The rice K+ channel OsK5.2 is necessary for rapid stomatal closure and contributes to shoot K+ supply from the root, thus highlighting the differing functional distribution of Shaker channels compared to Arabidopsis.
Abstract
The roles of potassium channels from the Shaker family in stomatal movements have been investigated by reverse genetics analyses in Arabidopsis (Arabidopsis thaliana), but corresponding information is lacking outside this model species. Rice (Oryza sativa) and other cereals possess stomata that are more complex than those of Arabidopsis. We examined the role of the outward Shaker K+ channel gene OsK5.2. Expression of the OsK5.2 gene (GUS reporter strategy) was observed in the whole stomatal complex (guard cells and subsidiary cells), root vasculature, and root cortex. In stomata, loss of OsK5.2 functional expression resulted in lack of time-dependent outward potassium currents in guard cells, higher rates of water loss through transpiration, and severe slowdown of stomatal closure. In line with the expression of OsK5.2 in the plant vasculature, mutant plants displayed a reduced K+ translocation from the root system toward the leaves via the xylem. The comparison between rice and Arabidopsis show that despite the strong conservation of Shaker family in plants, substantial differences can exist between the physiological roles of seemingly orthologous genes, as xylem loading depends on SKOR and stomatal closure on GORK in Arabidopsis, whereas both functions are executed by the single OsK5.2 Shaker in rice.
Since a waxy cuticle covers outer leaf tissues, water vapor diffusion into the atmosphere occurs mainly through the stomatal pores at the leaf surface. The size of the stomatal aperture is tightly regulated to optimize gas exchanges between the leaf inner tissues and the atmosphere, including CO2 intake for photosynthesis and water loss by transpiration (Lawson and Blatt, 2014). This is achieved by fine tuning of the turgor pressure of the two guard cells that surround the stomatal pore and involves a complex coordinated activity of transport systems at the guard cell plasma membrane and vacuolar membrane (Hedrich, 2012; Chen et al., 2012; Hills et al., 2012; Kollist et al., 2014). This control also affects long-distance transport of mineral nutrients from the roots, which take up these nutrients, to the aerial parts, to support plant growth (Marschner et al., 1996).
Potassium ion (K+), as a major inorganic constituent of the plant cells and the most abundant cation in the cytosol, is an essential macronutrient for growth and development. It is involved in various functions, including electrical neutralization of negative charges, control of cell membrane polarization, and osmoregulation (Clarkson and Hanson, 1980; Leigh and Wyn Jones, 1984). K+ is thus the main cation absorbed by the roots and circulating within the plant at the cellular or long-distance levels. In guard cells, it is well known as a major contributor, with Cl-, NO3− and malate, to the osmolarity (Raschke and Schnabl, 1978; Willmer and Fricker, 1996). Stomatal opening is initiated by activation of plasma membrane proton pumps in guard cells, which promotes K+ influx through voltage-gated inward K+ channels, as well as anion uptake through H+-anion symporters (Blatt, 1987a; Schroeder et al., 1987; Roelfsema and Prins, 1997; Talbott and Zeiger, 1998; Guo et al., 2003; Jezek and Blatt, 2017). Conversely, stomatal closure requires inhibition of proton pumping at the guard cell membrane and activation of both anion channels and voltage-gated outward K+ channels.
The molecular mechanisms responsible for inward and outward K+ fluxes across the plasma membrane have been extensively investigated in Arabidopsis (Arabidopsis thaliana). Shaker channel subunits, present as a nine-member family in Arabidopsis, have been shown to form the major pathways for these fluxes throughout the plant (Véry and Sentenac, 2003). In the Arabidopsis model species, four genes encoding Shaker channel subunits have been identified as playing a major role in root to shoot K+ translocation and in stomatal movements. The SKOR subunit, which is expressed in root pericycle and xylem parenchyma, forms outwardly rectifying channels involved in K+ secretion into the xylem sap (Gaymard et al., 1998). In stomata, the inward Shaker channel subunits KAT1 and KAT2 are involved in guard cell K+ uptake, and the outward Shaker channel GORK mediates guard cell K+ release (Ache et al., 2000; Pilot et al., 2001; Szyroki et al., 2001; Hosy et al., 2003; Lebaudy et al., 2008). Whereas these Shaker subunits have been deeply characterized in Arabidopsis, and the Shaker family, as a whole, can be considered as the best characterized family of plant membrane transport systems, little information at the molecular genetic level is yet available on this family outside Arabidopsis.
The stomatal complex in rice (Oryza sativa), the current model cereal, is very different from that of Arabidopsis (Itoh et al., 2005; Franks and Farquhar, 2007; Roelfsema and Hedrich, 2009). In rice, like in other cereals, it comprises two subsidiary cells in addition to the two guard cells. Substantial differences may thus exist between rice and Arabidopsis in stomatal functioning (Mumm et al., 2011). Rice possesses two putative outward Shaker channel subunits (Pilot et al., 2003; Véry et al., 2014), named OsK5.1 (or OsSKOR) and OsK5.2 (or OsGORK; Pilot et al., 2003; Kim et al., 2015). OsK5.1 was reported to be mainly expressed in root vasculature, just as SKOR in Arabidopsis (Kim et al., 2015). In contrast, OsK5.2 was found to be expressed both in roots and shoots (Kim et al., 2015). The expression pattern of this gene at the tissue level has, however, not been described. Here, we investigate the expression pattern and role of this rice outward Shaker gene. OsK5.2 is shown to play two important roles in rice plants: it mediates K+ translocation into the xylem sap toward the shoots, and it is involved in K+ release from guard cells and stomatal movements.
RESULTS
The Outward Shaker Channel Subfamily in Arabidopsis and Rice
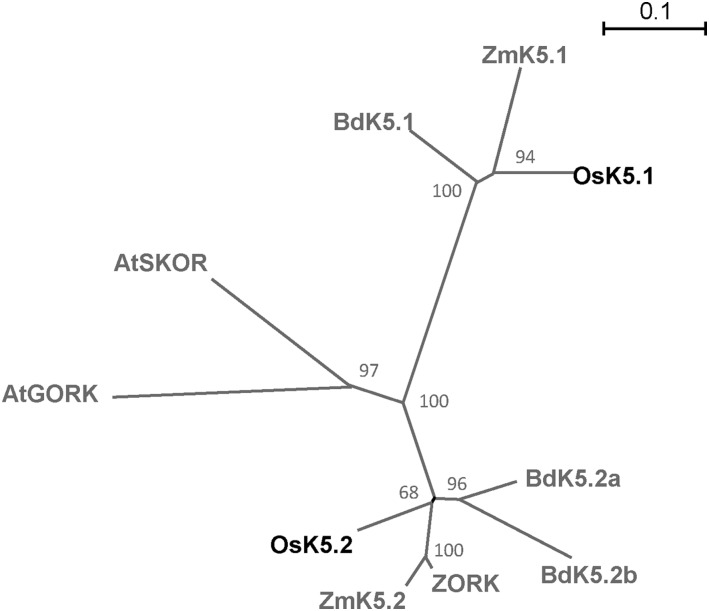
The Shaker group 5, which consists of the outward Shaker subunits, usually comprises two or three members in the plant genomes analyzed so far (Pilot et al., 2003; Véry et al., 2014). Figure 1 illustrates the phylogenetic relationships between the members of the Shaker group 5 present in Arabidopsis and in three monocots: rice, maize (Zea mays), and Brachypodium distachyon. Based on this phylogenetic tree, the rice OsK5.1 and OsK5.2 subunits appeared to belong to two different subgroups within the Shaker group 5. The distance between these subgroups was larger than that between GORK and SKOR from Arabidopsis.
Figure 1.
Phylogenetic analysis of outward Shaker channels in monocot species and Arabidopsis. The unrooted phylogenetic tree was generated with PhyML software (http://www.atgc-montpellier.fr/phyml/binaries.php) using the maximum likelihood method and 1,000 bootstrap replicates in Seaview application (http://doua.prabi.fr/software/seaview). The scale bar corresponds to a distance of 10 changes per 100 amino acid positions. The protein (GenBank) accession numbers are given in Supplemental Table S2. At, Arabidopsis thaliana; Bd, Brachypodium distachyon; Os, Oryza sativa (rice); Zm, Zea mays (maize).
Expression Pattern of OsK5.2
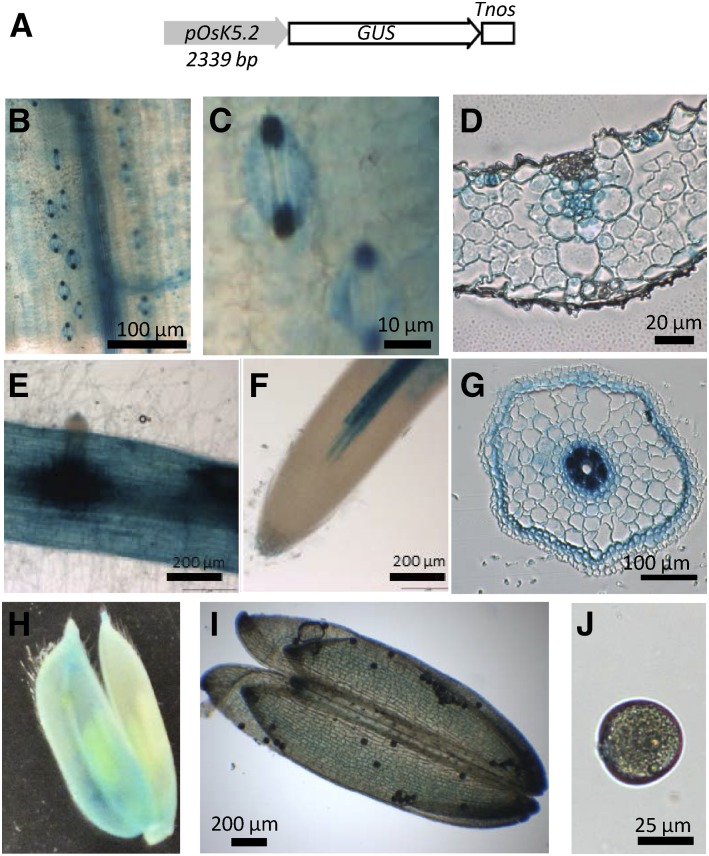
Transgenic rice plants expressing GUS under the control of the OsK5.2 promoter region (Fig. 2A) were generated to investigate the expression of this Shaker gene (Fig. 2, B–J). GUS staining revealed that OsK5.2 was expressed in vascular tissue of root (Fig. 2, E–G), like OsK5.1 (Kim et al., 2015), as well as in the shoot vascular tissue (Fig. 2, B and D). In addition, the OsK5.2 promoter was active in root cortical cells (except at the root tip; Fig. 2, E–G). Weak GUS staining was also observed in flowers, in the pollen sacs and grains (Fig. 2, H–J). Analysis of GUS staining in leaf epidermis revealed expression of OsK5.2 in stomata, the staining being stronger in guard cells than in subsidiary cells (Fig. 2, B–D).
Figure 2.
Expression pattern of OsK5.2 outward Shaker channel gene in rice. A, Promoter::GUS construct (promoter region size: 2,339 bp). B to J, GUS activity in transgenic rice plants, revealed by an overnight incubation in the presence of X-Gluc: leaf tissues (B), stomata (C), leaf cross section (D), root (E), root tip (F), root cross section (G), flower (H), anther (I), and pollen (J).
The following experiments were aimed at investigating the role of OsK5.2 in K+ translocation toward the shoots and in stomatal movements.
Production of osk5.2 Loss-of-Function Mutant Plants
Genotyping experiments performed on the progeny of the mutant lines ASJA08 and ASHF06 (Supplemental Fig. S1A) allowed us to identify osk5.2 homozygous mutant plants (Tos17 insertion alleles), as well as plants without insertion in the OsK5.2 gene, hereafter named wild-type controls. The offspring of the selected plants (osk5.2 mutants and wild-type controls) were used for in planta experiments. RT-PCR experiments on total RNA prepared from the osk5.2 mutants and the wild-type control plants confirmed the loss of function of OsK5.2 in the selected osk5.2 homozygous mutant lines (Supplemental Fig. S1B).
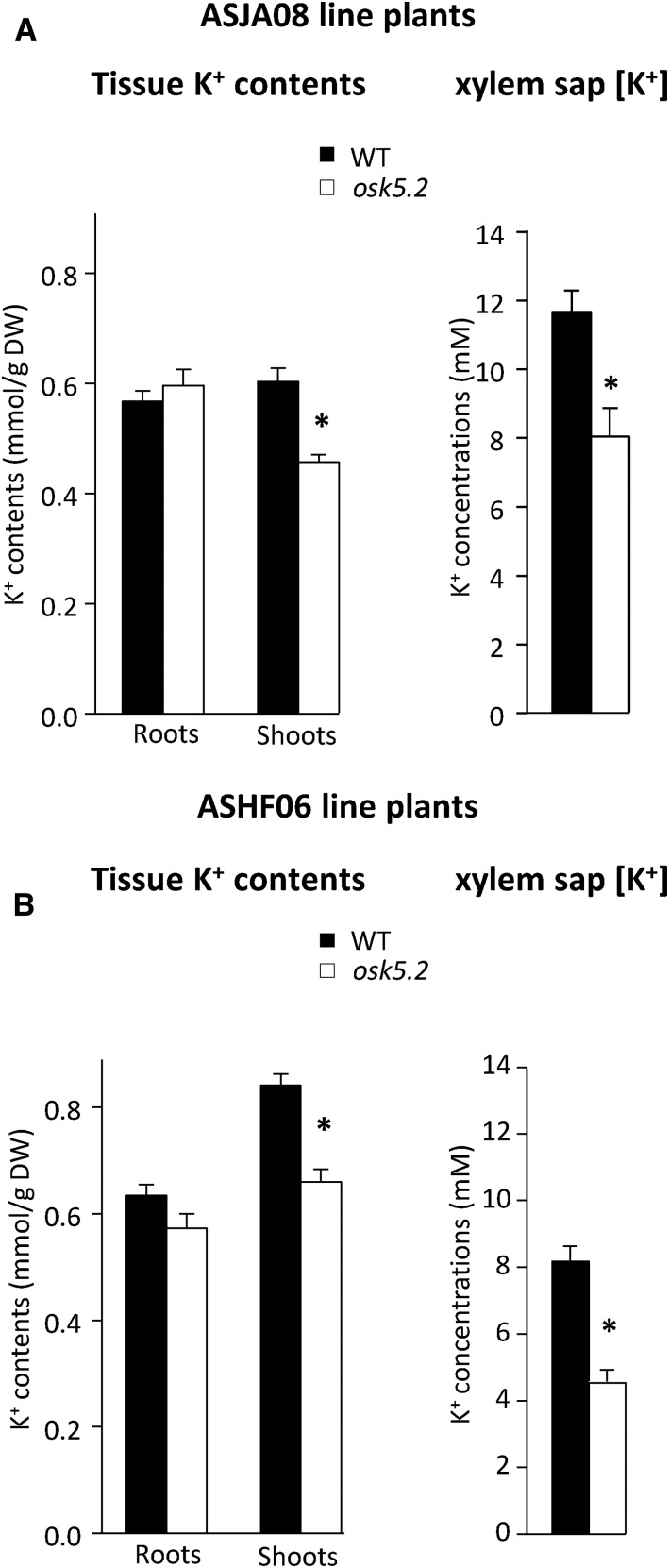
Impaired K+ Transport into the Xylem Sap and K+ Accumulation in Shoots in osk5.2 Mutant Plants
K+ was assayed in roots and shoots of 5-week-old plants hydroponically grown on standard Yoshida medium. Comparison of osk5.2 mutant plants with the corresponding wild-type plants revealed that the absence of OsK5.2 functional expression did not affect K+ accumulation in roots but resulted in significantly reduced K+ contents (by ∼25%) in shoots (Fig. 3, A and B, left). The K+ concentration measurements in xylem sap samples that were collected after leaf excision revealed lower values, by 30% to 40%, in the mutant plants (Fig. 3, A and B, right). Thus, the whole set of data indicated that the absence of OsK5.2 functional expression resulted in a decrease in K+ translocation from roots to shoots via the xylem sap.
Figure 3.
Tissue K+ contents and xylem sap K+ concentration in wild-type and osk5.2 mutant plants. Five-week-old plants were grown in standard Yoshida hydroponic solution. A and B, Root and shoot K+ contents (left) and xylem sap K+ concentration (right) in wild-type control (black bars) and osk5.2 mutant (white bars) plants issued from the initial ASJA08 (A) or ASHF06 (B) lines. Assayed xylem sap exudates were collected in wild-type control and osk5.2 mutant plants after 1-h exudation in the case of the plants issued from the ASJA08 line, and 2-h exudation in the case of the plants issued from the ASHF06 line. Data are means ± se (n = 3 or 4 for wild-type control plants, and 4 or 5 for osk5.2 mutant plants in A, and n = 4 or 6 for wild-type control plants, and 5 or 6 for osk5.2 mutant plants in B). An asterisk indicates that the corresponding difference in K+ content or concentration between the wild-type control and osk5.2 mutant plants were statistically significant (Student’s t test, P < 0.05).
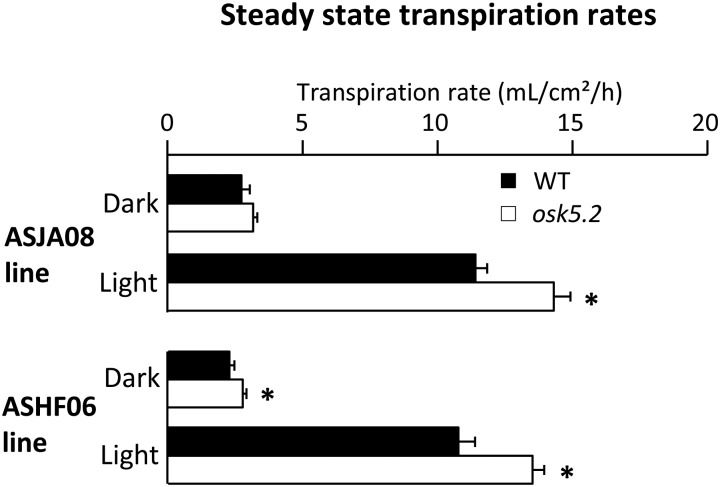
Increased Transpirational Water Loss Due to Impaired Stomatal Movements in osk5.2 Mutant Plants
Water consumption (essentially due to transpirational water loss) was monitored in osk5.2 mutant plants and the corresponding wild-type control plants hydroponically grown for 5 weeks on Yoshida medium using a multipotometer (Supplemental Fig. S2). Both mutant lines displayed larger steady-state rates of transpirational water loss than the corresponding wild-type plants in the light, by about 25% (Fig. 4).
Figure 4.
Steady-state transpiration rates in wild-type control and osk5.2 mutant plants. Five-week-old plants issued from the initial ASJA08 line (above) or ASHF06 line (below) were used for steady-state transpiration rate analyses in dark (∼5 h after light was switched off) and in light (∼3 h after light was switched on) conditions. The steady-state transpiration rate (in mL/cm2/h) was determined by dividing the average speed of water loss at steady state (mean of three values) by the total area of the plant aerial parts. Black and white bars represent wild-type control and osk5.2 mutant plants, respectively. Data are means ± se; n = 6 for wild-type control and 13 for osk5.2 mutant plants in the case of the plants issued from the ASJA08 line, and n = 7 for wild-type control and 9 for osk5.2 plant plants in the case of the plants issued from the ASHF06 line. An asterisk indicates that the corresponding difference between the wild-type control and mutant plants was statistically significant (Student’s t test, P < 0.05).
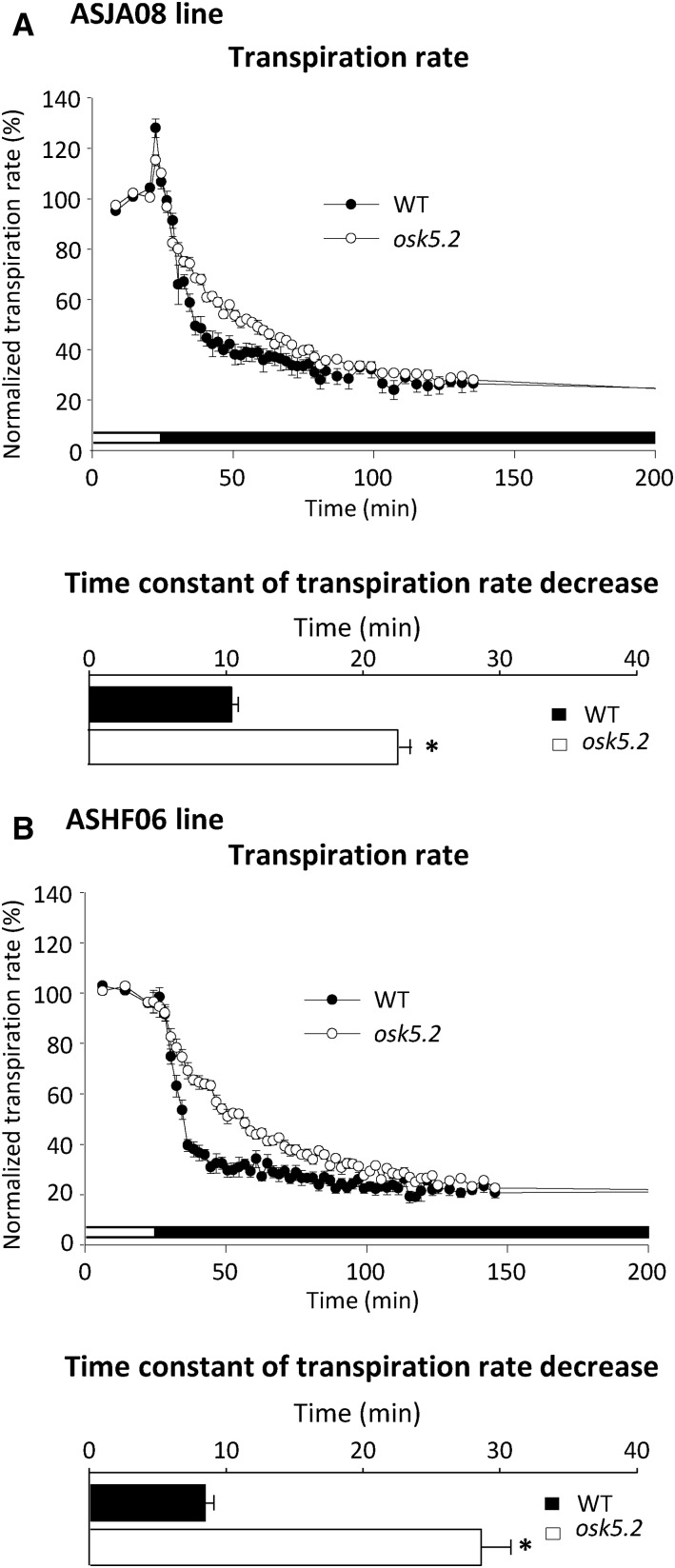
The transpiration rate is dependent on stomatal aperture (Pallas, 1965; Hosy et al., 2003). Light to dark transitions were used to provoke stomatal closure. The decrease in transpiration rate when light was switched off was slower in the mutant plants than in the corresponding wild-type control plants (Fig. 5, A and B, top), revealing a slower rate of dark-induced stomatal closure in absence of OsK5.2 functional expression. Fitting the decrease in transpiration rates with exponential functions yielded a time constant of ∼10 min for the wild-type control plants, and of 20 to 30 min for the mutants from the two osk5.2 lines (Fig. 5, A and B, bottom).
Figure 5.
Kinetics of the decrease in transpiration rate upon dark-induced stomatal closure in wild-type and osk5.2 mutant plants. A and B, Kinetics (top) and time constant (bottom) of the dark-induced decrease in transpiration rate in wild-type control (black circle, black square) and osk5.2 mutant (white circle, white square) plants issued from the initial ASJA08 (A) or ASHF06 (B) lines in standard Yoshida hydroponic solution. The light was switched off after 30 min of recording under light condition as indicated by the change from white to black in the bar below the graph. Transpiration rates were normalized by dividing absolute transpiration rates by the steady-state transpiration rate in light condition and are presented as percentages (%). Time constants were derived by fitting the experimental dark-induced transpiration rate decrease kinetics with an exponential function (least-squares fitting). Black and white bars correspond to wild-type control and osk5.2 mutant plants, respectively. Data are means ± se; n = 6 for wild-type control and 13 for osk5.2 mutant plants in A, and n = 7 for wild-type control and 9 for osk5.2 mutant plants in B. The asterisks indicate that the corresponding differences between the wild-type control and mutant plants were statistically significant (Student’s t test, P < 0.05).
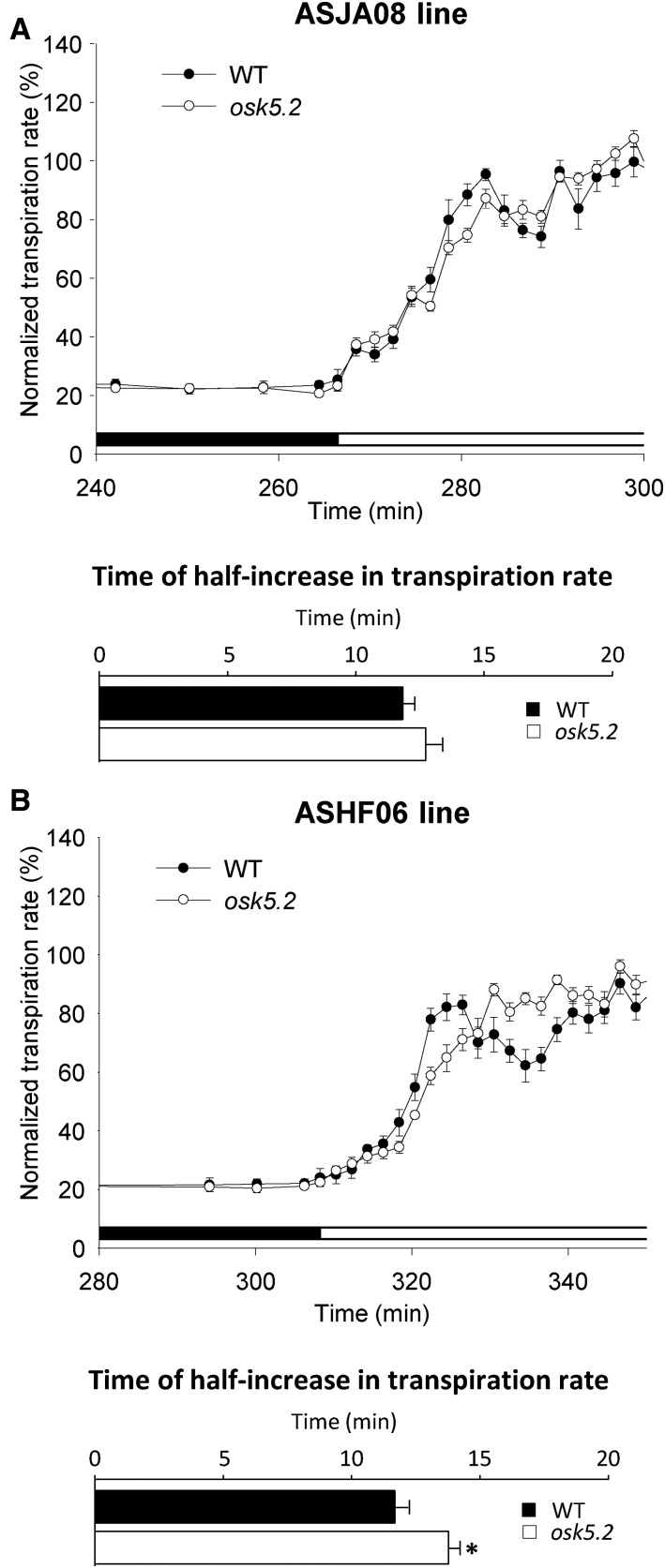
The kinetics of the light-induced increase in transpiration rate was also studied (Fig. 6). In wild-type plants, when light was switched on, the transpiration rate displayed a rapid increase, which was followed by a transient decrease. The osk5.2 mutant plants also displayed a rapid increase in transpiration, but the kinetics was slightly slower than in the wild-type plants (Fig. 6, A and B, bottom), and no following transient decrease in transpiration rate was observed (Fig. 6).
Figure 6.
Kinetics of the increase in transpiration rate upon light-induced stomatal opening after a long period of darkness in wild-type and osk5.2 mutant plants. A and B, Kinetics of light-induced increase in transpiration rate (top) and time of the corresponding half-increase in transpiration rate (bottom) in wild-type control (black circle, black square) and osk5.2 mutant (white circle, white square) plants from the initial ASJA08 (A) and ASHF06 (B) lines in standard Yoshida hydroponic solution. The change from black to white in the bar below the curves indicates the time at which light was switched on after the dark period. The normalized transpiration rate (%) was determined as described in Figure 5. Times of half-light–induced increase in transpiration rate were derived from the fit of the experimental data up to the end of the initial rapid phase of light-induced transpiration rate increase to a sigmoidal equation (least-squares fitting). Data are means ± se (n = 6 for wild-type control and 13 for osk5.2 mutant plants in A, and n = 7 for wild-type control and 9 for osk5.2 mutant plants in B). An asterisk indicates that the corresponding difference between the wild-type control and mutant plants in time of half-increase in transpiration rate was statistically significant (Student’s t test, P < 0.05).
Absence of Shaker-Type Outwardly Rectifying K+ Channel Activity in Guard Cells of osk5.2 Mutant Plants
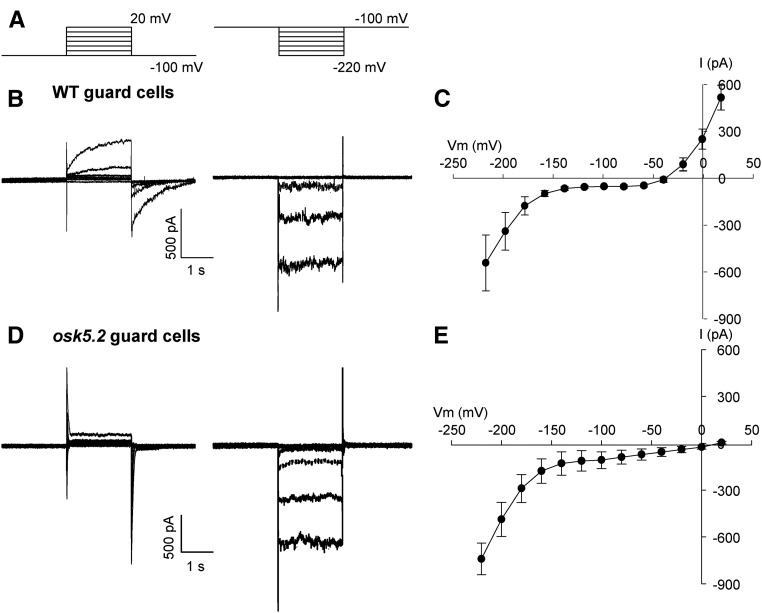
The consequences of OsK5.2 loss-of-function mutation on the K+ conductance of the guard cell membrane were analyzed in planta. Guard cells from 2-week-old plants were impaled with double-barreled electrodes (Blatt, 1990; Thiel et al., 1992; Roelfsema et al., 2001), and inward and outward currents through the membrane were elicited by voltage clamp pulses (Fig. 7A). In wild-type guard cells, depolarizing voltage pulses elicited slowly activating outward currents (Fig. 7B, left, and Fig. 7C), clearly reminiscent of those mediated by the outwardly rectifying K+ channels from the Shaker family (Gaymard et al., 1998; Hosy et al., 2003; Hedrich, 2012; Véry et al., 2014). Slowly activating outward currents were lacking in osk5.2 mutant plants (Fig. 7, D and E). In contrast, the inward conductance appeared to be very similar in osk5.2 mutant and wild-type guard cells (Fig. 7, B and D, right). This suggested that, although in planta voltage-clamp analyses can give rise to experimental drawbacks (Blatt, 1987b; Roelfsema et al., 2001), comparison between wild-type and mutant guard cell currents was valid in our conditions and thus that OsK5.2 encodes slowly activating, outwardly rectifying K+ channels in rice guard cells. It was also observed that the absence of OsK5.2 channel activity caused a positive shift of the free-running membrane potential (cross point with x axis in the current-voltage relationship), by about 50 mV (Fig. 7, C and E).
Figure 7.
Voltage-clamp experiments with guard cells in intact rice plants. A, Voltage-clamp protocols used to record either outward (left) or inward (right) currents in rice guard cells. Starting at a holding value of −100 mV, the voltage was shifted in 20-mV steps to either +20 mV, or −220 mV. B and D, Representative outward (left) and inward (right) current traces recorded in guard cells from ASJA08 line wild-type control (B) or osk5.2 mutant (D) plants. C and E, Average current-voltage relationships obtained in wild-type control (C) or mutant (E) guard cells. Data are means ± se (n = 6 in C, and n = 4 in E).
DISCUSSION
Comparison of the Expression Patterns of the Outward Shaker Genes in Rice and Arabidopsis
In rice, as in Arabidopsis, the outward Shaker channel group comprises two members. However, based on the phylogenetic tree displayed in Figure 1, OsK5.1 and OsK5.2 cannot be simply considered as the orthologs of the Arabidopsis outward Shaker channels SKOR and GORK. OsK5.2 and OsK5.1 belong to two separate subgroups within the group of outward Shaker channels from cereal plant species (Fig. 1).
In Arabidopsis, SKOR has been reported to be essentially expressed in the root vasculature (Gaymard et al., 1998), and GORK in root periphery cells (except in the root apex) and in stomata (Ache et al., 2000; Ivashikina et al., 2001). The expression pattern of OsK5.1 in rice is reminiscent of that of SKOR in Arabidopsis, with strong expression in root vasculature (Kim et al., 2015). In contrast, OsK5.2 displays high expression levels in both the root vasculature (Fig. 2, E–G) and the stomata (Fig. 2, B–D); thus, its expression pattern clearly encompasses the patterns reported for both SKOR and GORK in Arabidopsis (Gaymard et al., 1998; Ache et al., 2000; Ivashikina et al., 2001). It is not yet known whether the expression patterns of OsK5.1 and OsK5.2 are shared by genes belonging to the same outward Shaker subgroups from other monocots. The expression pattern of only one maize gene from the OsK5.2 subgroup has been partially characterized so far, by qRT-PCR analyses (Büchsenschütz et al., 2005). Like OsK5.2, this maize outward Shaker gene is expressed in both stomata and roots, and thus its expression pattern may be similar to that of OsK5.2.
Functional Properties of OsK5.2 Channels
Members of Shaker group 5 have been cloned in several plant species and successfully expressed in heterologous systems (Xenopus laevis oocyte essentially) for electrophysiological analysis of their functional properties (Véry et al., 2014). They all behave as slowly activating, outwardly rectifying, K+-selective channels. However, so far, all the channels for which such functional analyses have been reported belong to dicotyledonous species. We attempted to express OsK5.2 in X. laevis oocytes, but no exogenous current in the oocytes was detected using the classical two-electrode voltage-clamp technique (Véry et al., 1995; Tounsi et al., 2016). This might indicate that these outward K+ channels have to be coexpressed with regulatory protein partners to be active at the cell membrane, as reported for number of members of the inwardly rectifying Shaker channel group 1 from dicots (Xu et al., 2006; Honsbein et al., 2009; Cuéllar et al., 2010, 2013) and monocots (Boscari et al., 2009; Geiger et al., 2009).
Our voltage-clamp measurements with intact guard cells from wild-type plants and osk5.2 mutants (Fig. 7) indicate that OsK5.2 displays functional features reminiscent of group 5 Shaker channels as determined upon expression in heterologous systems (Ache et al., 2000; Langer et al., 2002; Johansson et al., 2006; Sano et al., 2007), especially a strong outward rectification, a slow activation upon membrane depolarization, and slow deactivation of the so-called tail currents when the membrane is repolarized. Finally, the fact that these Shaker-type outward currents were no longer observed in guard cells from the osk5.2 mutants indicates that OsK5.2 is the major contributor to the expression of such a channel activity at the guard cell membrane.
Comparison of the Roles of OsK5.2 and SKOR in K+ Translocation toward the Shoots
Both ASJA08 and ASHF06 osk5.2 loss-of-function mutations caused reduced leaf K+ contents, by 21% to 24% (Fig. 3). Taking into account that OsK5.2 displays expression in root vascular tissues, the simplest explanation is that OsK5.2-encoded channel activity contributes to K+ secretion into the xylem sap in roots, like SKOR in Arabidopsis, and thereby in K+ translocation toward the shoots. This hypothesis received support from the assay of K+ in xylem sap exudates from excised roots (Fig. 3), which revealed a marked concentration decrease (by >30%) in the osk5.2 loss-of-function mutants.
In Arabidopsis, the absence of SKOR channel activity due to a loss-of-function mutation has been shown to lead to a decrease in the concentration of K+ in the xylem sap by about 40%, and to a decrease in the accumulation of K+ in shoots by about 50% (Gaymard et al., 1998). Thus, the contribution of SKOR to the control of K+ translocation and accumulation in leaves seems a bit higher than that of OsK5.2 in rice. Since SKOR is the only outward Shaker channel gene expressed in the root stele, K+-permeable channels belonging to other families and/or other types of transport systems (e.g., from the KUP/HAK family; Han et al., 2016) have to significantly contribute to K+ secretion into the xylem sap in Arabidopsis. The situation may be different in rice, where both outward Shaker channel genes OsK5.1 and OsK5.2 are expressed in the plant vasculature (Fig. 2; Kim et al., 2015). In other words, besides OsK5.2, another outward Shaker channel, OsK5.1, could contribute to K+ translocation toward the shoots.
Comparison of the Roles of OsK5.2 and GORK in Control of Stomatal Aperture and Transpirational Water Loss
Altogether, these data indicate that OsK5.2 enhances the rate of stomatal closure by mediating K+ efflux through the guard cell membrane, just as its counterpart GORK in Arabidopsis (Hosy et al., 2003). The kinetics of dark-induced decrease in transpiration rate indicated that stomata can close in absence of this outwardly rectifying Shaker-type channel activity in rice, like in Arabidopsis, but the closure kinetics is slowed down. It is likely that poorly selective cation channels (Hedrich, 2012) substitute for Shaker channels as K+ efflux systems, but stomatal closure is then less efficient. The defect in stomatal closure in osk5.2 mutant plants is also likely to result from the large difference in free running membrane potential, by ∼50 mV, between osk5.2 and wild-type guard cells (Fig. 7). Indeed, in osk5.2 guard cells, the free-running voltage across the plasma membrane was shifted toward the reversal potential of anion channels, which was approximately +25 mV (as in Kollist et al., 2014). Thus, the driving force for anion efflux (difference between the free running membrane potential and reversal potential of anion channels) was reduced in osk5.2 guard cells. This is expected to cause a decrease in anion efflux, and thus in the rate of stomatal closure.
Besides its impact on the rate of dark-induced stomatal closure and steady-state stomatal aperture in darkness, the absence of Shaker-type outward K+ channel activity in rice is also likely to result in larger steady-state stomatal aperture in light, based on the observation that the steady-state transpiration rate was increased (Fig. 4). This would mean that OsK5.2 outwardly rectifying channel activity displays an inhibitory effect on steady-state stomatal aperture in light. The fact that the leaf K+ content was lower in the osk5.2 mutant plants (Fig. 3) is likely to lead to a decrease in turgor of the leaf cells. A decrease in the turgor of epidermal and subsidiary cells more important than that of guard cells could result in reduced back-pressure on stomatal guard cells and, thus, in increased steady-state stomatal aperture in the light. The impact of OsK5.2 activity on steady-state transpiration rate in light could be understood within the framework of this latter hypothesis.
CONCLUSION
By controlling the transpirational water loss at leaf surface, stomata also regulate the flux of water from roots to shoots within the xylem vasculature, and thereby contribute to the regulation of nutrient ion translocation toward the shoots. In Arabidopsis, various ion transport systems play a role in both ion translocation into the xylem sap toward the shoots and ion transport across the guard cell membrane and control of stomatal aperture. This is the case, for example, of the anion channel SLAH3 (Geiger et al., 2011; Cubero-Font et al., 2016), the nitrate transporter NRT1.1 (Guo et al., 2003; Wang et al., 2012), and the phosphate transporter PHO1 (Hamburger et al., 2002; Zimmerli et al., 2012). In contrast, regarding K+, some kind of specialization has occurred within the Shaker group 5 in Arabidopsis since K+ release from guard cells for stomatal closure has been strictly assigned to GORK, and K+ secretion into the xylem sap to SKOR. In rice, OsK5.2 contributes to both K+ release by guard cells for stomatal closure and K+ translocation toward the shoots. The physiological significance of such evolutionary differences would be interesting to clarify. The organization and the roles of the Shaker family are strongly conserved in plants, allowing one to assume functional equivalence between orthologs, but these data clearly indicate that the biological variability deserves to be investigated, since differences between plant species in the use of this gene family are likely to play a role in major physiological functions and in adaptation to environmental constraints.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rice Nipponbare cultivar (Oryza sativa var Nipponbare) was used in all the experiments. For transpiration analysis, seeds were germinated in petri dishes containing deionized water. One-week-old plants were then transferred onto Yoshida hydroponic solution in 10-liter containers [0.7 mm KNO3, 0.8 mm KH2PO4, 1.2 mm Ca(NO3)2, 0.5 mm (NH4)2SO4, 1.6 mm MgSO4, 60 µm Na2FeEDTA, 45 µm H3BO3, 20 µm MnSO4, 1.6 µm CuSO4, 1.4 µm ZnSO4, and 0.3 µm (NH4)6Mo7O24, pH adjusted to 5.5]. Plants were grown in growth chambers (day/night 12-h/12-h photoperiod, 28°C/25°C, photon flux density 500 µmol m−2 s−1, 80% relative humidity).
Construction of Reporter Gene Constructs and Transgenic Plants
OsK5.2 promoter region (2,339 bp) was amplified by PCR from rice Nipponbare genomic DNA using the primers listed in Supplemental Table S1. The PCR product was then cloned into pGemTeasy vector for sequencing before subcloning into destination vector. The promoter was then transferred upstream the GUS reporter gene in pCAMBIA1301 vector, where it replaced CAMV35S promoter. The resulting plasmid was introduced into Agrobacterium tumefaciens EHA105 strain for rice transformation, as described by Sallaud et al. (2003). Briefly, dehulked rice seeds were rinsed in ethanol 70% for 90 s and treated with disinfection solution (50 g/L of sodium hypochlorite) for 30 min. After sterilization, the seeds were transferred to a callus induction medium, leading to callus development from the scutellum region. Rice grains with developing calluses were incubated with bacteria suspension for 90 to 180 s and cocultured during 3 d. After the coculture period, developing calluses were washed with carbenicillin (500 mg/L), separated, divided into small pieces, and transferred to shoot induction medium containing hygromycin for transgenic tissue selection. Shoots regenerated from calluses were separated and transferred to rooting medium. Four-week-old plantlets were transferred to soil and grown in greenhouse for multiplication and selection of homozygous plants in the next generation using antibiotics leaf painting method (Cotsaftis et al., 2002).
Histochemical Analysis of GUS Activity
Leaf samples of 4-week-old plants and flowers were collected from homozygous plants grown in a greenhouse. Root samples were collected from 1-week-old plants grown in standard Yoshida hydroponic medium. All samples were submerged in GUS solution (50 mm phosphate buffer, pH 7, 2.5 mm ferricyanide, 2.5 mm ferrocyanide, 0.05% [v/v] Triton X-100, and 1 mm X-Gluc) contained in a 24-well plate, the leaves having been previously quickly divided into 1 × 1-cm pieces. The plate was placed under vacuum for 30 min to facilitate the penetration of GUS solution into the tissues, and then the samples were incubated overnight at 37°C. After the incubation step, green samples were washed with 70% ethanol solution to remove chlorophyll. Leaf samples were furthermore treated overnight with chloral hydrate (2.5 mg/L in 30% glycerol) before being observed under microscope (BH2; Olympus) for GUS activity detection.
Thin (8 µm thick) sections were prepared, using a RM 2165 microtome (Leica), from GUS-stained fixed samples (prefixation in 50 mm phosphate buffer, pH 7, 1.5% formaldehyde, and 0.05% Triton X-100; fixation in 75 mm phosphate buffer, 2% paraformaldehyde, and 0.5% glutaraldehyde) embedded in Technovit 7100 resin (Kulzer).
Selection of KO Mutant and Wild-Type Control Plants
Tos17 insertion mutant lines (cv Nipponbare background) displaying insertion in OsK5.2 gene were ordered from OTL collection (CIRAD-Genoscope, oryzatagline.cirad.fr/; Piffanelli et al., 2007). Homozygous mutant plants as well as “wild-type” plants for OsK5.2 gene were selected by PCR on genomic DNA extracted from plantlets issued from received seeds, using Phire Plant Direct PCR Kit (Thermo Fischer Scientific) and specific primers hybridizing on the target gene and on the Tos17. Total RNA of 2 plants selected in the progeny of a homozygous mutant, or of a plant displaying a wild-type genotype for OsK5.2 (hereafter generically named wild-type control plants), were isolated using RNeasy kit (Qiagen) and used as templates to synthesize total cDNA. PCR experiments, using primers hybridizing downstream from the Tos17 insertion sites (listed in Supplemental Table S1), allowed us to check the presence (in the wild-type control plants) or absence (in the osk5.2 mutants) of OsK5.2 transcripts.
Voltage-Clamp Recordings on Intact Plant Guard Cells
Two-electrode voltage-clamp experiments were performed on intact guard cells (Roelfsema et al., 2001; Mumm et al., 2011) of intact plants grown on standard Yoshida hydroponic solution for 2 weeks. A plant (wild-type control or homozygous osk5.2 mutant) was transferred into a 15-mL tube (Falcon type) containing about 5 mL of standard Yoshida solution, the tube being inclined so that the root system was fully immerged in the solution. Outside of the tube, the youngest fully developed leaf was attached to a Plexiglas block with double-sided adhesive tape, the plant remaining intact. The whole plant, attached with its leaf to the Plexiglass, was carefully mounted onto the table of an upright microscope (Axoiskop 2FS; Zeiss). About 0.5 to 1 mL of bath solution (5 mm potassium citrate, 5 mm KCl, and 0.1 mm CaCl2, pH 5.0) was placed between the leaf and the water immersion objective (Achroplan 40/0.80W; Zeiss), used to visualize the stomata. Stomatal opening was induced by light provided by the microscope lamp. Guard cells were impaled using double-barreled electrodes prepared from 1-mm-diameter glass capillaries (1403547; Hilgenberg) filled with 300 mm KCl. A glass capillary filled with 300 mm KCl and plugged with 300 mm KCl in 2% agarose was placed in the drop of solution between the leaf and the objective and served as a reference electrode. The series resistance present between leaf apoplast and reference electrode was estimated by measuring the resistance between two drops of bath solution placed on the leaf surface, at a distance of ∼1 cm. This approach revealed a series resistance (which was not different between wild type and osk5.2 mutant plants) of 1.3 ± 0.1 (n = 5) MΩ at the abaxial leaf face. With guard cell currents <1 nA (Fig. 7), such a series resistance leads to an overestimation of the applied voltage of <2 mV. The potential difference between the bath solution and guard cell apoplast (“surface” potential) was −10 ± 2 mV (n = 6). Because the series resistance and potential difference between the external solution and the apoplast appeared as low, no attempt was made to correct their impact on current-voltage plots. The Win WCP V5.1.6 software (J. Dempster, Strathclyde University, Glasgow, UK) was used to set up the protocol and record applied voltage and current traces, using an ITC-16 interface (Instrutech) and a GeneClamp 500 (Axon Instruments) amplifier.
Analysis of Stomatal Movements Induced by Light or Darkness
Wild-type control and homozygous osk5.2 mutant plants were grown in parallel in standard Yoshida hydroponic medium for 5 weeks. One day before the experiment, plants were transferred into a multipotometer device (Supplemental Fig. S2), the root system bathed in standard Yoshida hydroponic solution. On the day of the experiment, transpiration rates started to be recorded after 2 h under light condition. A dark treatment was imposed after 30 min of recording and was maintained for 4 h before switching back to light condition.
Analysis of K+ Content in Tissues and Xylem Sap
Root and shoot K+ contents were assayed in plants grown as described above for the analysis of stomatal movements, that is, hydroponic conditions for 5 weeks on standard Yoshida solution. After excision, roots and shoots were weighed, dried (60°C during 2 d), and weighed again. Ions were extracted from the tissues with 0.1 n HCl during 2 d. K+ content in the extracts was determined by flame spectrophotometry (SpectrAA 220FS; Varian).
K+ concentration in xylem sap was analyzed in 5-week-old plants grown in standard Yoshida hydroponic solution. Excision at 1 cm above the crown allowed collection of the xylem sap. Exudation duration before collection (identical in control and osk5.2 mutant plants) lasted for 1 to 2 h. Collected samples were diluted in 0.1 n HCl and the concentration of K+ was determined like that of tissue extracts.
Accession Numbers
Sequence data for OsK5.2 Shaker gene can be found in the GenBank library under accession number Os06g0250600.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Selection of knockout mutant plants for OsK5.2 gene.
Supplemental Figure S2. The multipotometer system used for plant transpiration rate monitoring.
Supplemental Table S1. Primer sequences used for cloning, genotyping, and verification of KO osk5.2 mutant plants.
Supplemental Table S2. GenBank accession numbers of Shaker polypeptides present in phylogenetic trees
Acknowledgments
We thank Nathalie Leonhardt, Thierry Simonneau, and Alain Vavasseur for fruitful discussions, Sonia Mohamed and Manuel Nieves-Cordones for help with genotyping, and Thanh Danh Nguyen, Thi Xuan Vo, and Binh Anh Thu Nguyen for help with analysis of raw transpiration rate data.
References
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MRG, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett 486: 93–98 [DOI] [PubMed] [Google Scholar]
- Boscari A, Clément M, Volkov V, Golldack D, Hybiak J, Miller AJ, Amtmann A, Fricke W (2009) Potassium channels in barley: cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ 32: 1761–1777 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (1987a) Electrical characteristics of stomatal guard cells: The contribution of ATP-dependent “electrogenic” transport revealed by current-voltage and difference-current-voltage analysis. J Membr Biol 98: 257–274 [Google Scholar]
- Blatt MR. (1987b) Electrical characteristics of stomatal guard cells: the ionic basis of the membrane potential and the consequence of potassium chlorides leakage from microelectrodes. Planta 170: 272–287 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (1990) Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta 180: 445–455 [DOI] [PubMed] [Google Scholar]
- Büchsenschütz K, Marten I, Becker D, Philippar K, Ache P, Hedrich R (2005) Differential expression of K+ channels between guard cells and subsidiary cells within the maize stomatal complex. Planta 222: 968–976 [DOI] [PubMed] [Google Scholar]
- Chen Z-H, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31: 239–298 [Google Scholar]
- Cotsaftis O, Sallaud C, Breitler JC, Meynard D, Greco R, Pereira A, Guiderdoni E (2002) Transposon-mediated generation of T-DNA-and marker-free rice plants expressing a Bt endotoxin gene. Mol Breed 10: 165–180 [Google Scholar]
- Cubero-Font P, Maierhofer T, Jaslan J, Rosales MA, Espartero J, Díaz-Rueda P, Müller HM, Hürter A-L, Al-Rasheid KA, Marten I, et al. (2016) Silent S-Type anion channel subunit SLAH1 gates SLAH3 open for chloride root-to-shoot translocation. Curr Biol 26: 2213–2220 [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Azeem F, Andrianteranagna M, Pascaud F, Verdeil JL, Sentenac H, Zimmermann S, Gaillard I (2013) Potassium transport in developing fleshy fruits: the grapevine inward K(+) channel VvK1.2 is activated by CIPK-CBL complexes and induced in ripening berry flesh cells. Plant J 73: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I (2010) A grapevine Shaker inward K(+) channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 61: 58–69 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud J-B, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, Anschuetz U, Dreyer I, Kudla J, Hedrich R (2009) Heteromeric AtKC1{middle dot}AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem 284: 21288–21295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. [DOI] [PubMed] [Google Scholar]
- Guo F-Q, Young J, Crawford NM (2003) The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 15: 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Wu W, Wu W-H, Wang Y (2016) Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol Plant 9: 437–446 [DOI] [PubMed] [Google Scholar]
- Hedrich R. (2012) Ion channels in plants. Physiol Rev 92: 1777–1811 [DOI] [PubMed] [Google Scholar]
- Hills A, Chen Z-H, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsbein A, Sokolovski S, Grefen C, Campanoni P, Pratelli R, Paneque M, Chen Z, Johansson I, Blatt MR (2009) A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21: 2859–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, Boucherez J, Lebaudy A, Bouchez D, Véry A-A, et al. (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Rice plant development: from zygote to spikelet. Plant Cell Physiol 46: 23–47 [DOI] [PubMed] [Google Scholar]
- Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R (2001) K(+) channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett 508: 463–469 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Wulfetange K, Porée F, Michard E, Gajdanowicz P, Lacombe B, Sentenac H, Thibaud JB, Mueller-Roeber B, Blatt MR, et al. (2006) External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J 46: 269–281 [DOI] [PubMed] [Google Scholar]
- Kim HY, Choi E-H, Min MK, Hwang H, Moon S-J, Yoon I, Byun M-O, Kim B-G (2015) Differential gene expression of two outward-rectifying shaker-like potassium channels OsSKOR and OsGORK in rice. J Plant Biol 58: 230–235 [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C, Regan S, Fromm J, Hedrich R (2002) Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant J 32: 997–1009 [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud J-B, Véry A-A, Simonneau T, Sentenac H (2008) Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc Natl Acad Sci USA 105: 5271–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R, Wyn Jones R (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97: 1–13 [Google Scholar]
- Marschner H, Kirkby EA, Cakmak I (1996) Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J Exp Bot 47: 1255–1263 [DOI] [PubMed] [Google Scholar]
- Mumm P, Wolf T, Fromm J, Roelfsema MRG, Marten I (2011) Cell type-specific regulation of ion channels within the maize stomatal complex. Plant Cell Physiol 52: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Piffanelli P, Droc G, Mieulet D, Lanau N, Bes M, Bourgeois E, Rouvière C, Gavory F, Cruaud C, Ghesquière A, et al. (2007) Large-scale characterization of Tos17 insertion sites in a rice T-DNA mutant library. Plant Mol Biol 65: 587-601. [DOI] [PubMed] [Google Scholar]
- Pallas JE Jr. (1965) Transpiration and stomatal opening with changes in carbon dioxide content of the air. Science 147: 171–173 [DOI] [PubMed] [Google Scholar]
- Pilot G, Lacombe B, Gaymard F, Cherel I, Boucherez J, Thibaud JB, Sentenac H (2001) Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J Biol Chem 276: 3215–3221 [DOI] [PubMed] [Google Scholar]
- Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H (2003) Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol 56: 418–434 [DOI] [PubMed] [Google Scholar]
- Raschke K, Schnabl H (1978) Availability of chloride affects the balance between potassium chloride and potassium malate in guard cells of Vicia faba L. Plant Physiol 62: 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MR, Prins HB (1997) Ion channels in guard cells of Arabidopsis thaliana (L.) Heynh. Planta 202: 18–27 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R(2009) Stomata. In Encyclopedia of Life Sciences (eLS). John Wiley & Sons, Chichester, UK, doi/10.1002/9780470015902.a0002075.pub2
- Roelfsema MRG, Steinmeyer R, Staal M, Hedrich R (2001) Single guard cell recordings in intact plants: light-induced hyperpolarization of the plasma membrane. Plant J 26: 1–13 [DOI] [PubMed] [Google Scholar]
- Sallaud C, Meynard D, van Boxtel J, Gay C, Bès M, Brizard J-P, Larmande P, Ortega D, Raynal M, Portefaix M, et al. (2003) Highly efficient production and characterization of T-DNA plants for rice ( Oryza sativa L.) functional genomics. Theor Appl Genet 106: 1396–1408 [DOI] [PubMed] [Google Scholar]
- Sano T, Becker D, Ivashikina N, Wegner LH, Zimmermann U, Roelfsema MRG, Nagata T, Hedrich R (2007) Plant cells must pass a K+ threshold to re-enter the cell cycle. Plant J 50: 401–413 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyroki A, Ivashikina N, Dietrich P, Roelfsema MRG, Ache P, Reintanz B, Deeken R, Godde M, Felle H, Steinmeyer R, et al. (2001) KAT1 is not essential for stomatal opening. Proc Natl Acad Sci USA 98: 2917–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott L, Zeiger E (1998) The role of sucrose in guard cell osmoregulation. J Exp Bot 49: 329–337 [Google Scholar]
- Thiel G, MacRobbie EA, Blatt MR (1992) Membrane transport in stomatal guard cells: the importance of voltage control. J Membr Biol 126: 1–18 [DOI] [PubMed] [Google Scholar]
- Tounsi S, Ben Amar S, Masmoudi K, Sentenac H, Brini F, Véry A-A (2016) Characterization of two HKT1;4 transporters from Triticum monococcum to elucidate the determinants of the wheat salt tolerance Nax1 QTL. Plant Cell Physiol 57: 2047–2057 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 171: 748–769 [DOI] [PubMed] [Google Scholar]
- Véry AA, Gaymard F, Bosseux C, Sentenac H, Thibaud JB (1995) Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J 7: 321–332 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Wang Y-Y, Hsu P-K, Tsay Y-F (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17: 458–467 [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker M (1996) Stomata. Topics in Plant Functional Biology, Vol 2. Chapman and Hall Publishers, London [Google Scholar]
- Xu J, Li H-D, Chen L-Q, Wang Y, Liu L-L, He L, Wu W-H (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Zimmerli C, Ribot C, Vavasseur A, Bauer H, Hedrich R, Poirier Y (2012) PHO1 expression in guard cells mediates the stomatal response to abscisic acid in Arabidopsis. Plant J 72: 199–211 [DOI] [PubMed] [Google Scholar]