Abstract
Unrelated donor cord blood (CB) transplantation (CBT) results in disease-free survival comparable to that of unrelated adult donor transplantation in patients with hematologic malignancies. Extension of allograft access to racial and ethnic minorities, rapid graft availability, flexibility of transplant date, and low risks of disabling chronic graft-versus-host disease (GVHD) and relapse are significant advantages of CBT, and multiple series have reported a low risk of late transplant-related mortality (TRM) post-transplant. Nonetheless, early post-transplant morbidity and TRM and the requirement for intensive early post-transplant management have slowed wide adoption of CBT. Targeted care strategies in CBT recipients can, however, mitigate early transplant complications and reduce transplant costs. Herein, we provide a practical “how to” guide to CBT for hematologic malignancies on behalf of the NMDP and the ASBMT CB Special Interest Group (SIG). It shares the best practices of 6 experienced United States transplant centers with a special interest in the use of CB as a hematopoietic stem cell source. We address donor search and unit selection, unit thaw and infusion, conditioning regimens, immune suppression, management of GVHD, opportunistic infections and other factors in supportive care appropriate for CBT. Meticulous attention to such details has improved CBT outcomes and will facilitate the success of CBT as a platform for future graft manipulations.
Keywords: allogeneic transplantation, cord blood transplantation, alternative donor
Introduction
Unrelated donor (URD) cord blood (CB) is well established as an allogeneic hematopoietic stem cell (HSC) source that extends allograft access. Volunteer donor searches are much less likely to identify a matched URD in patients of non-European and mixed descent due to diverse human leukocyte antigen (HLA) haplotypes, lower representation in URD registries, and an increased risk of poor donor availability. A recent NMDP study has demonstrated that while approximately 75% of white European patients are likely to identify an 8/8 HLA-matched URD, there are much lower rates for minority patients with availability for donation further compromising access1. In the setting of no suitable URD, either CB or haplo-identical related donor transplants are alternative options. Due to the lesser stringency of the HLA-match requirement, CB transplantation (CBT) has been shown to extend access to the majority of adults in all ancestry groups1,2. A further advantage of CB is its rapid availability permitting flexibility of scheduling the transplant date. This greatly facilitates the care of patients with high-risk malignancies and avoids the adverse effects of transplant delay3.
Multiple retrospective studies have demonstrated that CBT in experienced centers can achieve disease-free survival (DFS) rates comparable to the gold standard of HLA-matched unrelated donor transplants in patients with hematologic malignancies4–8. For example, the University of Minnesota (UMN)/Fred Hutchinson Cancer Research Center (FHCRC) reported comparable 5-year disease-free survival (DFS) after myeloablative matched related, matched URD, mismatched URD and double unit CB (dCB) transplantation4. Recent single center comparisons has demonstrated comparable DFS in recipients of 8/8 HLA-matched URD and CB transplants (Figures 1A/1B)6,8. These analyses are notable for the low rates of relapse after CBT. Moreover, the recent FHCRC analysis has shown a markedly reduced relapse rate after CBT as compared to URD transplantation in patients transplanted with minimal residual disease (Figure 1A)8. Mechanisms of relapse protection are under investigation9,10.
Figure 1. Comparison of survival after CBT compared to URD transplant recipients.

Figure 1A compares acute leukemia/MDS survival at FHCRC after unmodified adult donor allografts and CBT8. The hazard ratio (HR) for death in HLA-matched versus CB transplant recipients was 1.12 (95%CI: 0.77–1.63, p = 0.57), and the HR in HLA-mismatched versus CB transplant recipients was 1.91 (95%CI: 1.23–2.98, p = 0.004). The HR for relapse in HLA-matched versus CB transplant recipients was 1.95 (95%CI: 1.16–3.27, p = 0.01), and the HR in the HLA-mismatched versus CB transplant recipients was 1.97 (95%CI: 1.04–3.73, p = 0.04). Figure 1B is a MSKCC analysis demonstrating DFS in adult acute leukemia patients (dCBT compared to T-cell depleted unrelated donor allografts)6. In this analysis, CBT recipients had significantly higher DFS than HLA-mismatched URD recipients.
Single center and registry retrospective series are also emerging reporting disease-specific outcomes in patients with acute myelogenous leukemia (AML)11, 12, acute lymphoblastic leukemia13,14, myelodysplasia15, myelofibrosis16,17, non-Hodgkins or Hodgkin lymphoma18–20, and multiple myeloma21,22. In addition, a number of prospective studies have now been reported. For example, the Bone Marrow Transplant Clinical Trials Network (BMT CTN) randomized study of myeloablative CBT in children and adolescents with hematologic malignancies, while there was no difference in the 1-year overall survival of 73% (95%CI: 63–80) and 65% (95%CI: 56–74) after infusion of single-unit and double-unit grafts, respectively (Figure 2), the survival in both groups was high23. In a phase II multi-center adult myeloablative double-unit CBT trial, the 50% (95%CI: 37–63) 3-year DFS was comparable to that achieved after URD bone marrow or peripheral blood HSC transplantation24. In the non-myeloablative (NMA) setting, multi-center parallel BMT CTN phase II trials reported comparable DFS after double unit CBT (dCBT) and haplo-identical transplants, respectively25, and a randomized comparison is ongoing.
Figure 2. 1-year survival after pediatric myeloablative single versus double unit CBT23.

The survival in this BMT CTN randomized trial was no different in single versus double unit CBT recipients.
These results establish CBT as a standard therapy for patients with high risk hematologic malignancies. Moreover, a recent NMDP analysis of U.S. transplant outcomes has demonstrated that engraftment has improved after CBT (Figure 3)26 and CBT survival has improved with time27. Nonetheless, CBT in patients with hematologic malignancies will be further improved if the early post-transplant morbidity and transplant-related mortality (TRM) can be reduced. In addition to novel technologies (e.g ex vivo expansion) currently under development, TRM can be mitigated by expert transplant care and an extensive literature now supports many aspects of CBT practice. Recognition of the clinical challenges in CBT and review of the management strategies instituted by experienced transplant centers that have evolved over time can assist in the optimization of patient care and reduce costs. Review of areas of controversy will also highlight topics for further investigation and stimulate discussion and information exchange between centers. Thus, in a joint NMDP and ASBMT initiative we outline our “how we treat” at 6 expert U.S. transplant centers that have a special interest in CBT for the treatment of hematologic malignancies.
Figure 3. Neutrophil engraftment after myeloablative CBT in patients treated on the National Marrow Donor Program Cord Blood Access Protocol (10-CBA, ClinicalTrials.gov NCT01351545) with unlicensed units.
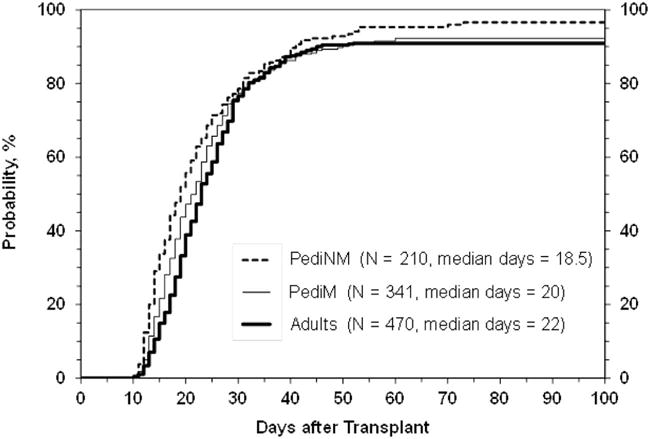
This figure demonstrates neutrophil engraftment in 1,021 patients transplanted between October 2011 and December 2014 with either single or double unit 4–6/6 HLA-matched CB grafts26. The cumulative incidence of engraftment at 42 days was 89% (95%CI: 86–91) in adults with malignancy, 88% (95%CI: 84–91) in children with malignancy, and 92% (95%CI: 88–95) in children with non-malignant disorders.
Patient selection
In our centers, disease eligibility definitions are largely similar to those for transplantation with other allogeneic stem cell sources with the exceptions of less common allograft indications such as advanced myelofibrosis or multiple myeloma, for example. Moreover, disease risk is a major determinant of DFS in all allograft recipients including CBT. Importantly, while CBT has been associated with robust graft-versus-leukemia (GVL)/graft-versus-malignancy (GVM) effects, as with all other HSC sources CBT in patients with acute leukemia in full morphologic relapse or aggressive chemo-refractory Non-Hodgkins lymphoma, for example, is associated with a low likelihood of success. Delay in transplant can increase patient risk due to worsening disease burden and/or therapy complications. Thus, an important consideration in the management of high-risk patients is that delay in proceeding to CBT due to failed URD searches can adversely affect survival.
Patients of advanced age (e.g. > 65 years), or those with extensive prior therapy, treatment complications or other serious comorbidities may not tolerate the longer cytopenias combined with calcineurin-inhibitor (CNI)-based graft-versus-host disease (GVHD) prophylaxis associated with CBT. Few centers would consider CBT in patients over 70 years whereas CB is an excellent graft source in children and adults with low hematopoietic cell transplant comorbidity index (HCT-CI) scores. For example, a recent analysis demonstrated an 81% 2-year PFS in adult dCBT recipients with hematologic malignancies (Figure 4). Thus, the patient’s score is critically important in determining CBT outcome28–30. HCT-CI calculation is recommended prior to selecting the conditioning regimen intensity for CBT (see Conditioning section). Furthermore, adequate creatinine clearance is critical to the success of CNI-based CBT. Center criteria for the upper age limit and the lower limit of organ function are shown in Table 1. In children, prior mechanical ventilation, deep tissue fungal or antibiotic-resistant bacterial infections, or very prolonged pre-transplant neutropenia are associated with increased TRM risk.
Figure 4. 2-year PFS by rDRI and aaHCT-CI (n = 110).

2-year PFS in adult CB recipients (median age 51 years) transplanted with cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 5–10 mg/kg, 400 cGy TBI and CSA/MMF for the treatment of acute leukemia/MDS or myeloproliferative disease (≤ 10% blasts pre-CBT), B-cell NHL or Hodgkin lymphoma (J. Barker, unpublished 2016).
Table 1.
Center practice for patient selection, conditioning regimens, GVHD prophylaxis and G-CSF use in patients with hematologic malignancies.
| Criteria | Boston | Duke | FHCRC | MDACC | MSKCC | UMN |
|---|---|---|---|---|---|---|
| Standard remission requirement: AML/MDS/MPD | AML in morphologic CR. MPD avoided. |
Children: < 5% blasts Adults: AML < 5% blasts. MDS/MPD < 10% blasts. |
< 5% blasts by morphology/flow cytometry. | AML in morphologic CR. | ≤ 10% blasts & not rapidly progressive disease. | Morphologic CR. MPD avoided. |
| Standard remission requirement: ALL/aggressive NHL | ALL in morphologic CR. NHL in CR or chemosensitive PR. |
Children: ALL in morphologic CR. Adults: ALL < 5%blasts. NHL in CR or chemosensitive PR. |
ALL < 5% blasts by morphology & flow. NHL in CR or chemo- sensitive PR. |
ALL in morphologic CR. NHL in CR or chemo- sensitive PR. |
ALL in morphologic CR. NHL in CR. |
ALL in morphologic CR. NHL CR or chemosensitive PR. |
| Remission requirement other NHL/HL | Chemo-sensitivity by CT or PET | |||||
| Upper age limit | Not defined | Not defined | < 70 years | ≤ 65 years | < 70 years | ≤ 75 years |
| Lower limit of acceptable organ function* | EF ≥ 50%. Spirometry/DLCOhb ≥50%. Bilirubin <1.5 ULN. Transaminases <3 × ULN. Creat. clearance ≥ 50 ml/min. |
EF ≥ 50%. Spirometry/DLCOhb ≥50%. Bilirubin <1.5 ULN. Transaminases <3 × ULN. Creat. clearance ≥ 60 ml/min. |
EF ≥ 45% if ablative (35% if NMA). Spirometry/DLCOhb ≥ 50% – ≥ 70% (depending on intensity) Bilirubin ≤ 2 × ULN. Transaminases < 3 × ULN. Creat. clearance ≥ 40–60 ml/min. |
EF ≥ 45% Spirometry/DLCOhb ≥ 50% Bilirubin < 1.5 ULN. Transaminases < 3 × ULN. Creat. clearance ≥ 60 ml/min. |
EF ≥ 35%. Spirometry/DLCOhb ≥ 40%. Bilirubin < 2.0 × ULN. Transaminases < 3 × ULN. Creat. clearance ≥ 40 ml/min. |
|
| High dose regimens | Cy 120/Flu 75/TBI 1200–1375 |
Children:
** Cy 120/Flu 75/TBI 1320 Adults: TBI 1350/Thio 10/Flu 160 |
Cy 120/Flu 75/TBI1320 | Flu 100/Clo 30/Bu (4 days)/TBI 200 | Cy 120/Flu 75/TBI 1375 |
Children:
*** Cy 120/Flu 75/TBI1320 Adults: Cy 120/Flu 75/TBI1320 |
| Intermediate dose regimens | Flu 180/Mel 100/TBI 200 | None | Treo 42/Flu 150–200/TBI 200 | Flu 160/Mel 140 | Cy 50/Flu 150/Thio 10/TBI 400 or Flu 150/Thio 10/Mel 100–140 or Mel 140/Flu 150. |
None |
| NMA regimens | Cy 50/Flu 150/TBI 200 | Cy 50/Flu 150/TBI 200–300 | Cy 50/Flu 150/TBI 200 | |||
| ATG inclusion | Yes | No | ATG including and excluding protocols | No | ATG including and excluding protocols | |
| GVHD Prophylaxis **** | Tacro IV/sirolimus | Tacro IV/MMF IV | CSA IV/MMF IV | Tacro IV/MMF IV | CSA IV/MMF IV | CSA IV/MMF IV or MMF IV/sirolimus |
| Day of G-CSF start | Day +5. |
Children: Day +1. Adults: Day +2. |
Day +1 | Day 0 | Day +7 | Day +5 |
Abbreviations: AML, acute myelogenous; MDS, myelodysplasia; MPD, myeloprolerative disease; ALL, acute lymphoblastc leukemia; NHL, non-Hodgkins lymphoma; HL, Hodgkin lymphoma; Non-MA, non-myeloablative; ATG, ant-thymocyte globulin; GVHD, graft-versus-host disease; G-CSF, granulocyte colony stimulating factor; Cy, cyclophosphamide; Flu, fludarabine; Thio, thiotepa; TBI, total body irradiation; Mel, melphalan; EF, ejection fraction; DLCOhb, diffusing capacity corrected for hemoglobin; ULN, upper limit of normal; creat., creatinine; tacro, tacrolimus; CSA, cyclosporine-A; MMF, mycophenolate mofetil.
ULN guideline for bilirubin does not apply to patients with Gilberts syndrome.
Alternatives for pediatric patients who are not candidates for radiation include Bu/Cy/ATG, Flu/Bu/Cy +/−ATG, Flu/Bu/Mel or low dose Flu/Cy/TBI. MSKCC has also investigated clofarabine based regimens.
Graft selection
Unrelated donor and CB searches
Efficient management of URD searches is mandatory in centers that prioritize an HLA-matched URD in the absence of a matched sibling donor. Our centers frequently prioritize a CB graft over a 7/8 HLA-matched URD and this practice is strongly supported by single center analyses4,6,8. Knowledge of the patient’s ancestry, use of computerized programs based upon population haplotype frequencies (eg the NMDP’s Haplogic and search prognosis tool31,32 and consultation with HLA experts can rapidly determine the likelihood of identifying an 8/8 HLA-matched URD as soon as the recipients HLA-typing and preliminary search results are obtained. Since prolonged URD searches and donor drives are very unlikely to result in the acquisition of a suitably matched URD if one is not identified within the first days-weeks of search initiation, futile URD searches must be avoided with timely consideration of other stem cell sources. If CBT is an alternative, URD and CB searches should be initiated simultaneously to guarantee a suitable graft. This is especially important for patients without multiple likely 8/8 matched URDs or those that require urgent transplantation. If a center is proficient in CB searches units can be identified, confirmatory typed and shipped within 1–3 weeks. Efficiency is enhanced if the center has clear criteria for unit selection. The CB unit characteristics considered by our 6 centers for patients with hematologic malignancies are summarized in Table 2.
Table 2.
Center criteria for CB unit selection in patients with hematologic malignancies.
| Criteria | Boston | Duke | FHCRC | MDACC | MSKCC | UMN |
|---|---|---|---|---|---|---|
| Resolution of HLA-typing | 8-allele HLA-A, -B, -C, -DRB1 |
|||||
| Donor-recipient HLA-match | ≥ 4/6 alleles | ≥ 4/6 HLA-A,-B antigens, -DRB1 alleles& ≥ 3/8 alleles | ≥ 4/6 HLA-A,-B antigens, -DRB1 alleles | ≥ 4/6 HLA-A,-B antigens, -DRB1 alleles & ≥ 3/8 alleles. | ≥ 4/6 HLA-A,- B antigens, - DRB1 alleles. 8 allele match grade also considered. | |
| Dose/kg: single unit* | Single unit grafts not done | TNC ≥ 2.5. CD34+ cells ≥ 1.5. |
TNC > 2.5. CD34+ cells > 2. |
TNC ≥2.5. CD34+ cells ≥1.5. |
TNC ≥2.5. CD34+ cells ≥1.5. |
TNC ≥ 2.5 if ≥5–6/6 & ≥5.0 if 4/6 HLA-match. CD34+ dose also considered. |
| Dose/kg/unit: double unit* | TNC ≥ 1.5/unit. | TNC ≥ 1.5/unit. CD34+ cells ≥ 1.0/unit. |
TNC ≥1.5/unit. CD34+ cells ≥2.0/unit. |
TNC ≥1.5/unit. CD34+ cells ≥1.0/unit. |
TNC ≥1.5/unit. CD34+ cells ≥1.0/unit. |
TNC ≥ 1.5/unit. |
| Avoidance of units against which recipient has DSA | Yes | Not if malignancy | Yes | Usually not if malignancy | Yes | |
| Bank of origin major criteria in selection | Yes | |||||
| Netcord-FACT accreditation considered | No | Yes | ||||
| Use of RBC replete units | Sometimes | Avoid | ||||
| Mandatory testing of attached segment for identity | Yes | Yes** | ||||
| Viability testing on thawed product (day 0) | Yes: % viable CD34+ cells by flow (7AAD) | |||||
| Back-up unit policy | Haploidentical donor identified if possible | 1–2 domestic units*** | No | 1–2 domestic units*** | 1 domestic CB unit or haploidentical donor*** | |
Abbreviations: HLA, human leukocyte antigen; DSA, donor specific antibodies FACT, Foundation for the Accreditation of Cellular Therapy; RBC, red blood cells; TNC, total nucleated cell.
TNC dose is × 107/kg; CD34+ cell dose is × 105/kg.
Rarely, if identity confirmation was not available emergency rapid serological HLA typing can be done at thaw.
Units are reserved but not shipped, and released at time of patient engraftment. Some centers will use backup CB units if there were problems with shipment or unit quality problems whereas a haplo-identical donor would be first choice in the event of graft failure.
Cell dose in unit selection
While total nucleated cell (TNC) dose is a well established determinant of engraftment and survival after CBT, the definition of what is an “adequately dosed” single unit is not truly established as CD34+ dose must be considered in unit selection as well as TNC dose and an adequate CD34+ dose has not been truly defined. This is especially as post-thaw CD34+ viability (i.e. unit quality) must be taken into account. From the standpoint of TNC dose, the pediatric randomized trial of single versus double-unit CBT (BMT CTN 0501) defined a sufficient cryopreserved TNC dose in a single unit as > 2.5 × 107/kg23. However, a registry study of 1,568 myeloablative single-unit CBT recipients found an increased TRM in recipients of units with a TNC < 3.0 × 107/kg33. Moreover, a New York Blood Center analysis of 1061 myeloablative single unit CBT recipients suggested that a higher TNC dose is required to compensate for an increasing degree of HLA-mismatch34.
As early as 2002, Wagner et al reported that in 102 4–6/6 HLA-matched single-unit CBT recipients, there was a higher probability of survival when units contained > 1.7 × 105 CD34+ cells/kg were infused35. More recently, the dominant unit infused viable CD34+ cell dose was reported as the only critical determinant of the speed and success of neutrophil engraftment after dCBT36. Neutrophil engraftment was markedly impaired in patients with a dominant unit infused viable CD34+ cell dose < 0.5 × 105/kg. An important finding of this study was that selecting units based on the cryopreserved CD34+ dose was feasible, cryopreserved CD34+ dose was a better predictor than TNC of post-thaw CD34+ dose, and some units selected on adequate TNC dose will contain dangerously low CD34+ cell doses. It should also be noted that a higher cryopreserved TNC dose in units that are not red blood cell (RBC) depleted does not necessarily reflect a higher progenitor cell content. Overall, most centers consider CD34+ cell dose as a critical factor in optimal unit selection (Table 2).
HLA-match in unit selection
Acceptable donor-recipient HLA-match in CBT has traditionally been at 4-6/6 HLA-A,-B antigens, -DRB1 alleles, although as shown in Figure 5, only considering the traditional 4–6/6 HLA- match grade permits the transplantation of units with a very high degree of HLA-mismatch37. Recently, a 1,568 patient registry study demonstrated a progressively increasing TRM with increasing 8-allele mismatch independent of TNC dose and patient age33. MDACC data have also shown increased TRM with increasing HLA-mismatch38. In contrast, a recent UMN analysis, for example, reported that a high degree of HLA-allele mismatch did not adversely affect transplant outcomes39, and such findings have also been reported in MSKCC and Spanish analyses30,40 and the CTN propsective trial23. Notably, a subset analysis in the UMN study suggested recipients of dCB grafts with a 2–5/10 HLA-allele match were associated with a reduced risk of relapse in patients with acute leukemia39.
Figure 5. Demonstration of the extent of mismatch at 8 HLA-alleles of CB units that are selected based on 4–6/6 HLA-A,-B antigen,-DRB1 allele donor-recipient HLA-match (n = 377)37.

The 4–6/6 HLA-A,-B antigen, -DRB1 allele donor-recipient match to the patient is shown. At high-resolution the median donor-recipient HLA-match at HLA-A,-B,-C,-DRB1 alleles was 5/8 (range 2–8/8). While 6/6 matched units (n = 10) were at least 5/8 HLA-allele matched, 5/6 units (n = 94) were as low as 3/8 matched, and 4/6 units (n = 96) were as low as 2/8 allele matched to the recipient.
Further investigation is required to resolve this controversy with the most critical question being how to trade-off high resolution HLA-match against the TNC and CD34+ dose. The lower limit of acceptable HLA-match at the allele level is also not known and it should be noted that the use of units that are 4/6 HLA-matched by traditional match criteria and 3–4/8 HLA-allele match extends transplant access for minority patients. At this time, many centers consider units that are at least 4/6 by traditional matching criteria and then select unit(s) for the graft based upon the match at 8 HLA-alleles since considering the donor-recipient HLA-A,-B,-C,-DRB1 allele match can help to avoid the most extreme mismatch. The varying center practices are detailed in Table 2.
Unit quality
Early in the practice of CBT unit quality was recognized as an important additional factor to that could influence engraftment. For example, a number of publications have shown the importance of post-thaw colony-forming unit (CFU) dose in engraftment success41,42. Additionally, units with a low percentage of viable CD34+ cells after thaw have been shown to have very poor engraftment potential43. Units from Netcord-Foundation for the Accreditation of Cellular Therapy (FACT) accredited Banks have been reported to have higher CD34+ cell recovery, and units from such Banks with standard cryovolumes of approximately 25 mls are more likely to have high post-thaw CD34+ cell viability36. As a result of center experience and such data, all of our 6 centers consider the Bank of origin in unit selection. If unit segment potency testing can be standardized44 and are shown to reflect unit content, such assays may assist in pre- and post-thaw quality assessment. In the interim, post-thaw assays of HSC potency43 are critical as they are available within hours of thaw at the transplant center making the shipment of a back-up unit possible in the unlikely event of a poor quality unit45. This is especially important in single unit CBT as poor graft potency on transplant day would dictate the need to immediately ship and infuse a back-up graft.
Selection of single versus double unit grafts
Single unit CBT is well established in pediatric patients. In the BMT CTN pediatric myeloablative CBT study that randomized single unit grafts with a TNC dose > 2.5 × 107/kg against the addition of a second unit showed similar neutrophil engraftment and 1-year overall survival (Figure 2)23. A recent European study had similar findings although in this study a higher TNC dose threshold was required to be eligible46. However, many adults and some larger sized children do not have access to adequately dosed single units. dCBT has been highly successful in such patients despite the fact that only one unit dominates in almost all recipients. In a Center for International Blood and Marrow Transplant Registry (CIBMTR) study in adult CBT recipients with acute leukemia, despite double unit recipients receiving grafts that were predominantly comprised of 2 inadequately dosed units, their engraftment and survival was comparable to single-unit recipients who received a TNC ≥ 2.5 × 107/kg. This and other studies47 suggest that double unit grafts successfully extend the application of CBT in adults with inadequate single units.
A recent analysis of 129 dCBT recipients has shown that in patients with a low dosed dominant unit (infused viable CD34+ cell dose < 1.2 × 105/kg), a higher infused TNC dose in the non-dominant unit was associated with improved neutrophil recovery. This observation suggests that despite the non-engrafting unit not contributing to hematopoiesis it may have a facilitatory effect upon engraftment48. Moreover, while it is not definitively established, retrospective and randomized studies have suggested dCBT may be associated with a reduced risk of relapse19,46,49–51 with one analysis additionally demonstrating dCBT is cost effective as compared to single unit CBT51. These findings support the continued practice of dCBT in many adult patients. Unit selection principles for single unit and dCB grafts are described in Table 2 and Figure 7. Importantly, if a double unit graft is selected, the same unit selection principles with adequate CD34+ dose should apply to each unit as either unit could engraft and the engrafting unit cannot be predicted at the time of selection. Additionally, no data exists to date that supports incorporating unit-unit HLA-match into double unit graft selection52.
Figure 7.

Summary of the current measures to optimize the practice of CBT in patients with hematologic malignancies. The emphasis is on patient and unit selection, conditioning intensity, GVHD prophylaxis, transplant day management, and early post-transplant care.
Other considerations
Whether other factors beyond cell dose, HLA-match and unit quality are important is not established. The issues with red cell content are discussed in the Thaw and Infusion section. The relative importance of avoidance of units against which the patient may have donor specific anti-HLA antibodies (DSA) is controversial with conflicting studies showing that they may53,54 or may not55,56 be important. Further investigation is required to resolve this question taking into account factors such as the patient’s prior therapy (that could influence risk for T-cell mediated rejection), the intensity of the conditioning regimen and use of ATG, the type of graft, and the number of antibodies, their titer and complement fixation. It is appropriate to avoid units targeted by high titer DSA in patients without prior immunosuppressive therapy at increased risk of graft rejection. How to trade-off avoidance of units against which the patient has DSA versus the donor-recipient HLA-match and cell dose of the selected unit(s) has not been determined.
Currently, there is no established evidence that suggests ABO match should be considered in CB graft selection. The age of the unit is also not established as a unit selection criterion although the quality of unit processing and standardization of progenitor measurements may be better in more recent years. Policies concerning infectious disease markers and hemoglobinopathy screening have previously been described57. Nearly all centers consider confirmatory HLA typing of an attached segment as mandatory to confirm unit identity. In the U.S., most centers do not take unit licensure or cost into account. The influence of KIR alloreactivity on outcomes is highly controversial58–64 and NK considerations have not been incorporated into unit selection practices at this time. Similarly, the influence of gene polymorphisms of the immune response65 requires further investigation. The biologic effects of NIMA are also of great interest but consideration of these is often not logistically feasible as it is contingent on obtaining the maternal typing66–68.
Efficient CB search management
To track the selection process, relevant unit characteristics (the Bank of origin, TNC and CD34+ cell dose, HLA-match, cryovolume, year of cryopreservation, licensure status and FACT-Netcord or AABB accreditation and other information of interest) can be summarized in a one page Search Summary (Figure 6). Once all information and the confirmatory typing of units of interest are obtained, the graft and back-up unit(s) can easily be selected. Some banks now also provide a measure of potency testing at the time of confirmatory typing although this practice has not been standardized.
Figure 6. Example of a CB Search Summary Report.

Patient demographics, the best available URD, the date that the search was most recently run, and the patient’s current weight are indicated. The details of units of interest including the bank of origin, the unit volume (to reflect processing type and RBC depletion), collection date, TNC dose, CD34+ cell dose (listed here as × 103/kg), traditional 4–6/6 HLA-match, and 8 allele HLA-match are listed. ABO/Rh group and donor gender are listed for future reference. The loci of mismatch are shown (lower case indicates HLA-allele mismatch only and upper case indicates a full HLA-antigen mismatch). All listed mismatches are bidirectional unless otherwise indicated. The Bank Netcord-FACT accreditation, licensure, problems with unit availability (e.g. reserved on another patient’s search), or other issues making the unit ineligible (and therefore requiring a Declaration of Urgent Medical Need in the U.S.) are listed under “comments”. When all information is available, the unit rank is assigned in the first column (unit 1 if a single unit graft, or 1a and 1b if a double unit graft). Back-up units are reserved at domestic bank(s).
Conditioning
CBT for hematologic malignancies was initially performed after high dose myeloablative conditioning regimens incorporating anti-thymocyte globulin (ATG) with cyclosporine-A and corticosteroids to mitigate the risks of graft failure and GVHD with the use of HLA-mismatched grafts. In the year 2000, in an effort to mitigate post-transplant opportunistic infections, UMN investigators developed a regimen using cyclophosphamide 120 mg/kg, fludarabine 75 mg/m2 and total body irradiation 1320 cGy with cyclosporine and mycophenolate mofetil (MMF) for GVHD prophylaxis69. Thus, fludarabine replaced ATG and MMF replaced corticosteroids. This regimen has been associated with high levels of engraftment and DFS in pediatric and younger adult recipients24,69. Moreover, that outcomes of single and double-unit CBT recipients are similar in children suggest that conditioning and immunosuppression are as important as the characteristics of the graft.
A significant limitation of high dose regimens is that they can be extremely toxic in adults24, especially in the setting of delayed engraftment. Consequently, to tailor conditioning intensity to recipient age and the HCT-CI many adult centers have developed reduced toxicity intermediate intensity regimens to substitute for high dose conditioning (Table 1). Such regimens remain myeloblative but are better tolerated than high dose chemotherapy-radiation and are also more potent than non-myeloablative (NMA) conditioning facilitating engraftment and improving disease control. These can routinely be offered to older adult patients with lower comorbidity scores (Figure 4). In an effort to avoid TBI some centers have developed chemotherapy-only based regimens with success70. In the NMA setting, the most established regimen is cyclophosphamide 50 mg/kg, fludarabine 150–200 mg/m2, and 200 cGy of total body irradiation71,72. NMA regimens have been associated with an increased risk of rejection in patients without prior immunosuppressive chemotherapy71 as well as higher rates of relapse25. They may, therefore, be most appropriate for diseases in which the main efficacy of the allograft is based on a GVL effect such as indolent lymphomas, or older adults and those with multiple comorbidities or extensive prior therapy unable to tolerate more intensive regimens.
GVHD Prophylaxis
Effective prophylaxis against GVHD is a critical component of CBT supportive care. While multiple approaches including a calcineurin-inhibitor (CNI), methotrexate, corticosteroids, and ATG were explored in the first decade of CBT, the preferred regimen used by most centers today utilizes a CNI for 6–9 months with MMF for 45–180 days. In pediatric patients receiving single units for transplantation, this regimen is associated with a 13% (95%CI: 7–20) incidence of grade II-IV acute GVHD and 9% (95%CI: 4–14) incidence of extensive chronic GVHD, despite transplantation with HLA-mismatched grafts23.
While the reduced incidence of disabling chronic GVHD is a major advantage of CBT as compared to URD transplants4,23,73,74, acute GVHD can still be a cause of significant morbidity and mortality. For example, grade II-IV acute GVHD rates of over 50% have been reported in dCBT recipients transplanted with CNI/MMF immunosuppression without ATG4,73. While much of this is grade II, these results suggest augmented acute GVHD prophylaxis is appropriate (Table 1). Numerous strategies to reduce acute GVHD are under development. These include selecting units based on high-resolution donor-recipient match to avoid extreme mismatch73,75. Optimizing cyclosporine-A levels early post-transplant is critical76,77. Two recent analyses have independently identified the MMF dose as a critical determinant of acute GVHD and support intensified MMF dosing as the new standard in MMF-based CBT78,79. The monitoring of mycophenolic acid trough levels may also be of use79. Extending the duration of MMF prophylaxis (eg > 100 days) has been investigated in recent years, and tailoring patient immunosuppression according to GVHD serum biomarkers could also be of use in the future80.
In vivo T-cell depletion of the CB allograft with ATG is an alternative strategy to prevent GVHD and has been associated with reduced rates of GVHD81. In patients with hematologic malignancies, ATG inclusion is highly controversial, however. While a CIBMTR myeloablative CBT study analyzing children with ALL showed comparable 3-year DFS regardless of ATG inclusion82, other series have shown multiple disadvantages. Viral infections such as Epstein-Barr virus (EBV)-post-transplant lymphoproliferative disease (PTLD) are significantly increased83. Moreover, immune recovery is notably delayed84 likely accounting for multiple reports of increased TRM and/or increased relapse in children and adults85–88. However, the biological effects of ATG are altered by multiple factors (e.g. patient age, diagnosis, conditioning intensity and pre-transplant lymphocyte count as well as by ATG dose, brand and timing of administration relative to graft infusion). This complicates the assessment of its role in CBT. The work of Admiraal et al suggest the safety of ATG-based regimens can be enhanced by performing ATG pharmacokinetics or dosing based on pharmacokinetic principles to greatly reduce or eliminate post-transplant ATG exposure and thus enhance immune reconstitution and disease-free survival88,89.
Thaw and infusion
Transplant centers must have a standard of practice for the receipt of CB grafts to ensure appropriate handling upon arrival and storage until the day of transplantation. The center should have prior knowledge of the container, cryovolume, RBC content, and type of access ports on the cryopreservation bag. Thaw techniques vary at experienced centers but should take the following into consideration: age of the patient, distance from the site of thaw to the patient, RBC content of the unit, ABO match of the unit if a RBC containing unit is utilized, size of the patient and relative load of dimethyl sulfoxide (DMSO), and the planned final unit volume.
Practices utilized at our centers include dilution with Dextran 40 with 25% human serum albumin followed by centrifugation (wash)90 or dextran-albumin dilution only (Table 3). Wash has the advantage of longer product stability, and DMSO/cellular debris removal but is associated with increased graft manipulation, technologist time, and the potential for cell loss. Automated wash devices may have an advantage over manual washing from the standpoint of post-wash viability. Dilution is faster, easier, and is not associated with mechanical cell loss but must be administered with increased attention to time from thaw to infusion, DMSO associated infusion reactions and volume overload91.
Table 3.
Overview of guidelines for thaw and infusion of RBC-depleted CB grafts by transplant center.
| Criteria | Boston | Duke | FHCRC | MDACC | MSKCC | UMN |
|---|---|---|---|---|---|---|
| Manual wash, automated wash or dilution only | Manual wash | Automated wash | Dilution if recipient > 20 kg. Otherwise manual wash. | Automated wash | Dilution if recipient > 20 kg*. Otherwise manual wash. | Automated wash |
| Final volume | As clinically appropriate |
Children: < 5 ml/kg Adults: 50 ml |
8-fold dilution | ~ 50 ml | 8-fold dilution | ~ 100 ml |
| Pre-medication | Diphenhydramine | |||||
| Hydrocort | AMP Hydrocort | AMP Hydrocort | Hydrocort | AMP Lorazepam Hydrocort | AMP | |
| Hydration | 500 mls prior to infusion |
Children: Twice maintenance for 4–6 hours. Adults: maintenance fluids. |
Twice maintenance 4–6 hours pre- & 24 hours post-CBT | Twice maintenance 2 hours pre- & 4 post-CBT | Twice maintenance 4–6 hours pre- & 12 hours post-CBT if units not washed. Furosemide to maintain fluid balance. | 2–6 hours pre- & 12 hours post- CBT |
| Minimum infusion time | ~ 45 minutes/unit |
Children: ~ 15 minutes Adults: ~ 45 minutes |
~ 30 minutes/unit | ~ 30–45 minutes/unit | Infusion by gravity for small children. Otherwise ~ 45 minutes/unit | |
| Treatment of hypertension | Individualized to patient | IV hydralazine | IV hydralazine + furosemide | Antihypertensive +/− furosemide | IV hydralazine ± furosemide | As clinically indicated |
Abbreviations: AMP, acetaminophen; Hydrocort, hydrocortisone.
Diluent used: 5:1 ratio of 10% dextran 40 (molecular weight 40,000) and 25% human serum albumin.
With either technique adequate dilution (including prior to wash) in a controlled environment is critical. For these reasons our centers do not perform bedside thaw. Importantly, life-threatening infusion reactions have been reported with non-washed RBC-replete units92. Dilution may be inadequate to mitigate severe infusional toxicity93 and yet RBC replete units require considerable expertise to wash given the markedly increased risk for cell loss. Therefore, such units are avoided by most centers. Post-thaw rapid testing of hematopoietic potency is ideal to ensure the infusion of a product with high engraftment potential43.
Infection prophylaxis, monitoring and therapy
Supportive care to prevent or treat opportunistic infections until neutrophil and immune recovery has occurred is critical in CBT. Center practices are summarized in Table 4.
Table 4.
Overview of guidelines for infection prophylaxis and monitoring early post-CBT by transplant center.
| Criteria | Boston | Duke | FHCRC | MDACC | MSKCC | UMN |
|---|---|---|---|---|---|---|
| Prophylaxis | ||||||
| Bacterial | Levofloxacin or Ciprofloxacin |
Children: none. Adults: ciprofloxacin day −2. |
Levofloxacin starting with neutropenia | Levofloxacin or ciprofloxacin day −1 to engraftment. | Vancomycin/ciprofloxacin day −2 or at neutropenia. | Levofloxacin |
| Fungal | Fluconazole or nothing. | Voriconazole from day 0. Micafungin if azoles not tolerated. | Fluconazole starting with conditioning. | Voriconazole or posaconazole day −1 until off significant immune suppression. | Micafungin from admit, switch to voriconazole/posaconazole as tolerated after day +7. Micafungin if azoles not tolerated. | Fluconazole or voriconazole in selected patients |
| Viral | Acyclovir or Famciclovir | Acyclovir | Valacyclovir 2 gm TID | Valacyclovir from day −1 until 12 months | Acyclovir (gancyclovir during conditioning if recipient CMV+) | Acyclovir |
| Pneumocystis active drug: Tri/sulpha, pentamidine or atovaquone | During conditioning. Resume after day 30 | Pre-conditioning. Resume day 30 | Tri/sulfa at start of conditioning to day −2. Resume when engrafted (by day 30) |
Tri/sulfa start day 30 | During conditoning. Resume day 30. | Tri/sulfa start day +28 |
| Toxoplasmosis active drug Tri/sulpha or atovaquone or pyramethamine | During conditioning. Resume after day 30. |
Children: After completion of conditioning. Adults: Preconditioning. resume day 30. |
Pyramethamine or Tri/sulfa. | During conditioning. Resume day 14. | ||
| Monitoring by quantitative PCR | ||||||
| CMV | Weekly | Weekly day 0–100 then as clinically indicated | Weekly from day 0–100, then weekly to 1 year | Twice weekly from day 14–100. | Twice weekly from day 14–60. If CMV+ then weekly till 100. Then less frequent per patient risk. | Weekly to day 100. Then as clinically indicated (e.g if GVHD) |
| HHV-6 | Weekly | Weekly day 0–100 then as clinically indicated | As clinically indicated | Weekly from day 14–100. | Once-twice weekly from day 14–60. | If clinically indicated |
| Adenovirus | Yes at DFCI/No at Mass. General | As clinically indicated | Weekly from day 14–100. | Weekly from day 14–60. | No | |
| EBV | Weekly | As clinically indicated | Weekly from day 14–100. Then as clinically indicated (e.g if GVHD). | Once-twice weekly from day 14–60 then weekly till day 100. Then as clinically indicated (e.g if GVHD). | If ATG, weekly day 30–180 | |
| Toxo-plasmosis | None | As clinically indicated | None | Weekly until discharge if seropositive recipient. | Twice weekly day +14–60 if recipient seropositive. | None |
Abbreviations: CMV, cytomegalovirus; HHV-6, human herpes virus-6; EBV, Epstein-Bar virus; PCR, polymerase chain reaction; Tri/sulfa, Trimethoprim/sulphamethozole.
Bacterial prophylaxis and treatment of febrile neutropenia
Knowledge of the sensitivities of prior bacteremias and documentation of bacterial colonization [e.g. methicillin resistant Staphylococcal aureus, Vancomycin-resistant Enterococcus (VRE), and multi-resistant gram negative bacteria] are important to guide antibiotic choices. A current standard in neutropenic patients is to administer prophylaxis and treat fever according to transplant center patterns (and sensitivities of prior infections, if applicable). VRE is a common cause of pre-engraftment bacteremia94 and VRE active antibiotics can be given pre-emptively at febrile neutropenia onset in colonized patients or at the time of notification of Gram-positive bacteremia.
Fungal prophylaxis
Fungal prophylaxis, often with extended spectrum azoles, is standard in most centers. Due to the risk of significant drug interactions extended spectrum azoles should be avoided during the preparative regimen, however, and careful CNI drug monitoring must be performed when using these drugs early post-transplant77. No prophylaxis or an echinocandin can be used until switching to an extended spectrum azole (eg voriconazole or posaconazole) post-transplant and our centers have a low threshold for a non-contrast chest CT scan to evaluate patients for occult fungal infections. Given transplant success is contingent on maintenance of therapeutic CNI dosing, early post-transplant avoidance of the nephrotoxicity of amphotericin is appropriate.
Viral prophylaxis, monitoring and therapy
Cytomegalovirus (CMV) reactivation in CMV seropositive CBT recipients is as high as 100% in patients monitored by sensitive PCR95. CMV disease rates vary by series, and CMV infection can potentially increase TRM risk95–97. Our centers perform once or twice weekly PCR monitoring in seropositive patients early post-CBT, and due to the risk of life-threatening CMV disease with delayed therapy most centers will commence pre-emptive therapy at first-second low level PCR detection95,97. CMV infection is treated with foscarnet before myeloid recovery and ganciclovir or valgancyclovir after myeloid engraftment for treatment of CMV viremia. New agents are needed as delayed myeloid recovery can limit early use of ganciclovir/valgancyclovir and the risk of nephrotoxicity complicates the combined use of CNI and foscarnet.
While human herpes virus-6 (HHV-6) viremia is common after CBT, the clinical impact of HHV-6 reactivation is controversial with the reported incidence of encephalitis varying from < 2% to 9.9%98,99. Many centers perform PCR monitoring to facilitate early detection of high level viremia and prompt recognition of end-organ disease although pre-emptive foscarnet therapy in uncomplicated viremia may not be warranted99. Further investigation of the risk-benefit of HHV-6 viremia therapy and standardization of PCR testing is required. While CBT can be a risk factor for adenovirus viremia, there is no agreement concerning the utility of prospective viremia surveillance. Brincidofovir has significant anti-adenovirus activity. The gastro-intestinal toxicity of this drug must be weighed against the nephrotoxicity of intravenous cidofovir.
EBV reactivation and PTLD risk is markedly increased with ATG-based conditioning regimens and such transplants require close monitoring for EBV reactivation and pre-emptive therapy with rituximab83. A very low incidence of EBV-associated PTLD has been reported in CBT recipients transplanted without ATG although it may occur and some centers will monitor for EBV viremia. This is especially appropriate in patients on high dose or prolonged systemic therapy for GVHD or patients with documented poor T-cell recovery.
Pneumocystis jiroveci (PCP)/Toxoplasmosis prophylaxis
All our centers commence PCP prophylaxis by one month post-CBT. Most use either inhaled pentamidine or atovaquone until adequate hematopoiesis will permit use of trimethoprim-sulphur. Patients should be screened pre-transplant for toxoplasmosis exposure. Those who are IgG seropositive for toxoplasmosis are at significant risk for life-threatening infection and prophylaxis is appropriate. Pre-transplant IgM seropositivity requires highly specialized management. Practices vary concerning the use of PCR surveillance for toxoplasmosis reactivation early post-CBT but seropositive recipients are at risk.
Intravenous immune globulin supplementation (IVIg)
Most centers administer repletion for hypogammaglobinemia although the threshold for replacement varies by center (in adults IgG < 400 mg/dL or lower as guided by infection risk and in children < 10% lower than the lower limit of normal for age for young children). Prophylaxis should be continued until there is evidence of functional B-cell recovery.
Duration of infection prophylaxis
No data are available to guide the duration of prophylaxis for fungal, viral and PCP/toxoplasmosis infections. Importantly, prophylaxis until the patient is off immunosuppression and has achieved some basic measures of immune recovery is appropriate (as indicated by a CD4 count > 200/microL, for example). This time period can vary widely and will be influenced by ATG-based in vivo T-cell depletion and ongoing GVHD therapy. Clinically validated measures of functional immune reconstitution are needed. All patients require post-transplant immunization with all relevant protein conjugate and live vaccines and vaccine responses in CBT recipients have been reported100.
Management of Delayed Engraftment and Graft Failure
Single center series have reported sustained donor neutrophil engraftment rates of 95%36 that are comparable to those of adult donor bone marrow transplantation. Nonetheless, CBT is associated with delayed engraftment and an increased risk of graft failure as compared to PBSC allografts. As graft failure is associated with a very high lethality risk every effort must be made to avoid this complication by rigorous unit selection, optimized thaw, immediate post-thaw potency evaluation and optimized conditioning and immune suppression. Access to reserved “ready to ship” back-up unit(s) is prudent. Use of granulocyte-colony-stimulating factor early post-transplant to facilitate neutrophil recovery is standard (Table 1).
Prompt diagnosis of graft failure is also critical. Early evaluation can permit emergency intervention. Standard chimerism evaluation is by quantitative PCR for informative polymorphic short tandem repeat regions and centers assess engraftment between days 21 and no later than day 28. Lack of signs of white cell count recovery should trigger rapid processing of chimerism assays. Bone marrow chimerism > 90% donor 21 days post-CBT has been shown to be associated with a high likelihood of sustained engraftment following dCBT101 although clinical graft failure may still occur in patients who are 100% donor chimeric especially in the setting of severe illness. In patients without white cell count recovery and who lack evidence of progressive donor engraftment in serial chimerism testing most centers would commence workup for second transplant as of approximately day 35. While optimal preparative regimens for second transplant are not established they are usually fludarabine or cyclophosphamide and ATG is often included. Additionally, using a haplo-identical donor for rescue of patients with graft failure after CBT is preferred by some centers.
GVHD Diagnosis and Therapy
While some studies have reported the skin is the most commonly involved organ in acute GVHD23,102, other centers have found that the gastro-intestinal (GI) tract is the most frequently affected73,103. CBT mediated GVHD may be more corticosteroid responsive than that mediated by adult donor grafts and initial treatment of the skin with lower doses of prednisone or of the upper GI tract with poorly absorbed corticosteroids can be effective73. Lower GI acute GVHD is most likely to mediate TRM and requires prompt diagnosis and more intensive therapy. When diarrhea is present, rapid evaluation for infection (C. difficile or viruses) should be performed recognizing some patients can have both GVHD and infection. Therapy of lower gut disease must not be delayed. Interestingly, a number of analyses have suggested development of acute GVHD is not associated with a decrement in survival104,105. Multiple series have reported low incidences of moderate or severe disabling chronic GVHD4,73,74,103 and increased corticosteroid responsiveness106 after CBT. This represents a major advantage of this HSC source from the standpoint of quality of life and should be the subject of long-term CBT outcome analyses.
Other Complications
Pre-engraftment syndrome
Pre-engraftment syndrome (PES) manifests as unexplained fever > 38.3 C (101 F) in the absence of infection. It is sometimes accompanied by an erythematous rash. It occurs before or at neutrophil recovery (median onset approximately 9 days ranging 5–12 days) and is a recognized complication of both single and double-unit CBT107,108. As patients can develop severe capillary leak syndrome resulting in hypoxia and renal impairment and potentially multi-organ failure, PES requires prompt recognition and therapy. PES is highly corticosteroid responsive and short course intravenous methylprednisolone daily (eg 1 mg/kg for 3 days) with or without taper is the accepted therapy.
Autoimmune hemolysis and immune thrombocytopenic purpura
Autoimmune hemolysis (AH) and immune thrombocytopenic purpura (ITP) are also reported complications of CBT and may occur at a higher rate than adult donor allografts. AH/ITP can be both abrupt in onset and life-threatening109–111. High rates of immune cytopenias have been reported in pediatric patients undergoing CBT in the first year of life112. Early intervention with corticosteroids and rituximab at diagnosis is effective and early rituximab administration is a corticosteroid sparing strategy in patients with severe disease111.
New Technologies
Extensive investigation of strategies to enhance myeloid engraftment is underway113,114. Expansion methods that are currently in clinical trials include ex vivo expansion of stem/progenitor cells utilizing culture in the presence of Notch ligand115, a mesenchymal co-culture system116, and culture with the addition of small molecules (nicotinamide117, StemRegenin-1118 and UM171119). Pre-clinical expansion systems that are soon to be evaluated in the clinical setting include an endothelial-based expansion system120. Concerns that real-time expansion of the graft could cause undue transplant delay has led to investigation of the provision of “off-the-shelf” previously expanded CB-derived myeloid progenitors115. The addition of a third-party CD34+ cell selected myeloid bridge121,122 is another alternative although in vivo T-cell depletion appears to be necessary to promote a myeloid bridge123. Enhanced homing is an alternative approach and fucosylation is currently under investigation124. In addition to enhancing myeloid recovery, strategies to prevent and treat viral infections in CBT recipients through the provision of cytotoxic T-cells are also under development114,125. The infusion of third-party CB-derived T-regulatory cells is being investigated as novel GVHD prophylaxis126, and third-party EBV-specific cytotoxic T lymphocytes can be effective therapy for EBV-PTLD127.
Conclusions
CBT is an established therapy for the treatment of patients with hematologic malignancies. Recognition of commonly encountered complications of CBT will further improve upon CBT success. Importantly, the goal to reduce TRM is realistic given the increasing global CB inventory and center experience as well as efficient management of donor searches to include early recognition of poor or futile URD searches and optimized unit selection. Further improvement has been realized with the development of novel conditioning regimens tailored to patient diagnosis and comorbidities, multiple new strategies to speed engraftment, augmented GVHD prophylaxis, prompt GVHD diagnosis and therapy, and improved early treatment of opportunistic infections (Figure 7). While the cost of CB grafts and supportive care early after transplantation remain a source of concern for many centers, graft costs should be placed in perspective. All alternative donor allograft strategies for patients with hematologic malignancies are expensive, and true cost comparisons must take into account long-term patient care including, for example, the cost of management of severe chronic GVHD, the cost of relapse, or the cost of relapse prevention measures such as the addition of post-transplant maintenance or cellular therapies. Only long-term outcome analyses with quality of life measures will be able to determine the value and cost of CBT as compared to other therapies. Additionally, improved graft availability, informed graft selection, and emerging techniques enhancing engraftment are likely to improve outcomes and decrease CBT costs. Finally, collaborative multi-center studies, information exchange between centers worldwide, and efforts of special interest groups will hasten progress in the optimized care of CBT recipients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions. All authors wrote the manuscript.
Conflict of Interest. All authors have no financial conflicts of interest to disclose. J.K. and E.J.S. and are medical directors of CB banks.
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16(11):1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iori AP, Valle V, Piciocchi A, et al. Concurrent search for unrelated cord and volunteer donor in high-risk acute lymphoblastic leukemia. Ann Hematol. 2012;91(6):941–948. doi: 10.1007/s00277-011-1392-z. [DOI] [PubMed] [Google Scholar]
- 4.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119(23):5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015;21(11):1985–1993. doi: 10.1016/j.bbmt.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warlick ED, Peffault de Latour R, Shanley R, et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Biol Blood Marrow Transplant. 2015;21(2):357–363. doi: 10.1016/j.bbmt.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016;375(10):944–953. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. 2015;126(26):2882–2891. doi: 10.1182/blood-2015-06-654780. [DOI] [PubMed] [Google Scholar]
- 10.Lamers CH, Wijers R, van Bergen CA, et al. CD4+ T-cell alloreactivity towards mismatched HLA-class II alleles early after double umbilical cord blood transplantation (dUCBT) Blood. 2016;128(17):2165–2174. doi: 10.1182/blood-2016-06-718619. [DOI] [PubMed] [Google Scholar]
- 11.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014;20(6):816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks DI, Woo KA, Zhong X, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–328. doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucunduva L, Ruggeri A, Sanz G, et al. Risk factors for outcomes after unrelated cord blood transplantation for adults with acute lymphoblastic leukemia: a report on behalf of Eurocord and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):887–894. doi: 10.1038/bmt.2014.72. [DOI] [PubMed] [Google Scholar]
- 15.Robin M, Sanz GF, Ionescu I, et al. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia. 2011;25(1):75–81. doi: 10.1038/leu.2010.219. [DOI] [PubMed] [Google Scholar]
- 16.Robin M, Giannotti F, Deconinck E, et al. Unrelated cord blood transplantation for patients with primary or secondary myelofibrosis. Biol Blood Marrow Transplant. 2014;20(11):1841–1846. doi: 10.1016/j.bbmt.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Takagi S, Ota Y, Uchida N, et al. Successful engraftment after reduced-intensity umbilical cord blood transplantation for myelofibrosis. Blood. 2010;116(4):649–652. doi: 10.1182/blood-2009-11-252601. [DOI] [PubMed] [Google Scholar]
- 18.Bachanova V, Burns LJ, Wang T, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50(2):197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 20.Thompson PA, Perera T, Marin D, et al. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leuk Lymphoma. 2015:1–9. doi: 10.3109/10428194.2015.1105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paviglianiti A, Xavier E, Ruggeri A, et al. Outcomes of unrelated cord blood transplantation in patients with multiple myeloma: a survey on behalf of Eurocord, the Cord Blood Committee of Cellular Therapy and Immunobiology Working Party, and the Chronic Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2016 doi: 10.3324/haematol.2015.138917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura K, Takamatsu H, Ikeda T, et al. Cord Blood Transplantation for Multiple Myeloma: A Study from the Multiple Myeloma Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(7):1291–1298. doi: 10.1016/j.bbmt.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JE, Jr, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685–1694. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker JN, Fei M, Karanes C, et al. Results of a prospective multicentre myeloablative double-unit cord blood transplantation trial in adult patients with acute leukaemia and myelodysplasia. Br J Haematol. 2015;168(3):405–412. doi: 10.1111/bjh.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballen K, Logan B, Chitphakdithai P, Spellman S, Adams A, Drexler R, Duffy M, Kemp A, King R, Delaney C, Shpall E, Kurtzberg, Babic A, Confer D, Miller J. Excellent Outcomes in 1589 Patients Receiving Umbilical Cord Blood Transplantation Using Unlicensed Units from a Centralized Cord Blood Registry. Biol Blood Marrow Transplant. 2017;23(3):S170. [Google Scholar]
- 27.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver D, Milano F, Delaney C, Sorror M, Salit R. Comorbidity Index in the Context of Cord Blood Transplantation: An Accurate Predictor of Treatment Related Mortality? Blood. 2014;124(21):1205. [Google Scholar]
- 30.Kosuri S, Hilden P, Devlin S, Yoo Y, Lauer E, Peled J, Avecilla S, Castro-Malaspina H, Dahi P, Giralt S, Gyurkocza B, Jakubowski A, Meagher R, Papadopoulos E, Perales M, Reich L, Sauter C, Scaradavou A, Shaffer B, Tamari R, van den Brink M, Young J, Ponce D, Barker J. High Progression-Free Survival (PFS) in Adult Double Unit Cord Blood (dCB) Transplant Recipients with High Risk Disease after a Novel Intermediate Intensity Conditioning Regimen. Biol Blood Marrow Transplant. 2016;2(3):s76–77. [Google Scholar]
- 31.Dehn J, Setterholm M, Buck K, et al. HapLogic: A Predictive Human Leukocyte Antigen-Matching Algorithm to Enhance Rapid Identification of the Optimal Unrelated Hematopoietic Stem Cell Sources for Transplantation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Wadsworth K, Albrecht M, Fonstad R, Spellman S, Maiers M, Dehn J. Unrelated donor search prognostic score to support early HLA consultation and clinical decisions. Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.162. [DOI] [PubMed] [Google Scholar]
- 33.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA-match on transplant outcome in 1061 cord blood recipients with hematological malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 36.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905–2912. doi: 10.1182/blood-2014-03-566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahi PB, Ponce DM, Devlin S, et al. Donor-recipient allele-level HLA matching of unrelated cord blood units reveals high degrees of mismatch and alters graft selection. Bone Marrow Transplant. 2014;49(9):1184–1186. doi: 10.1038/bmt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oran B, Cao K, Saliba RM, et al. Better allele-level matching improves transplant-related mortality after double cord blood transplantation. Haematologica. 2015;100(10):1361–1370. doi: 10.3324/haematol.2015.127787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunstein CG, Petersdorf EW, DeFor TE, et al. Impact of Allele-Level HLA Mismatch on Outcomes in Recipients of Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2016;22(3):487–492. doi: 10.1016/j.bbmt.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz J, Jaramillo FJ, Planelles D, et al. Impact on outcomes of human leukocyte antigen matching by allele-level typing in adults with acute myeloid leukemia undergoing umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2014;20(1):106–110. [PubMed] [Google Scholar]
- 41.Migliaccio AR, Adamson JW, Stevens CE, Dobrila NL, Carrier CM, Rubinstein P. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96(8):2717–2722. [PubMed] [Google Scholar]
- 42.Page KM, Zhang L, Mendizabal A, et al. Total colony-forming units are a strong, independent predictor of neutrophil and platelet engraftment after unrelated umbilical cord blood transplantation: a single-center analysis of 435 cord blood transplants. Biol Blood Marrow Transplant. 2011;17(9):1362–1374. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Scaradavou A, Smith KM, Hawke R, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–508. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoulars K, Noldner P, Troy JD, et al. Development and validation of a rapid, aldehyde dehydrogenase bright-based cord blood potency assay. Blood. 2016;127(19):2346–2354. doi: 10.1182/blood-2015-08-666990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponce DM, Lubin M, Gonzales AM, et al. The use of back-up units to enhance the safety of unrelated donor cord blood transplantation. Biol Blood Marrow Transplant. 2012;18(4):648–651. doi: 10.1016/j.bbmt.2011.12.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127(26):3450–3457. doi: 10.1182/blood-2016-01-694349. [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri A, Sanz G, Bittencourt H, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28(4):779–786. doi: 10.1038/leu.2013.259. [DOI] [PubMed] [Google Scholar]
- 48.Purtill D, Stevens CE, Lubin M, et al. Association between Nondominant Unit Total Nucleated Cell Dose and Engraftment in Myeloablative Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(11):1981–1984. doi: 10.1016/j.bbmt.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47(7):924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labopin M, Ruggeri A, Gorin NC, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99(3):535–540. doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunstein C, Zhang MJ, Barker J, et al. The effect of inter-unit HLA matching in double umbilical cord blood transplantation for acute leukemia. Haematologica. 2017 doi: 10.3324/haematol.2016.158584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takanashi M, Atsuta Y, Fujiwara Kea. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 55.Brunstein CG, Noreen H, DeFor TE, Maurer D, Miller JS, Wagner JE. Anti-HLA antibodies in double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17(11):1704–1708. doi: 10.1016/j.bbmt.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahi PB, Barone J, Devlin SM, et al. Sustained donor engraftment in recipients of double-unit cord blood transplantation is possible despite donor-specific human leukoctye antigen antibodies. Biol Blood Marrow Transplant. 2014;20(5):735–739. doi: 10.1016/j.bbmt.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brunstein CG, Wagner JE, Weisdorf DJ, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–5634. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka J, Morishima Y, Takahashi Y, et al. Effects of KIR ligand incompatibility on clinical outcomes of umbilical cord blood transplantation without ATG for acute leukemia in complete remission. Blood Cancer J. 2013;3:e164. doi: 10.1038/bcj.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rocha V, Ruggeri A, Spellman S, et al. Killer Cell Immunoglobulin-Like Receptor-Ligand Matching and Outcomes after Unrelated Cord Blood Transplantation in Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2016;22(7):1284–1289. doi: 10.1016/j.bbmt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garfall A, Kim HT, Sun L, et al. KIR ligand incompatibility is not associated with relapse reduction after double umbilical cord blood transplantation. Bone Marrow Transplant. 2013;48(7):1000–1002. doi: 10.1038/bmt.2012.272. [DOI] [PubMed] [Google Scholar]
- 63.Sekine T, Marin D, Cao K, et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood. 2016;128(2):297–312. doi: 10.1182/blood-2016-03-706317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rettman P, Malard F, Legrand N, et al. Impact of KIR/HLA genetic combinations on double umbilical cord blood transplantation outcomes. Results of a French multicentric retrospective study on behalf of the Societe Francophone de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) and the Societe Francophone d’Histocompatibilite et d’Immunogenetique (SFHI) Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.151. [DOI] [PubMed] [Google Scholar]
- 65.Cunha R, Zago MA, Querol S, et al. Impact of CTLA4 genotype and other immune response gene polymorphisms on outcomes after single umbilical cord blood transplantation. Blood. 2017;129(4):525–532. doi: 10.1182/blood-2016-06-722249. [DOI] [PubMed] [Google Scholar]
- 66.van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to non-inherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci U S A. 2009;106(47):19952–19957. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van der Zanden HG, Van Rood JJ, Oudshoorn M, et al. Noninherited maternal antigens identify acceptable HLA mismatches: benefit to patients and cost-effectiveness for cord blood banks. Biol Blood Marrow Transplant. 2014;20(11):1791–1795. doi: 10.1016/j.bbmt.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Rocha V, Spellman S, Zhang MJ, et al. Effect of HLA-Matching Recipients to Donor Noninherited Maternal Antigens on Outcomes after Mismatched Umbilical Cord Blood Transplantation for Hematologic Malignancy. Biol Blood Marrow Transplant. 2012 doi: 10.1016/j.bbmt.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 70.Sanz J, Boluda JC, Martin C, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transplant. 2012;47(10):1287–1293. doi: 10.1038/bmt.2012.13. [DOI] [PubMed] [Google Scholar]
- 71.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 72.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after non-myeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponce DM, Gonzales A, Lubin M, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant. 2013;19(6):904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutman JA, Ross K, Smith C, et al. Chronic graft versus host disease burden and late transplant complications are lower following adult double cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transplant. 2016;51(12):1588–1593. doi: 10.1038/bmt.2016.186. [DOI] [PubMed] [Google Scholar]
- 75.Lazaryan A, Weisdorf DJ, DeFor T, et al. Risk Factors for Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation with Umbilical Cord Blood and Matched Sibling Donors. Biol Blood Marrow Transplant. 2016;22(1):134–140. doi: 10.1016/j.bbmt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogosheske JR, Fargen AD, DeFor TE, et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. Bone Marrow Transplant. 2014;49(1):122–125. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatt V, Lin A, Beyer K, et al. Analysis of Cyclosporine A Levels Supports New Dosing Guidelines in Adult Double-Unit Cord Blood Transplant Recipients to Optimize Immunosuppression Early Post-Transplant. Biol Blood Marrow Transplant. 2016;22(8):1533–1534. doi: 10.1016/j.bbmt.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Bejanyan N, Rogosheske J, DeFor T, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):926–933. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harnicar S, Ponce DM, Hilden P, et al. Intensified Mycophenolate Mofetil Dosing and Higher Mycophenolic Acid Trough Levels Reduce Severe Acute Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):920–925. doi: 10.1016/j.bbmt.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ponce DM, Hilden P, Mumaw C, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199–205. doi: 10.1182/blood-2014-06-584789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46(5):659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ponce DM, Eapen M, Sparapani R, et al. In Vivo T Cell Depletion with Myeloablative Regimens on Outcomes after Cord Blood Transplantation for Acute Lymphoblastic Leukemia in Children. Biol Blood Marrow Transplant. 2015;21(12):2173–2179. doi: 10.1016/j.bbmt.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunstein CG, Weisdorf DJ, Defor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of anti-thymocyte globulin to a non-myeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2(5):e194–203. doi: 10.1016/S2352-3026(15)00045-9. [DOI] [PubMed] [Google Scholar]
- 85.Pascal L, Mohty M, Ruggeri A, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50(1):45–50. doi: 10.1038/bmt.2014.216. [DOI] [PubMed] [Google Scholar]
- 86.Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126(8):1027–1032. doi: 10.1182/blood-2014-09-599241. [DOI] [PubMed] [Google Scholar]
- 87.Zheng C, Luan Z, Fang J, et al. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high-risk or advanced hematological malignancies. Biol Blood Marrow Transplant. 2015;21(4):707–712. doi: 10.1016/j.bbmt.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 88.Admiraal R, Lindemans CA, van Kesteren C, et al. Excellent T-cell reconstitution and survival provided ATG exposure is low or absent after pediatric cord blood transplantation. Blood. 2016 doi: 10.1182/blood-2016-06-721936. [DOI] [PubMed] [Google Scholar]
- 89.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Population pharmacokinetic modeling of Thymoglobulin((R)) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. 2015;54(4):435–446. doi: 10.1007/s40262-014-0214-6. [DOI] [PubMed] [Google Scholar]
- 90.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92(22):10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dahi PB, Ponce DM, Devlin S, et al. “No wash” albumin-dextran dilution for double-unit cord blood transplantation is safe with high rates of sustained donor engraftment. Biol Blood Marrow Transplant. 2014;20(4):490–494. doi: 10.1016/j.bbmt.2013.12.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barker JN, Scaradavou A. Response: the controversy of red cell replete cord blood units. Blood. 2011;118(2):480. [Google Scholar]
- 93.Barker JN, Abboud M, Rice RD, et al. A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15(12):1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17(10):1460–1471. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dahi PB, Perales MA, Devlin SM, et al. Incidence, nature and mortality of cytomegalovirus infection after double-unit cord blood transplant. Leuk Lymphoma. 2015;56(6):1799–1805. doi: 10.3109/10428194.2014.963079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):215–222. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118(20):5689–5696. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill JA, Koo S, Guzman Suarez BB, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638–1648. doi: 10.1016/j.bbmt.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olson AL, Dahi PB, Zheng J, et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant. 2014;20(6):787–793. doi: 10.1016/j.bbmt.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shah GL, Shune L, Purtill D, et al. Robust Vaccine Responses in Adult and Pediatric Cord Blood Transplantation Recipients Treated for Hematologic Malignancies. Biol Blood Marrow Transplant. 2015;21(12):2160–2166. doi: 10.1016/j.bbmt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avery S, Voss MH, Gonzales AM, et al. Importance of day 21 BM chimerism in sustained neutrophil engraftment following double-unit cord blood transplantation. Bone Marrow Transplant. 2012;47(8):1056–1060. doi: 10.1038/bmt.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alsultan A, Giller RH, Gao D, et al. GVHD after unrelated cord blood transplant in children: characteristics, severity, risk factors and influence on outcome. Bone Marrow Transplant. 2011;46(5):668–675. doi: 10.1038/bmt.2010.174. [DOI] [PubMed] [Google Scholar]
- 104.Lazaryan A, Weisdorf DJ, DeFor T, et al. Risk Factors for Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation with Umbilical Cord Blood and Matched Related Donors. Biol Blood Marrow Transplant. 2016;22(1):134–140. doi: 10.1016/j.bbmt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanda J, Morishima Y, Terakura S, et al. Impact of graft-versus-host disease on outcomes after unrelated cord blood transplantation. Leukemia. 2016 doi: 10.1038/leu.2016.288. [DOI] [PubMed] [Google Scholar]
- 106.Arora M, Nagaraj S, Wagner JE, et al. Chronic graft versus host disease following unrelated donor hematopoietic stem cell transplantation: higher response rate in recipients of unrelated donor umbilical cord blood. Biol Blood Marrow Transplant. 2007;13:1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 107.Patel KJ, Rice RD, Hawke R, et al. Pre-engraftment syndrome after double-unit cord blood transplantation: a distinct syndrome not associated with acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;16(3):435–440. doi: 10.1016/j.bbmt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kanda J, Kaynar L, Kanda Y, et al. Pre-engraftment syndrome after myeloablative dual umbilical cord blood transplantation: risk factors and response to treatment. Bone Marrow Transplant. 2013;48(7):926–931. doi: 10.1038/bmt.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daikeler T, Labopin M, Ruggeri A, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2013;121(6):1059–1064. doi: 10.1182/blood-2012-07-445965. [DOI] [PubMed] [Google Scholar]
- 110.Sanz J, Arango M, Carpio N, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. 2014;49(8):1084–1088. doi: 10.1038/bmt.2014.107. [DOI] [PubMed] [Google Scholar]
- 111.Bhatt V, Shune L, Lauer E, et al. Autoimmune hemolysis and immune thrombocytopenic purpura after cord blood transplantation may be life-threatening and warrants early therapy with rituximab. Bone Marrow Transplant. 2016;51(12):1579–1583. doi: 10.1038/bmt.2016.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Page KM, Mendizabal AM, Prasad VK, et al. Posttransplant autoimmune hemolytic anemia and other autoimmune cytopenias are increased in very young infants undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2008;14(10):1108–1117. doi: 10.1016/j.bbmt.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horwitz ME, Frassoni F. Improving the outcome of umbilical cord blood transplantation through ex vivo expansion or graft manipulation. Cytotherapy. 2015;17(6):730–738. doi: 10.1016/j.jcyt.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Thompson PA, Rezvani K, Hosing CM, et al. Umbilical cord blood graft engineering: challenges and opportunities. Bone Marrow Transplant. 2015;50(Suppl 2):S55–62. doi: 10.1038/bmt.2015.97. [DOI] [PubMed] [Google Scholar]
- 115.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124(7):3121–3128. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wagner JE, Jr, Brunstein CG, Boitano AE, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2016;18(1):144–155. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43(5):365–373. doi: 10.1038/bmt.2008.329. [DOI] [PubMed] [Google Scholar]
- 122.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lindemans CA, Te Boome LC, Admiraal R, et al. Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(10):1839–1845. doi: 10.1016/j.bbmt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Popat U, Mehta RS, Rezvani K, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood. 2015;125(19):2885–2892. doi: 10.1182/blood-2015-01-607366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanley PJ, Melenhorst JJ, Nikiforow S, et al. CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo. Sci Transl Med. 2015;7(285):285ra263. doi: 10.1126/scitranslmed.aaa2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–1051. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of Epstein-Barr virus (EBV)-associated post-transplant lymphoma after cord blood transplant using third-party EBV specific cytotoxic T-lymphocytes. Blood. 2010;116(23):5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]


