Starch in Arabidopsis leaves is increasingly liable to degradation with time after dawn, so that accumulation slows and turnover in response to falling light accelerates as the day proceeds.
Abstract
We investigated whether starch degradation occurs at the same time as starch synthesis in Arabidopsis (Arabidopsis thaliana) leaves in the light. Starch accumulated in a linear fashion for about 12 h after dawn, then accumulation slowed and content plateaued. Following decreases in light intensity, the rate of accumulation of starch declined in proportion to the decline in photosynthesis if the decrease occurred <10 h after dawn, but accumulation ceased or loss of starch occurred if the same decrease in light intensity was imposed more than 10 h after dawn. These changes in starch accumulation patterns after prolonged periods in the light occurred at both high and low starch contents and were not related to time-dependent changes in either the rate of photosynthesis or the partitioning of assimilate between starch and Suc, as assessed from metabolite measurements and 14CO2 pulse experiments. Instead, measurements of incorporation of 13C from 13CO2 into starch and of levels of the starch degradation product maltose showed that substantial starch degradation occurred simultaneously with synthesis at time points >14 h after dawn and in response to decreases in light intensity that occurred >10 h after dawn. Starch measurements in circadian clock mutants suggested that the clock influences the timing of onset of degradation. We conclude that the propensity for leaf starch to be degraded increases with time after dawn. The importance of this phenomenon for efficient use of carbon for growth in long days and for prevention of starvation during twilight is discussed.
Light provides the energy for photosynthesis and plant growth. However, plants grow in a world where light alternates with darkness every 24-h cycle and daylength changes with the season. Irradiance also varies in a broadly sinusoidal manner each day, with climatic conditions imposing additional, unpredictable, fluctuations during the day, and from one day to the next. In many plants, starch buffers metabolism and growth against the daily alternation of light and darkness (Smith and Stitt, 2007; Graf and Smith, 2011; Stitt and Zeeman, 2012). Part of the newly fixed carbon (C) is accumulated as starch in the light period and remobilized to support metabolism and growth during the night. The rates of starch synthesis and degradation are adjusted to daylength, so that synthesis is increased and the rate of degradation is decreased as photoperiods are shortened (Smith and Stitt, 2007; Gibon et al., 2009; Sulpice et al., 2014). These responses ensure that starch reserves last until the end of the night. Growth in short days is thus maximized by mechanisms that avoid deleterious periods of C starvation at the end of the night and ensure that all of the C assimilated in the light period is used for growth by the start of the following day. These mechanisms result in a C use efficiency (the fraction of assimilated C used in growth over a day-night cycle) that is relatively constant over day lengths from 4 to 12 h. In longer days (16 h of light or more), more C is available, controls over starch turnover are relaxed, and substantial amounts of starch remain at dawn, and C use efficiency is much lower than in short days (Sulpice et al., 2014; Baerenfaller et al., 2015).
Experiments that underpin our present understanding of the control of starch metabolism over the day-night cycle have been performed in controlled conditions in which the start and end of the light period consist of instantaneous transitions between full light and complete darkness (square-wave light regimes; Annunziata et al., 2017). Most researchers have assumed that starch degradation does not occur during the light period (but see Supplemental Text S1 for fuller discussion), is triggered by the onset of darkness, and ceases at dawn (Rasse and Tocquin, 2006; Kötting et al., 2010; Weise et al., 2011; Thormählen et al., 2013). Furthermore, research has been done almost exclusively in short days (≤12h). These simplifying conditions and assumptions have helped to reveal the basic mechanisms that control starch turnover through the day-night cycle. However, it remains unclear how starch turnover is controlled in long days, or during twilight when starch degradation can occur at the same time as photosynthesis (Servaites et al., 1989; Fondy et al., 1989; see below).
The simplifying assumption that leaf starch degradation is low or absent during the light period is supported by pulse-chase experiments with 14CO2, which failed to detect significant starch degradation in standard light periods in pea (Pisum sativum; Kruger et al., 1983), pepper (Capsicum annuum; Grange, 1984), wild-type Arabidopsis (Arabidopsis thaliana; Walters et al., 2004; Zeeman et al., 2002), and tobacco (Nicotiana tabacum; Häusler et al., 1998) leaves (limitations of this approach are discussed in Supplemental Text S1). However, starch degradation may occur during long periods in continuous light, in some mutants with chloroplast export deficiencies, and in response to acute abiotic stress. Rather than increasing at a constant rate, starch content oscillated with a period of ∼24 h following transfer to continuous light in leaves of pea (Kruger et al., 1983), bean (Phaseolus vulgaris; Weise et al., 2006), and Arabidopsis (Lu et al., 2005). In Arabidopsis and bean leaves, levels of the starch degradation product maltose also varied with a similar periodicity. Simultaneous synthesis and degradation of starch in the light was observed in spinach (Spinacia oleracea) chloroplasts, following a protocol for chloroplast preparation that involved illumination for 14 to 16 h (Stitt and Heldt, 1981). Arabidopsis mutants with deficiencies in starch degradation accumulate higher levels of starch in constant light than wild-type plants, implying that starch levels in these conditions are determined by simultaneous synthesis and degradation (Caspar et al., 1991; Baslam et al., 2017). In triose phosphate transporter mutants of Arabidopsis and tobacco, the block in export of carbon from the chloroplast in the light is bypassed through starch turnover and export of starch degradation products (Häusler et al., 1998; Walters et al., 2004). Imposition of strong osmotic stress or photorespiratory conditions (zero CO2 and elevated O2) also rapidly induces starch degradation in the light during normal photoperiods (Weise et al., 2006; Thalmann et al., 2016).
Arabidopsis plants grown in very long photoperiods (16–18 h) display a plateauing of starch content toward the end of the light period (Hädrich et al., 2012; Sulpice et al., 2014; Figueroa et al., 2016), consistent with the possibility that starch degradation is initiated before the end of the light period. When Arabidopsis plants were grown in a 12-h photoperiod under natural light or artificial light with a sinusoidal profile, leaf starch content also plateaued during the last 2 to 3 h of the day (Annunziata et al., 2017). Starch content actually decreased in sugar beet (Beta vulgaris) and bean leaves as light intensity decreased at the end of the light period in a near-natural sinusoidal light regime (Servaites et al., 1989; Fondy et al., 1989). Net starch loss commenced during the decline in light intensity in the later stages of a (14 h) light period (which for convenience we refer to as twilight) and could be detected when the rate of photosynthesis was still substantial (about half of its midday peak value).
The onset of starch degradation in the light in long days and during twilight in short days could potentially improve the C use efficiency of the plant, relative to a situation in which starch degradation occurs only in complete darkness. In short photoperiods, there is likely to be a premium on accumulating starch throughout the entire light period, in order to provide enough reserves for the ensuing long night. However, in a long photoperiod, this strategy might result in accumulation of more starch than can be consumed in the short night. We hypothesize that in such conditions it would be advantageous to commence starch degradation before dusk to supplement the supply of sugars from current photosynthesis for export and growth. Remobilization of fixed C from starch reserves in the light would allow more of the available light energy (i.e. ATP and reducing power) to be used for biosynthesis of amino acids and other structural precursors required for growth. Even in relatively short days, the onset of starch degradation during twilight rather than after dark could ensure a steady supply of sugars during the transition from day to night. To test these hypotheses and to further our understanding of starch turnover in natural light conditions, we investigated the reasons for the plateauing of starch content in leaves of Arabidopsis plants grown in long days and the impact on starch accumulation of a simulated twilight imposed at different times of day.
RESULTS
Starch Accumulation Slows or Halts in Extended Light Periods, Independently of Changes in Photosynthesis and Suc Synthesis
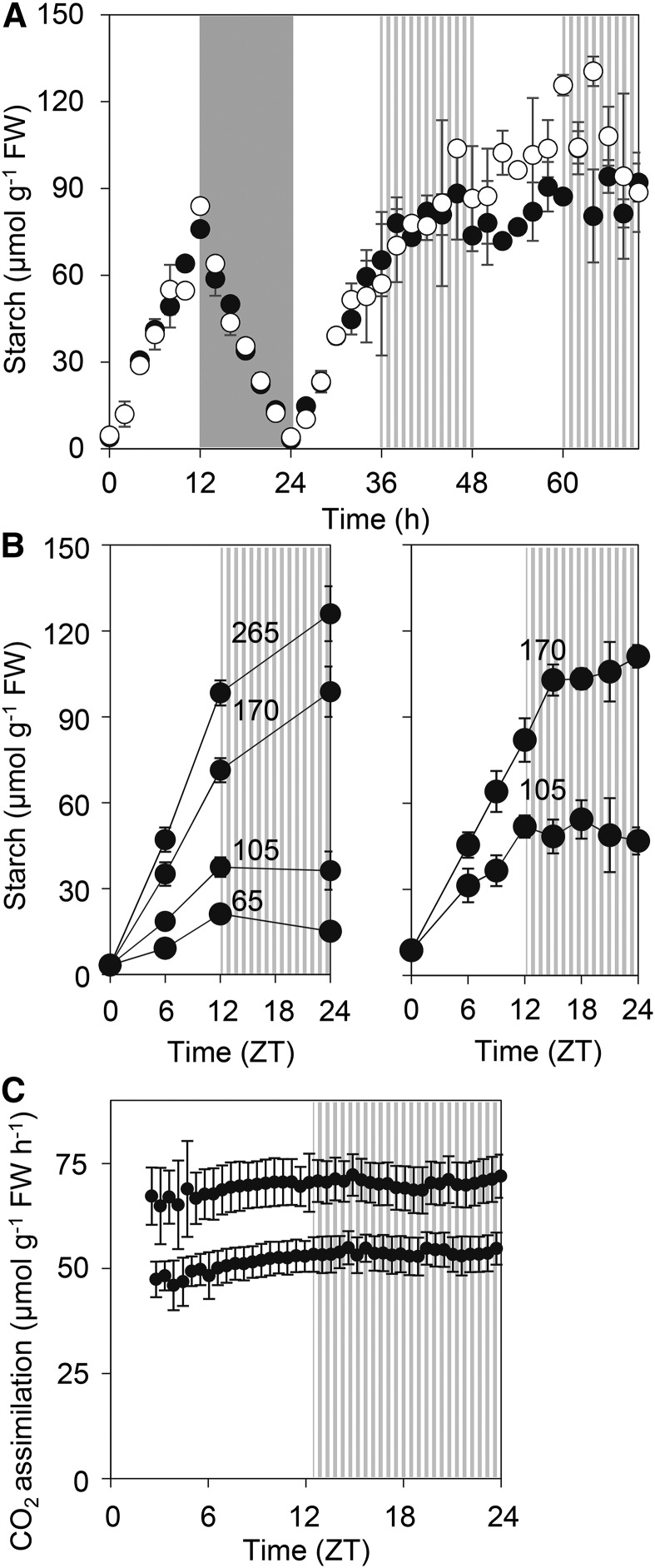
When wild-type Col-0 and Ws-2 plants grown in 12-h-light/12-h-dark cycles were transferred to continuous light at the light intensity at which they were grown, starch accumulation was linear for the first 14 h but then slowed markedly. After 24 h of continuous light, there was no further accumulation of starch (Fig. 1; Supplemental Fig. S1). We reasoned that the slowing of starch accumulation might reflect either feedback regulation of starch synthesis due to a high starch content or a time-dependent effect on starch metabolism. To distinguish between these possibilities, plants were transferred to continuous light at a range of intensities that resulted in very different starch contents after 12 h. In all cases, starch accumulation slowed markedly or starch content declined during the first subjective night (ZT12-24; ZT is Zeitgeber Time, i.e. time after last dawn; Fig. 1B). Thus, the decline in starch accumulation after ZT12 occurred regardless of the starch content at that point (values ranged from 20 to 100 µmol g−1 fresh weight [FW] at ZT12; Fig. 1B), making it likely the decline is due to a time-dependent effect on starch metabolism.
Figure 1.
Starch accumulation and photosynthesis following transfer to continuous light. A, Wild-type plants (black symbols, Col-0; white symbols, Ws-2) were grown for 21 d in 12-h-light/12-h-dark cycles at 160 µmol quanta m−2 s−1 and then transferred at dawn to continuous light of the same intensity. Plants were harvested and assayed for starch at 2-h intervals during the day preceding transfer and for 48 h of continuous light. The gray zone marks an actual night, and striped zones represent subjective nights. Values are as µmol hexose equivalents and are means ± sd of measurements on two pots of plants, each of which contained five individual rosettes. B, Wild-type (Col-0) plants were grown for 21 d in 12-h-light/12-h-dark cycles at 140 µmol quanta m−2 s−1 and then transferred at dawn to continuous light at a range of intensities (values in µmol quanta m−2 s−1 indicated on the graphs) and harvested and assayed for starch at intervals over 24 h. The striped zone marks the subjective night. Values are means ± sd of measurements on six individual rosettes. The left and right graphs are outcomes of two independent experiments. C, Rates of photosynthesis of plants grown for 21 d at in 12-h-light/12-h-dark cycles at 150 µmol quanta m−2 s−1 and then transferred at dawn to continuous light at 105 (upper data set) or 65 (lower data set) µmol quanta m−2 s−1. Measurements were made in the growth chamber on individual whole rosettes, using the apparatus described by Kölling et al. (2015b). The striped zone marks the subjective night. Values are means ± sd of measurements on six (upper data set) or four (lower data set) individual rosettes.
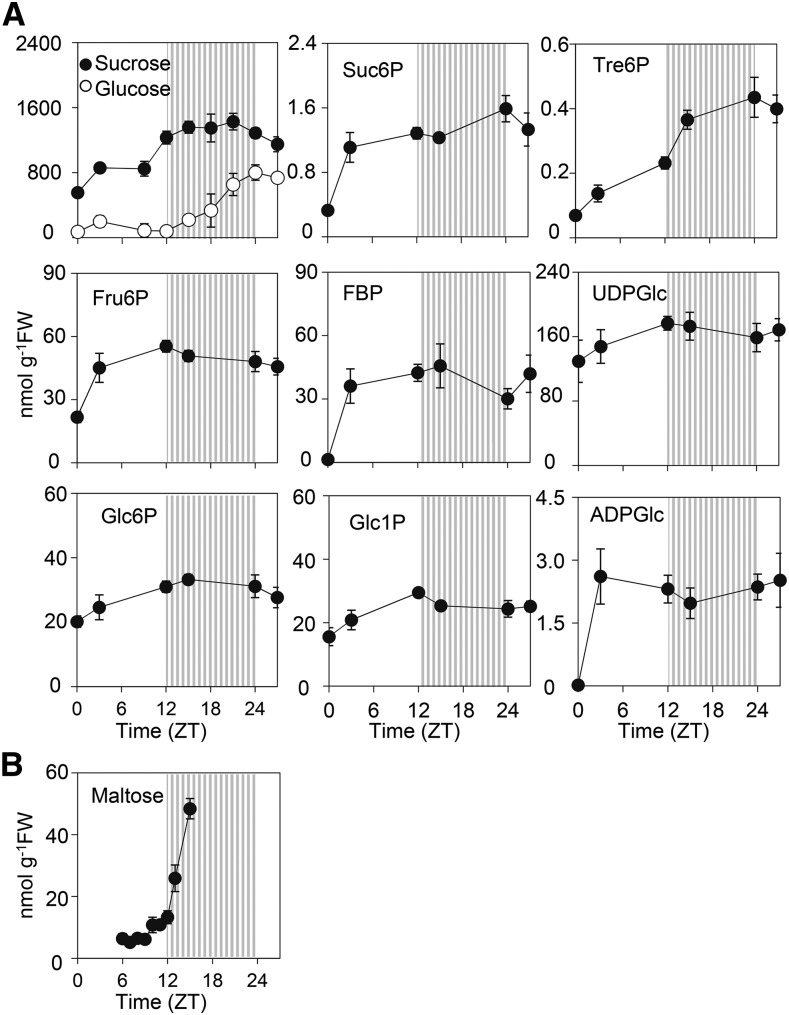
We investigated first whether the decline coincided with changes in either the rate of CO2 assimilation or levels of metabolites involved in Suc and starch synthesis. Independent measurements of CO2 assimilation using different methods provided no evidence for significant, consistent changes in the rate of photosynthesis over the first 24 to 36 h in continuous light (Fig. 1C; Supplemental Fig. S1C). Measurements of metabolites involved in Suc and starch synthesis revealed no substantial changes following transfer to continuous light (Fig. 2). Suc levels rose up to ZT15 and then plateaued, and levels of the Suc signaling metabolite trehalose 6-phosphate (Yadav et al., 2014) behaved similarly. Levels of hexose phosphates, Fru 1,6-bisphosphate, UDPGlc, and Suc 6′-phosphate rose immediately after dawn, then rose slightly or showed little change between ZT3 and ZT27. Levels of the substrate for starch synthesis, ADPGlc, showed an initial steep rise after dawn and remained high with no consistent change between ZT9 and ZT27. Given the importance of ADPglucose pyrophosphorylase in controlling the rate of starch synthesis (Stitt et al., 2010), the rather constant levels of ADPGlc suggest that the decline in starch accumulation after ZT12 is not due to a reduced rate of starch synthesis. Taken as a whole, these data provide no evidence for major changes in carbon assimilation and partitioning over the period in which starch accumulation declines, although they do not rule out such changes. To investigate this phenomenon further, we examined whether the decline in starch accumulation is associated with the initiation of starch degradation.
Figure 2.
Changes in intermediates of starch and Suc metabolism in plants under continuous light. A, Plants were grown for 21 d at 160 µmol quanta m−2 s−1 and then transferred at dawn to continuous light of 90 µmol m−2 s−1. Plants were harvested and assayed for metabolites at intervals over 27 h. The striped zone represents the subjective night. Values are as nmol hexose and are means ± sd of measurements on five pots of plants, each of which contained five individuals. Values for Suc and Glc are from a different experiment from other values. B, As for A, except plants were grown at 140 µmol m−2 s−1 and transferred to continuous light of this intensity.
The Decline in Starch Accumulation Is Associated with the Simultaneous Synthesis and Degradation of Starch
We used 13CO2 labeling experiments to examine whether and when starch turnover occurs in plants transferred to continuous light. Plants were grown in 12-h-light (160 µmol quanta m−2 s−1), 12-h-dark cycles, transferred to continuous light also at 160 µmol quanta m−2 s−1, and supplied with 13CO2 for periods of up to 4 h. For each labeling period, the total amount of starch accumulated was compared with the amount of 13C that accumulated in starch. The principle of this approach is as follows. If starch degradation occurs at the same time as synthesis and removes previously synthesized 12C starch as well as or instead of newly synthesized 13C starch, the total accumulation of starch over the labeling period will be less than the amount of 13C starch accumulated. Thus, if the amount of 13C starch exceeds the total amount of starch accumulated, starch synthesis and degradation occurred simultaneously during that period. However, if the amount of 13C starch and the total amount of starch accumulated are the same, this could mean either that no starch degradation occurred over the labeling period or that degradation occurred but removed only newly synthesized 13C starch. More information on interpretation of results of 13CO2 labeling experiments is presented in Supplemental Text S1.
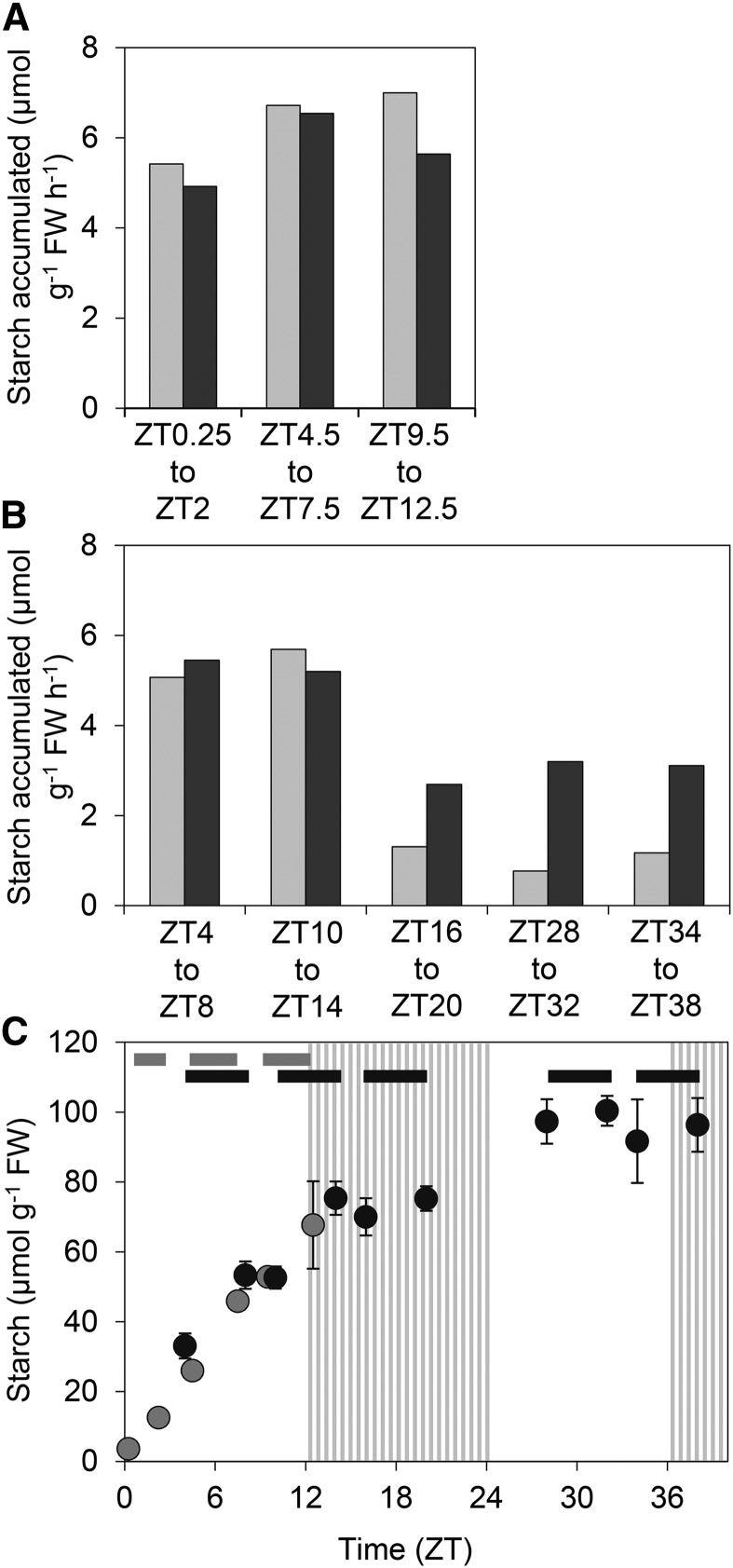
In a first experiment, 13CO2 was supplied for three periods of 1.75 or 3 h between dawn (ZT0) and ZT12.5 (Fig. 3). Rates of starch accumulation were similar in each of the three periods. Within each period, the total amount of starch accumulated and the amount of 13C starch accumulated were similar (values differing by <20%). In a second experiment, 13CO2 was supplied for periods of 4 h over an extended time course that encompassed the first subjective night, the first subjective day, and the second subjective night (ZT4 to ZT42; Fig. 3B, timing of labeling periods shown in Fig. 3C). For the first two periods, ZT4-8 and ZT10-14, results were similar to those of the first experiment. Rates of starch accumulation were about 5 µmol g−1 FW h−1, and similar levels of total starch and 13C starch accumulated. Results were substantially different for labeling periods from ZT16 onwards. Rates of starch accumulation were 75 to 85% lower than for earlier labeling periods. The amount of 13C starch accumulated was also lower than in earlier labeling periods, but by <50%. Thus, the amount of 13C starch accumulated greatly exceeded the total amount of starch accumulated in each of the labeling periods from ZT16 onwards. These data indicate a radical and sustained shift in starch metabolism during the first subjective night. They provide no evidence for the occurrence of starch degradation prior to ZT14, but they clearly show that there is simultaneous synthesis and degradation after this point.
Figure 3.
Labeling of starch during pulses of 13CO2. Plants were grown for 21 d in 12-h-light/12-h-dark cycles at 160 µmol m−2 s−1. On the day of the experiment, starting at dawn, plants were transferred to continuous light. A, Rate of accumulation of total starch (light gray bars) and 13C starch (dark gray bars) during pulses of 13CO2 over the time intervals indicated (see also C). Rates were calculated from measurements of total starch content and 13C starch content at the beginning and end of each pulse (see Supplemental Text S1, Part I). Original data are in Supplemental Table S1A. B, As for A, but an independent experiment over an extended period in continuous light. Original data are in Supplemental Table S1B. C, Total starch accumulation over the periods of the experiments shown in A (light-gray symbols) and B (dark-gray symbols). Bars at the top indicate the pulse periods in A (gray) and B (black). Striped zones represent the subjective nights. Values are means ± sd of measurements on three or four pots of plants, each of which contained five individuals.
The higher rate of 13C starch accumulation in the ZT4 to ZT8 and ZT10 to ZT14 hour labeling periods than in subsequent labeling periods could reflect either a lower rate of synthesis during the later periods or the onset of degradation of newly synthesized 13C starch as well as 12C starch. The fact that ADPGlc levels remain high and unaltered for at least 33 h following transfer to continuous light (Fig. 2A; Supplemental Fig. S1D) suggests that degradation of 13C starch rather than a lower rate of starch synthesis is the likely explanation.
To check that the onset of starch degradation after 14 h in continuous light was triggered by a time-dependent signal rather than by a high starch content, we performed a further experiment in which plants grown in 12-h-light (160 µmol quanta m−2 s−1), 12-h-dark cycles were transferred to a lower level of continuous light (90 µmol quanta m−2 s−1), resulting in a much lower starch content during each labeling period (Supplemental Fig. S1). Overall, the results were very similar to those from the experiment in continuous light of higher intensity. The amount of 13C starch accumulated was similar to the total amount of starch accumulated during a labeling period in the subjective day (ZT3 to ZT9), but greatly exceeded the total amount of starch accumulated during labeling periods after ZT14 (Supplemental Fig. S1A). The rate of 13C accumulation was much lower in the later labeling periods than in the labeling period ZT3 to ZT9, even though the rate of CO2 assimilation and levels of ADPGlc and Glc6P remained high (Supplemental Fig. S1, C–E), suggesting that starch synthesis was sustained but degradation of both 12C and 13C starch occurred in these labeling periods.
To provide further information about the onset of starch degradation in continuous light, we measured maltose levels in leaves. Maltose is a major product of leaf starch degradation, and levels provide an indication of whether starch degradation is occurring (Weise et al., 2004; Niittylä et al., 2004; Smith and Stitt, 2007; Stitt and Zeeman, 2012). Levels are low during the day and elevated at night when plants are grown in day-night cycles (Niittylä et al., 2004; Weise et al., 2006; Pal et al., 2013). The rise at night is attenuated in plants in which starch degradation is inhibited by trehalose 6-phosphate (Martins et al., 2013), and maltose rises in the light when starch degradation is triggered by acute photorespiratory conditions (Weise et al., 2006) or acute osmotic stress (Thalmann et al., 2016). Upon transfer to continuous light, maltose levels remained low up to ZT9, rose slightly from ZT9 to ZT12, then rose abruptly after ZT12, increasing by 5-fold in the first subjective night (Fig. 2B). These data are consistent with an acceleration of starch degradation at a point after ZT12 and may indicate that limited degradation is initiated in the later part of a 12-h day.
The Onset of Starch Degradation in Continuous Light Is Influenced by the Circadian Clock
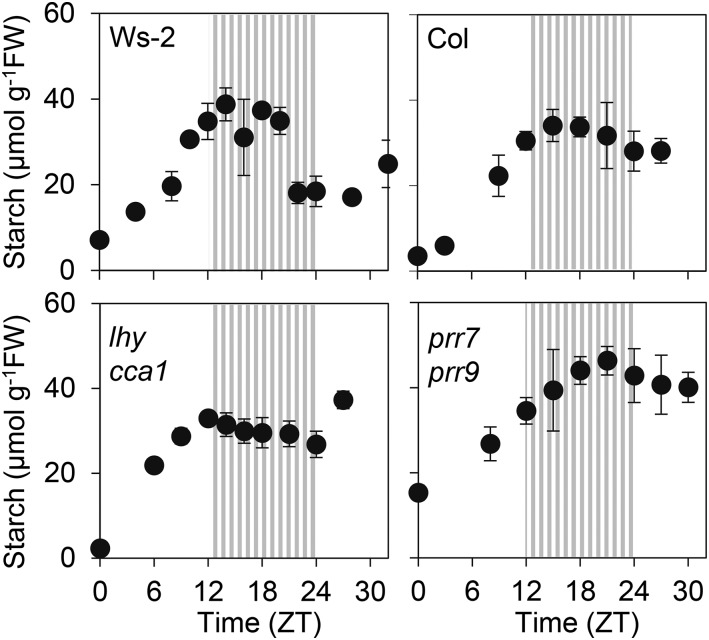
As a first step to discover the mechanism that triggers starch degradation after long periods in the light, we investigated a possible role for the circadian clock. The clock is already known to play a central role in starch metabolism, in that it controls the rate of starch degradation during the night (Graf et al., 2010; Graf and Smith, 2011; Scialdone et al., 2013). To establish whether normal clock function is important for the timing of onset of starch degradation in continuous light, we compared starch accumulation patterns following transfer to continuous light in wild-type plants and clock mutants. The clock mutants were the short-period mutant cca1 lhy (Alabadí et al., 2002; Mizoguchi et al., 2002) and the long-period mutant prr7 prr9 (Farré et al., 2005; Salomé and McClung, 2005). These mutants are in the Ws-2 and Col-0 backgrounds, respectively. Both mutants have altered diel patterns of primary carbohydrate metabolism. The cca1 lhy mutant degrades starch too quickly during the night, so that starch reserves are exhausted before dawn (Graf et al., 2010; Scialdone et al., 2013). The prr7 prr9 mutant maintains a higher ratio of starch to Suc synthesis during the day than wild-type plants (Kölling et al., 2015a). We grew plants in 12-h-light (160 µmol quanta m−2 s−1) 12-h-dark cycles then transferred them to continuous light at 90 µmol quanta m−2 s−1. Starch content plateaued by ZT15 and subsequently declined in Col-0 and Ws-2 wild-type plants (see Supplemental Fig. S1B for data from an independent experiment for Col-0). The time at which starch accumulation plateaued was clearly different in cca1 lhy and prr7 prr9 plants from that in wild-type plants. Whereas linear starch accumulation continued until about ZT15 in wild-type plants, it continued only until ZT9-12 in cca1 lhy plants and until at least ZT18 in prr7 prr9 plants (Fig. 4). These data suggest that the circadian clock may play a role in the timing of onset of starch degradation in the light.
Figure 4.
Starch accumulation in continuous light in wild-type plants and two clock mutants. Plants were grown in 12-h-light/12-h-dark cycles at 160 µmol quanta m−2 s−1. On the day of the experiment, plants were transferred to continuous light of 90 µmol m−2 s−1 at dawn. cca1 lhy plants were 15 d old, Ws-2 plants were 12 d old, Col-0 plants were 21 d old, and prr7 prr9 plants were 28 d old on the day of the experiment. The cca1 lhy mutant is in the Ws-2 background; the prr7 prr9 mutant is in the Col-0 background. Striped zones represent the subjective night. Values are means ± sd of measurements on five biological replicates. Replicates consisted of pooled material from a single, 10-cm pot, which contained 10 to 12 plants for cca1 lhy and five plants for other genotypes.
A Decrease in Light Level Triggers Starch Degradation at ZT12, But Not at ZT6
To investigate the possibility that starch degradation is initiated during twilight in Arabidopsis plants, we mimicked twilight by a step reduction in light intensity. Use of a step rather than a continuous decline in light enabled analysis of metabolic effects in a stable state rather than in continuously changing conditions. In plants grown in 12-h-light/12-h-dark cycles, the effect on starch accumulation of a decrease from 170 to 105 µmol quanta m−2 s−1 depended on the time of day at which the decrease occurred (Fig. 5). When the decrease occurred at ZT6, starch accumulation continued but at a lower rate than at the original light intensity. This rate was comparable with the rate of starch accumulation in plants held continuously from dawn at the lower light intensity. However, when the decrease in light intensity occurred at ZT12, there was a net loss of starch over 6 h at the lower light intensity. By contrast, plants held continuously from dawn at the lower light intensity accumulated starch linearly up to 12 h, followed by a marked slowing down in starch accumulation (Fig. 5A). The effects of decreases in light intensity at ZT6 and ZT12 were reproducible in independent experiments (examples in Supplemental Fig. S2, A and B). These data suggest that starch degradation was triggered by the decrease in light intensity at ZT12, but was not triggered, or triggered to a much lesser extent, by the decrease in light intensity at ZT6.
Figure 5.
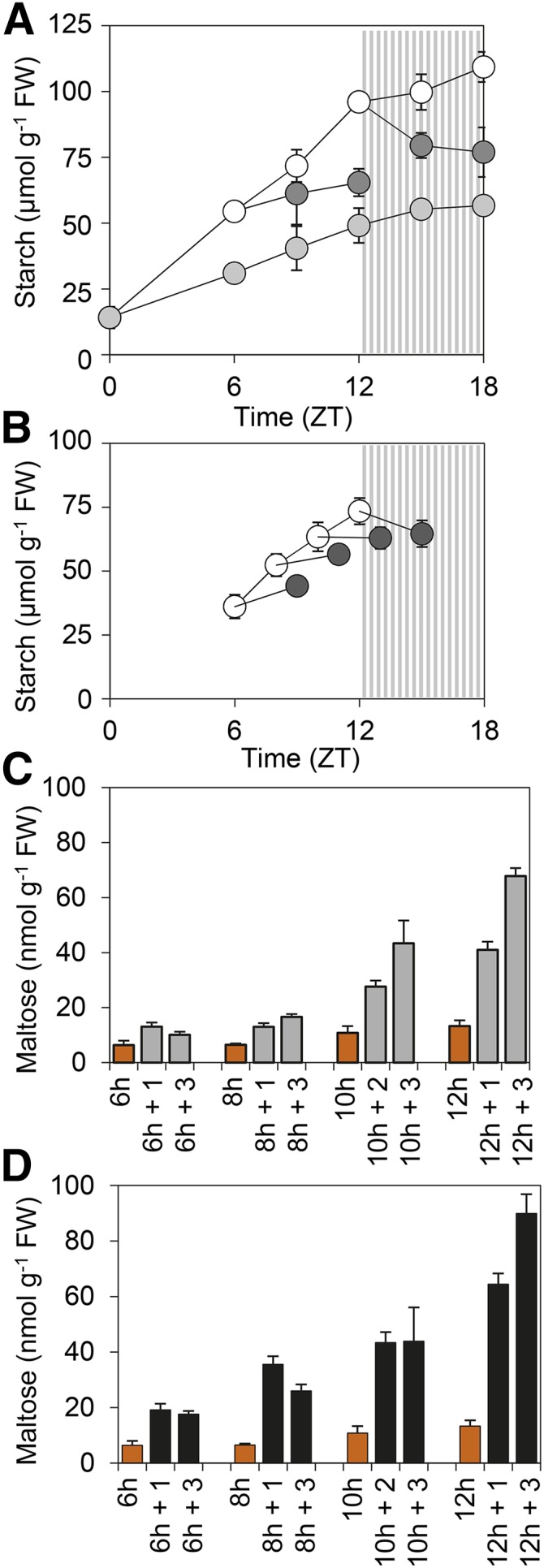
Starch and maltose accumulation in response to decreases in light intensity. Plants grown under 12-h-light/12-h-dark at ∼150 µmol m−2 s−1 for 21 d were transferred to continuous light at dawn and then subjected to decreases in light intensity at specific time points. A, Starch contents of plants subjected to a decrease in light intensity from 170 (white symbols) to 105 µmol m−2 s−1 at ZT6 and ZT12 (dark-gray symbols) or subjected to 105 µmol m−2 s−1 continuously from dawn (light-gray symbols). B, Starch contents of plants subjected to a reduction in irradiance from 105 (white symbols) to 65 µmol m−2 s−1 at ZT6, 8, 10, or 12 (dark-gray symbols). C, Maltose contents of plants subjected to the same reductions in light intensity as in B, harvested immediately prior to the reduction (orange) or at 1 or 3 h following the reduction (gray). D, As for C except plants were subjected to complete darkness at ZT6, 8, 10, or 12 and harvested at 1 or 3 h following the onset of darkness (black). Striped zones in A and B represent the subjective night. Values are means ± sd of measurements on six rosettes.
To obtain more precise information about the time of day at which a decrease in light intensity would trigger starch degradation, the impact on starch content of decreases in light intensity at ZT6, 8, 10, and 12 was monitored. Lower light intensities were used (plants initially at 105 µmol quanta m−2 s−1, light decreased to 65 µmol quanta m−2 s−1) to achieve lower starch contents and, hence, more accurate measurements. Whereas the decrease in light intensity at ZT12 again resulted in net starch loss, there was no net loss following decreases in light intensity at ZT6, 8, and 10. However, the extent of starch accumulation following the decrease in light intensity declined progressively. There was substantial accumulation in the 3 h following the decrease in light intensity at ZT6, but the rate of accumulation was lower following the decrease in light intensity at ZT8 and there was no net accumulation following the decrease at ZT10 (Fig. 5B). This effect was observed in two further, independent experiments (example in Supplemental Fig. S2C). Across the three data sets there were statistically significant increases in starch content in the 3 h following decreases in light intensity at ZT6 and ZT8 (P < 10−9 and P = 7.3 × 10−6, respectively), no significant change following the decrease at ZT10 (P = 0.98), and a significant decrease in starch content following the decrease in light intensity at ZT12 (P = 0.004; tested by two-way ANOVA with experiment and conditions as factors, followed by Tukey’s range test). These data are consistent with starch degradation being activated to a progressively greater extent by decreases in light intensity at progressively later points in the day.
We measured maltose, the main product of starch degradation, to assess whether the decline in starch accumulation following decreases in light intensity did indeed reflect increasing starch degradation (Fig. 5C). At 105 µmol quanta m−2 s−1, maltose levels were very low for most of the light period, rose slightly at ZT10-12, and then rose strongly after this point (similar changes in maltose levels were seen in plants grown at 140 µmol quanta m−2 s−1; Figure 2B). Compared to the level at 105 µmol quanta m−2 s−1, decreases in light intensity at ZT6 and ZT8 resulted in increases in maltose of about 2-fold in the first hour, and little further change in the next 2 h. By contrast, following decreases in light intensity at ZT10 and ZT12 maltose levels rose 4- to 5-fold over 3 h (Fig. 5C). Controls in which plants were subject to darkness rather than a decrease in light intensity at the same time points confirmed that maltose production following decreases in light intensity was not limited by the capacity for starch degradation: Darkness resulted in a 3- to 5-fold rise in maltose within an hour at all time points (Fig. 5D). Notably, the increase in maltose content in the hour following darkness was much higher at later than at earlier time points. For earlier time points, maltose levels at 3 h after darkness were considerably higher than those 3 h after a decrease in light intensity (∼10 and 18 nmol g−1 FW for a decrease in light intensity and darkness, respectively, at ZT6; 17 and 26 nmol g−1 FW for a decrease in light intensity and darkness, respectively, at ZT8). For the later time points, maltose levels 3 h after a decrease in light intensity and darkness were more comparable (∼43 nmol g−1 FW for both a decrease in light intensity and darkness at ZT10; 68 and 89 nmol g−1 FW for a decrease in light intensity and darkness, respectively, at ZT12). These data are consistent with the activation of starch degradation in response to decreases in light intensity that occur later in the day than ZT8. By ZT10, it appears that a decrease in light intensity can trigger a rate of starch degradation comparable with that triggered by the onset of darkness.
We investigated whether progressive changes in the sensitivity of photosynthesis or photoassimilate partitioning to decreases in light intensity could be responsible for the different responses of starch accumulation when light is lowered at different times of day. Continuous measurement of photosynthesis revealed that a decrease in light intensity at ZT6 and ZT12 caused identical reductions in the rate of CO2 assimilation to new levels that were maintained over at least the next 6 h (Supplemental Fig. S2D). Thus, the different effects of decreases in light intensity on starch accumulation at these two times of day are not secondary consequences of changes in the response of photosynthesis.
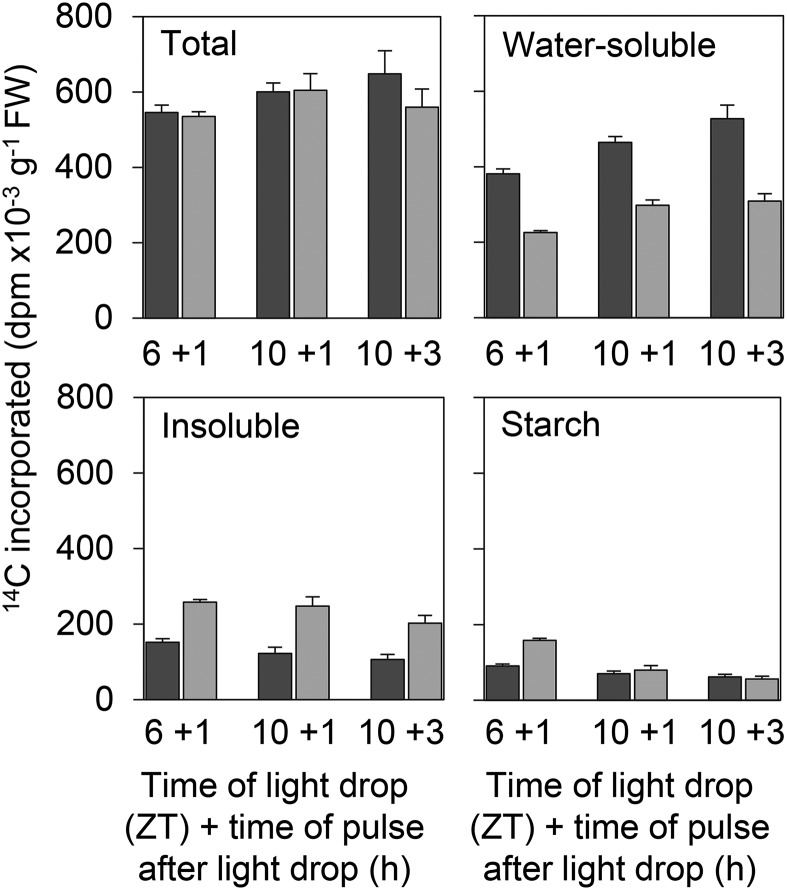
To assess whether the effect of decreases in light intensity on partitioning of photosynthate into Suc is different at different times of day, rosettes were subjected to a decrease in light intensity at either ZT6 or ZT10. They were given a 5-min pulse of 14CO2 and a subsequent 30-min chase period in air at three time points: at 1 h after the decrease in light intensity at ZT6 and at 1 and 3 h after the decrease in light intensity at ZT10. There was relatively little variation between the time points in the total amount of 14C incorporated (Fig. 6), consistent with an unchanged photosynthetic rate. The 14C content of the water-soluble (including sugars, Calvin-Benson cycle intermediates, and organic and amino acids) and insoluble (starch, protein, and cell walls) fractions showed broadly similar trends at the three time points: 14C in water soluble compounds fell during the chase, while 14C in insoluble compounds rose. However, incorporation of 14C into starch was different at the two time points. Less 14C was incorporated in pulses after the ZT10 decrease in light intensity than in the pulse after the ZT6 decrease in light intensity, and whereas 14C in starch rose by 60% during the chase after the ZT6 decrease there was no rise in 14C in starch during chases after the ZT10 decrease.
Figure 6.
Incorporation of 14C into leaf fractions during pulse-chase experiments on plants subjected to decreases in light intensity. Plants grown in 12-h-light/12-h-dark cycles for 21 d at 140 µmol quanta m−2 s−1 were transferred to continuous light of 105 µmol quanta m−2 s−1 at dawn and then subjected to decreases in light intensity to 65 µmol quanta m−2 s−1 at either ZT6 or ZT10. Either 1 or 3 h after decreases in light intensity, plants were supplied with 14CO2 for 5 min followed by a 30-min chase in 12CO2. Plants harvested immediately after the pulse (dark-gray bars) or after the pulse and chase (light-gray bars) were extracted and fractionated, and fractions were assayed for 14C. Graphs show total 14C in leaf, 14C contents of the water-soluble and insoluble fractions, and the 14C content of starch, which formed part of the Insoluble fraction. Values are means ± sd of measurements on four rosettes, except for the 10 +1 and 10 +3 points for which there were three measurements.
In an independent experiment, we examined the 14C content of the neutral subfraction of the water-soluble material (expected to be largely Suc) and of starch following 5-min pulses of 14CO2 1 h after decreases in light intensity at ZT6 and ZT11 (Table I). There was no statistically significant difference between the two time points with respect to total 14C incorporated, 14C in the soluble fraction, 14C in the ethanol-soluble fraction (largely lipids), and 14C in Suc. However, the 14C content of starch following the pulse 1 h after the ZT11 decrease in light intensity was markedly lower than that after the earlier pulse. Taken as a whole, these data confirm that decreases in light intensity at ZT6 and ZT10/ZT11 have very different effects on starch metabolism. Both the reduced labeling of starch during the pulse and the lack of increase in its 14C content during the chase at the later time points could be explained by the loss of newly synthesized starch through degradation at ZT10/ZT11 but not at ZT6.
Table I. Incorporation of 14C into leaf fractions following decreases in light intensity.
Plants grown in 12-h-light/12-h-dark cycles were transferred to continuous light of 105 µmol m−2 s−1 at dawn and then subjected to a decrease in light intensity to 65 µmol m−2 s−1 at either ZT6 or ZT11. At 1 h after the decrease in light intensity, plants were supplied with 14CO2 for 5 min and then immediately harvested, extracted, and fractioned, and fractions were assayed for 14C. Values are means of measurements on four rosettes. Asterisks indicate values for ZT11 that are significantly different from those for ZT6 (Student’s t test, P < 0.05).
| Time of Decrease in Light Intensity |
14C Incorporated (dpm × 10−3 g−1
FW) |
|||||
|---|---|---|---|---|---|---|
| Total in Tissue | Water-Soluble |
Insoluble |
Ethanol-Soluble | |||
| Total | Neutral | Total | Starch | |||
| ZT6 | 1,032 ± 178 | 874 ± 26 | 163 ± 16 | 140 ± 20 | 94 ± 14 | 18 ± 1 |
| ZT11 | 991 ± 145 | 879 ± 60 | 166 ± 21 | 98 ± 5* | 57 ± 6* | 14 ± 2 |
DISCUSSION
Two direct conclusions arise from our results. First, starch degradation commences in the light in long days. Starch synthesis is accompanied by substantial degradation at times after about ZT14, resulting in a plateauing of starch content. Second, decreases in light intensity at points after ZT8 trigger starch degradation. Subsequent starch synthesis at the lower light intensity is accompanied by significant degradation, so that starch content may actually decline in response to a decrease in light intensity after ZT8. Below, we discuss these phenomena separately and consider their implications for plant growth in long days and in natural light. We then propose that the two phenomena may actually be manifestations of a single process—a gradual increase in the propensity for starch degradation with time in the light. This proposal has profound implications for our understanding of the control of leaf starch turnover and, hence, the availability of carbohydrate for growth across the day-night cycle.
Starch Turnover in Long Light Periods May Optimize Diel Carbon Use Efficiency
Our investigation of starch turnover in continuous light stemmed from the observation that starch content plateaus toward the end of the day in Arabidopsis plants grown in long days (Fig. 1; Hädrich et al., 2012; Sulpice et al., 2014; Figueroa et al., 2016). Leaf starch content also plateaus rather than continuing to rise when plants are exposed to long light periods or continuous light in tobacco, tomato (Solanum lycopersicum), sugar beet, pepper, and soybean (Glycine max; Fondy and Geiger, 1982, 1985; Hewitt et al., 1985; Li et al., 1992; Dorais et al., 1996; Masuda and Murage, 1998; Haque et al., 2015). However, the extent and timing of plateauing of starch content in continuous light varies considerably between studies. For example, plateauing of starch content occurred in 16-h days in some previous studies of Arabidopsis (Carrari et al., 2005; Li et al., 2009) but was not observed in others (Lu et al., 2005; Zhang et al., 2005; Szydlowski et al., 2009).
The plateauing of starch content in continuous light after ZT14 might in principle be due to a decline in the rate of CO2 assimilation or a shift in partitioning of assimilate away from starch and toward Suc. We suggest that neither of these explanations is likely. First, the rate of CO2 assimilation did not decline with time in continuous light (Fig. 1). Second, there was little change in levels of Suc and intermediates of Suc synthesis (Suc6P, hexose monophosphates, FBP, and UDPGlc) over the period in which starch accumulation declined (Fig. 2; Pal et al., 2013; Martins et al., 2013; Figueroa et al., 2016). Third, the level of ADPGlc, which is the dedicated substrate for starch synthesis, changed very little over the period in which starch accumulation plateaued.
Our data provide unequivocal evidence for the occurrence of starch degradation at the same time as synthesis after ZT14. Analysis of starch from leaves provided with 13CO2 showed that unlabeled (12C) starch was lost at the same time as 13C starch was synthesized during labeling periods from ZT14 onwards (Fig. 3). The data do not allow us to determine unambiguously whether starch degradation occurred only on older, unlabeled regions of the granule, or whether newly synthesized 13C starch was also removed by degradation during the labeling period. The fact that incorporation of 13C into starch over the labeling period was much lower after than before ZT14 could mean that newly synthesized as well as older starch was degraded during labeling periods from ZT14 onwards. While this observation could also be interpreted as a reduced rate of synthesis after ZT14, our data argue against this explanation (see above). We also note the possibility that there is spatial variation across the rosette or individual leaves in the extent or timing of onset of starch degradation in the light. Our experiments were conducted on whole rosettes: Further work will be required to discover whether, for example, there are differences between expanding and mature leaves in the pattern of starch turnover in the light. However, our previous analyses of starch content at dawn and dusk in Arabidopsis leaf 6 at the division and expansion stages revealed a pattern of starch turnover similar to those in mature leaves and the whole rosette (Baerenfaller et al., 2015).
The 13CO2 labeling experiments provide no evidence for substantial starch degradation before ZT14. It is possible that degradation occurred at earlier points but was confined to newly synthesized 13C labeled starch. The elevation of maltose content from ZT10 onwards (Fig. 4) is consistent with an earlier onset of degradation. Taken as a whole, our data indicate that a major change in the occurrence and/or magnitude of starch degradation takes place by about ZT14 and that this onset or increase in starch degradation is the major or sole cause of the plateauing of starch accumulation in continuous light.
Our conclusions from 13CO2 labeling experiments are at odds with those of Baslam et al. (2017), who measured 13C enrichment of starch (δ13C) following pulse-chase experiments with 13CO2 from ZT4 onwards. These authors concluded that substantial starch degradation and resynthesis occurs at the same time as new synthesis throughout light periods. We discuss why our conclusions are different from those of Baslam et al. (2017) in Supplemental Text S1.
A previous investigation of the reasons for plateauing of starch content in long days concluded that a change in partitioning between starch and Suc rather than the onset of starch degradation was responsible (Li et al., 1992). Starch accumulation slowed in sugar beet leaves in the light after ZT14. However, 14CO2 pulse-chase experiments provided no evidence for starch degradation: Label accumulated in starch during either short (10 min) or long (12 h) pulses remained in starch during subsequent long (up to 12 h) chases in the light. The authors used indirect means to estimate the rates of Suc synthesis and export, both of which appeared to increase after ZT14. They suggested that an unknown mechanism increasingly diverted newly assimilated C toward Suc, leading to a reduced flux of C into starch synthesis rather than the onset of degradation after prolonged periods in the light (Li et al., 1992). It is possible that this pulse-chase experiment failed to detect starch degradation because 14C starch was buried within the starch granule beneath newly synthesized 12C starch during the chase and thus protected from degradation occurring at the granule surface (Zeeman et al., 2002). However, it is also possible that the cause of the commonly observed cessation of starch accumulation in continuous light differs between species.
The onset of starch degradation in Arabidopsis leaves toward the end of a long photoperiod is consistent with the idea that C storage and C use efficiency are relaxed under long-day relative to short-day conditions (Baerenfaller et al., 2015; Sulpice et al., 2014). In long days, only a small amount of stored C is required for nighttime maintenance. There is a low ratio of starch to Suc synthesis during the light period and a relatively incomplete mobilization of stored starch by dawn (Sulpice et al., 2014). We propose that the onset of starch degradation toward the end of long photoperiods also reflects the low requirement for stored C in these conditions. Starch accumulated in the latter part of a long day cannot be fully used during the succeeding short night. Starch degradation prior to the onset of the night allows some of this accumulated C to be used for export and leaf cell maintenance during the day, when light energy is available. The decrease in partitioning of photoassimilate to starch proposed to occur in the latter part of long photoperiods in sugar beet leaves (Li et al., 1992) would effectively serve the same function: Assimilated C is diverted from storage to immediate use when the ensuing night is anticipated to be short.
Starch Turnover during Twilight May Optimize Suc Availability across the Day-Night Transition
Our investigation of starch turnover in response to decreases in light intensity, mimicking twilight, stemmed from the observation that starch content of sugar beet leaves started to decline during twilight in simulated natural light conditions (Fondy et al., 1989; Servaites et al., 1989), while the rate of photosynthesis was still substantial. This contrasted with a square-wave light regime with the same day length (14 h) in which starch accumulation slowed down at the end of the day but was not reversed prior to the onset of darkness (Fondy and Geiger, 1985). These data indicate that degradation of starch may be triggered in response to falling light levels at the end of the day.
Our data show that a similar phenomenon occurs in Arabidopsis leaves. Decreases in light intensity at ZT6 simply resulted in starch accumulation falling to the same rate as that in plants held from dawn at the lower light level. However decreases in light intensity at time points after ZT8 resulted either in rates of starch accumulation that were lower than those of plants held continuously at the lower light intensity or in net loss of starch. Taken together with the fact that maltose accumulated following decreases in light intensity at ZT10 and ZT12 but not ZT6 and ZT8, these data imply that starch degradation is accelerated by decreases in light intensity from ZT10 onwards.
Decreases in light intensity from ZT10 onwards appear to have a specific stimulatory effect on starch degradation, rather than promoting starch degradation as a secondary consequence of changes in photosynthesis or Suc synthesis. We showed that the rates of both CO2 assimilation and Suc synthesis respond identically to decreases in light intensity regardless of whether they occur at ZT6 or ZT10-ZT12.
Our data do not preclude the possibility that decreases in light intensity after ZT10 also result in a larger reduction of the rate of starch synthesis than decreases at earlier times in the light period. The marked decline between decreases in light intensity at ZT6 and ZT10 in the incorporation of 14C from 14CO2 into starch might be interpreted as a reduction in starch synthesis, but might alternatively be explained by turnover of newly synthesized starch at the granule surface from ZT10 onwards.
The onset of starch degradation in response to declining light intensity at the end of the day may be important for the maintenance of carbon availability during the light-dark transition in natural lighting conditions. If starch degradation were to be triggered only by complete darkness, Suc availability would decline dramatically during twilight as the rate of photosynthesis falls, potentially leading to transient starvation prior to the establishment of maximum rates of starch degradation after dark. Triggering of starch degradation as light levels decline during twilight potentially ensures that a supply of Suc from starch degradation replaces the supply of Suc from photosynthesis without major perturbations in Suc levels.
This proposal about the importance of the onset of starch degradation during twilight is consistent with the outcomes of our recent study of diel changes in leaf metabolites in natural, artificial sinusoidal, and artificial square-wave light regimes (Annunziata et al., 2017). In line with previous studies (Zeeman and ap Rees, 1999; Martins et al., 2013; Pal et al., 2013), we showed that Suc levels undergo a marked, transient dip at the onset of darkness in square-wave light regimes. This pattern was also seen for the Suc precursor Suc 6-P, other intermediates of primary carbon metabolism (Annunziata et al., 2017), and numbers of large polysomes (Pal et al., 2013), implying the existence of a short period of general starvation following an instantaneous onset of darkness. By contrast, levels of Suc and related metabolites were barely affected by dusk in sinusoidal light regimes. A similar pattern was seen for sugar beet leaves in sinusoidal light regimes: Fondy et al. (1989) showed that Suc levels were essentially unaltered during a gradual light-dark transition in which starch degradation increased as the rate of photosynthesis fell.
Starch Turnover in Long Days and in Twilight May Reflect an Increasing Propensity for Starch Degradation with Time after Dawn
The apparently different timings of onset of starch degradation in constant light (after ZT14) and the onset of starch degradation in response to decreases in light intensity (after ZT8) might suggest these are independent phenomena. However, it is also possible that some degradation in constant light occurs before ZT14 and was not detected in our experiments. Thus, the onset of starch degradation following decreases in light intensity before ZT14 might represent an enhancement of a phenomenon that is already present prior to the decrease in light intensity. Consistent with this possibility, we observed a small increase in maltose in constant light from ZT10 onwards. A small rise in maltose between ZT8 and ZT12 was also seen in some previous studies of Arabidopsis grown in 12-h-light/12-h-dark cycles (Hädrich et al., 2012; Martins et al., 2013), although maltose levels were constant over the light period in other studies (Pal et al., 2013). In the discussion below, we have assumed that degradation in constant light and in response to decreases in light intensity has the same cause: an increase with time after dawn in either the capacity for starch degradation or the susceptibility of starch to degradation.
As discussed above, the onset of degradation in the light does not appear to be a secondary consequence of changes with time in the rate of photosynthesis or the partitioning of primary assimilates nor is it a specific function of starch content. An alternative explanation is that the propensity for starch degradation in the light increases as a direct function of time after dawn, under the control of the circadian clock or another endogenous timing mechanism. We showed previously that the circadian clock determines the rate of starch degradation at night (Graf et al., 2010; Scialdone et al., 2013). At the onset of darkness, information about time remaining until dawn, derived from the clock, is integrated with information about starch content to set a constant rate of degradation that will result in the consumption of almost all of the stored starch by dawn. This mechanism explains our observation that subjecting plants to darkness at intervals between ZT6 and ZT12 results in progressively greater rises in maltose content: The rate of degradation set following darkness at ZT6 is expected to be only one-third of the rate set following darkness at ZT12 (plants at ZT6 have half the starch content and a 50% longer night than plants at ZT12). We consider below whether the circadian clock could also determine the timing of onset of starch degradation in the light and the mechanism by which degradation in the light may be initiated.
As a first approach to assessing the role of the clock in the onset of starch degradation in constant light, we examined whether the plateauing of starch degradation in constant light occurred at a different point in cca1 lhy and prr7 prr9 mutants from that in wild-type plants. The cca1 lhy mutant lacks MYB transcription factors that constitute the “dawn loop” of the circadian clock and has a short period phenotype (Alabadí et al., 2002; Mizoguchi et al., 2002). The prr7 prr9 mutant lacks pseudoresponse regulator components of the circadian clock that are expressed during the day and repress LHY and CCA1 expression: It has a long period phenotype (Salomé and McClung, 2005). Both mutants showed shifts in the time when starch content plateaus in constant light relative to the timing in wild-type plants, in the direction expected from their altered period phenotypes: Plateauing occurred early in the cca1 lhy mutant and late in the prr7 prr9 mutant. However, these results do not constitute robust evidence of a role for the clock in the timing of onset of starch degradation. Both mutants have complex phenotypes with direct and indirect consequences for starch turnover, and different starch contents at the start of the day from wild-type plants. The lhy cca1 mutant grows much more slowly than wild-type plants. It undergoes starvation at the end of each night and has an altered pattern of partitioning of newly assimilated carbon during the day (Mizoguchi et al., 2002; Graf and Smith, 2011; Kölling et al., 2015a). Further research is required to assess the importance of the circadian clock in the timing of onset of starch degradation in the light.
What might bring about an increase in the capacity for starch degradation or the susceptibility of starch to degradation? An obvious possibility is an increase with time after dawn in the activity of enzyme(s) involved in starch degradation. It is relatively unlikely that such an increase would be brought about at the level of gene expression. Although transcript levels for many enzymes of starch degradation rise with time after dawn, there is evidence for enzymes including glucan, water dikinase, α-amylase 3 (AMY3), and the glucanotransferase DPE2 that the amount of protein varies very little over the day-night cycle (Smith et al., 2004; Lu et al., 2005; Yu et al., 2005; Skeffington et al., 2014). In general terms, marked diel fluctuations in amounts of enzymes of primary metabolism are unlikely because of their high abundance and long half-lives (Gibon et al., 2004; Piques et al., 2009; Baerenfaller et al., 2012; Nelson et al., 2014). An intriguing exception is the major starch-degrading enzyme BAM3, which was recently shown to have a very short half-life of only 0.43 d (Li et al., 2017). The significance of its fast turnover time for the control of starch degradation remains to be explored.
A second major exception is granule-bound starch synthase (GBSS), responsible for the synthesis of the amylose component of the starch granule. GBSS transcript levels rise at the end of the night, and there is rapid synthesis of the protein early in the day. All of the protein becomes incorporated into the starch granule. Transcript levels fall and GBSS synthesis is very strongly reduced in the latter part of the day. As the granule is degraded at night, GBSS is released and appears to be immediately degraded (Smith et al., 2004; Ortiz-Marchena et al., 2014; Seung et al., 2015). These observations raise the possibility that the granule surface is increasingly susceptible to degradation as the day proceeds because its amylose:amylopectin ratio changes. However, studies of amylose-free gbss mutants of Arabidopsis do not support this idea. The mutants have the same starch contents as wild-type plants at the end of the day and the end of the night (Seung et al., 2015; Feike et al., 2016).
Three further proteins are known to influence the propensity for starch degradation during the day. The ESV1 protein is essential for both normal control of the rate of starch degradation at night and the susceptibility of starch granules to degradation during the day. Leaves of the esv1 mutant have accelerated rates of degradation at night and low rates of starch accumulation accompanied by elevated maltose contents during the day (Feike et al., 2016). The function of ESV1 is unknown, but it may directly influence the susceptibility of the granule matrix to degradation. However, there is currently no evidence that its activity is modulated in wild-type plants in a manner that might lead to increasing susceptibility of starch to degradation with time in the light.
A recent study shows that transcription of genes encoding AMY3 and the β-amylase BAM1 is strongly elevated under osmotic stress, leading to large increases in enzyme activity and the onset of starch degradation in the light (Thalmann et al., 2016). However neither enzyme is required for diel starch metabolism in mesophyll cells in square wave light regimes in the absence of abiotic stress (Yu et al., 2005; Fulton et al., 2008; Horrer et al., 2016).
Two other means can be suggested by which the propensity for starch degradation could increase with time in the light. First, activities of starch-degrading enzymes may increase through the day by means of posttranslational modifications. For example, several starch-degrading enzymes are known to be phosphorylated, including BAM1 and AMY3, although the importance of phosphorylation for activity has not been established (Thalmann et al., 2016). Second, there may be progressive changes in the properties of starch granules that directly influence their susceptibility to degradation. The obvious candidate in this context is the degree of phosphorylation of the starch granule surface. The presence of phosphate groups on Glc residues within amylopectin chains profoundly influences the rate of starch degradation by β-amylases (Hejazi et al., 2008), probably because these groups reduce the level of crystalline organization of the granule matrix and thus open the surface to attack (Hansen et al., 2009). Phosphate groups are added by two glucan water dikinases: water dikinase and PWD. Both enzymes are required for normal rates of starch degradation. Intriguingly, the level of phosphorylation of the starch granule (measured as the 6-phosphate content per unit Glc) increases markedly during the light period then falls at night (Scialdone et al., 2013). This pattern potentially generates a diel change in the accessibility of the granule surface to hydrolytic enzymes; hence, it might account for the increased propensity for starch degradation with time in the light.
The increasing propensity for starch degradation with time in the light is reminiscent of the fact that the rate of starch degradation following the onset of darkness increases with time after dawn (Graf et al., 2010; Scialdone et al., 2013). It is pertinent to ask whether these phenomena are related. Our previous studies of the control of starch degradation have been predicated on the idea that starch degradation occurs only in darkness. The results above reveal that starch actually becomes more liable to degradation—either through changing degradative capacity or changes in the properties of starch—with time in the light. We speculate that the increasing propensity for degradation with time in the light may be a factor in setting the rate of degradation following the onset of darkness. Thus, if darkness comes early (e.g. at ZT8 in plants entrained to 12-h days), the susceptibility of starch to degradation at this point will be much lower than if darkness comes at ZT12. These are important considerations for efforts to discover the mechanism that sets the rate of starch degradation at night.
The discovery that starch degradation increases with time in the light and is accelerated by decreases in light intensity raises major questions about how degradation is initiated and controlled. The answers are likely to be complex and may involve control of both protein activity/function and the spatial interactions between these proteins and the surfaces of starch granules.
MATERIALS AND METHODS
Plant Material
Plants of Arabidopsis (Arabidopsis thaliana) were grown in soil in controlled environment rooms. Unless otherwise stated, conditions up to the point of experimentation were 12 h light, 12 h dark, 20 to 22°C, 140 to 170 µmol quanta m−2 s−1, and 75% relative humidity. Mutants cca1 lhy and prr7 prr9 were kindly provided by Andrew Millar (University of Edinburgh) and were in the Ws-2 (Wassilewskija-2) and Col-0 (Columbia-0) backgrounds, respectively.
Metabolite Analysis
Rosettes were harvested and frozen immediately in liquid nitrogen. Sugars and starch were extracted and measured enzymatically (Stitt et al., 1989). Starch values were expressed as µmol hexose equivalents. Other metabolites except for maltose were extracted and measured using high-performance anion-exchange chromatography coupled to tandem mass spectrometry (Lunn et al., 2006; modified as in Figueroa et al., 2016). For maltose, rosettes were extracted sequentially in hot 80% (v/v) and 50% (v/v) ethanol. Soluble extracts were combined, filtered (45 µm), dried down, and then resuspended and treated with prokaryotic trehalase (Megazyme) in 50 mm KH2PO4, pH 6.8, 1 mm MgCl2, and 10 mm NaCl at 35°C for 1h. Following heat denaturation of trehalase (80°C, 15 min) and vacuum drying, the sugars in the extract were derivatized by sequential incubation with 50 µL of methoxyamine hydrochloride at 30°C for 90 min then 100 µL of N-methyl-N-trimethylsilyltrifluoroacetamide at 37°C for 30 min. Samples were held at room temperature for 2 h before injection and analysis by GC-MS (Agilent 6800 GC-MS system). The validity of the assay was checked by spiking tissue prior to extraction with various amounts of 12C and 13C maltose: This confirmed that recovery was in the region of 90% and peak identification was correct.
Measurement of Photosynthesis
Gas-exchange measurements were made by infrared CO2 analysis using either the LI-6400-17 whole-plant chamber (LI-COR Biosciences; Supplemental Fig. S1) or the whole-plant chambers described by Kölling et al. (2015b) (Fig. 1; Supplemental Fig. S2).
13CO2 Labeling Experiments
13CO2 labeling was carried out as described by Ishihara et al. (2015). Three-week-old plants were placed in advance of the experiment in a Plexiglas chamber inside a controlled environment cabinet under the conditions described in the text and legends. They were supplied with a premixed air stream containing 450 µL L−1 CO2 (∼99% 12CO2 natural abundance) or 13CO2, 21% (v/v) oxygen, and 79% (v/v) nitrogen at a flow rate of 5 L min−1. Plants were harvested by opening the lid of the labeling chamber, removing the plants and submerging them in liquid N2. Each replicate consisted of five plants grown in a single 10-cm pot. Following grinding in liquid nitrogen, material was extracted twice for 30 min at 80°C with 80% (v/v) ethanol/10 mm MES, pH 5.9, and once with 50% (v/v) ethanol/10 mm MES, pH 5.9. The insoluble material was pelleted by centrifugation as described by Cross et al. (2006). Starch in insoluble material was solubilized by heating in 0.1 m NaOH. Following neutralization, starch was digested to Glc with α-amylase and α-amyloglucosidase. For total starch content, Glc was assayed enzymatically. For 13C starch content, Glc was converted to Glc6P by incubation with hexokinase and excess ATP, and 13C in Glc6P was measured by LC-MS/MS (Arrivault et al., 2009; Supplemental Text S1).
14CO2 Labeling Experiments
14CO2 labeling was carried out on whole plants using the Plexiglas chamber described by Kölling et al. (2015a, 2015b).Three-week-old plants were placed in the chamber in advance of the experiment, inside a fume cupboard at ∼20°C under the lighting conditions described in the text and legends. After sealing the chamber, 75 μCi 14CO2 was supplied by injection of lactic acid into NaH14CO3 (specific activity 2.2 TBq mmol−1; CO2 release increased concentration in the chamber by ∼8 ppm). After 5 min, the chamber was opened and rosettes for “pulse” measurements were excised and plunged into hot ethanol. Samples for “chase” measurements were harvested 30 min later. Extraction and fractionation of the tissue and measurement of 14C were performed as described by Kölling et al. (2013).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Starch turnover, photosynthesis, and starch-related metabolites in plants transferred to low continuous light.
Supplemental Figure S2. Effects of decreases in light intensity on starch accumulation and the rate of photosynthesis.
Supplemental Table S1. Content of starch and 13C starch at the start and end of 13CO2 labeling periods.
Supplemental Text S1. Additional information on the measurement of 13C starch accumulation, interpretation of the results, and commentary on the debate about whether leaf starch is simultaneously synthesized and degraded in the light.
Acknowledgments
We thank Andrew Millar (University of Edinburgh) for the kind gift of seeds of mutants cca1 lhy and prr7 prr9, Paul Brett (Metabolite Service, John Innes Centre) for his help with maltose assays, and the John Innes Centre Horticultural Services and Christin Abel (MPIMP) for assistance with plant growth.
Glossary
- FW
fresh weight
- GBSS
granule-bound starch synthase
Footnotes
Articles can be viewed without a subscription.
This work was supported by the European Commission FP7 Collaborative Project TiMet (contract 245143 to O.F., V.M., and A.F.), by BBSRC Institute Strategic Programme Grant BB/J004561/1 to the John Innes Centre (O.F., A.M.S.), by the Max-Planck Society, Germany (H.I., D.S., S.A., R.F., J.E.L., M.S.), by ETH (Zurich), and by SystemsX (Switzerland) grant “Plant Growth in a Changing Environment” (G.M.G., S.C.Z.).
References
- Alabadí D, Yanovsky MJ, Más P, Harmer SL, Kay SA (2002) Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol 12: 757–761 [DOI] [PubMed] [Google Scholar]
- Annunziata MG, Apelt F, Carillo P, Krause U, Feil R, Mengin V, Lauxmann MA, Koehl K, Nikoloski Z, Stitt M, Lunn JE (2017) Getting back to nature: a reality check for experiments in controlled environments. J Exp Bot (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Guenther M, Ivakov A, Feil R, Vosloh D, van Dongen JT, Sulpice R, Stitt M (2009) Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. Plant J 59: 826–839 [DOI] [PubMed] [Google Scholar]
- Baerenfaller K, Massonnet C, Hennig L, Russenberger D, Sulpice R, Walsh S, Stitt M, Granier C, Gruissem W (2015) A long photoperiod relaxes energy management in Arabidopsis leaf six. Curr Plant Biol 2: 34–45 [Google Scholar]
- Baerenfaller K, Massonnet C, Walsh S, Baginsky S, Bühlmann P, Hennig L, Hirsch-Hoffmann M, Howell KA, Kahlau S, Radziejwoski A, et al. (2012) Systems-based analysis of Arabidopsis leaf growth reveals adaptation to water deficit. Mol Syst Biol 8: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslam M, Baroja-Fernández E, Ricarte-Bermejo A, Sánchez-López ÁM, Aranjuelo I, Bahaji A, Muñoz FJ, Almagro G, Pujol P, Galarza R, Teixidor P, Pozueta-Romero J (2017) Genetic and isotope ratio mass spectrometric evidence for the occurrence of starch degradation and cycling in illuminated Arabidopsis leaves. PLoS One 12: e0171245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Coll-Garcia D, Schauer N, Lytovchenko A, Palacios-Rojas N, Balbo I, Rosso M, Fernie AR (2005) Deficiency of a plastidial adenylate kinase in Arabidopsis results in elevated photosynthetic amino acid biosynthesis and enhanced growth. Plant Physiol 137: 70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C (1991) Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol 95: 1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorais M, Yelle S, Gosselin A (1996) Influence of extended photoperiod on photosynthate partitioning and export in tomato and pepper plants. N Z J Crop Hortic Sci 24: 29–37 [Google Scholar]
- Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA (2005) Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol 15: 47–54 [DOI] [PubMed] [Google Scholar]
- Feike D, Seung D, Graf A, Bischof S, Ellick T, Coiro M, Soyk S, Eicke S, Mettler-Altmann T, Lu KJ, et al. (2016) The starch granule-associated protein EARLY STARVATION1 (ESV1) is required for the control of starch degradation in Arabidopsis thaliana leaves. Plant Cell 28: 1472–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Feil R, Ishihara H, Watanabe M, Kölling K, Krause U, Höhne M, Encke B, Plaxton WC, Zeeman SC, et al. (2016) Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J 85: 410–423 [DOI] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR (1982) Diurnal pattern of translocation and carbohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiol 70: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR (1985) Diurnal changes in allocation of newly fixed carbon in exporting sugar beet leaves. Plant Physiol 78: 753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR, Servaites JC (1989) Photosynthesis, carbohydrate metabolism, and export in Beta vulgaris L. and Phaseolus vulgaris L. during square and sinusoidal light regimes. Plant Physiol 89: 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, Vaughan CK, Li J, Francisco P, Gil M, Reinhold H, Eicke S, Messerli G, et al. (2008) β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell 20: 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bläsing O, Hannemann J, Carillo P, Höhne M, Cross J, Selbig J, Stitt M (2004) A robot-based platform to measure multiple enzyme activities using a set of cycling assays: comparison of changes of enzyme activities and transcript levels in Arabidopsis during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A, Smith AM (2011) Starch and the clock: the dark side of plant productivity. Trends Plant Sci 16: 169–175 [DOI] [PubMed] [Google Scholar]
- Grange RI. (1984) The extent of starch turnover in mature pepper leaves in the light. Ann Bot (Lond) 54: 289–292 [Google Scholar]
- Hädrich N, Hendriks JHM, Kötting O, Arrivault S, Feil R, Zeeman SC, Gibon Y, Schulze WX, Stitt M, Lunn JE (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J 70: 231–242 [DOI] [PubMed] [Google Scholar]
- Hansen PI, Spraul M, Dvortsak P, Larsen FH, Blennow A, Motawia MS, Engelsen SB (2009) Starch phosphorylation--maltosidic restrains upon 3′- and 6′-phosphorylation investigated by chemical synthesis, molecular dynamics and NMR spectroscopy. Biopolymers 91: 179–193 [DOI] [PubMed] [Google Scholar]
- Haque MS, Kjaer KH, Rosenqvist E, Ottosen CO (2015) Continuous light increases growth, daily carbon gain, antioxidants, and alters carbohydrate metabolism in a cultivated and a wild tomato species. Front Plant Sci 6: 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Schulz B, Flügge UI (1998) Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204: 366–376 [DOI] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Haebel S, Edner C, Paris O, Frohberg C, Steup M, Ritte G (2008) Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J 55: 323–334 [DOI] [PubMed] [Google Scholar]
- Hewitt JD, Casey LL, Zobel RW (1985) Effect of day length and night temperature on starch accumulation and degradation of soybean. Ann Bot (Lond) 56: 513–522 [Google Scholar]
- Horrer D, Flütsch S, Pazmino D, Matthews JSA, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol 26: 362–370 [DOI] [PubMed] [Google Scholar]
- Ishihara H, Obata T, Sulpice R, Fernie AR, Stitt M (2015) Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein. Plant Physiol 168: 74–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling K, George GM, Künzli R, Flütsch P, Zeeman SC (2015b) A whole-plant chamber system for parallel gas exchange measurements of Arabidopsis and other herbaceous species. Plant Methods 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling K, Müller A, Flütsch P, Zeeman SC (2013) A device for single leaf labelling with CO2 isotopes to study carbon allocation and partitioning in Arabidopsis thaliana. Plant Methods 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling K, Thalmann M, Müller A, Jenny C, Zeeman SC (2015a) Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ 38: 1965–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kötting O, Kossmann J, Zeeman SC, Lloyd JR (2010) Regulation of starch metabolism: the age of enlightenment? Curr Opin Plant Biol 13: 321–329 [DOI] [PubMed] [Google Scholar]
- Kruger NJ, Bulpin PV, ap Rees T (1983) The extent of starch degradation in the light in pea leaves. Planta 157: 271–273 [DOI] [PubMed] [Google Scholar]
- Li B, Geiger DR, Shieh WJ (1992) Evidence for circadian regulation of starch and sucrose synthesis in sugar beet leaves. Plant Physiol 99: 1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Foster CM, Gan Q, Nettleton D, James MG, Myers AM, Wurtele ES (2009) Identification of the novel protein QQS as a component of the starch metabolic network in Arabidopsis leaves. Plant J 58: 485–498 [DOI] [PubMed] [Google Scholar]
- Li L, Nelson CJ, Trösch J, Castleden I, Huang S, Millar AH (2017) Protein degradation rate in Arabidopsis thaliana leaf growth and development. Plant Cell 29: 207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol 138: 2280–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins MCM, Hejazi M, Fettke J, Steup M, Feil R, Krause U, Arrivault S, Vosloh D, Figueroa CM, Ivakov A, et al. (2013) Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiol 163: 1142–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Murage EN (1998) Continuous fluorescent illumination enhances growth and fruiting of pepper. J Japan Hort Sci 67: 862–865 [Google Scholar]
- Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, Carré IA, Coupland G (2002) LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell 2: 629–641 [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Alexova R, Jacoby RP, Millar AH (2014) Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol 166: 91–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Ortiz-Marchena MI, Albi T, Lucas-Reina E, Said FE, Romero-Campero FJ, Cano B, Ruiz MT, Romero JM, Valverde F (2014) Photoperiodic control of carbon distribution during the floral transition in Arabidopsis. Plant Cell 26: 565–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Liput M, Piques M, Ishihara H, Obata T, Martins MCM, Sulpice R, van Dongen JT, Fernie AR, Yadav UP, et al. (2013) Diurnal changes of polysome loading track sucrose content in the rosette of wild-type arabidopsis and the starchless pgm mutant. Plant Physiol 162: 1246–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piques M, Schulze WX, Höhne M, Usadel B, Gibon Y, Rohwer J, Stitt M (2009) Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Mol Syst Biol 5: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse DP, Tocquin P (2006) Leaf carbohydrate controls over Arabidopsis growth and response to elevated CO2: an experimentally based model. New Phytol 172: 500–513 [DOI] [PubMed] [Google Scholar]
- Salomé PA, McClung CR (2005) PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell 17: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialdone A, Mugford ST, Feike D, Skeffington A, Borrill P, Graf A, Smith AM, Howard M (2013) Arabidopsis plants perform arithmetic division to prevent starvation at night. eLife 2: e00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites JC, Geiger DR, Tucci MA, Fondy BR (1989) Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol 89: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung D, Soyk S, Coiro M, Maier BA, Eicke S, Zeeman SC (2015) PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol 13: e1002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeffington AW, Graf A, Duxbury Z, Gruissem W, Smith AM (2014) Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Plant Physiol 165: 866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman SC, Smith AM (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Heldt HW (1981) Simultaneous synthesis and degradation of starch in spinach chloroplasts in the light. Biochim Biophys Acta 638: 1–11 [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Determination of metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174: 518–552 [Google Scholar]
- Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M (2014) Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant 7: 137–155 [DOI] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21: 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann M, Pazmino D, Seung D, Horrer D, Nigro A, Meier T, Kölling K, Pfeifhofer HW, Zeeman SC, Santelia D (2016) Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 28: 1860–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich SM, Massot V, Hümmer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P (2013) Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ 36: 16–29 [DOI] [PubMed] [Google Scholar]
- Walters RG, Ibrahim DG, Horton P, Kruger NJ (2004) A mutant of Arabidopsis lacking the triose-phosphate/phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiol 135: 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Schrader SM, Kleinbeck KR, Sharkey TD (2006) Carbon balance and circadian regulation of hydrolytic and phosphorolytic breakdown of transitory starch. Plant Physiol 141: 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Weber AP, Sharkey TD (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218: 474–482 [DOI] [PubMed] [Google Scholar]
- Weise SE, van Wijk KJ, Sharkey TD (2011) The role of transitory starch in C(3), CAM, and C(4) metabolism and opportunities for engineering leaf starch accumulation. J Exp Bot 62: 3109–3118 [DOI] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, Lunn JE (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue WL, Hegemann B, Tung SY, Umemoto T, Chapple A, et al. (2005) α-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem 280: 9773–9779 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, ap Rees T (1999) Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ 22: 1445–1453 [Google Scholar]
- Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM (2002) Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol 129: 516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Myers AM, James MG (2005) Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol 138: 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]