Substrate catalysis by cinnamyl alcohol dehydrogenases from Sorghum bicolor deduced from crystal structures, site-directed mutagenesis, and kinetics.
Abstract
Cinnamyl alcohol dehydrogenase (CAD) catalyzes the final step in monolignol biosynthesis, reducing sinapaldehyde, coniferaldehyde, and p-coumaraldehyde to their corresponding alcohols in an NADPH-dependent manner. Because of its terminal location in monolignol biosynthesis, the variation in substrate specificity and activity of CAD can result in significant changes in overall composition and amount of lignin. Our in-depth characterization of two major CAD isoforms, SbCAD2 (Brown midrib 6 [bmr6]) and SbCAD4, in lignifying tissues of sorghum (Sorghum bicolor), a strategic plant for generating renewable chemicals and fuels, indicates their similarity in both structure and activity to Arabidopsis (Arabidopsis thaliana) CAD5 and Populus tremuloides sinapyl alcohol dehydrogenase, respectively. This first crystal structure of a monocot CAD combined with enzyme kinetic data and a catalytic model supported by site-directed mutagenesis allows full comparison with dicot CADs and elucidates the potential signature sequence for their substrate specificity and activity. The L119W/G301F-SbCAD4 double mutant displayed its substrate preference in the order coniferaldehyde > p-coumaraldehyde > sinapaldehyde, with higher catalytic efficiency than that of both wild-type SbCAD4 and SbCAD2. As SbCAD4 is the only major CAD isoform in bmr6 mutants, replacing SbCAD4 with L119W/G301F-SbCAD4 in bmr6 plants could produce a phenotype that is more amenable to biomass processing.
Lignin is formed by the oxidative polymerization of monolignols, a series of hydroxycinnamyl alcohols, and related compounds whereby the radicals are generated by either laccases or peroxidases (Ralph et al., 2004; Zhao et al., 2013; Moural et al., 2017). Monolignol precursors are synthesized from l-Phe via the general phenylpropanoid pathway, and the resulting hydroxycinnamoyl-CoA thioesters are then converted via a series of dedicated enzymatic reactions to produce p-coumaryl, coniferyl, and sinapyl alcohols, which, respectively, give rise to p-hydroxyphenyl, guaiacyl, and syringyl residues in the lignin polymer (Vanholme et al., 2010). Lignin ensures that secondary cell walls of xylem are hydrophobic, facilitating water transport. Lignin also strengthens the cell walls of fibers and provides a mechanical barrier against pests and pathogens.
Manipulation of the monolignol biosynthetic pathway can alter plant lignification in vivo, allowing for more efficient conversion of plant lignocellulosic biomass, either in the rumen of animals (Cherney et al., 1988; Fontaine et al., 2003) or in a biorefinery that produces ethanol or other renewable chemicals (Zeng et al., 2014; Vermerris and Abril, 2015; Mottiar et al., 2016). Alteration of the syringyl/guaiacyl (S/G) ratio has been shown to increase saccharification yields, with a high S/G ratio generally improving biomass conversion efficiency in dicots and a low S/G ratio generally enhancing saccharification in monocots (Chen and Dixon, 2007; Shadle et al., 2007; Fu et al., 2011; Studer et al., 2011; Vermerris et al., 2007; Van Acker et al., 2013; Sattler et al., 2014). Simultaneous reduction of both syringyl and guaiacyl residues also has been shown to increase saccharification efficiency, providing multiple avenues by which to increase plant saccharification efficiency and bioethanol yields (Chen and Dixon, 2007; Shadle et al., 2007; Van Acker et al., 2013).
Cinnamyl alcohol dehydrogenase (CAD; EC 1.1.1.195) is responsible for the final step involved in monolignol biosynthesis, and this enzyme exhibits high substrate promiscuity (Baucher et al., 1996). CAD enzymes are both Zn2+ dependent and NADP(H) dependent and generally exhibit preference for aldehyde reduction over the reverse reaction of alcohol oxidation (Sattler et al., 2009). Controlling the activity of CAD, therefore, can provide an effective mechanism for generating plants with different lignin content and composition.
Mutants with reduced CAD activity are among the earliest mutants described in the literature. The brown midrib1 (bm1) mutant of maize (Zea mays; Eyster, 1926), named after its characteristic brown vascular tissue in the leaves, was shown to accumulate cinnamaldehydes in its lignin (Kuc and Nelson, 1964), which could be explained by the reduced CAD activity (Halpin et al., 1998), resulting from a point mutation in the ZmCAD2 gene (Chen et al., 2012). The sorghum (Sorghum bicolor) brown midrib6 (bmr6) mutant (Porter et al., 1978) also accumulates cinnamaldehydes in its lignin (Pillonel et al., 1991) as the result of a nonsense mutation in the SbCAD2 gene (Saballos et al., 2009; Sattler et al., 2009). Mutants with reduced CAD activity also have been described in pearl millet (Pennisetum glaucum; Cherney et al., 1988), loblolly pine (Pinus taeda; Ralph et al., 1997), Arabidopsis (Arabidopsis thaliana; Kim et al., 2004; Youn et al., 2006), rice (Oryza sativa; Zhang et al., 2006), and Brachypodium distachyon (Bouvier d’Yvoire et al., 2013).
Of the 59 bmr mutants with reduced lignin content and altered lignin subunit composition that have been characterized in sorghum (Porter et al., 1978; Vermerris et al., 2007; Xin et al., 2008; Sattler et al., 2009, 2012; Saballos et al., 2012; Scully et al., 2016), 12 have been shown to harbor bmr6 mutant alleles (Saballos et al., 2008; Scully et al., 2016). The remaining mutants have been classified into six other allelic groups. Three bmr genes have been cloned (Bout and Vermerris, 2003; Saballos et al., 2009, 2012; Sattler et al., 2009, 2012; Scully et al., 2016). The cell walls of some bmr mutants, including bmr6, have been shown to be more amenable to the thermochemical pretreatments utilized for cellulosic ethanol production (Saballos et al., 2008; Dien et al., 2009; Sattler et al., 2012, 2014; Scully et al., 2016).
CAD in sorghum is encoded by a multigene family (Saballos et al., 2009), of which two isoforms are expressed in lignifying tissues, SbCAD2 and SbCAD4, that have different substrate specificity and activity (Saballos et al., 2009; Sattler et al., 2009). SbCAD4 is expressed at a significantly lower level than SbCAD2 but has been shown to act in a compensatory role in bmr6 plants (Sattler et al., 2009). Little is known about SbCAD4, however, indicating a need for a detailed biochemical characterization of this enzyme to fully understand the final step in monolignol biosynthesis in sorghum.
We recently described the detailed structural and enzymatic analyses of the enzymes hydroxycinnamoyl transferase (Walker et al., 2013), caffeic acid O-methyltransferase (Green et al., 2014), caffeoyl-CoA O-methyltransferase (Walker et al., 2016), cinnamoyl-CoA reductase (Sattler et al., 2017), and peroxidase (Moural et al., 2017) in sorghum and switchgrass (Panicum virgatum). These studies identified amino acid residues critical for substrate binding and catalytic activity and indicated the utility of an enzyme engineering approach to altering the substrate affinity of monolignol biosynthetic enzymes, rather than gene-silencing methods, with the aim to avoid deleterious phenotypes that render the plant unfit for large-scale agricultural production while still imparting traits favorable for biofuel production. Moreover, the precise engineering of sorghum enzymes is expected to face fewer regulatory challenges than transcriptional control via antisense RNA or RNA interference and offers greater control over lignin biosynthesis than the arbitrary mutations in chemically mutagenized crops. Several technologies currently exist to utilize structural knowledge to modify existing sorghum genes, including zinc-finger nucleases (Shukla et al., 2009), transcription activator-like effector nucleases (Cermak et al., 2011), and the CRISPR/Cas9 system (Jinek et al., 2012; Jiang et al., 2013).
In this report, we have investigated both SbCAD2 and SbCAD4 and compare them with other well-characterized CAD and related enzymes (Li et al., 2001; Bomati and Noel, 2005). These findings will be of value for the development of high-biomass sorghum genotypes that can be efficiently converted to fermentable sugars. Combined with sorghum’s low input requirements and tolerance of adverse environmental conditions (Rooney et al., 2007; Propheter et al., 2010; Gill et al., 2014), this approach can contribute to a more sustainable production of bio-based fuels and chemicals.
RESULTS
Global Structure and Oligomeric Structure
The recombinant SbCAD4 was crystallized in the monoclinic space group, P21, and its structure at 1.8 Å resolution was determined (Protein Data Bank [PDB] no. 5VKT; Table I). The asymmetric unit of the SbCAD4 crystal lattice was composed of two molecules of SbCAD4 (Fig. 1). Those two molecules are connected through a continuous 12-stranded β-sheet, of which six β-strands were from each SbCAD4 subunit being assembled in a pseudo-2-fold-related manner, hinting at its dimeric nature. In order to determine the exact oligomeric status of SbCAD4 in solution, the coordinates of the SbCAD4 crystal structure were submitted to PISA (Krissinel and Henrick, 2007). The results indicated that the formation of such a dimer as exists in the crystal lattice is related to the free energy change of −84.1 kcal mol−1, which strongly supports the dimeric nature of SbCAD4 in vivo. This oligomerization of SbCAD4 is analogous to that observed for AtCAD5 (Youn et al., 2006) and sinapyl alcohol dehydrogenase (SAD) from Populus tremuloides (PotSAD; Bomati and Noel, 2005) and other zinc (Zn)-containing medium chain dehydrogenase/reductases (MDRs) from higher plants and mammals. Significantly, one SbCAD4 molecule in this tightly associated dimer was in its apo-form, and the other molecule was associated with an NADP+ that was available from the crystallization buffer. The two subunits displayed no major differences in their secondary structures.
Table I. Crystallographic data for SbCAD4.
| Parameter | SbCAD4 (PDB No. 5VKT) |
|---|---|
| Data collectiona | |
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 55.597, 74.280, 97.288 |
| α, β, γ (°) | 90.000, 96.741, 90.000 |
| Resolution (Å) | 45.68–1.83 (1.85–1.83) |
| Wavelength (Å) | 1.00 |
| Asymmetric unit | 2 |
| Total reflections | 128,308 (1,794) |
| Completeness (%) | 97.4 (73.5) |
| I/σI | 9.2 (3.5) |
| CC1/2b | 0.994 (0.555) |
| Redundancy | 1.8 |
| Rmeasc | 0.146 (0.809) |
| Rpim | 0.077 (0.514) |
| Refinement | |
| Resolution (Å) | 45.68–1.83 (1.85–1.83) |
| Unique reflections | 67,962 (1,256) |
| Rwork/Rfreed | 0.1633/0.1871 (0.2718/0.3052) |
| B factors (Å2) | |
| All atoms | 22.40 |
| Solvent | 33.5 |
| Root mean square deviations | |
| Bonds (Å) | 0.003 |
| Angles (°) | 0.568 |
| Ramachandrans (%) | |
| Favored | 96.61 |
| Outliers | 0.14 |
| No. of atoms | |
| Protein and ligand | 10,513 |
| Water | 884 |
Numbers in parentheses refer to the highest resolution shell.
CC1/2 is the correlation between two data sets each based on half of the data, as defined by Karplus and Diederichs (2012).
Rmeas is the multiplicity-weighted merging R factor.
Rfree was calculated as for Rcryst using 5% of the data that was excluded from refinement.
Figure 1.
A, Ribbon diagram representing the crystal structure of SbCAD4. Protein chains are shown as blue and magenta ribbons, NADP+ is shown as orange spheres, and Zn2+ is shown as green spheres. B, Superposition of the two SbCAD4 subunits. The nucleotide-binding domains of each subunit are superposed, and the conformational difference is marked as an arrow at the C-terminal ends. The subunit in a form of NADP+ complex is shown in green, the unbound subunit is shown in blue, and NADP+ is shown as purple sticks. Molecular graphics images were produced using the UCSF Chimera package.
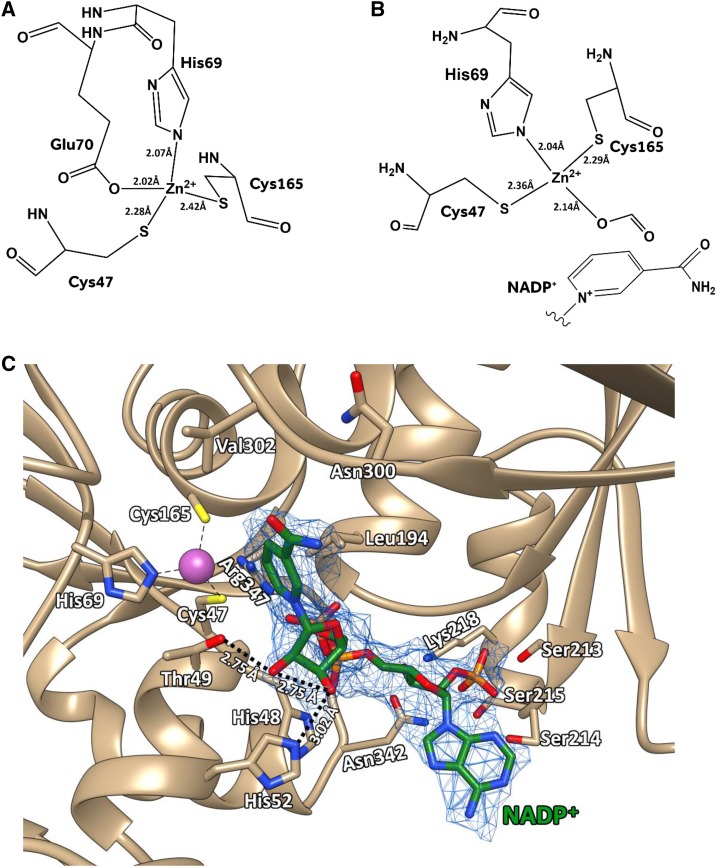
Like other Zn2+-dependent alcohol dehydrogenases (Brändén et al., 1973), each SbCAD4 subunit is composed of two distinct domains: a smaller Rossman-like fold (residues 166–303) that is the nucleotide-binding domain and a larger catalytic domain (residues 1–165 and 304–360). The cavity or cleft between those two domains exhibited in the apo-form was significantly larger than that of the NADP+ complex form. Thus, open and closed conformations are designated for describing those two subunits through this report (Fig. 1B). The core of the larger catalytic domain was constituted of a mixed β-sheet in the order β3-β4-β5-β6-β10-β18-β19, which was flanked by two antiparallel β-sheets (β1-β2 and β7-β8-β9) and seven α-helices (α1, α2, α3, α4, α5, α13, and α14). Similar to AtCAD5 (Youn et al., 2006) and PotSAD (Bomati and Noel, 2005), the larger domain contained two Zn2+ ions, one structural Zn2+ and one catalytic Zn2+, with the latter being located at the bottom of the hydrophobic substrate-binding pocket inside the above-mentioned cleft. Although the coordination of the structural Zn2+ ion was identical between the open and closed subunits, the two subunits displayed a significant difference in coordinating the catalytic Zn2+ ion. The catalytic Zn2+ ion of the open form was coordinated by the side chain of Cys-47, His-69, and Cys-165 together with the Oε1 atom of Glu-70 as the fourth coordination that is located opposite to the substrate-binding pocket (Fig. 2A). On the other hand, the catalytic Zn2+ ion in the closed form was coordinated by Cys-47, His-69, and Cys-165, but the oxygen atom of formic acid, which was in the crystallization buffer, replaced the side chain of Glu-70 (Fig. 2B). In the closed form, Glu-70 instead established a salt bridge with the nearby guanidinium side chain of Arg-347, which prevented its side chain from having any direct interaction with the catalytic Zn2+ ion. When the catalytic domains of the apo-form (open state) and the NADPH complex form (closed state) were superimposed, the corresponding positions of their catalytic Zn2+ ions were 1.9 Å apart from each other, probably due to the association of NADPH altering the coordination of this ion through the domain opening.
Figure 2.
Representations of the SbCAD4 active site. A, Coordination of catalytic Zn2+ of the open form subunit. In the absence of NADP+, Cys-47, His-69, Glu-70, and Cys-165 form tetrahedral coordination around the catalytic Zn2+. B, Coordination of catalytic Zn2+ of the closed form subunit. In the presence of NADP+, the position of the catalytic Zn2+ was shifted toward NADP+. Coordination between the zinc ion and the carboxylic oxygen of Glu-70 was replaced with formate. C, NADP+ in the SbCAD4 active site. The proton-shuttling system is shown as dotted lines, and the bound NADP+ is shown as green sticks. Molecular graphics images were produced using the UCSF Chimera package.
The Rossman-like fold of the smaller domain was established by a six-stranded parallel β-sheet and six α-helices arranged in the order of α7-β12-α8-β13-α9-β14-α10-β15-α11-β16-α12-β17, where the last two β-strands (β16 and β17) establish the dimeric interface. The observed dimer in the crystal lattice of SbCAD4 was established through pseudo-2-fold symmetry between two subunits and was driven primarily by the formation of the above-mentioned continuous β-sheet through interaction between two β17 strands of smaller domain from each subunit (Fig. 1). This intersubunit interaction extended the β-sheet and established the NADP(H)-binding platform. In addition, there were substantial interactions between β16-α12-β17 (residues 267−299) of each subunit, which allowed for the formation of a hydrophobic pocket created by the side chains of Leu-267, Gly-271, Val-274, Val-275, Val-276, Gly-277, Ala-278, Leu-283, Leu-285, Ala-287, Ala-289, Ile-290, Ile-291, Gly-293, Gly-294, Val-297, Ala-298, and Gly-299. There also were two intersubunit hydrogen bonds between the side chains of Gln-176/Tyr-177 from one subunit and the side chain of Arg-296 from the other subunit.
NADP(H)-Binding Pocket
One NADP+ molecule in its syn-conformation was located at the cleft between the nucleotide-binding domain and the catalytic domain of the closed subunit, and its nicotinamide ring was faced in an orientation for the A-face hydride transfer (Figs. 1 and 2C). Significantly, the corresponding position in the apo-form subunit was populated with a large number of water molecules, which, when they are freed upon NADP(H) binding, likely provide a substantial entropic contribution. Careful inspection of this NADPH-bound subunit revealed that affinity for NADP(H) was provided by the residues from both the nucleotide-binding domain and the catalytic domain of SbCAD4. The larger domain contributed the side chains of His-52 and Thr-49, which established hydrogen bonds with the 3′-OH and 2′-OH, respectively, of the nicotinamide ribose moiety of NADP+ as well as the side chains of His-48 and Arg-347, which established electrostatic interactions with the pyrophosphate group of NADP+. The smaller domain also established a pair of hydrogen bonds with the pyrophosphate group of the bound NADP+ molecule through the backbone nitrogen atoms of both Gly-193 and Leu-194. The 3′-OH of the adenosyl ribose of NADP+ was anchored through a hydrogen bond with the amide side chain of Asn-342 and the amino moiety of Lys-218. The smaller domain also interacts with the 2′ phosphate of the adenosine moiety via the hydroxyl side chains of three consecutive Ser residues, Ser-213, Ser-214, and Ser-215. The hinge region between the two domains of SbCAD4 also interacts with both the oxygen and the nitrogen of the amide moiety of the nicotinamide ring of NADP+ through the backbone carbonyl oxygen of Asn-300 and the backbone nitrogen of Val-302, respectively. Asn-300 and Val-302 are located at the hinge region and appear to be responsible for the conformational switch between the open and closed states of SbCAD4, providing a mechanism for a domain closure upon NADP(H) binding.
Substrate-Binding Pockets
SbCAD4
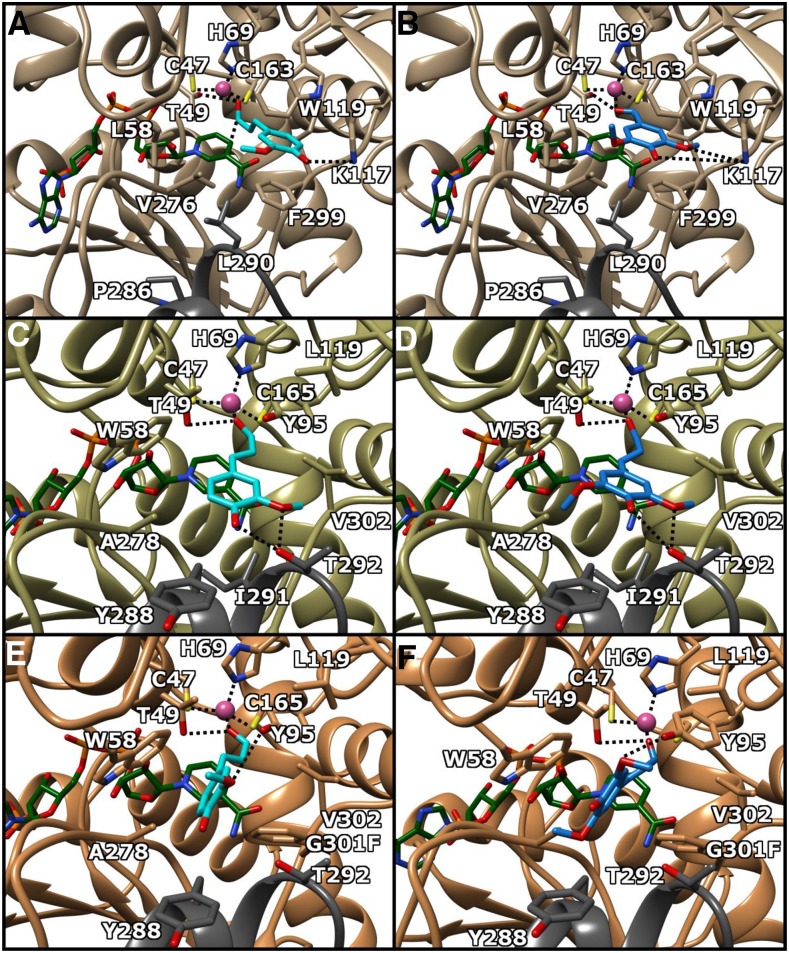
In spite of numerous trials for both cocrystallization and diffusion approaches, we were not able to establish diffraction-quality crystals for the ternary complex for SbCAD4, as in the case of Arabidopsis CAD5 (AtCAD5) previously (Youn et al., 2006). Thus, the plausible binding mode for three aldehyde substrates, p-coumaraldehyde, coniferaldehyde, and sinapaldehyde, was pursued by in silico substrate-docking approaches, which were performed with AutoDock Vina (Trott and Olson, 2010). Molecular docking was carried out with the closed form SbCAD4 crystal structure, which is in complex with NADP+, after removing the formic acid that was coordinated to the catalytic Zn2+ ion. The docking result shows that the aldehydic oxygen of the docked substrates was placed in the exact identical position where the removed crystallographic formic acid was found. In this conformation and position, the measured distances between the aldehydic oxygen and the catalytic Zn2+ and the hydroxyl side chain of Thr-49 were 2.4 and 2.93 Å, respectively. In addition, the carbonyl carbon of the bound substrate was positioned on top of the re-face of the nicotinamide ring of the bound NADP+ molecule, 2.94 Å away from the C4 atom of the nicotinamide ring, where its pro-R hydrogen is at a proper distance for hydride transfer (Fig. 3, C and D).
Figure 3.
Active sites of SbCAD2, SbCAD4, and G301F-SbCAD4. A, SbCAD2 bound to coniferaldehyde. B, SbCAD2 bound to sinapaldehyde. C, SbCAD4 bound to coniferaldehyde. D, SbCAD4 bound to sinapaldehyde. E, G301F-SbCAD4 bound to coniferaldehyde. F, G301F-SbCAD4 bound to sinapaldehyde. NADPH is shown as green sticks, coniferaldehyde as cyan sticks, sinapaldehyde as blue sticks, and Zn2+ as a purple sphere. Dark gray residues denote a dimeric subunit. Molecular interactions are denoted by dotted lines. Molecular graphics images were produced using the UCSF Chimera package.
The observed substrate-binding pocket of SbCAD4 was relatively hydrophobic in nature, surrounded by 12 residues, including nine residues from one subunit, Thr-49, Val-53, Trp-58, Tyr-95, Leu-119, Val-276, Ala-278, Gly-301, and Val-302, and three residues from the other subunit, Tyr-288, Ile-291, and Thr-292. The nonpolar hydrocarbon body of the docked substrate positions itself in this hydrophobic pocket where aldehydic and p-hydroxyl oxygen atoms establish hydrogen bonds with hydroxy groups of Thr-49 and Thr-292. In addition, the hydroxy group of Thr-292 forms an additional hydrogen bond with the m-methoxy oxygen of the docked coniferaldehyde and sinapaldehyde. Binding affinities for p-coumaraldehyde, coniferaldehyde, and sinapaldehyde in those docked positions were calculated to be −5.1, −5.8, and −5.9 kcal mol−1, respectively. Judging by the binding positions and conformations, the hydroxyl groups of Tyr-95 and Tyr-288, two other polar residues in the substrate-binding pocket, were unable to form hydrogen bonds with bound substrates.
SbCAD2
In order to compare the structures of two major CADs in lignifying tissues of sorghum, SbCAD2 and SbCAD4, the homology model for SbCAD2 was built using SWISS-model (Guex et al., 2009) with the coordinates of the SbCAD4 crystal structure. As expected from the high sequence similarity (65.7%) and identity (51.5%) between them, as reported before (Saballos et al., 2009), the three-dimensional backbone structures of these two SbCADs displayed no significant difference. Moreover, all NADP(H)-binding residues identified in the crystal structures of SbCAD4 and AtCAD5 (PDB no. 2CF6; Youn et al., 2006) were conserved in identical positions. However, the residues constituting the substrate-binding pocket of SbCAD2 and SbCAD4 differ significantly. The active site of SbCAD4 has more bulky residues on the same side of the binding pocket as the catalytic Thr (Thr-49), whereas most of the bulky residues in the SbCAD2 active site are located opposite to the catalytic Thr, except for the SbCAD4 Tyr-95, which is located opposite the catalytic Thr. Thus, the positions and conformations of both coniferaldehyde and sinapaldehyde were pursued by manually placing them in the active site of the closed form SbCAD2 homology model, where the aldehydic oxygen of the coniferaldehyde (or sinapaldehyde) was positioned for Zn2+ coordination and within a hydrogen bond distance from the hydroxyl side chain of Thr-49, as shown in SbCAD4. The resulting coordinates of dimeric SbCAD2 containing coniferaldehyde (or sinapaldehyde) were relaxed using the energy-minimization software MOZYME (MOPAC 2016; Stewart Computational Chemistry). The binding mode of sinapaldehyde indicated that the affinity of SbCAD2 for sinapaldehyde (Fig. 3B) was contributed mainly by the indole and phenyl side chains of Trp-119 and Phe-299, which are at the equivalent positions of less bulky Leu-119 and Gly-301 of SbCAD4, respectively (Fig. 3D). In addition, the 4-hydroxyl group and methoxyl oxygen of sinapaldehyde were positioned for a proper interaction with the side chain of Lys-117. The methyl ends of the sinapaldehyde methoxy moieties also establish a hydrophobic interaction with the side chains of Ile-300 and Val-276, which correspond to Val-302 and Ala-278 in SbCAD4. Similar to SbCAD4, the aldehydic oxygen of sinapaldehyde was properly coordinated to both the active site Zn2+ and the hydroxyl side chain of Thr-49 (Fig. 3, A and B).
Enzyme Kinetics of Wild-Type SbCAD2 and SbCAD4 and Mutants
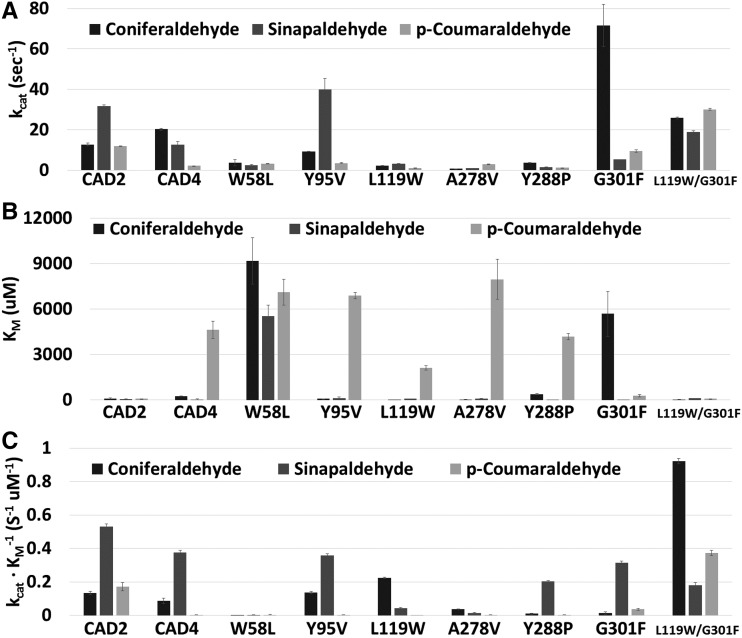
Enzyme kinetic assays with the wild-type SbCAD2 and SbCAD4 were carried out in the presence of sinapaldehyde, coniferaldehyde, and p-coumaraldehyde. Although the Km value of SbCAD4 for p-coumaraldehyde was 66.4 times larger than that of SbCAD2, the kcat values for SbCAD2 indicated higher turnover rates for both sinapaldehyde and p-coumaraldehyde than for SbCAD4 but a lower turnover rate for coniferaldehyde than for SbCAD4. Thus, SbCAD2 is a more efficient reductase than SbCAD4 for all three tested substrates, especially for p-coumaraldehyde, considering kcat/Km values (Table II).
Table II. Enzyme kinetic parameters for wild-type SbCAD2, SbCAD4, and SbCAD4 mutants.
| Kinetic Parameters | Wild-Type SbCAD2 | Wild-Type SbCAD4 | W58L | Y95V | L119W | A278V | Y288P | G301F | L119W/G301F |
|---|---|---|---|---|---|---|---|---|---|
| p-Coumaraldehyde | |||||||||
| kcat (s−1) | 11.93 | 2.17 | 3.27 | 3.59 | 1.08 | 3.03 | 1.19 | 9.50 | 30.16 |
| Km (μm) | 69.75 | 4,633.23 | 7,101.66 | 6,882.54 | 2,115.01 | 7,966.01 | 4,178.84 | 267.98 | 80.88 |
| Ki (μm) | 32,672.92 | 20,585.15 | 10,021.79 | 7,633.01 | 4.3E+123 | 31,916.86 | 7.5E+113 | 98.93 | 160.03 |
| kcat/Km (s−1 μm−1) | 0.17 | 4.7E-04 | 4.6E-04 | 5.2E-04 | 5.1E-04 | 3.8E-04 | 2.8E-04 | 0.04 | 0.37 |
| Coniferaldehyde | |||||||||
| kcat (s−1) | 12.77 | 20.28 | 3.68 | 9.26 | 2.33 | 0.76 | 3.76 | 71.57 | 25.83 |
| Km (μm) | 95.18 | 233.20 | 9,173.72 | 67.96 | 10.38 | 21.38 | 386.73 | 5,679.29 | 28.01 |
| Ki (μm) | 122.62 | 295.12 | 12.07 | 427.20 | 4,171.92 | 6,030.88 | 710.89 | 16.20 | 11,472.15 |
| kcat/Km (s−1 μm−1) | 0.13 | 0.09 | 4.0E-04 | 0.14 | 0.22 | 0.04 | 0.01 | 0.01 | 0.92 |
| Sinapaldehyde | |||||||||
| kcat (s−1) | 31.75 | 12.54 | 2.50 | 39.97 | 3.32 | 1.05 | 1.59 | 5.29 | 18.98 |
| Km (μm) | 59.76 | 33.34 | 5,522.36 | 111.95 | 78.31 | 79.31 | 7.82 | 16.83 | 105.19 |
| Ki (μm) | 83.01 | 111.75 | 1,455.74 | 37.39 | 1,056.37 | 2,912.79 | 1,391.65 | 1,232.67 | 68,075.58 |
| kcat/Km (s−1 μm−1) | 0.53 | 0.38 | 4.5E-04 | 0.36 | 0.04 | 0.01 | 0.20 | 0.31 | 0.18 |
In order to investigate the role of individual residues in the active site pocket of SbCAD2 and SbCAD4 for the apparent difference in activity, single amino acid substitutions were generated in SbCAD4 through site-directed mutagenesis to systematically mimic the residues of the active site in SbCAD2. The result of enzyme kinetic assays for those mutants showed that most single mutant enzymes displayed Km values similar to those observed in the wild-type SbCAD4, except for W58L-SbCAD4 and G301F-SbCAD4 (Table II). Both mutant enzymes displayed slightly elevated Km values for p-coumaraldehyde but decreased Km for coniferaldehyde compared with those values of wild-type SbCAD4. On the other hand, L119W displayed a slightly reduced Km value for both p-coumaraldehyde and coniferaldehyde. Uniquely, G301F-SbCAD4 displayed a reduced Km for p-coumaraldehyde but a 24.4-fold increase in Km for coniferaldehyde. W58L-SbCAD4 exhibited large increases in Km for all three substrates. Most of those single mutant enzymes displayed diminished kcat values for all three substrates, except for Y95V- and G301F-SbCAD4s. G301F displayed 5.6 and 3.5 times higher kcat values for coniferaldehyde than those of SbCAD2 and SbCAD4, respectively. On the other hand, Y95V displayed 1.3 and 3.2 times higher kcat values uniquely for sinapaldehyde than those of SbCAD2 and SbCAD4, respectively. Overall, the calculated kcat/Km indicated that L119W-SbCAD4 becomes more efficient toward coniferaldehyde due to the very small Km value, with substantially decreased efficiency for both sinapaldehyde and p-coumaraldehyde, which is a new characteristic differentiating it from both wild-type SbCAD2 and SbCAD4. On the other hand, both Y288P-SbCAD4 and G301F-SbCAD4 become more dedicated toward sinapaldehyde, with substantially decreased efficiency for coniferaldehyde.
As both L119W and G301F exhibited favorable Km and kcat toward coniferaldehyde, respectively, an L119W/G301F double mutant was tested. Not only did L119W/G301F-SbCAD4 show elevated kcat for all three substrates compared with its wild-type version, this double mutant also displayed lower Km for both coniferaldehyde and p-coumaraldehyde and a slightly increased value for sinapaldehyde. Notably, improvement of kcat and Km values for p-coumaraldehyde were most significant. Overall, compared with that of wild-type SbCAD4, L119W/G301F-SbCAD4 displayed 10- and 800-fold increases in its catalytic efficiency for coniferaldehyde and p-coumaraldehyde, respectively, and a 50% decrease in catalytic efficiency for sinapaldehyde. Significantly, L119W/G301F-SbCAD4 favored coniferaldehyde as a substrate and showed highest catalytic efficiency for both coniferaldehyde and p-coumaraldehyde among all the wild type and mutants that were analyzed (Fig. 4).
Figure 4.
Substrate preference of wild-type SbCAD2, SbCAD4, and SbCAD4 mutants. The enzyme kinetics parameters kcat (A), Km (B), and kcat/Km (C) are compared. SbCAD2 prefers sinapaldehyde over coniferaldehyde and p-coumaraldehyde. SbCAD4 prefers coniferaldehyde, in terms of kcat, but SbCAD4 is more efficient with sinapaldehyde. Seven SbCAD4 mutants were compared. Near-total loss of activity was observed for the W58L and A278V mutants. Y95V (also Y95C; Supplemental Data S1) displayed similar behavior to the wild type. Among single mutants, L119W was the most efficient at catalyzing coniferaldehyde reduction. Y288P displayed a lower turnover rate. G301F exhibited a substantial change with coniferaldehyde, but due to elevation of both kcat and Km, lowered catalytic efficiency was observed. The L119W/G301F double mutant showed high preference toward coniferaldehyde based on all three parameters.
SbCAD2/CAD4-Like Sequences in Other Plants
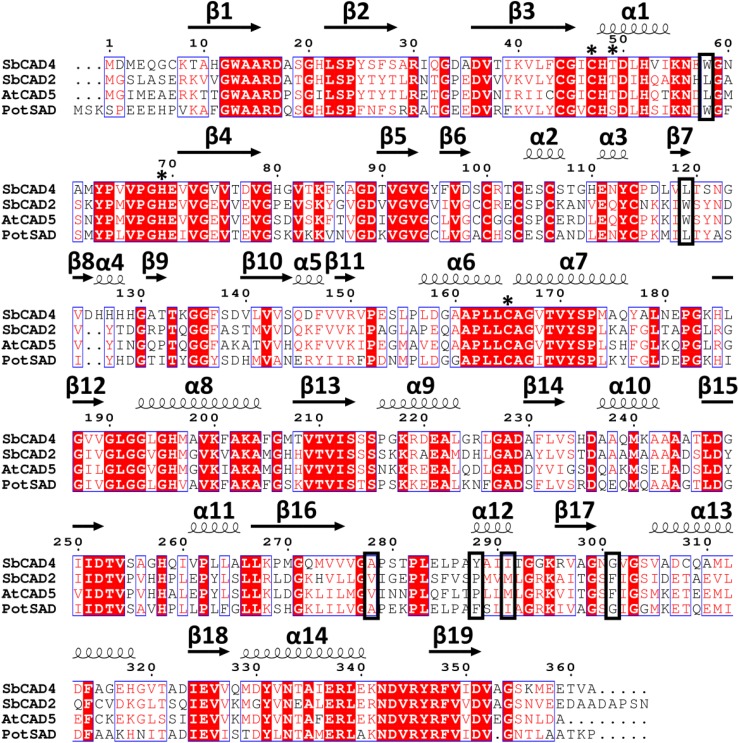
A BLAST search in nonredundant GenBank was performed using the SbCAD4 sequence as a query, which identified CAD enzymes from maize (Zea mays; 91%), foxtail millet (Setaria italica; 87%), barley (Hordeum vulgare; 82%), Brachypodium distachyon (83%), and perennial ryegrass (Lolium perenne; 81%). An additional uncharacterized CAD from sorghum also was identified (identity, 81%; query coverage, 98%), indicating that these SbCAD4-like sequences may belong to a unique functional subclass of the MDR superfamily. Among all aligned enzymes, both the structural and catalytic Zn2+-binding sites were fully conserved. These enzymes additionally had fully conserved NADP(H)-binding sites, including the 213SSS215 motif that indicates NADP(H) specificity. Thr-49 and His-52, identified from our crystal structure to have a probable role in the SbCAD4 proton shuttle, also were fully conserved among these enzymes. Both SbCAD4 and the ZmCAD identified in the BLAST search contained the 126HHHH129 region, which is an insertion unique to these two CADs and forms the basis for α4, interrupting the short β8 and β9 regions. The effect of this sequence on catalytic efficiency or substrate specificity is unclear, but it may impart unique functional characteristics to these two CAD enzymes. On the other hand, a BLAST search to identify proteins with similar amino acid sequences in the PDB yielded PotSAD (PDB no. 1YQD) as the highest match for SbCAD4, with 61% identity. SbCAD4 showed relatively low identity with AtCAD5 (51% identity; Fig. 5).
Figure 5.
Amino acid sequence alignment for SbCAD2, SbCAD4, AtCAD5, and PotSAD. SbCAD2 (accession no. BAF42789) and SbCAD4 (XP_002462348) show the greatest similarity to AtCAD5 (NP_195149) and PotSAD (AAK58693), respectively. Residues required for catalysis are marked by asterisks. Other active site residues that seem to indicate SAD/CAD substrate specificity are boxed. Multiple sequence alignment was performed with the ESPript software package.
A BLAST search also was performed using the SbCAD2 sequence as a query, identifying CAD2-like enzymes from monocots such as various Miscanthus spp. (Miscanthus lutarioriparius [97%], Miscanthus giganteus [96%], Miscanthus floridulus [96%], and Miscanthus sinensis [96%]), maize (96%), panicgrass (Dichanthelium oligosanthes; 96%) and foxtail millet (92%). The CAD identified in the search from maize is a different CAD enzyme than that identified using the SbCAD4 sequence as a query. Unlike for the SbCAD4-like sequences, there was little variation in the SbCAD2-like sequences, with fully conserved NADP(H)-binding and aldehyde-binding sites, indicating that this subclass of the MDR superfamily is significantly more conserved, likely owing to members’ higher catalytic efficiency. A BLAST search in the PDB showed that the highest match for SbCAD2 was AtCAD5 (PDB no. 2CF5), with 73% identity, indicating that SbCAD2 is somewhat closer to AtCAD5 than PotSAD (52%; Fig. 5).
DISCUSSION
The sequence motif 188GXGGV(L)G193 has been used to identify or predict general CAD enzyme activity thus far. Based on the accumulated structures of CADs, including SbCAD2 and SbCAD4, it appears responsible to add three more motifs in order to confidently predict CAD activity. These include (1) 44CGXCX[ST]49, (2) 63YPXVPGHE70, and (3) 161APLLCAGXTVY171, which contain critical and conserved residues for CAD activity, Ser/Thr-49, His-69, and Cys-165, respectively.
Basis for Kinetic Differences between SbCAD2 and SbCAD4
The catalytic efficiency of both SbCAD2 and SbCAD4 for coniferaldehyde is lower relative to sinapaldehyde mainly due to the larger Km for coniferaldehyde, although the kcat value of SbCAD4 for coniferaldehyde is even greater than that for sinapaldehyde (Fig. 4). The binding modes for both coniferaldehyde and sinapaldehyde in SbCAD2 indicated that the hydrophobic pocket formed by side chains of Leu-58, Val-276, and Pro-286 interacts with the methyl group of the m-methoxy moiety of coniferaldehyde, along with the side chains of Trp-119 and Phe-299. In addition, its p-hydroxy group forms hydrogen bonding with the amine group of Lys-117 (Fig. 3A). Significantly, sinapaldehyde establishes extra polar interaction between its second m-methoxy oxygen and the Lys-117 side chain, reducing its Km value (Fig. 3B). Similarly, sinapaldehyde in SbCAD4 establishes an extra interaction in addition to those interactions that coniferaldehyde forms. In detail, the p-hydroxyl and m-methoxyl oxygens of coniferaldehyde form a hydrogen bond and a polar interaction with the hydroxyl group of Thr-292, respectively, and sinapaldehyde establishes extra hydrophobic interactions between the methyl group of the extra m-methoxyl moiety and the methyl side chain of Ala-278 (Fig. 3, C and D).
Our enzyme kinetic data also indicated that the catalytic efficiency of SbCAD4 is lower than that of SbCAD2 for all three substrates, in accordance with the noticeable difference in their constituting residues of the substrate-binding pocket (Fig. 4). Unlike the binding pocket of SbCAD2, which offers stable π-stacking with the docked substrates, the corresponding aromatic residues of SbCAD4 are not able to provide such an interaction with substrates. In addition, Tyr-95 of SbCAD4, which corresponds to Val-95 of SbCAD2, is somewhat distanced from the aromatic ring of the substrate, and Tyr-288 of SbCAD2 faces outward from the pocket (Fig. 3, C and D). The lack of interaction with bound p-coumaraldehyde in SbCAD4 is more severe, as the only significant interaction is between the p-hydroxyl moiety of the substrate and the side chain of Thr-292, which corresponds to Leu-290 of SbCAD2, to hold the substrate in place. This lack of interaction is consistent with a large Km value of SbCAD4 for p-coumaraldehyde.
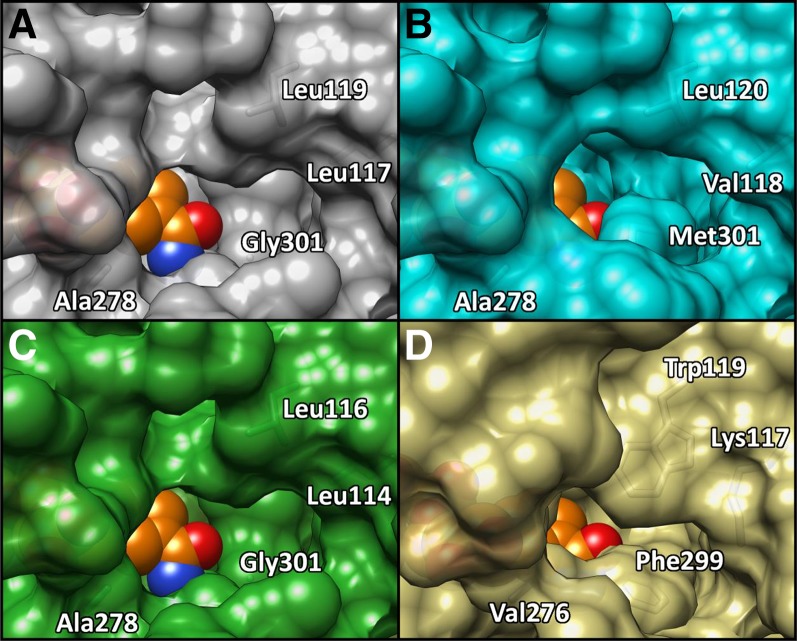
The apparent differences of the binding pocket between SbCAD2 and SbCAD4 were similarly observed between the binding pockets of AtCAD5 and PotSAD. Careful inspection of the residues constituting the substrate-binding site (Fig. 3) suggests that there are unique conservation patterns among those two groups of CAD enzymes, which could serve as a signature sequence to subdivide their activities. Among the 12 residues that constitute the substrate-binding pocket, six residues showed systematic differences (Fig. 5). SbCAD2 and AtCAD5 have Leu-58, Trp-119, Val-276, Pro-286, Met-289, and Phe-299; whereas SbCAD4 and PotSAD have Trp-58, Leu-119, Ala-278, Tyr/Phe-288, Ile-291, and Gly-301 at these corresponding positions.
In order to correlate those observed sequence patterns with their enzymatic activities, site-directed mutagenesis for each residue was performed and the enzyme activities of the modified enzymes were analyzed. In SbCAD2, Leu-58-Val-276-Pro-286 together establish a hydrophobic binding region covering both nicotinamide and the m-methoxyl moiety of substrates. The corresponding triad in SbCAD4 is Trp-58-Ala-278-Tyr-288. Despite apparent differences in their sequences, the three unique residues of each triad provide a complementary surface for both nicotinamide and substrate. Thus, both W58L-SbCAD4 and A278V-SbCAD4 fail to provide an adequate triad. Our structural data for SbCAD4 indicate that its indole side chain of Trp-58 is in close proximity to the aromatic ring of the docked substrates. Its imperative role can be inferred from the consistent presence of Trp at position 58 in PotSAD, SbCAD4, and SbCAD8s (Fig. 6). In addition, the necessity of Trp-58 is further supported by the kinetic data of the W58L-SbCAD4 mutant, which nearly abolished the catalytic efficiency. The A278V-SbCAD4 mutation also results in steric hindrance between nicotinamide/ribose and the aromatic group of docked substrates, as the larger Val residue pushes ribose away. As a result of the increased distance of hydride transfer, the enzyme turnover rate is decreased for all three substrates, which causes diminished catalytic efficiency.
Figure 6.
DNA sequence alignment for sorghum CADs. Sequences of SbCAD4 (Sb02g024190), SbCAD2 (Sb04g005950), SbCAD4-2 (Sb10g006300), SbCAD4-3 (Sb10g006290), SbCAD4-4 (Sb10g006280), SbCAD4-5 (Sb10g006270), SbCAD5 (Sb07g006090), SbCAD6 (Sb06g001430), SbCAD7 (Sb06g028240), SbCAD8-1 (Sb02g024220), and SbCAD8-2 (Sb02g024210) are aligned. Asterisks are used to indicate residues required for Zn2+. Boxes indicate active site residues that have potential roles in substrate affinity and catalysis. The region of higher conservation is depicted with gray shading. Multiple sequence alignment was performed with the ESPript software package.
On the other hand, the L119W-SbCAD4 mutation imitating Trp-119 of SbCAD2 had a distinct consequence. A bulkier indole side chain protrudes farther into the substrate-binding pocket, providing extra hydrophobicity to the docked aromatic ring of the substrates. The elevated catalytic efficiency for coniferaldehyde and p-coumaraldehyde of L119W-SbCAD4 is due to a lowered Km (Table II). The additional hydrophobic interaction with the L119W mutation resulted in a higher affinity to coniferaldehyde. However, the extra m-methoxyl group on sinapaldehyde clashes with the same indole side chain of Trp-119, increasing its Km value. Overall, this L119W-SbCAD4 mutant could provide new substrate selectivity toward coniferaldehyde, which could generate a higher amount of the guaiacyl subunit and produce less recalcitrant lignin polymers in grasses.
Gly-301 of SbCAD4 is positioned at the bottom platform of the substrate-binding pocket, in between the two residues that hold the nicotinamide moiety of NADPH in place. The amidic nitrogen and oxygen of the nicotinamide form a salt bridge with the backbone oxygen and nitrogen atom of Asn-300 and Val-302, respectively. The G301F mutation introduces steric hindrance, altering the mode of substrate binding. Upon AutoDock experiments, a bulky side chain of Phe-301 creates significant steric hindrance at the base of the original binding site, and substrates were pushed closer to Trp-58 with an ∼90°-rotated aromatic group, forming edge-to-face stacking with the mutated Phe-301 residue. Consequently, the ring of substrates was oriented in parallel with a side chain of Trp-58. The hydrogen bond between the p-hydroxyl group and the hydroxyl side chain of Thr-292, as observed in the crystal structure of the wild-type enzyme, was no longer present. However, for both coniferaldehyde and sinapaldehyde, a new interaction was observed between the m-methoxyl oxygen and the side chain of Tyr-95 (Fig. 3, E and F). The estimated binding energy for p-coumaraldehyde, coniferaldehyde, and sinapaldehyde was calculated to be −5.8, −3.8, and −5.3 kcal mol−1, respectively. The Km values of the G301F mutant for p-coumaraldehyde and sinapaldehyde were decreased, probably due to extra π-interaction. According to the AutoDock calculation, coniferaldehyde with one methoxyl group binds with its aromatic ring sandwiched between Trp-58 and Phe-301 and both hydroxyl and methoxyl groups interacting with the side chain of Tyr-95. However, the position and conformation of bound coniferaldehyde put its aldehyde group face slightly away from the catalytic pocket. Unlike the other two substrates, docked coniferaldehyde displayed lower affinity (−3.8 kcal mol−1), being consistent with its elevated Km. However, coniferaldehyde in the same position puts the aldehydic oxygen 0.1 Å closer to catalytic hydroxy group of Thr-49, resulting in elevated kcat.
The last residue of the triad in SbCAD4, Tyr-288, is located on the solvent-exposed face of α12 that is positioned in the dimerization interface and contributes to the active site pocket of the dimer partner subunit. Significantly, the α12 of SbCAD4 and PotSAD have two more residues than the α12 observed in SbCAD2 and AtCAD5. This difference in length of α12 causes SbCAD4/PotSAD to have its Tyr-288/Phe-288 at the position of Pro-286 of SbCAD2 (Fig. 5), which somewhat contributes to narrowing the opening for substrate entrance. Significantly, α12 is located right upstream of the NADPH-binding loop, and the side chain of Tyr-288 connects the loop to α12. Introducing the Y288P mutation changes the angle between the loop and the helix, which generates a direct impact on the position of the loop. Accordingly, the Y288P mutation in SbCAD4 resulted in a slower turnover rate.
There is one unique residue, Tyr-95, in the substrate-binding pocket of SbCAD4 near Leu-119. Corresponding residues in PotSAD and other bona fide CADs, such as SbCAD2 and AtCAD5, are either Val or Cys, both of which have shorter side chains than Tyr. The structure of SbCAD2 reveals that a nearby residue, Trp-119, likely compensates for the lack of the aromatic ring at this position, as similarly shown before (Saathoff et al., 2012). The Y95C-SbCAD4 mutant has shown almost identical behavior (Supplemental Data S1) as the wild-type SbCAD4. Similarly, Y95V-SbCAD4 exhibited increased kcat for sinapaldehyde, but due to a corresponding increase in Km, the overall catalytic efficiency of Y95V remained similar to its wild-type version. A close examination of the crystal structure of SbCAD4 shows that Tyr-95 would be too far to have π-stacking with the phenyl group of a substrate. In addition, the closest atoms, Cβ and Cγ of the aldehyde group, would lie in a slanted way from the para-carbon of Tyr-95, indicating a marginal role of Tyr-95 in substrate binding. However, comparative kinetics studies of the wild-type SbCAD4, L119W-SbCAD4, and Y95V-SbCAD4 indicate that the presence of both bulky residues, Tyr-95 and Trp-119, causes unfavorable steric hindrance to sinapaldehyde, as mentioned above.
Addition of the G301F mutation to the L119W-SbCAD4 mutant can position coniferaldehyde at a catalytically favorable position, and its side chain of Trp-119 can hydrophobically stabilize the binding of the aromatic group of coniferaldehyde and p-coumaraldehyde. In addition, this L119W/G301F-SbCAD4 double mutant uses the two bulky residues, Tyr-95 and Trp-119, to eschew the binding of sinapaldehyde. As predicted, kinetics results show that L119W/G301F-SbCAD4 demonstrates its decreased binding affinity (increased Km) for sinapaldehyde and increased binding affinity and turnover rate for both coniferaldehyde and p-coumaraldehyde. Previously, a similar double mutant study was reported for L122W/G302F-PotSAD (Bomati and Noel, 2005), with catalytic efficiency of 0.49 μm−1 s−1. Comparing the catalytic efficiency of those two double mutants, one each from a dicot and a monocot, L119W/G301F-SbCAD4 (0.92 μm−1 s−1) is nearly twice as efficient than L122W/G302F-PotSAD. Close examination of the two crystallographic structures shows that the space between Leu-119 and Gly-301 of SbCAD4 is occupied by the side chain of Tyr-95, where the same space between Leu-122 and Gly-302 of PotSAD is occupied by the side chain of Cys-98. As mentioned above, having two bulky residues in the Tyr-95 and Trp-119 positions could make the active site pocket tighter (or more compact), allowing a lesser degree of freedom for bound substrates, which might contribute favorable binding for coniferaldehyde and p-coumaraldehyde and unfavorable binding for sinapaldehyde. Our structural and enzymatic data for L119W/G301F-SbCAD4 can provide a model for monocot CADs favoring coniferaldehyde and p-coumaraldehyde, which can lead to decreased amounts of the syringyl unit in lignin content, yielding softer lignin.
Reaction Mechanism
In the absence of NADPH (or NAPD+), the two domains of SbCAD4 (and SbCAD2) are in their open conformation and the carboxyl oxygen of Glu-70 (Glu-66 for SbCAD2) participates in the coordination of the catalytic Zn2+ (step A in Fig. 7). Thus, a diffused-in substrate does not participate in Zn2+ coordination. Instead, the side chain of Thr-49 and the catalytic Zn2+ provide a hydrogen bond and electrostatic interaction, respectively, to the aldehyde group of the substrates (step B1). Upon binding of NADPH to the apo-form SbCAD4 or its substrate binary complex, the resulting domain closure with concomitant dissociation of the carboxyl oxygen of Glu-70 from the Zn2+ brings the carbonyl oxygen of the substrate into a proper position for Zn2+ coordination, establishing a productive ternary complex (step C). As shown in the crystal structure of SbCAD4 and other MDHs such as FurX (Kang et al., 2012), in the event of NADPH association to the apo-form SbCAD2/SbCAD4, the two domains become closed and a water or other oxygenic molecule in the solution, as shown in our crystal structures, will replace the Glu-70’s Zn2+ coordination (step B2). In the closed state, diffused-in coniferaldehyde replaces this water molecule and locates its carbonyl oxygen in the Zn2+-coordinating position, establishing a productive ternary complex (step C). In this switching event for Zn2+ coordination between substrate and a water molecule, Glu-70 may not be involved, which would require a domain opening. Thus, it is tempting to speculate a random-order mechanism for SbCAD2/SbCAD4. Isotope exchange and kinetic analysis of YADH1 point to a random-order mechanism for the formation of the enzyme-ethanol-NAD+ complex (Silverstein and Boyer, 1964; Dickinson and Monger, 1973; Plapp, 2010); however, the mechanism has not been widely accepted due to the lack of supporting structural evidence. In addition, the substrate molecule can readily diffuse into the NADPH-SbCAD4 binary complex (step B2); thus, the reaction could still kinetically follow compulsory order, as HLADH and other animal liver ADHs do (Theorell, 1967; Plapp, 2010).
Figure 7.
Proposed random-order reaction mechanism for SbCAD2 and SbCAD4. Step A is a representation of the unbound active site. After a random-order binding of an aldehydic substrate (step B1) and NADPH (step B2), a ternary complex is formed (step C). After reduction of the aldehyde (step D), products are dissociated in a random order (steps E1 and E2).
In both plausible routes (step B1 or B2) of ternary complex formation (step C), the aldehydic oxygen of the substrate maintains a hydrogen bond with the hydroxyl side chain of Thr-49. The hydroxyl group of Thr-49 consequently acts as a general acid for the Zn2+-coordinated oxygen of the substrate. The closed conformation of SbCAD4 (step C) allows a pro-R hydride to be transferred from the C4 atom of NADPH to the C1 atom of the aldehyde group, producing a corresponding alcohol (step D) through a previously proposed hydrogen-tunneling event (Kohen et al., 1999). The fully closed conformation at step C can establish stability and efficiency in the proton network and hydrogen tunneling, facilitating conversion to step E2 via step D (Fig. 7). In that built-in proton-channeling system in SbCAD2/SbCAD4 as in AtCAD5 (Youn et al., 2006) and PotSAD (Bomati and Noel, 2005), a proton can be channeled into the side chain of Thr-49 from the 2′-OH of the nicotinamide ribose, followed by a transfer from the 3′-OH of the nicotinamide to the 2′-OH, and completed with a proton transfer from the side chain of His-52 to the nicotinamide ribose 3′-OH. His-52 is solvent exposed and is highly conserved in MDR enzymes (Youn et al., 2006). The side chain of Glu-57, which coordinates His-52, may have a role as a final relay in the proton shuttle, as has been suggested previously (Youn et al., 2006), although it has been reported that both His-52 and Glu-57 are nonessential for catalysis (Lee et al., 2013). Mutating His-52 to Ala would allow for access of the nicotinamide ribose 3′-OH to the solvent, allowing the strongly basic alkoxide moiety to deprotonate a water molecule to complete the catalytic cycle. To this extent, His-52 likely plays a role in the SbCAD2/SbCAD4 proton shuttle, although this role can be accomplished by other mechanistic means. Upon reduction of substrate, the nicotinamide ring of a remaining NADP+ molecule becomes mobile and loses its affinity, probably due to electrostatic repulsion between its positive charge and Zn2+. That is, the oxidized nicotinamide ring dissociates the binding pocket first, followed by the ribose and adenine diphosphoribose, as in previous proposals (Kovaleva and Plapp, 2005).
SbCAD2/CAD4-Like Sequences in Sorghum
To gain information about their plausible activities, the sequences of SbCAD4 and SbCAD2 were aligned with nine putative sorghum CAD enzymes that contain all the required residues to coordinate both catalytic and structural Zn2+ ions (Saballos et al., 2009; Fig. 6). SbCAD4-2, SbCAD4-3, SbCAD4-4, and SbCAD4-5 contained an Ala rather than a Ser or Thr at the position of the catalytic Thr-49 in SbCAD4, indicating that the catalytic efficiencies of these enzymes are very likely compromised. Comparing residues that were identified as indicating SbCAD4 or SbCAD2 character by our previous alignment showed that the remaining enzymes, SbCAD5, SbCAD6, SbCAD7, SbCAD8-1, and SbCAD8-2, generally resembled SbCAD4 and, thus, are more likely SbCAD4-like enzymes rather than SbCAD2-like enzymes. Furthermore, all those five enzymes contained a Trp residue at the position of Trp-58 in SbCAD4 (Fig. 6).
Both SbCAD8-1 and SbCAD8-2 contained a Tyr at the corresponding position to the Tyr-95 of SbCAD4, whereas SbCAD5, SbCAD6, and SbCAD7 contained a Cys at this position (Fig. 6). Even though Tyr-95 is a discernible polar residue in a mainly hydrophobic substrate-binding pocket, insignificant kinetic differences caused by single mutations on Tyr-95 to either Val or Cys show that a residue at the 95 position alone is not significantly involved in the enzymatic activity.
Only SbCAD8-2 had a Leu at a position homologous to the SbCAD4 Leu-117, with SbCAD5 and SbCAD7 having an Ile at this position and SbCAD6 and SbCAD8-1 having a Val. These are conservative substitutions, which likely do not contribute any significant changes in active site character. At the position of Leu-119 in SbCAD4, which is conserved in both SbCAD6 and SbCAD8-2, SbCAD5 and SbCAD7 contained a Phe and SbCAD8-1 contained a Gln. Due to the proximity of Leu-119 to the active site, these nonconservative substitutions likely would negatively impact substrate affinity by further crowding the active sites of these CADs. In addition, SbCAD6 and SbCAD8-2 contained an Ala at a position homologous to the SbCAD4 Ala-278, but SbCAD5 and SbCAD7 had a Leu and SbCAD8-1 had a Gly at this position, although the impact of these substitutions on the catalytic efficiency of the enzyme is unclear. It is possible that the Leu present in SbCAD5 and SbCAD7 may adopt a conformation that extends close enough to the active site to reduce activity by sterically crowding the active site. The SbCAD4 Gly-301 is preserved in SbCAD8-2 but is substituted to Cys in SbCAD5 and SbCAD7, to Met in SbCAD6, and to Ser in SbCAD8-1. The position of Gly-301 in the active site is directly adjacent to the bound substrate, and the substitution to the hydrophobic Met in SbCAD6 may accommodate coniferaldehyde binding but would likely sterically block sinapaldehyde activity. The substitutions to the more polar Cys in SbCAD5 and SbCAD7 as well as to the polar Ser in SbCAD8-1 would likely reduce enzyme activity, due to the position of these residues relative to the hydrophobic aromatic ring in the predicted coniferaldehyde and sinapaldehyde positions. Overall, it is plausible that SbCAD5, SbCAD7, and SbCAD8-1 have even lower activity than SbCAD4. However, the activity of SbCAD6 and SbCAD8-2 might be similar to that of SbCAD4, with SbCAD4 having slightly different substrate specificity (Fig. 8).
Figure 8.
Surface representation of the active site opening of highly homologous SbCAD isozymes. Active site opening to nicotinamide of SbCAD4 (A), SbCAD6 (B), SbCAD8-2 (C), and SbCAD2 (D) is shown. SbCAD4 and SbCAD8-2 have nearly identical surfaces around the active site opening. SbCAD6 has a narrower opening due to Met-301 but is mostly homologous to SbCAD4. Compared with SbCAD4, SbCAD6, and SbCAD8-2, SbCAD2 has a significantly tighter opening due to bulky residues such as Lys-117, Trp-119, and Phe-299. NADP+ is shown as orange spheres, and red/blue color indicates oxygen/nitrogen. Molecular graphics images were produced using the UCSF Chimera package.
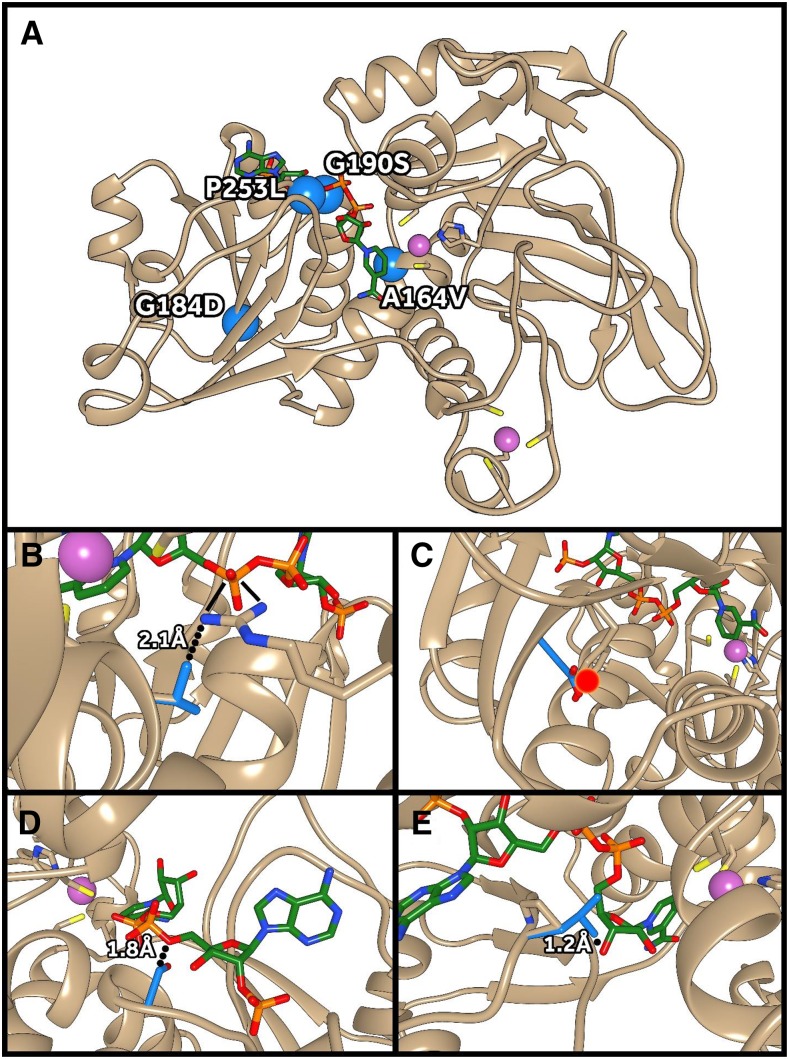
The bmr6 Mutations
Four missense (A164V, G184D, G190S, and P253L) and two nonsense (Q132stop and Q321stop) mutations, as well as a frameshift mutation that results in the bmr6 phenotype, have been identified previously (Saballos et al., 2009; Sattler et al., 2009; Scully et al., 2016). Using our crystal structure of SbCAD4 and the homology model of SbCAD2, the structural consequences of those six bmr6 mutations were (re)interpreted (Fig. 9). Three of those four missense mutations (G184D, G190S, and P253L) are located in the NADP(H)-binding domain of SbCAD2. The A164V mutation, which is adjacent to the Zn2+-coordinating side chain of Cys-163 (Fig. 9), will produce a steric clash with the backbone of the highly conserved 188GXGGV(L)G193 motif that is known to ensure the proper folding of the NADP(H)-binding domain of all known CADs. The extended polar side chain upon G184D mutation would significantly disrupt the overall or local fold of the NADP(H)-binding domain due to its major steric clash with the neighboring Tyr-247 side chain, interrupting an existing hydrogen bond between β10 and β13. Probably due to this folding instability, the G184D SbCAD2 mutant’s protein does not accumulate either in Escherichia coli or in sorghum (Scully et al., 2016). The Gly residue in the G190S mutation (bmr6-3) is part of the 188GXGGV(L)G193 motif, and the polar substitution upon mutation results in a direct steric clash with the diphosphate linker of the bound NADPH. In addition, the altered character in this flexible region of the G190S mutant may very well severely reduce the affinity for NADP(H), as indicated previously (Saballos et al., 2009). The P253L mutation also is located in the NADP(H)-binding domain and occurs adjacent to Val-252, which coordinates the adenosine of NADP(H). Thus, this mutation not only has the potential to cause a steric clash with bound NADP(H) directly but also alters backbone flexibility, potentially bringing the side chain of Val-252 out of the proper orientation for NADP(H) coordination. On the other hand, the two nonsense mutations, Q132stop and Q321stop, result in the loss of significant portions of SbCAD2, both of which are not detectable in planta (Scully et al., 2016). The frameshift mutation as noticed in bmr6-27 could completely abolish the integrity of SbCAD2.
Figure 9.
Structural impact of bmr6 mutations on SbCAD2. A, The locations of bmr6 missense mutations are indicated by blue spheres. B, The A164V mutation results in a close contact with Arg-354, involved in NADP(H) binding. C, The G184D mutation causes a close contact with Tyr-247, disrupting protein folding. D, The G190S mutation causes a close contact with NADP(H). E, The P253L mutation causes a close contact with NADP(H). Zn2+ ions are shown in purple, wild-type SbCAD2 side chains in tan, bmr6 mutant side chains in blue, and NADP+ in green. Molecular graphics images were produced using the UCSF Chimera package.
CONCLUSION
As CADs catalyze the final step of the monolignol biosynthetic pathway, this in-depth understanding of their substrate preferences can provide a strategy for reducing the syringyl content of lignin beyond what has been observed in bmr6 while maintaining a level of lignin needed for plant growth and defense. As most CADs and SAD that have been characterized to date are catalytically more efficient in the reduction of sinapaldehyde than coniferaldehyde, a further reduction in the proportion of syringyl residues, with the aim of further improving biomass conversion, could be accomplished by down-regulating both SbCAD2 and SbCAD4. This strategy has the risk of reducing the lignin concentration to levels that will no longer support normal growth and development, as has been observed in several double and triple bm mutants of maize (Vermerris et al., 2010) and sorghum (Dien et al., 2009). Our site-directed mutagenesis based on the crystal structures and homology models of SbCAD2, SbCAD4, AtCAD5, and PotSAD revealed several unique mutants that have altered substrate specificity. Among those, the L119W/G301F-SbCAD4 mutant displayed its substrate preference in the order coniferaldehyde > p-coumaraldehyde > sinapaldehyde. In addition, this double mutant showed higher catalytic efficiency than both wild-type SbCAD4 and SbCAD2. As SbCAD4 is the only major isoform responsible for generating monolignols in those bmr6 mutants that have inactive or minimally active SbCAD2 enzymes, replacing SbCAD4 with L119W/G301F-SbCAD4 in bmr6 mutants is expected to result in plants with lignin that displays the desirably low S/G ratio but that may not suffer a negative impact on plant growth and development. Given the similarities in cell wall composition among the grasses and the identification of SbCAD2 and SbCAD4 enzymes in several other grass species (e.g. Miscanthus spp.), this strategy may not apply only to sorghum but also may show promise in other monocot biomass crops.
MATERIALS AND METHODS
Chemicals and General
Analytical-grade chemicals were obtained from Sigma-Aldrich, Thermo Fisher, and Alfa-Aesar. Crystallization screens were obtained from Hampton Research. An Isolera One flash chromatography system (Biotage) with prepacked silica gel cartridges was used for model compound separation. Preparative thin-layer chromatography (TLC) plates (1 mm thickness, 20 cm × 20 cm, silica gel, normal phase) from Analtech also were used for the purification. Bruker Biospin AVANCE 500-MHz (equipped with a cryogenically cooled 5-mm TCI 1H/13C/15N gradient probe) and 700-MHz (equipped with a 5-mm QCI 1H/31P/13C/15N cryoprobe) spectrometers with inverse geometry (proton coil closest to the sample) were used at 300K. The 1D NMR (1H, 13C, and DEPT-135) and 2D NMR (HMQC, HMBC, and COSY) experiments used standard Bruker pulse programs. We used Bruker’s Topspin 3.5pl6 (Mac) software to process the spectra.
Recombinant Enzyme Expression and Purification
Cloning of both SbCAD2 and SbCAD4 has been described previously (Sattler et al., 2009). For the expression of recombinant SbCAD4, 200 mL of Luria-Bertani medium containing 100 µg mL−1 kanamycin and 34 µg mL−1 chloramphenicol was inoculated with a freezer stock of Escherichia coli Rosetta cells (EMD Millipore) containing the pET30a-SbCAD4 construct and grown overnight at 37°C while shaking. This culture was used to inoculate 3 L of Luria-Bertani medium, which was grown to an OD600 of 0.6 at 37°C with shaking. The cells were then brought to 20°C with shaking, and isopropyl β-thiogalactopyranoside was added to a final concentration of 1 mm. The culture was grown at 20°C while shaking for an additional 18 h. Cells were collected by centrifugation at 6,000 rpm for 12 min at 4°C. The cell pellet was resuspended in 40 mL of lysis buffer (20 mm Tris, pH 8, 150 mm NaCl, and 10 mm imidazole) and sonicated five times with 15-s pulses (model 450 sonifier; Branson Ultrasonics). The lysate was cleared by centrifugation at 15,000 rpm for 25 min. Cleared supernatant was applied to 15 mL of nickel-nitrilotriacetate agarose (Qiagen), equilibrated with lysis buffer, and placed into a gravity-flow column. The column was washed with 20 column volumes of washing buffer (20 mm Tris-HCl, pH 8, 300 mm NaCl, and 20 mm imidazole), and protein was eluted with elution buffer (20 mm Tris-HCl, pH 8, 300 mm NaCl, and 250 mm imidazole). Column fractions containing SbCAD4 were desalted and concentrated into buffer A (20 mm Tris-HCl, pH 8, and 1 mm DTT) using an Amicon 8050 ultrafiltration cell with a 10-kD cutoff membrane (Millipore). Concentrated protein was applied to a Mono-Q column (GE Healthcare) that was preequilibrated with buffer A using a flow rate of 2 mL min−1. SbCAD4 was eluted from the column with 300 mm NaCl applied in a linear gradient. The fractions containing SbCAD4 were pooled, and buffer was exchanged into 20 mm HEPES, pH 7.5. The expression and purification of SbCAD2 were performed in an identical manner to SbCAD4, except that induction was performed at 22°C for 15 h.
Site-directed mutations were created in the SbCAD4 coding region by PCR-based amplification using Phusion High-Fidelity DNA polymerase (New England Biolabs). The amplification was performed using complementary plus- and minus-strand oligonucleotides containing the target mutations and was followed by DpnI (New England Biolabs) digestion to degrade the template prior to the transformation of competent E. coli Rosetta cells (EMD Millipore). Both mutations were confirmed by DNA sequencing (GENEWIZ).
Crystallization and Structure Determination
Crystals of SbCAD4 were grown through the hanging-drop, vapor-diffusion method. The purified SbCAD4 was concentrated to 20 mg mL−1 in 20 mm HEPES, pH 7.5, and then 1 mm NADP+ was mixed with an equal volume of reservoir solution and equilibrated against the same solution at 4°C. The reservoir solution contained 2% (v/v) Tacsimate, pH 7, 5% (v/v) 2-propanol, 0.1 m imidazole, pH 7, and 8% (w/v) polyethylene glycol 3,350. Crystals of SbCAD4 appeared within 7 d. Adequate cryoprotection was achieved by passing crystals through a small drop of storage buffer/mother liquor mixture, which was brought to a final concentration of 15% (v/v) glycerol. SbCAD4 was crystallized in the space group P21 and had unit cell dimensions a = 55.604 Å, b = 74.277 Å, c = 97.293 Å, α = γ = 90°, β = 96.741°. Data were collected to 1.83 Å at the Berkeley Advanced Light Source (beamline 8.2.1) with an exposure time of 1 s and a detector distance of 270 mm. Crystals were transferred to a cryoprotectant solution that contained the reservoir solution as well as 12.5% (v/v) glycerol, prior to data collection at 100 K. Diffraction data were scaled using the program HKL2000 (Otwinowski and Minor, 1997).
Phasing and Refinement
The initial phasing of diffraction data was performed by molecular replacement with the Phaser program in the PHENIX package using Populus tremuloides SAD as a search model (Bomati and Noel, 2005; Terwilliger et al., 2008; Adams et al., 2010). The Autobuild program in the PHENIX package was used to generate an initial structure after phasing (Terwilliger et al., 2008; Adams et al., 2010). NADP+ and Zn2+ were placed manually into the resulting model, and missing amino acid regions were fitted manually using the program Coot and refined using PHENIX (Adams et al., 2010; Emsley et al., 2010). Following refinement, Rwork was 16.3%, Rfree was 19.6%, and root mean square deviations from ideal geometry of the model were 0.007 Å for bonds and 1.173° for angles. The statistics for the diffraction data are listed in Table I.
Enzyme Kinetics
Enzyme kinetic assays were performed in a 70-µL reaction volume of 20 mm sodium phosphate buffer (pH 6.5) containing 5 mm NADPH, 0, 10, 20, 40, 50, 100, 150, 200, 400, 500, 800, 1,000, 3,000, and 5,000 µm aldehydic substrates (p-coumaraldehyde, coniferaldehyde, or sinapaldehyde), 5 mm 2-mercaptoethanol, and 100 nm enzyme. Reactions were initiated by the addition of NADPH, incubated at 30°C for 60 s, and quenched by the addition of 30 µL of glacial acetic acid. Reaction products were separated by HPLC (Hitachi Elite LaChrom L-2100; Hitachi High-Tech) operating at a flow rate of 1 mL min−1, with a gradient of solvent A (0.1% trifluoroacetic acid in deionized water) and solvent B (100% acetonitrile) varying from 95% A and 5% B to 30% A and 70% B over a period of 20 min. Products were quantified by detection at 260 nm using the Hitachi Elite LaChrom L-2400 detector. All data points were triplicated. Each data set was fitted with a substrate inhibition equation (Eszes et al., 1996):
 |
Molecular Docking of Substrate to Wild-Type and G301F SbCAD4
To monitor differences in their substrate-binding mode between the wild type and the G301F mutant of SbCAD4, in silico substrate-docking experiments were performed with AutoDock Vina (Trott and Olson, 2010). Prior to a docking calculation, the formic acid molecule was removed from the active site of the SbCAD4 crystal structure. Partial charge (q) of the tricoordinated Zn2+ ion was manually changed to 1.639 (Ahmadi and Fattaji, 2011). Once the substrate-binding site was confirmed by a blind search where the search box contained a whole dimeric SbCAD4, both binding affinity and modes of the aldehydic substrates were determined by an exhaustive search within the active site pocket.
Chemical Synthesis
Despite being commercially available, coniferyl alcohol and sinapyl alcohol were synthesized as we described in a previous publication to ensure high quality (Kim and Ralph, 2005). The corresponding aldehydes were obtained from Sigma-Aldrich as the starting materials.
As p-coumaraldehyde is not commercially available, it was synthesized via p-coumaryl alcohol that was itself synthesized from p-coumaric acid based on a previous method (Quideau and Ralph, 1992). p-Coumaric acid (4-hydroxycinnamic acid; 50 g, 0.31 mol) was dissolved in methanol (400 mL) and stirred at room temperature. Acetyl chloride (40 mL) was carefully added to the reaction solution and stirred overnight. The reaction solution was dried using an evaporator. A small volume of methanol was added several times and dried each time. White or slightly purple crystals (41 g, 0.23 mol, 74%) formed after drying. The obtained methyl p-coumarate was reduced directly without recrystallization. The methyl p-coumarate (30 g, 0.17 mol) was dissolved in tetrahydrofuran (THF; 500 mL), and lithium aluminum hydride (LiAlH4; 12.9 g, 2 equivalents) was added at room temperature. The reaction was stirred for 6 h, and reaction completion was monitored by TLC. The reaction flask was placed in an ice bath before quenching with ethyl acetate and left for 30 min until gas evolution ceased. Saturated aqueous ammonium chloride (NH4Cl) was added slowly to ensure no further CO2 evolution. The reaction solution changed color to gray and was extracted three times with ethyl acetate (200 mL). The obtained product solution was evaporated until the solvent was reduced to half of its initial level. Anhydrous magnesium sulfate (MgSO4) was added into the solution, and the product solution was filtered through a silica bed in a sintered glass filter. Solid products were obtained after evaporation, and white crystals (24.4 g, 0.16 mol, 96%) were obtained after recrystallization from ethyl acetate/petroleum ether. 1H-NMR (acetone-d6) δ 4.20 (2H, bd, J = 5.2 Hz, γ), 6.21 (1H, dt, J = 15.8, 5.5 Hz, β), 6.51 (1H, bd, J = 15.9 Hz, α), 6.80 (2H, m, 3, 5), 7.31 (2H, m, 2, 6); 13C-NMR (acetone-d6) δ 63.47 (γ), 116.19 (3, 5), 127.67 (β), 128.33 (2, 6), 129.73 (1), 130.29 (α), 157.78 (4).
The p-coumaraldehyde was synthesized from p-coumaryl alcohol. A total of 2 g (13.3 mmol) was dissolved in dioxane, 0.8 molar equivalents of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) was added, and the mixture was stirred overnight. Solid DDQH2 was removed by a thin silica bed in a sintered glass filter, and the filtrate was dried. The residue was dissolved in ethyl acetate and washed with water, sodium bicarbonate (0.4 m), and saturated NH4Cl. The resulting solution was dried over anhydrous MgSO4. After recrystallization in CH2Cl2, a dark brown solid (0.784 g, 5.3 mmol, 40%) was obtained. 1H-NMR (acetone-d6) δ 6.62 (1H, dd, J = 15.8, 7.7 Hz, 8), 6.94 (2H, m, 3, 5), 7.58 (1H, d, J = 15.8 Hz, 7), 7.61 (2H, m, 2, 6), 9.64 (1H, d, J = 7.7 Hz, 9); 13C-NMR (acetone-d6) δ 116.84 (3, 5), 126.73 (1), 126.96 (8), 131.48 (2, 6), 153.64 (7), 161.24 (4), 193.81 (9).
The 5-hydroxyconiferyl alcohol and 5-hydroxyconiferaldehyde were synthesized from 5-hydroxybenzaldehyde. Acetylated 5-hydroxybenzaldehyde was prepared in acetic anhydride/pyridine first, and this was used as the starting material for the Horner-Wadsworth-Emmons (Wittig-Horner) reaction. Sodium hydride (315.4 mg, 13.14 mmol) was suspended in anhydrous THF. Triethyl phosphonoacetate (1.43 mL, 7.32 mmol) was added via syringe under argon. The reaction mixture was stirred at room temperature for 20 min, after which a solution of the diacetate 5-hydroxybenzaldehyde (1.6 g, 6.57 mmol) in anhydrous THF was added. This reaction mixture was stirred at room temperature for another 20 min and subsequently quenched with NH4Cl, extracted with ethyl acetate, and dried over MgSO4, and the solvent evaporated. A mixture of monoacetate and diacetate ethyl 5-hydroxyferulate was obtained as a pale-yellow solid in 93% yield (1.96 g, 6.11 mmol). The diacetate ethyl 5-hydroxyferulate was deacetylated with pyrrolidine (Lu and Ralph, 1998) before reduction.
The 5-hydroxyconiferyl alcohol was prepared using LiAlH4 (Wang et al., 2009). The ethyl 5-hydroxyferulate (384.7 mg, 1.62 mmol) from the previous step was dissolved in 50 mL of THF. LiAlH4 (91.98 mg, 2.42 mmol; 1.5 eq.) and benzyl chloride (278.9 μL, 2.42 mmol) were added slowly into the reaction mixture at room temperature and stirred for 1.5 h. The reaction mixture was quenched with NH4Cl, extracted with ethyl acetate, and evaporated. The crude product was purified with TLC (CHCl3:methanol, 10:1, v/v) to obtain the clean product (70.3 mg, 0.36 mmol, 22%). 1H-NMR (acetone-d6) δ 3.81 (3H, s, OMe), 4.18 (2H, dd, J = 5.5 Hz, γ), 6.18 (1H, dt, J = 15.8, 5.5 Hz, β), 6.42 (1H, dt, J = 15.8, 1.5 Hz, α), 6.57 (1H, d, J = 1.7 Hz, 6), 6.59 (1H, d, J = 1.7 Hz, 2); 13C-NMR (acetone-d6) δ 56.27 (OMe), 63.31 (γ), 102.34 (2), 107.93 (6), 128.13 (β), 129.40 (1), 130.61 (α), 134.38 (4), 146.18 (5), 148.92 (3).
5-Hydroxyconiferaldehyde was synthesized via the acetate-protected alcohol without deacetylation (Quideau and Ralph, 1992). Thus, the diacetate of ethyl 5-hydroxyferulate (1 g, 3.1 mmol) in anhydrous THF (50 mL) was reduced with 1 m diisobutylaluminum hydride (18.63 mL, 18.63 mmol) at 0°C with stirring under argon. The reaction mixture was worked up by quenching with NH4Cl and extracted with ethyl acetate before evaporation to produce the 4,5-diacetate of 5-hydroxyconiferyl alcohol. Flash-column chromatography was used to purify the product (780 mg, 2.79 mmol, 90%). The diacetate of 5-hydroxyconiferaldehyde was prepared using DDQ in the same way as described for the preparation of p-coumaraldehyde, and it was deacetylated with pyrrolidine to produce 5-hydroxyconiferaldehyde as the final product in low yield (7.2 mg, 0.037 mmol, 0.11%). NMR was used to confirm product identity based on comparison with previously reported data (Li et al., 2001). 1H-NMR (acetone-d6) δ 3.88 (3H, s, OMe), 6.60 (1H, dd, J = 15.8, 7.75 Hz, 8), 6.88 (1H, d, J = 1.8 Hz, 6), 6.96 (1H, d, J = 1.8 Hz, 2), 7.50 (1H, d, J = 15.8 Hz, 7), 9.61 (1H, d, J = 7.75 Hz, 9).
Supplemental Data
The following supplemental materials are available.
Supplemental Data S1. Michaelis-Menten curve of wild-type SbCAD2, SbCAD4, and various SbCAD4 mutants.
Glossary
- S/G
syringyl/guaiacyl
- TLC
thin-layer chromatography
- THF
tetrahydrofuran
Footnotes
This work was supported by the National Science Foundation (grant no. DBI 0959778 to C.K.), the National Institutes of Health (grant no. 1R01GM11125401 to C.K.), and the M.J. Murdock Charitable Trust (to C.K.); by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office, and sponsored by the U.S. DOE’s International Affairs (grant no. DE-PI0000031 to W.V.); by the U.S. Department of Agriculture’s Biomass Research and Development Initiative (grant no. 2011-1006-30358 to W.V.); and by the U.S. Department of Agriculture National Institute of Food and Agriculture (AFRI grant no. 2011-67009-30026 to S.E.S. and CRIS project grant no. 3042-21220-032-00D). J.R. and H.K. were funded by the U.S. DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494).
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M, Fattaji A (2011) On the binding of Mg2+, Ca2+, Zn2+ and Cu+ metal cations to 2′-deoxyguanosine: changes on sugar puckering and strength of the N-glycosidic bond. Scientia Iranica 18: 1343–1352 [Google Scholar]
- Baucher M, Chabbert B, Pilate G, Van Doorsselaere J, Tollier MT, Petit-Conil M, Cornu D, Monties B, Van Montagu M, Inze D, et al. (1996) Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol 112: 1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomati EK, Noel JP (2005) Structural and kinetic basis for substrate selectivity in Populus tremuloides sinapyl alcohol dehydrogenase. Plant Cell 17: 1598–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bout S, Vermerris W (2003) A candidate-gene approach to clone the sorghum Brown midrib gene encoding caffeic acid O-methyltransferase. Mol Genet Genomics 269: 205–214 [DOI] [PubMed] [Google Scholar]
- Bouvier d’Yvoire M, Bouchabke-Coussa O, Voorend W, Antelme S, Cézard L, Legée F, Lebris P, Legay S, Whitehead C, McQueen-Mason SJ, et al. (2013) Disrupting the cinnamyl alcohol dehydrogenase 1 gene (BdCAD1) leads to altered lignification and improved saccharification in Brachypodium distachyon. Plant J 73: 496–508 [DOI] [PubMed] [Google Scholar]
- Brändén CI, Eklund H, Nordström B, Boiwe T, Söderlund G, Zeppezauer E, Ohlsson I, Akeson A (1973) Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci USA 70: 2439–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25: 759–761 [DOI] [PubMed] [Google Scholar]
- Chen W, VanOpdorp N, Fitzl D, Tewari J, Friedemann P, Greene T, Thompson S, Kumpatla S, Zheng P (2012) Transposon insertion in a cinnamyl alcohol dehydrogenase gene is responsible for a brown midrib1 mutation in maize. Plant Mol Biol 80: 289–297 [DOI] [PubMed] [Google Scholar]
- Cherney J, Johnson K, Volenec J, Anliker K (1988) Chemical composition of herbaceous grass and legume species grown for maximum biomass production. Biomass 17: 215–238 [Google Scholar]
- Dickinson FM, Monger GP (1973) A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J 131: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien BS, Sarath G, Pedersen JF, Sattler SE, Chen H, Funnell-Harris DL, Nichols NN, Cotta MA (2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. BioEnergy Res 2: 153–164 [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eszes CM, Sessions RB, Clarke AR, Moreton KM, Holbrook JJ (1996) Removal of substrate inhibition in a lactate dehydrogenase from human muscle by a single residue change. FEBS Lett 399: 193–197 [DOI] [PubMed] [Google Scholar]
- Eyster WH. (1926) Chromosome VIII in maize. Science 64: 22. [DOI] [PubMed] [Google Scholar]
- Fontaine AS, Bout S, Barrière Y, Vermerris W (2003) Variation in cell wall composition among forage maize (Zea mays L.) inbred lines and its impact on digestibility: analysis of neutral detergent fiber composition by pyrolysis-gas chromatography-mass spectrometry. J Agric Food Chem 51: 8080–8087 [DOI] [PubMed] [Google Scholar]
- Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M Jr, Chen F, Foston M, Ragauskas A, Bouton J, et al. (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc Natl Acad Sci USA 108: 3803–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JR, Burks PS, Staggenborg SA, Odvody GN, Heiniger RW, Macoon B, Moore KJ, Barrett M, Rooney WL (2014) Yield results and stability analysis from the sorghum regional biomass feedstock trial. Bioenerg Res 7: 1026–1034 [Google Scholar]
- Green AR, Lewis KM, Barr JT, Jones JP, Lu F, Ralph J, Vermerris W, Sattler SE, Kang C (2014) Determination of the structure and catalytic mechanism of Sorghum bicolor caffeic acid O-methyltransferase and the structural impact of three brown midrib12 mutations. Plant Physiol 165: 1440–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis (Suppl 1) 30: S162–S173 [DOI] [PubMed] [Google Scholar]
- Halpin C, Holt K, Chojecki J, Oliver D, Chabbert B, Monties B, Edwards K, Barakate A, Foxon GA (1998) Brown-midrib maize (bm1): a mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J 14: 545–553 [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Hayes R, Sanchez EJ, Webb BN, Li Q, Hooper T, Nissen MS, Xun L (2012) Furfural reduction mechanism of a zinc-dependent alcohol dehydrogenase from Cupriavidus necator JMP134. Mol Microbiol 83: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus PA, Diederichs K (2012) Linking crystallographic model and data quality. Science 336: 1030–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ralph J (2005) Simplified preparation of coniferyl and sinapyl alcohols. J Agric Food Chem 53: 3693–3695 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim MR, Bedgar DL, Moinuddin SG, Cardenas CL, Davin LB, Kang C, Lewis NG (2004) Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc Natl Acad Sci USA 101: 1455–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen A, Cannio R, Bartolucci S, Klinman JP (1999) Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature 399: 496–499 [DOI] [PubMed] [Google Scholar]
- Kovaleva EG, Plapp BV (2005) Deprotonation of the horse liver alcohol dehydrogenase-NAD+ complex controls formation of the ternary complexes. Biochemistry 44: 12797–12808 [DOI] [PubMed] [Google Scholar]
- Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372: 774–797 [DOI] [PubMed] [Google Scholar]
- Kuc J, Nelson OE (1964) The abnormal lignins produced by the brown-midrib mutants of maize. I. The brown-midrib-1 mutant. Arch Biochem Biophys 105: 103–113 [DOI] [PubMed] [Google Scholar]
- Lee C, Bedgar DL, Davin LB, Lewis NG (2013) Assessment of a putative proton relay in Arabidopsis cinnamyl alcohol dehydrogenase catalysis. Org Biomol Chem 11: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Li L, Cheng XF, Leshkevich J, Umezawa T, Harding SA, Chiang VL (2001) The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell 13: 1567–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Ralph J (1998) Highly selective syntheses of coniferyl and sinapyl alcohols. J Agric Food Chem 46: 1794–1796 [Google Scholar]
- Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD (2016) Designer lignins: harnessing the plasticity of lignification. Curr Opin Biotechnol 37: 190–200 [DOI] [PubMed] [Google Scholar]
- Moural TW, Lewis KM, Barnaba C, Zhu F, Palmer NA, Sarath G, Scully ED, Jones JP, Sattler SE, Kang C (2017) Characterization of class III peroxidases from switchgrass. Plant Physiol 173: 417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol 276: 307–326 [DOI] [PubMed] [Google Scholar]
- Pillonel C, Mulder MM, Boon JJ, Forster B, Binder A (1991) Involvement of cinnamyl-alcohol dehydrogenase in the control of lignin formation in Sorghum bicolor L. Moench. Planta 185: 538–544 [DOI] [PubMed] [Google Scholar]
- Plapp BV. (2010) Conformational changes and catalysis by alcohol dehydrogenase. Arch Biochem Biophys 493: 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Axtell J, Lechtenberg V, Colenbrander V (1978) Phenotype, fiber composition, and in vitro dry matter disappearance of chemically induced brown midrib (bmr) mutants of sorghum. Crop Sci 18: 205–208 [Google Scholar]
- Propheter JL, Staggenborg SA, Wu X, Wang D (2010) Performance of annual and perennial biofuel crops: yield during the first two years. Agron J 102: 806–814 [Google Scholar]
- Quideau S, Ralph J (1992) Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J Agric Food Chem 40: 1108–1110 [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Ralph J, MacKay JJ, Hatfield RD, O’Malley DM, Whetten RW, Sederoff RR (1997) Abnormal lignin in a loblolly pine mutant. Science 277: 235–239 [DOI] [PubMed] [Google Scholar]
- Rooney WL, Blumenthal J, Bean B, Mullet JE (2007) Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod Biorefin 1: 147–157 [Google Scholar]
- Saathoff AJ, Hargrove MS, Haas EJ, Tobias CM, Twigg P, Sattler S, Sarath G (2012) Switchgrass PviCAD1: Understanding residues important for substrate preferences and activity. Appl Biochem Biotechnol 168: 1086–1100 [DOI] [PubMed] [Google Scholar]
- Saballos A, Ejeta G, Sanchez E, Kang C, Vermerris W (2009) A genomewide analysis of the cinnamyl alcohol dehydrogenase family in sorghum [Sorghum bicolor (L.) Moench] identifies SbCAD2 as the brown midrib6 gene. Genetics 181: 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saballos A, Sattler SE, Sanchez E, Foster TP, Xin Z, Kang C, Pedersen JF, Vermerris W (2012) Brown midrib2 (Bmr2) encodes the major 4-coumarate:coenzyme A ligase involved in lignin biosynthesis in sorghum (Sorghum bicolor (L.) Moench). Plant J 70: 818–830 [DOI] [PubMed] [Google Scholar]
- Saballos A, Vermerris W, Rivera L, Ejeta G (2008) Allelic association, chemical characterization and saccharification properties of brown midrib mutants of sorghum (Sorghum bicolor (L.) Moench). BioEnergy Res 1: 193–204 [Google Scholar]
- Sattler SE, Palmer NA, Saballos A, Greene AM, Xin ZG, Sarath G, Vermerris W, Pedersen JF (2012) Identification and characterization of four missense mutations in brown midrib12 (Bmr12), the caffeic O-methyltranferase (COMT) of sorghum. Bioenerg Res 5: 855–865 [Google Scholar]
- Sattler SE, Saathoff AJ, Haas EJ, Palmer NA, Funnell-Harris DL, Sarath G, Pedersen JF (2009) A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phenotype. Plant Physiol 150: 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Saballos A, Xin Z, Funnell-Harris DL, Vermerris W, Pedersen JF (2014) Characterization of novel sorghum brown midrib mutants from an EMS-mutagenized population. G3 (Bethesda) 4: 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SA, Walker AM, Vermerris W, Sattler SE, Kang C (2017) Structural and biochemical characterization of cinnamoyl-CoA reductases. Plant Physiol 173: 1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully ED, Gries T, Funnell-Harris DL, Xin Z, Kovacs FA, Vermerris W, Sattler SE (2016) Characterization of novel Brown midrib 6 mutations affecting lignin biosynthesis in sorghum. J Integr Plant Biol 58: 136–149 [DOI] [PubMed] [Google Scholar]
- Shadle G, Chen F, Srinivasa Reddy MS, Jackson L, Nakashima J, Dixon RA (2007) Down-regulation of hydroxycinnamoyl CoA:shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry 68: 1521–1529 [DOI] [PubMed] [Google Scholar]
- Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, et al. (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459: 437–441 [DOI] [PubMed] [Google Scholar]
- Silverstein E, Boyer PD (1964) Equilibrium reaction rates and the mechanisms of live and yeast alcohol dehydrogenase. J Biol Chem 239: 3908–3914 [PubMed] [Google Scholar]
- Studer MH, DeMartini JD, Davis MF, Sykes RW, Davison B, Keller M, Tuskan GA, Wyman CE (2011) Lignin content in natural Populus variants affects sugar release. Proc Natl Acad Sci USA 108: 6300–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Grosse-Kunstleve RW, Afonine PV, Moriarty NW, Zwart PH, Hung LW, Read RJ, Adams PD (2008) Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr 64: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell H. (1967) Function and structure of liver alcohol dehydrogenase. Harvey Lect 61: 17–41 [PubMed] [Google Scholar]
- Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker R, Vanholme R, Storme V, Mortimer JC, Dupree P, Boerjan W (2013) Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnol Biofuels 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermerris W, Abril A (2015) Enhancing cellulose utilization for fuels and chemicals by genetic modification of plant cell wall architecture. Curr Opin Biotechnol 32: 104–112 [DOI] [PubMed] [Google Scholar]
- Vermerris W, Saballos A, Ejeta G, Mosier N, Ladisch M, Carpita N (2007) Molecular breeding to enhance ethanol production from corn and sorghum stover. Crop Sci 47: S142–S152 [Google Scholar]
- Vermerris W, Sherman DM, McIntyre LM (2010) Phenotypic plasticity in cell walls of maize brown midrib mutants is limited by lignin composition. J Exp Bot 61: 2479–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AM, Hayes RP, Youn B, Vermerris W, Sattler SE, Kang C (2013) Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyltransferase and its structural relationship to other coenzyme A-dependent transferases and synthases. Plant Physiol 162: 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AM, Sattler SA, Regner M, Jones JP, Ralph J, Vermerris W, Sattler SE, Kang C (2016) The structure and catalytic mechanism of Sorghum bicolor caffeoyl-CoA O-methyltransferase. Plant Physiol 172: 78–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Xue J, Zhao YYZ (2009) A novel and efficient procedure for the preparation of allylic alcohols from α,β-unsaturated carboxylic esters using LiAlH4/BnCl. Tetrahedron Lett 50: 413–415 [Google Scholar]
- Xin Z, Wang ML, Barkley NA, Burow G, Franks C, Pederson G, Burke J (2008) Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biol 8: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn B, Camacho R, Moinuddin SGA, Lee C, Davin LB, Lewis NG, Kang C (2006) Crystal structures and catalytic mechanism of the Arabidopsis cinnamyl alcohol dehydrogenases AtCAD5 and AtCAD4. Org Biomol Chem 4: 1687–1697 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Zhao S, Yang S, Ding SY (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27: 38–45 [DOI] [PubMed] [Google Scholar]
- Zhang K, Qian Q, Huang Z, Wang Y, Li M, Hong L, Zeng D, Gu M, Chu C, Cheng Z (2006) GOLD HULL AND INTERNODE2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol 140: 972–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Nakashima J, Chen F, Yin Y, Fu C, Yun J, Shao H, Wang X, Wang ZY, Dixon RA (2013) Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 25: 3976–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]