Abstract
Herein, we report an unprecedented transition metal-free, coupling of indoles with aryl halides. The reaction is promoted by KOtBu and is regioselective for C3 over nitrogen. The use of degassed solvents devoid of oxygen is necessary for the success of the transformation. Preliminary studies implicate a hybrid mechanism that involves both aryne intermediates and non-propagative radical processes. Electron transfer is also a distinct possibility. These conclusions were substantiated by EPR data, isotopic labeling studies, and the use of radical scavengers and electron transfer inhibitors.
Keywords: indole, transition metal-free, cross-coupling, radical, aryne
Graphical Abstract
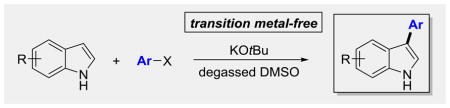
Metals? What metals? Regioselective transition metal-free cross-coupling of aryl halides with indoles at C3 has been reported. The reaction proceeds through an unusual hybrid radical/aryne mechanism.
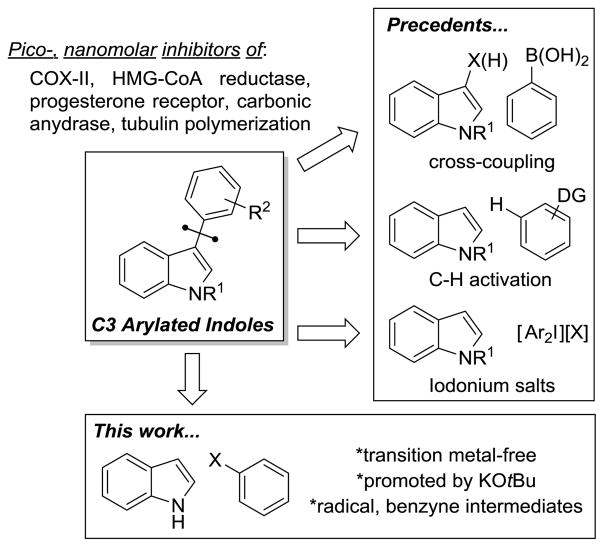
Indoles with aromatic substituents at C3 are an important class of compounds, many of which possess potent biological activity. For instance, fluvastatin is an FDA-approved HMG-CoA reductase inhibitor that is used in the clinic for lowering LDL cholesterol.[1] Other C3 arylated indoles exhibit pico- to nanomolar inhibitory actions against a wide range of biological targets and processes such as the progesterone receptor,[2] COX-II enzyme,[3] carbonic anhydrase I and II,[4] and tubulin polymerization,[5] etc.
The direct introduction of aromatic groups onto an extant indole nucleus at C3 is typically carried out by transition metal-catalyzed cross-coupling reactions (Figure 1).[2,6–14] Methods utilizing C–H activation have also been reported.[15–18] While transition metal-free processes are rare, they have been accomplished through the use of hypervalent iodine reagents,[19–21] diazonium salts,[22] or by means of electrochemistry.[23–25]
Figure 1.
Our method, and literature precedents for preparing C3 arylated indoles, highly potent biologically active compounds.
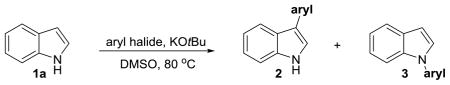
Our group has a longstanding interest in the reaction of indoles and related heterocycles.[26–29] Herein, we disclose a new approach for the intermolecular C3 arylation of unprotected indoles with simple aryl halides. The reaction is promoted by KOtBu and does not require the use of transition metal catalysts. Preliminary mechanistic investigations point towards an unusual hybrid reaction pathway that exhibits features of both radical and aryne intermediates. Although Daugulis and Tu have reported a limited number of base-promoted C2- and N-selective arylation reactions of indoles,[30–32] there appear to be very few precedents for C3-selective variants[32,33] and none that proceed by the proposed hybrid radical/aryne mechanism.
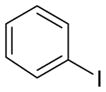
Optimization studies were carried out on the reaction between indole (1a) and iodobenzene (Table 1). A brief solvent survey identified DMF and DMSO as the only effective solvents (entries 1–5). A reaction performed in the dark resulted in similar yield and selectivity (entry 7 vs 5). Importantly, we observed that the use of degassed solvents is critical to the success of the reaction. Failure to degas the solvent, or the use of DSMO that was saturated with O2, resulted in the formation of little to no 2a (entry 8, 9 vs 5). Interestingly, for both entries 8 and 9 the N-arylated product 3a was still formed. Moreover, KOtBu is the only base that was effective (entry 10–12 vs 5). Bromobenezene and chlorobenzene were less efficient while the use of fluorobenzene and phenyltriflate gave only small amounts of 3a (entries 13–16 vs 5). In each case, the desired product 2a was isolated along with varying amounts of 3a.
Table 1.
Optimization Studies
| Entry[a] | Ar–X | Base | Solvent | Temp (°C) | Yield (%) | |
|---|---|---|---|---|---|---|
| 2a | 3a | |||||
| 1 | PhI | KOtBu | PhMe | 80 | 0 | 0 |
| 2 | PhI | KOtBu | THF | 60 | 0 | 0 |
| 3 | PhI | KOtBu | MeCN | 80 | 0 | 0 |
| 4[b] | PhI | KOtBu | DMF | 80 | 58 | 17 |
| 5 | PhI | KOtBu | DMSO | 80 | 70 | 11 |
| 6[c] | PhI | KOtBu | DMSO | 80 | 33 | 49 |
| 7[d] | PhI | KOtBu | DMSO | 80 | 63 | 18 |
| 8[e] | PhI | KOtBu | DMSO | 80 | 34 | 25 |
| 9[f] | PhI | KOtBu | DMSO | 80 | 0 | 39 |
| 10 | PhI | NaOtBu | DMSO | 80 | 0 | trace |
| 11 | PhI | KHMDS | DMSO | 80 | 0 | trace |
| 12 | PhI | LDA | DMSO | 80 | 0 | 12 |
| 13 | PhBr | KOtBu | DMSO | 80 | 64 | 15 |
| 14 | PhCl | KOtBu | DMSO | 80 | 52 | 11 |
| 15 | PhF | KOtBu | DMSO | 80 | 0 | 10 |
| 16 | PhOTf | KOtBu | DMSO | 80 | 0 | 21 |
Conditions: 2 equiv indole, 1 equiv aryl halide, 4 equiv KOtBu [0.3] M, all solvents were rigorously degassed by freeze/pump/thaw.
time = 24 h,
Conditions: 1 equiv indole, 3 equiv aryl halide, 4 equiv KOtBu, [0.3] M.
Reaction in the absence of light.
solvent not degassed, N2 atmosphere,
solvent saturated with O2, also O2 atmosphere.
Table 2 describes the scope of the arylation reaction with respect to the aryl halide. This method is compatible with alkoxy, nitro, and amine functional groups (entries 2–3, 7–9) as well as heterocyclic aryl halides (entries 4, 6). For all entries in which meta-substituted aryl halides were used (entries 2–5, 8, 11), we observed only ipso substituted products (>95:5 meta vs all others). In contrast, the use of ortho-iodoanisole and ortho-iodotoluene resulted in cine substitution for both substrates, giving once again the meta isomer as the major product (>95:5 meta vs all others; entries 7, 10). The use of para-substituted and benzofuranyl-based compounds furnished mixtures of regioisomers with respect to the aryl halide (entries 6, 9, 12). These regioselectivities are consistent with the aryne distortion model espoused by Garg and Houk.[34,35] With the exception of nitro, naphthyl, and benzofuran-derived aryl halides, the reactions were accompanied by various amounts of N-arylated products that were readily removed by standard silica flash chromatograph. The reaction carried out on a 1.2 g scale also proceeded as expected (entry 1b). We cannot rule out the possibility that some lower yielding entries are due, in part, to the reaction between DMSO and arynes.[36,37]
Table 2.
Scope of Aryl Halide
| ||||
|---|---|---|---|---|
| entry | Ar–X | product | % yield 2 | % yield 3 |
| 1a |
 4 |
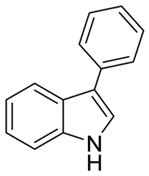 2a |
70% | 11% |
| 1b | 61% (1.2 gram scale) | 14% | ||
| 2 |
 5 |
 2b |
47% (>95:5 meta/para) | not observed |
| 3 |
 6 |
 2c |
26% (>95:5 meta/para) | 26% |
| 4 |
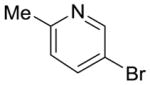 7 |
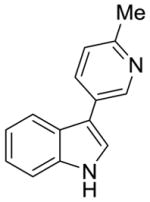 2d |
42% (>95:5 meta/para) | 21% |
| 5 |
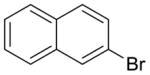 8 |
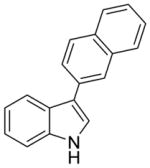 2e |
44% (>95:5 meta/para) | not observed |
| 6 |
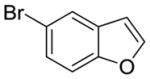 9 |
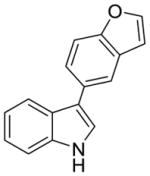 2f |
44% (3.5:1 C5/C4 on benzofuran) | not observed |
| 7 |
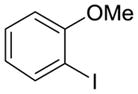 10 |
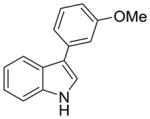 2g |
76% (>95:5 meta/para) | 13% |
| 8 |
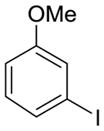 11 |
2g | 68% (>95:5 meta/para) | 14% |
| 9 |
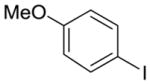 12 |
2g | 56% (1:1 meta/para) | 14% |
|
| ||||
| 10 |
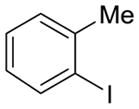 13 |
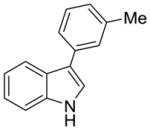 2h |
52% (>95:5 meta/para) | 13% |
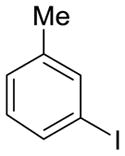
| 11 |
 14 |
2h | 59% (3.5:1 meta/para) | 15% |
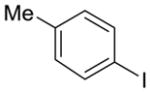
| 12 |
 15 |
2h | 40% (1:1 meta/para) | 13% |
Conditions: 2 equiv indole, 1 equiv aryl halide, 4 equiv KOtBu, in DMSO at 80 °C for 35 h. Percentages represent isolated yields.
The formation of cine substitution products (entries 7, 9–10, 12) is indicative of a pathway involving aryne intermediates. Notably, this data also precludes the possibility that the arylation is catalyzed by trace metal impurities since cross-coupling reactions promoted by transition metals would be expected to give exclusively ipso-substituted products. Further evidence of arynes was obtained when, under optimized conditions, we were able to trap the putative benzyne intermediate generated from PhI with either 3,4-dimethoxy phenol or morpholine to give diphenyl ether and N-phenylmorpholine, respectively. Finally, a control experiment utilizing 2-(TMS)phenyltriflate plus CsF to generate benzyne, in the presence of indole, KOtBu and degassed DMSO, resulted in none of the desired C3 or N-arylated products.
We then explored the scope of the reaction with respect to the indole coupling partner. Table 3 demonstrates that the method is tolerant to alkyl, alkoxy, and boron substituents on the 2, 5, and 7-positions of indole. The reaction of 1g furnished pinacol boronate ester 2n (entry 6), which we anticipate can be used as a handle for further functionalization. Attempts with 7-azaindole afforded only the N-arylation product. Other groups like –CN, –OH, –NH2 on indole or Ar–X were not compatible.
Table 3.
Scope of Indole
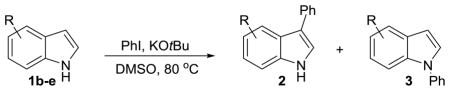
| ||||
|---|---|---|---|---|
| entry | indole | product | % yield 2 | % yield 3 |
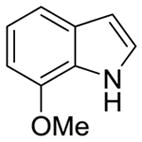
| 1 |
 1b |
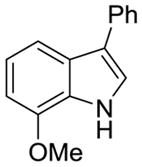 2i |
66% | 5% |
| 2 |
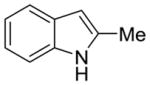 1c |
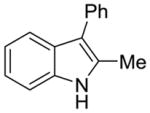 2j |
47% | 9% |
| 3 |
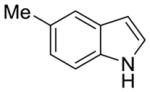 1d |
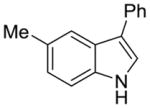 2k |
51% | 10% |
| 4 |
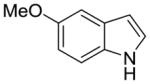 1e |
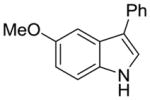 2l |
70% | 10% |
| 5 |
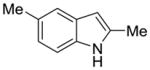 1f |
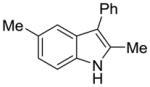 2m |
64% | 14% |
| 6 |
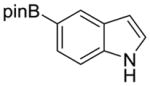 1g |
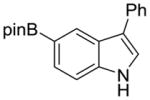 2n |
61% | 17% |
Conditions: 2 equiv indole, 1 equiv PhI, 4 equiv KOtBu
As illustrated in Eq 1 and 2, the reaction is also amenable to the use of pyrroles. As before, the use of oxygen-free DMSO is critical since failure to degas the solvent lead to recovery of pyrrole but complete consumption of PhI. These results represent a significant departure from the literature in which both Wittig and Larock report that pyrroles react with arynes by (4+2) cycloaddition.[38–40]
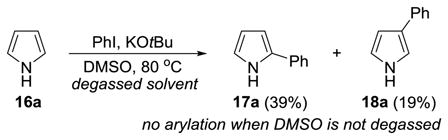 |
(Eq 1) |
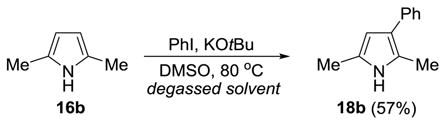 |
(Eq 2) |
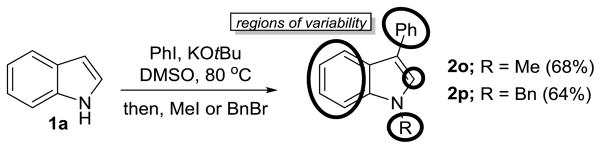
We then demonstrated the synthesis of C3-arylated–N-alkylated products 2o–p in a single pot by means of sequential arylation followed by quenching with either MeI or BnBr, respectively (Scheme 1). This transformation constitutes a three-component coupling reaction in which each starting material can be independently varied. We expect that this method can be used to rapidly generate diverse libraries of C3-arylated, N-alkylated indolyl compounds for use in fragment-based drug discovery programs.[41]
Scheme 1.
Three-Component Coupling for Library Synthesis in Fragment-Based Drug Discovery
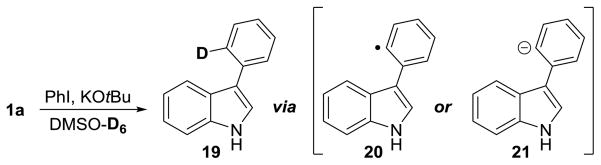
Performing the reaction in deuterated DMSO provided some insight into the mechanism. As shown in Scheme 2, the use of degassed DMSO-D6 as solvent resulted in approximately 76% deuterium incorporation at the position indicated in 19. This may arise as a result of deuterium abstraction from the solvent by either radical 20 or carbanion 21.
Scheme 2.
Isotopic-Labelling Studies
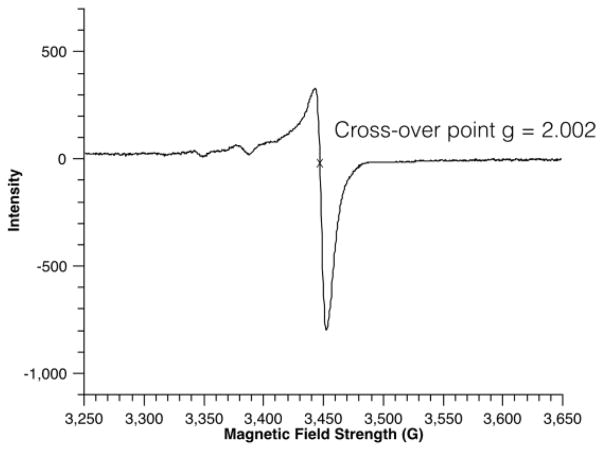
Moreover, the necessity of using oxygen-free solvents led us to suspect the involvement of free radicals. An EPR experiment carried out on a frozen aliquot of an incomplete reaction between 1a and PhI confirmed the presence of free radicals.[42] From our calculations, the g factor of the cross-over point of the peak was found to be 2.002, which is diagnostic of the free radical nature of an unpaired electron (Figure 2). EPR experiments on the following control reactions: 1) indole + KOtBu in DMSO, and 2) PhI + KOtBu in DMSO did not lead to any detectable signals, indicating the intermediacy of radical species only in the presence of all three reaction components.
Figure 2.
EPR spectrum of arylation reaction between 1a and PhI at 77 K.
The addition of radical scavengers such as galvinoxyl and TEMPO (Table 4, entry 1 and 2) resulted in moderate but noticeably lower yields of 2a. The partial inhibitory effect of radical scavengers suggests that while radical intermediates are involved, the overall reaction may proceed by a non-chain radical mechanism as described by Zhang and Liu et al.[43] As mentioned above, oxygen also appears to have a deleterious and concentration-dependent effect on the efficiency of C3 arylation (Table 1, entries 8–9, Eq 1 and 2). The fact that O2 leads to greater amounts of N-arylated products is consistent with literature precedent.[30] In contrast, the electron transfer inhibitor p-dinitrobenzene, completely suppresses C3 arylation (Table 4, entry 4), with only minimal amounts of N-arylated product formed. This result strongly implicates the role of electron transfer processes in the reaction mechanism.
Table 4.
Effect of Radical Scavenger and Electron Transfer Inhibitors as Additives.
| Entry | Additive | % yield 2a | % yield 3a |
|---|---|---|---|
| 1 | None | 70 | 11 |
| 2 | TEMPO | 52 | 23 |
| 3 | galvinoxyl | 45 | 18 |
| 4 | 1,4-dinitrobenzene | 9 | 0 |
Reaction between 1a and PhI under standard conditions. Isolated yields.
Because arynes are not typically sensitive to oxygen, our data is suggestive of a mechanism that is more complex than the polar reaction between indoles and arynes.[44] There is a growing body of literature in which KOtBu plus associated ligands are capable of promoting transition metal-free cross-coupling reactions between arenes and aryl halides.[45–57] Computational[58,59] and empirical studies implicate radical intermediates and electron transfer processes. While the findings from these reports may, in part, be applicable to our work, the formation of cine substitution products in the title reaction necessitates that aryne intermediates be considered alongside. Although we do not know the precise mechanism by which this reported arylation proceeds, nor can we exclude the possibility of competing pathways, the available data points towards the involvement of both aryne and either radical species and/or electron transfer processes.
In conclusion, we have described the transition metal-free, regioselective arylation of indoles at C3 with aryl halides. The reaction is promoted by KOtBu and the use of degassed solvent is critical. The yields and scope with respect to both the indole and aryl halide components are good. We also demonstrated that C-arylated, N-alkylated products can be produced in a one-pot transformation. This reaction is unusual in that mechanistic studies indicate the involvement of both aryne and radical intermediates.
Acknowledgments
J.W. acknowledges the generous support of the NIH NIGMS (1R01GM111638). We thank Prof. Neil Garg (UCLA), Prof. Dean E. Wilcox (Dartmouth College) for helpful discussions, and the Glueck group (Dartmouth College) for the use of their glove box.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Langtry HD, Markham A. Drugs. 1999;57:583–606. doi: 10.2165/00003495-199957040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Richardson TI, Clarke CA, Yu KL, Yee YK, Bleisch TJ, Lopez JE, Jones SA, Hughes NE, Muehl BS, Lugar CW, et al. ACS Med Chem Lett. 2011;2:148–153. doi: 10.1021/ml100220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu W, Guo Z, Chu F, Bai A, Yi X, Cheng G, Li J. Bioorg Med Chem. 2003;11:1153–1160. doi: 10.1016/s0968-0896(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 4.Güzel Ö, Maresca A, Hall RA, Scozzafava A, Mastrolorenzo A, Mühlschlegel FA, Supuran CT. Bioorg Med Chem Lett. 2010;20:2508–2511. doi: 10.1016/j.bmcl.2010.02.103. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HZ, Drewe J, Tseng B, Kasibhatla S, Cai SX. Bioorg Med Chem. 2004;12:3649–3655. doi: 10.1016/j.bmc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Amat M, Hadida S, Pshenichnyi G, Bosch J. J Org Chem. 1997;62:3158–3175. doi: 10.1021/jo962169u. [DOI] [PubMed] [Google Scholar]
- 7.Malapel-Andrieu B, Mérour JY. Tetrahedron. 1998;54:11079–11094. [Google Scholar]
- 8.Lane BS, Brown MA, Sames D. J Am Chem Soc. 2005;127:8050–8057. doi: 10.1021/ja043273t. [DOI] [PubMed] [Google Scholar]
- 9.Phipps RJ, Grimster NP, Gaunt MJ. J Am Chem Soc. 2008;130:8172–8174. doi: 10.1021/ja801767s. [DOI] [PubMed] [Google Scholar]
- 10.Cornella J, Lu P, Larrosa I. Org Lett. 2009;11:5506–5509. doi: 10.1021/ol902304n. [DOI] [PubMed] [Google Scholar]
- 11.Join B, Yamamoto T, Itami K. Angew Chem Int Ed. 2009;48:3644–3647. doi: 10.1002/anie.200806358. [DOI] [PubMed] [Google Scholar]
- 12.Mésangeau C, Amata E, Alsharif W, Seminerio MJ, Robson MJ, Matsumoto RR, Poupaert JH, McCurdy CR. Eur J Med Chem. 2011;46:5154–5161. doi: 10.1016/j.ejmech.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Guo S, Li K, Qu J, Yuan H, Hua Q, Chen B. Adv Synth Catal. 2013;355:711–715. [Google Scholar]
- 14.Modha SG, Greaney MF. J Am Chem Soc. 2015;137:1416–1419. doi: 10.1021/ja5124754. [DOI] [PubMed] [Google Scholar]
- 15.Stuart DR, Fagnou K. Science. 2007;316:1172–1175. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]
- 16.Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org Lett. 2007;9:3137–3139. doi: 10.1021/ol071308z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart DR, Villemure E, Fagnou K. J Am Chem Soc. 2007;129:12072–12073. doi: 10.1021/ja0745862. [DOI] [PubMed] [Google Scholar]
- 18.He CY, Fan S, Zhang X. J Am Chem Soc. 2010;132:12850–12852. doi: 10.1021/ja106046p. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Wang D. Tetrahedron Lett. 2010;51:2004–2006. [Google Scholar]
- 20.Ackermann L, Dell’Acqua M, Fenner S, Vicente R, Sandmann R. Org Lett. 2011;13:2358–2360. doi: 10.1021/ol200601e. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto K, Sakamoto K, Ohnishi Y, Miyamoto T, Ito M, Dohi T, Kita Y. Chem - Eur J. 2013;19:8726–8731. doi: 10.1002/chem.201301028. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YP, Feng XL, Yang YS, Cao BX. Tetrahedron Lett. 2016;57:2298–2302. [Google Scholar]
- 23.Chahma M, Combellas C, Thiébault A. Synthesis. 1994;1994:366–368. [Google Scholar]
- 24.Chahma M, Combellas C, Thiébault A. J Org Chem. 1995;60:8015–8022. [Google Scholar]
- 25.Morofuji T, Shimizu A, Yoshida J. Angew Chem Int Ed. 2012;51:7259–7262. doi: 10.1002/anie.201202788. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Li H, Hughes RP, Wu J. Angew Chem Int Ed. 2012;51:10390–10393. doi: 10.1002/anie.201205238. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Wu J. Angew Chem Int Ed. 2013;52:4637–4640. doi: 10.1002/anie.201209810. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Hughes RP, Wu J. J Am Chem Soc. 2014;136:6288–6296. doi: 10.1021/ja412435b. [DOI] [PubMed] [Google Scholar]
- 29.DiPoto MC, Hughes RP, Wu J. J Am Chem Soc. 2015;137:14861–14864. doi: 10.1021/jacs.5b10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Wang M, Fan CA, Zhang FM, Tu YQ. Org Lett. 2003;5:3515–3517. doi: 10.1021/ol0353868. [DOI] [PubMed] [Google Scholar]
- 31.Truong T, Daugulis O. J Am Chem Soc. 2011;133:4243–4245. doi: 10.1021/ja200184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong T, Mesgar M, Le KKA, Daugulis O. J Am Chem Soc. 2014;136:8568–8576. doi: 10.1021/ja504886x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehne ME, Kitagawa T. J Org Chem. 1964;29:1270–1273. [Google Scholar]
- 34.Cheong PHY, Paton RS, Bronner SM, Im GYJ, Garg NK, Houk KN. J Am Chem Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina JM, Mackey JL, Garg NK, Houk KN. J Am Chem Soc. 2014;136:15798–15805. doi: 10.1021/ja5099935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu FL, Chen JR, Zou YQ, Wei Q, Xiao WJ. Org Lett. 2014;16:3768–3771. doi: 10.1021/ol501638x. [DOI] [PubMed] [Google Scholar]
- 37.Li HY, Xing LJ, Lou MM, Wang H, Liu RH, Wang B. Org Lett. 2015;17:1098–1101. doi: 10.1021/ol5036326. [DOI] [PubMed] [Google Scholar]
- 38.Wittig G, Behnisch W. Chem Ber. 1958;91:2358–2365. [Google Scholar]
- 39.Wittig G, Reichel B. Chem Ber. 1963;96:2851–2858. [Google Scholar]
- 40.Liu Z, Larock RC. J Org Chem. 2006;71:3198–3209. doi: 10.1021/jo0602221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray CW, Rees DC. Angew Chem Int Ed. 2016;55:488–492. doi: 10.1002/anie.201506783. [DOI] [PubMed] [Google Scholar]
- 42.Chen WC, Hsu YC, Shih WC, Lee CY, Chuang WH, Tsai YF, Chen PPY, Ong TG. Chem Commun. 2012;48:6702. doi: 10.1039/c2cc32519e. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Yang D, Liu Y. J Org Chem. 1993;58:224–227. [Google Scholar]
- 44.García-López JA, Greaney MF. Chem Soc Rev. 2016;45:6766–6798. doi: 10.1039/c6cs00220j. [DOI] [PubMed] [Google Scholar]
- 45.Yanagisawa S, Ueda K, Taniguchi T, Itami K. Org Lett. 2008;10:4673–4676. doi: 10.1021/ol8019764. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Cao H, Zhang H, Zhang H, Chung KH, He C, Wang H, Kwong FY, Lei A. J Am Chem Soc. 2010;132:16737–16740. doi: 10.1021/ja103050x. [DOI] [PubMed] [Google Scholar]
- 47.Roman DS, Takahashi Y, Charette AB. Org Lett. 2011;13:3242–3245. doi: 10.1021/ol201160s. [DOI] [PubMed] [Google Scholar]
- 48.Shirakawa E, Itoh K, Higashino T, Hayashi T. J Am Chem Soc. 2010;132:15537–15539. doi: 10.1021/ja1080822. [DOI] [PubMed] [Google Scholar]
- 49.Rueping M, Leiendecker M, Das A, Poisson T, Bui L. Chem Commun. 2011;47:10629. doi: 10.1039/c1cc14297f. [DOI] [PubMed] [Google Scholar]
- 50.Shirakawa E, Zhang X, Hayashi T. Angew Chem Int Ed. 2011;50:4671–4674. doi: 10.1002/anie.201008220. [DOI] [PubMed] [Google Scholar]
- 51.Studer A, Curran DP. Angew Chem Int Ed. 2011;50:5018–5022. doi: 10.1002/anie.201101597. [DOI] [PubMed] [Google Scholar]
- 52.Sun CL, Gu YF, Huang WP, Shi ZJ. Chem Commun. 2011;47:9813. doi: 10.1039/c1cc13907j. [DOI] [PubMed] [Google Scholar]
- 53.Yanagisawa S, Itami K. ChemCatChem. 2011;3:827–829. [Google Scholar]
- 54.Yong GP, She WL, Zhang YM, Li YZ. Chem Commun. 2011;47:11766. doi: 10.1039/c1cc14420k. [DOI] [PubMed] [Google Scholar]
- 55.Pieber B, Cantillo D, Kappe CO. Chem - Eur J. 2012;18:5047–5055. doi: 10.1002/chem.201103748. [DOI] [PubMed] [Google Scholar]
- 56.Tanimoro K, Ueno M, Takeda K, Kirihata M, Tanimori S. J Org Chem. 2012;77:7844–7849. doi: 10.1021/jo3008594. [DOI] [PubMed] [Google Scholar]
- 57.Vaillard VA, Guastavino JF, Budén ME, Bardagí JI, Barolo SM, Rossi RA. J Org Chem. 2012;77:1507–1519. doi: 10.1021/jo202386b. [DOI] [PubMed] [Google Scholar]
- 58.Zhou S, Anderson GM, Mondal B, Doni E, Ironmonger V, Kranz M, Tuttle T, Murphy JA. Chem Sci. 2014;5:476–482. [Google Scholar]
- 59.Barham JP, Coulthard G, Emery KJ, Doni E, Cumine F, Nocera G, John MP, Berlouis LEA, McGuire T, Tuttle T, Murphy JA. J Am Chem Soc. 2016;138:7402–7410. doi: 10.1021/jacs.6b03282. [DOI] [PubMed] [Google Scholar]