRK-IX.2 interacts with RbohD, we investigated if LecRK-IX.2 could phosphorylate RbohD in vitro. Unexpectedly, the kinase activity assay showed that LecRK-IX.2 was unable to directly phosphorylate RbohD’s N terminus (Fig. 6D). In contrast, BIK1 strongly phosphorylated the RbohD N terminus, as reported previously (Kadota et al., 2014; Li et al., 2014). These data imply that LecRK-IX.2 may recruit other kinase(s) to phosphorylate RbohD.
LecRK-IX.2 Recruits CPKs to Phosphorylate RbohD
Although BIK1 is able to phosphorylate RbohD’s N terminus, bik1 mutants but not BIK1-overexpressing plants accumulate SA (Veronese et al., 2006). Thus, it is unlikely that BIK1 is responsible for LecRK-IX.2-mediated SA accumulation. Previously, it has been shown that some CPKs can phosphorylate RbohD’s N terminus at sites promoting enhanced ROS production. For example, CPK5 phosphorylates RbohD at Ser-39, Ser-148, Ser-163, and Ser-347, resulting in enhanced SA-mediated resistance to Pst, and the expression of constitutively active CPK5-VK leads to cell death in Arabidopsis and N. benthamiana (Dubiella et al., 2013). Notably, CPK4/5/6/11 are critical positive regulators in flg22 signaling (Boudsocq et al., 2010). Therefore, we investigated if LecRK-IX.2 recruits CPKs to phosphorylate RbohD. We chose CPK5/6/11 and performed MBP pull-down assays using recombinant LecRK-IX.2-CD. All tested CPKs interacted with LecRK-IX.2-CD (Fig. 7A). Next, we performed split-luciferase assays to examine if LecRK-IX.2 (K379R) could interact with CPKs in vivo (Fig. 7B). We used LecRK-IX.2 (K379R) to avoid the induction of cell death. These results showed that LecRK-IX.2 interacts with CPK/5/6/11 in vitro and in vivo. As LecRK-IX.2 (K379R) can still interact with CPKs, we conclude that the kinase activity of LecRK-IX.2 is not required for the interactions, but it is required for the induction of cell death.
Figure 7.
LecRK-IX.2 interacts with CPKs. A, LecRK-IX.2-CD interacts with CPKs in vitro by MBP pull-down assays. The recombinant MBP-LecRK-IX.2 cytosolic domain and His-CPKs were subjected to MBP pull-down analysis. Coomassie Brilliant Blue (CBB) shows the protein abundance. B, LecRK-IX.2 interacts with CPKs in N. benthamiana by split luciferase assays. N. benthamiana leaves were coinfiltrated with 35S::LecRK-IX.2 (K379R)-Nluc and 35S::CPKs-Cluc. Luciferase complementation imaging assays were performed 2 d later. This experiment was repeated three times with similar results. RLU, Relative light units.
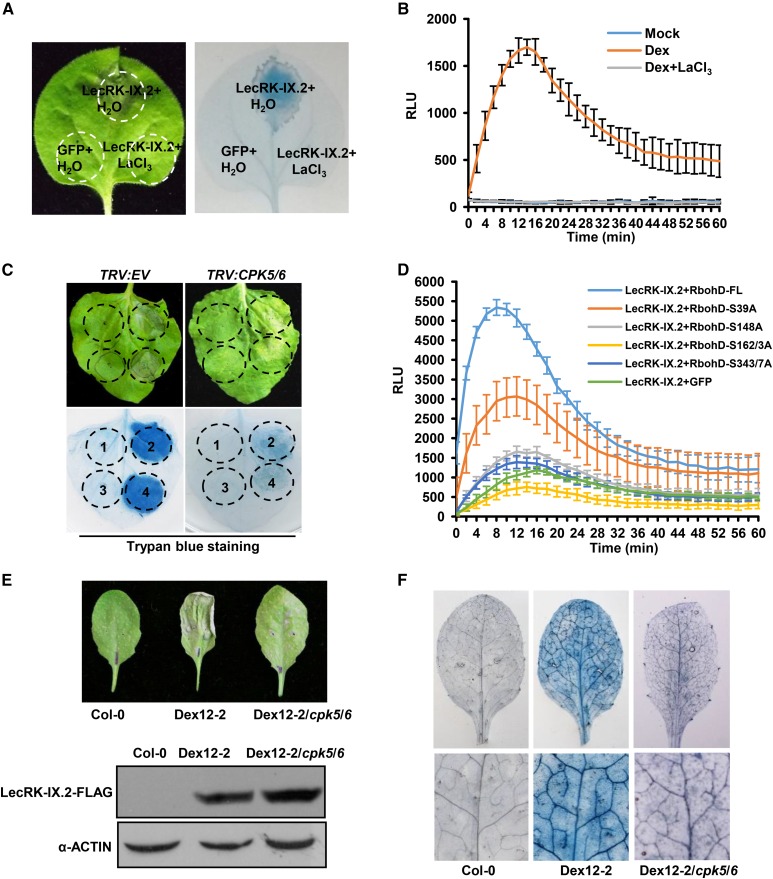
It is known that CPK activation requires calcium (Ca2+) influx. We found that the Ca2+ channel blocker LaCl3 not only inhibited LecRK-IX.2-mediated cell death but also inhibited H2O2 accumulation in plant leaves (Fig. 8, A and B). Next, we silenced two major CPKs, NbCPK5 and NbCPK6, in N. benthamiana using virus-induced gene silencing (VIGS) according to Kobayashi et al. (2007) and then transiently expressed LecRK-IX.2 in the leaves. Transient expression of LecRK-IX.2 showed significantly compromised cell death in CPK-silenced plants, indicating that CPKs were required for LecRK-IX.2-induced cell death (Fig. 8C). The silencing efficiency of NbCPK5/6 and the expression of LecRK-IX.2-T7 were confirmed by qRT-PCR and western blotting, respectively (Supplemental Fig. S5). In order to evaluate the biological relevance of CPKs for LecRK-IX.2-induced cell death in N. benthamiana, we further mutated the CPK-dependent phosphorylation sites of RbohD (Dubiella et al., 2013) and detected their responses to LecRK-IX.2 treatments. We found that all the mutants of RbohD were compromised in LecRK-IX.2-induced ROS production when compared with wild-type RbohD (Fig. 8D).
Figure 8.
LecRK-IX.2-mediated cell death requires Ca2+ influx and CPKs. A, The LecRK-IX.2-induced cell death phenotype requires Ca2+ influx. N. benthamiana leaves transiently expressed 35S::GFP-FLAG and 35S::LecRK-IX.2-T7 and then were infiltrated with water or 1 mm LaCl3 24 h later. The leaves were photographed at 2 dpi and were stained with Trypan Blue. The experiments were repeated three times with similar results. B, LaCl3 inhibits LecRK-IX.2-induced ROS in N. benthamiana. Plant leaves transiently expressed Dex::LecRK-IX.2. Dex alone or with 1 mm LaCl3 was applied to leaves 48 hpi. A luminol-based assay for ROS production was performed 2 h after Dex and LaCl3 treatment. Values are means ± sd (n = 3). RLU, Relative light units. C, LecRK-IX.2-induced cell death requires CPKs in N. benthamiana. The transcription of NbCPK5 and NbCPK6 was knocked down by VIGS in N. benthamiana. The silenced plants transiently expressed 35S::GFP and 35S::LecRK-IX.2 with different concentrations of A. tumefaciens. Sites 1 and 3 were infiltrated with 35S::GFP at concentrations of 1 × 108 and 2 × 108 cfu mL−1. Sites 2 and 4 were infiltrated with 35S::LecRK-IX.2 at concentrations of 1 × 108 and 2 × 108 cfu mL−1. Plant leaves were subjected to Trypan Blue staining and photographed at 48 hpi. The experiments were repeated three times with similar results. D, CPK5-dependent RbohD phosphorylation is required for LecRK-IX.2-induced ROS generation. Dex::LecRK-IX.2 and different variants of 35S::RbohD-T7 were coexpressed in N. benthamiana. After 48 h, 30 µm Dex was applied to leaves. Plant leaves were subjected to a luminol-based assay to measure ROS production 2 h after Dex treatment. GFP was used as a negative control. Values are means ± sd (n = 6). Six discs were from different leaves. E and F, LecRK-IX.2-induced cell death is compromised in cpk5/6. A Dex12-2 plant was crossed with cpk5/6 double mutant plants. Homozygous lines of Dex12-2/cpk5/6 were treated with 3 μm Dex. The gels at bottom show equal expression of the proteins before leaf collapse. Leaves were photographed and stained with Trypan Blue 72 h later (F).
To confirm the roles of CPKs in LecRK-IX.2-induced cell death in Arabidopsis, we crossed Dex::LecRK-IX.2 (Col-0) with cpk5/6 double mutant plants. The homozygous lines of Dex::LecRK-IX.2/cpk5/6 displayed a reduced cell death phenotype after treatments with 3 μm Dex (Fig. 8, E and F). The above results collectively showed that CPKs were involved in LecRK-IX.2-induced cell death in both N. benthamiana and Arabidopsis.
DISCUSSION
In Arabidopsis, L-type LecRKs form a family of 45 genes, and several of them have been implicated in plant defense (Wang et al., 2014). For example, LecRK-I.9 is required for full resistance to Pst in Arabidopsis and confers resistance to Phytophthora spp. in N. benthamiana (Bouwmeester et al., 2011; Balagué et al., 2016; Wang et al., 2016). LecRK-VI.2 is required for the full activation of PTI, and lecrk-VI.2 mutants show reduced expression of PTI marker genes, impaired callose deposition, and defective stomatal closure. Thus, the lecrk-VI.2 mutants displayed compromised resistance to Pst and the necrotrophic bacterial pathogen Pectobacterium carotovorum (Singh et al., 2012). However, LecRK-V.5 negatively regulates stomatal immunity, and lecrk-V.5 mutants exhibited enhanced resistance to surface inoculation with virulent Pst (Desclos-Theveniau et al., 2012). These data imply that LecRKs play diverse and sometimes distinct roles in plant immunity.
Previously, it was demonstrated that the L-type lectin RLKs LecRK-IX.1 and LecRK-IX.2 are functional analogs in regulating disease resistance to Phytophthora spp. and that overexpression of LecRK-IX.1 or LecRK-IX.2 leads to cell death in plants (Wang et al., 2015). Our data show that LecRK-IX.2 transcription was induced by bacterial pathogens as well as flg22 treatment (Figs. 1A and 2A). Furthermore, we demonstrate that LecRK-IX.2 contributes to disease resistance to the bacterial pathogen Pst DC3000 (Fig. 2; Supplemental Fig. S3), suggesting that LecRK-IX.2 plays more roles in disease resistance. Although it is worthwhile to examine the disease resistance of lecrk-IX.1 or lecrk-IX.2 to various pathogens, it is safe to conclude that, similar to LecRK-VI.2, LecRK-IX.2 likely acts in PTI to defend pathogen infection, including Phytophthora spp. and Pst DC3000.
It is well known that flg22 treatment can induce SA accumulation in Arabidopsis (Tsuda et al., 2013). However, the underlying mechanism is not fully understood (Desclos-Theveniau et al., 2012; Tsuda et al., 2013). Our results showed that lecrk-IX.2 mutants were compromised in flg22-induced SA signaling (Fig. 3). In contrast, overexpression of LecRK-IX.2 led to SA accumulation and SAR (Fig. 4, A and B; Supplemental Fig. S3F), demonstrating that LecRK-IX.2 contributes to flg22-induced SA signaling in Arabidopsis. Therefore, we discovered an important component that probably acts downstream of FLS2 signaling but upstream of SA signaling, bridging that gap between PTI and SA signaling activation.
Interestingly, we found that LecRK-IX.2-mediated SA induction relies on RbohD. It is known that H2O2 treatment induces SA accumulation in plants (Dubiella et al., 2013). SA application also induces H2O2 production and cell death (Chen et al., 1993; Gawroński et al., 2014). Thus, SA-H2O2 appears to propagate a mutual activation circuit to amplify disease signaling and is essential for cell death induction and SAR. Dubiella et al. (2013) reported that H2O2 production relied on RbohD activation, likely through phosphorylation. Interestingly, we found that LecRK-IX.2 was unable to phosphorylate RbohD in vitro, although they interacted physically and RbohD was genetically required for LecRK-IX.2-induced cell death (Fig. 6). Several protein kinases have been shown to be able to phosphorylate RbohD, including BIK1 and CPKs (Dubiella et al., 2013; Kadota et al., 2014; Li et al., 2014). We show that CPKs may be recruited by LecRK-IX.2 to phosphorylate RbohD.
Notably, BIK1-mediated RbohD phosphorylation does not depend on Ca2+ (Kadota et al., 2014; Li et al., 2014), whereas LecRK-IX.2-induced cell death requires Ca2+ influx (Fig. 8, A and B), indicating that the mechanism of CPK-mediated RbohD phosphorylation is different from BIK1. Other CPKs, such as AtCPK1, also may be involved in SA accumulation, as overexpression of AtCPK1 in Arabidopsis leads to the accumulation of SA and enhanced disease resistance (Coca and San Segundo, 2010). However, another CPK, CPK28, interacts with and phosphorylates BIK1 but acts as a negative regulator of immune signaling (Monaghan et al., 2014), indicating that only a subset of CPKs are positively involved in SA accumulation. Our data show that LecRK-IX.2 may recruit CPKs to phosphorylate RbohD upon flg22 perception (Figs. 7 and 8). It has been shown that CPKs are able to phosphorylate RbohD (Dubiella et al., 2013). CPK5 phosphorylates RbohD at Ser-39, Ser-148, Ser-163, and Ser-347, and these sites are required for CPK5-induced ROS production in N. benthamiana (Dubiella et al., 2013). Importantly, these sites also are required for LecRK-IX.2-induced ROS production (Fig. 8D), further supporting our hypothesis that LecRK-IX.2 or LecRK-IX.2-like RLKs may recruit CPKs to phosphorylate RbohD and eventually induce SA biosynthesis (Fig. 9).
Figure 9.
Working model of the LecRK-IX.2-mediated immune response. When plants perceive pathogen infection, some LecRKs are activated, leading to PTI activation. In addition, the activated LecRKs recruit CPKs to phosphorylate RbohD, resulting in PTI activation, ROS production, and the subsequent ROS-triggered SA accumulation. As a result, plant disease resistance is activated.
Many pathogens secrete effectors to suppress plant SA signaling and attenuate host immunity. The obligate Arabidopsis downy mildew pathogen Hyaloperonospora arabidopsidis secretes the effector HaRxL44 to degrade the mediator subunit 19a, a positive regulator of plant immunity, to attenuate SA-dependent gene expression, leading to enhanced host susceptibility (Caillaud et al., 2013). Similarly, the bacterial pathogen Xanthomonas campestris pv vesicatoria secretes the type III effector protein XopJ to interfere with SA-dependent defense responses by inhibiting the proteasome in pepper (Capsicum annuum) plants (Üstün et al., 2013). These results indicate that SA signaling is one of the major virulence targets of pathogen effectors. It is worth noting that Pst DC3000 suppresses some LecRK expression when compared with Pst DC3000 ΔhrcC (Fig. 1A; Supplemental Fig. S1), suggesting that some bacterial effector(s) may interfere with LecRK transcription. Further investigating the effect of bacterial effector(s) on LecRK transcription or protein stabilization will advance our knowledge of how virulence pathogens overcome LecRK-mediated disease resistance.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) lecrk-IX.2 (SALK_111817) and lecrk-IX.1 (SALK_149005) T-DNA knockout lines were ordered from the Arabidopsis Biological Resource Center. The cpk5/6 double mutant was described previously (Boudsocq et al., 2010). Plants were grown at 23°C under 10 h of light/14 h of dark for 4 weeks. Transgenic plants were generated via the floral dip transformation procedure (Bent, 2006). The LecRK-IX.2 open reading frame was PCR amplified and cloned into the pMD-1 binary vector with a 35S promoter and pTA7001 vector with a Dex-inducible promoter, respectively. The transgenic plants were selected with 25 μg mL−1 kanamycin for 35S::LecRK-IX.2 and 25 μg mL−1 hygromycin for Dex::LecRK-IX.2. To generate Dex::LecRK-IX.2/sid2 and Dex::LecRK-IX.2/rbohd lines, homozygous Dex::LecRK-IX.2 plants were crossed to sid2 and rbohd (Tsuda et al., 2013; Kadota et al., 2014; Li et al., 2014). Homozygous lines were used for all experiments. Site mutation of LecRK-IX.2 was performed following the procedure of the Transformer Site-Directed Mutagenesis Kit (TaKaRa) and was confirmed by sequencing.
Bacterial Inoculation Assay
Pseudomonas syringae pv tomato (Pst) DC3000 was grown on NYGA medium at 28°C for 2 d. Five-week-old Arabidopsis leaves were syringe infiltrated with 5 × 104 cfu mL−1 Pst DC3000 bacteria in 10 mm MgCl2. The bacterial titers were determined by growth curve analysis as described by Liu et al. (2011). To determine the bacterial growth in Dex::LecRK-IX.2 plants, the plants were pretreated with 0.3 μm Dex, and bacteria were infiltrated to plant leaves 24 h after Dex treatment. Callose deposition assays were performed as described by Gimenez-Ibanez et al. (2009).
qRT-PCR Assay
Arabidopsis and Nicotiana benthamiana leaves were collected in 1.5-mL RNase-free centrifuge tubes and frozen in liquid nitrogen at the indicated time points. Total RNA was extracted by the Trizol method (Invitrogen) and digested by DNase I (TaKaRa) to remove genomic DNA. Two micrograms of RNA was used for reverse transcription. SYBR premix Taq (Toyobo) was used for qRT-PCR. Reactions were detected by the Bio-Rad system. The Arabidopsis ACTIN2 gene and the N. benthamiana EF1α gene were used as internal controls.
Trypan Blue Staining and DAB Staining
The Arabidopsis or N. benthamiana leaves were boiled for 1 to 5 min in a 1:1 mixture of ethanol and staining solution (10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, and 10 mg of Trypan Blue, dissolved in 10 mL of distilled water) for staining. The leaves were then destained in 2.5 g mL−1 chloral hydrate in distilled water overnight.
For DAB staining, the infected plant leaves were immersed in a 1% solution of DAB in Tris-HCl buffer (pH 6.5). After vacuum infiltration for 10 min, the samples were incubated at room temperature for 20 h in the dark. When the brown spots appeared clearly, samples were bleached by boiling in ethanol:lactic acid:glycerol (1:1:1, v/v/v) for 15 min. Images were captured using a microscope (Olympus BX51).
SA Measurement
For the SA content assays, Dex-treated plant leaves were harvested at 0, 24, and 48 hpi. For each sample, 100 mg of fresh tissue was homogenized in liquid nitrogen and extracted following the method described previously (Yang et al., 2017). Six nanograms of d6-SA (Olomouc) was used as the internal standard. Endogenous SA was purified and measured by an Agilent 1200 HPLC device coupled with an Agilent 6410 triple quadrupole mass spectrometer equipped with an electrospray interface. The mass spectrometer was operated in negative mode, and multiple reaction monitoring of ion pairs was used for the analysis of d6-SA (142.1 > 97.6) and endogenous SA (136.9 > 93). Experiments were performed with three independent biological replicates.
Split-Luciferase Complementation Assay
The split-luciferase complementation assay was performed as described by Chen et al. (2008). Agrobacterium tumefaciens (strain EHA105) carrying the indicated Nluc and Cluc constructs was mixed and infiltrated into 4-week-old N. benthamiana leaves using a 1-mL needleless syringe. Two days after infiltration, N. benthamiana leaves were rubbed with 0.5 mm luciferin and kept in the dark for 5 min to quench the fluorescence. A cooled CCD imaging apparatus (Roper Scientific) was used to capture luciferase images.
GST and MBP Pull-Down Assays
The LecRK-IX.2 cytosolic domain was cloned into pMAl-C4X and pGEX-4T-1 using a one-step cloning kit (Vazyme Biotech) and expressed in Escherichia coli strain BL21 to produce MBP-LecRK-IX.2-CD and GST-LecRK-IX.2-CD recombinant proteins. Protein purification and pull-down assays were performed with the method described by Liu et al. (2011). The pull-down assay of MBP-LecRK-IX.2 and His-CPKs was performed with the method described by Lee et al. (2003).
Coimmunoprecipitation and Immunoblot Analysis
N. benthamiana leaves were used for A. tumefaciens-mediated transient protein expression. Typically, the samples were harvested at 48 hpi. The plant leaves were ground in liquid nitrogen and extracted in extraction/wash buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.1% Triton X-100, 0.2% Nonidet P-40, 1 mm DTT, and 1× complete protease inhibitor (Roche). The homogenate was centrifuged at 15,000g for 20 min. Anti-FLAG antibody-conjugated agarose beads (Sigma) were added to the supernatant. After incubation at 4°C on an end-over-end shaker for 1.5 h, the beads were spun down at 1,500g for 2 min and washed with wash buffer at least five times. The bound proteins were eluted by 1.5× Laemmli loading buffer and resolved by 12% SDS-PAGE and then subjected to immunoblot analysis.
In Vitro Kinase Activity Assays
The recombinant MBP-LecRK-IX.2-CD protein was used for kinase activity assays with His-RbohD N terminus or myelin basic protein as substrate. GST-BIK1 served as the positive control. Kinase activity assays were performed according to the method described by Liu et al. (2011).
VIGS in N. benthamiana
For VIGS, A. tumefaciens strain GV3101 harboring pTRV2::NbCPK5/6 and pTRV1 were mixed at a 1:1 ratio to a final OD of 1 and then coinfiltrated into 2-week-old N. benthamiana leaves. pTRV2::NbPDS was used as a positive control to evaluate the silencing efficiency as described by Liu et al. (2002).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers RBOHD (NP_199602), CPK5 (NP_195257), and CPK6 (NP_565411).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. L-type lectin RLK genes are differentially induced by pathogens and flg22 treatments.
Supplemental Figure S2. Dex::LecRK-IX.2 transgenic plants treated by a low concentration of Dex mimic the 35S::LecRK-IX.2 phenotype.
Supplemental Figure S3. Overexpression of LecRK-IX.2 activates PTI and enhances disease resistance.
Supplemental Figure S4. Overexpression of LecRK-IX.2 activates SA-mediated disease responses.
Supplemental Figure S5. NbCPK5 and NbCPK6 expression was silenced by VIGS.
Supplemental Table S1. Members of the LecRK-IX.2 protein complex identified by mass spectrometry.
Supplemental Table S2. Primers used in the experiments.
Acknowledgments
We thank Weixing Shan of Northwest A&F University for critical reading of the article and Huishan Guo at the Institute of Microbiology, Chinese Academy of Sciences, for offering the NahG transgenic N. benthamiana plants.
Glossary
- PRR
pattern recognition receptor
- PAMP
pathogen-associated molecular pattern
- PTI
PAMP-triggered immunity
- ROS
reactive oxygen species
- SA
salicylic acid
- Col-0
Columbia-0
- Dex
dexamethasone
- SAR
systemic acquired resistance
- hpi
hours postinoculation
- VIGS
virus-induced gene silencing
- cfu
colony-forming units
- dpi
days postinoculation
Footnotes
This study was supported by the Chinese Academy of Sciences (Strategic Priority Research Program grant no. XDB11020300), the Natural Science Foundation of China (grant nos. 31570252 and 31500220), the start-up fund of the ‘One Hundred Talents’ program of the Chinese Academy of Sciences, and by grants from the State Key Laboratory of Plant Genomics (grant no. O8KF021011) to J.L. and the National Institutes of Health (grant no. R01-GM092772) to G.C.
References
- Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB (2006) Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc Natl Acad Sci USA 103: 2851–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué C, Gouget A, Bouchez O, Souriac C, Haget N, Boutet-Mercey S, Govers F, Roby D, Canut H (2016) The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling. Mol Plant Pathol doi/ 10.1111/mpp.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A. (2006) Arabidopsis thaliana floral dip transformation method. Methods Mol Biol 343: 87–103 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, de Sain M, Weide R, Gouget A, Klamer S, Canut H, Govers F (2011) The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog 7: e1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, Govers F (2009) Arabidopsis L-type lectin receptor kinases: phylogeny, classification, and expression profiles. J Exp Bot 60: 4383–4396 [DOI] [PubMed] [Google Scholar]
- Caillaud MC, Asai S, Rallapalli G, Piquerez S, Fabro G, Jones JDG (2013) A downy mildew effector attenuates salicylic acid-triggered immunity in Arabidopsis by interacting with the host mediator complex. PLoS Biol 11: e1001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YR, Liang Y, Tanaka K, Nguyen CT, Jedrzejczak RP, Joachimiak A, Stacey G (2014) The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife 3: 03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol 146: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G (2014) Identification of a plant receptor for extracellular ATP. Science 343: 290–294 [DOI] [PubMed] [Google Scholar]
- Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 63: 526–540 [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16: 537–552 [DOI] [PubMed] [Google Scholar]
- Desclos-Theveniau M, Arnaud D, Huang TY, Lin GJC, Chen WY, Lin YC, Zimmerli L (2012) The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei dit Frey N, Garcia AV, Bigeard J, Zaag R, Bueso E, Garmier M, Pateyron S, deTauzia-Moreau ML, Brunaud V, Balzergue S, et al. (2014) Functional analysis of Arabidopsis immune-related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome Biol 15: R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64: 839–863 [DOI] [PubMed] [Google Scholar]
- Gawroński P, Witoń D, Vashutina K, Bederska M, Betliński B, Rusaczonek A, Karpiński S (2014) Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol Plant 7: 1151–1166 [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19: 423–429 [DOI] [PubMed] [Google Scholar]
- Göhre V, Spallek T, Häweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18: 1824–1832 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JDG, Shirasu K, Menke F, Jones A, et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D (2005) Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121: 749–759 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Cho HS, Yoon GM, Ahn JW, Kim HH, Pai HS (2003) Interaction of NtCDPK1 calcium-dependent protein kinase with NtRpn3 regulatory subunit of the 26S proteasome in Nicotiana tabacum. Plant J 33: 825–840 [DOI] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Lin ZJ, Coaker G (2011) A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe 9: 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P (2010) A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA 107: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Zipfel C (2015) Targeting of plant pattern recognition receptor-triggered immunity by bacterial type-III secretion system effectors. Curr Opin Microbiol 23: 14–22 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J, Matschi S, Shorinola O, Rovenich H, Matei A, Segonzac C, Malinovsky FG, Rathjen JP, MacLean D, Romeis T, et al. (2014) The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16: 605–615 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley KF, Martin GB (2003) Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu Rev Phytopathol 41: 215–243 [DOI] [PubMed] [Google Scholar]
- Rosebrock TR, Zeng L, Brady JJ, Abramovitch RB, Xiao F, Martin GB (2007) A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448: 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kuo YC, Mishra S, Tsai CH, Chien CC, Chen CW, Desclos-Theveniau M, Chu PW, Schulze B, Chinchilla D, et al. (2012) The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24: 1256–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F (2013) Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet 9: e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F (2008) Interplay between MAMP-triggered and SA-mediated defense responses. Plant J 53: 763–775 [DOI] [PubMed] [Google Scholar]
- Üstün S, Bartetzko V, Börnke F (2013) The Xanthomonas campestris type III effector XopJ targets the host cell proteasome to suppress salicylic-acid mediated plant defence. PLoS Pathog 9: e1003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid N, Macovei A, Tuteja N (2013) Knights in action: lectin receptor-like kinases in plant development and stress responses. Mol Plant 6: 1405–1418 [DOI] [PubMed] [Google Scholar]
- Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T (2006) The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18: 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bouwmeester K, Beseh P, Shan W, Govers F (2014) Phenotypic analyses of Arabidopsis T-DNA insertion lines and expression profiling reveal that multiple L-type lectin receptor kinases are involved in plant immunity. Mol Plant Microbe Interact 27: 1390–1402 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cordewener JHG, America AH, Shan W, Bouwmeester K, Govers F (2015) Arabidopsis lectin receptor kinases LecRK-IX.1 and LecRK-IX.2 are functional analogs in regulating Phytophthora resistance and plant cell death. Mol Plant Microbe Interact 28: 1032–1048 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nsibo DL, Juhar HM, Govers F, Bouwmeester K (2016) Ectopic expression of Arabidopsis L-type lectin receptor kinase genes LecRK-I.9 and LecRK-IX.1 in Nicotiana benthamiana confers Phytophthora resistance. Plant Cell Rep 35: 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, et al. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol 18: 74–80 [DOI] [PubMed] [Google Scholar]
- Yang C, Li W, Cao JD, Meng FW, Yu YQ, Huang JK, Jiang L, Liu MX, Zhang ZG, Chen XW, et al. (2017) Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J 89: 338–353 [DOI] [PubMed] [Google Scholar]
- Zipfel C. (2014) Plant pattern-recognition receptors. Trends Immunol 35: 345–351 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]