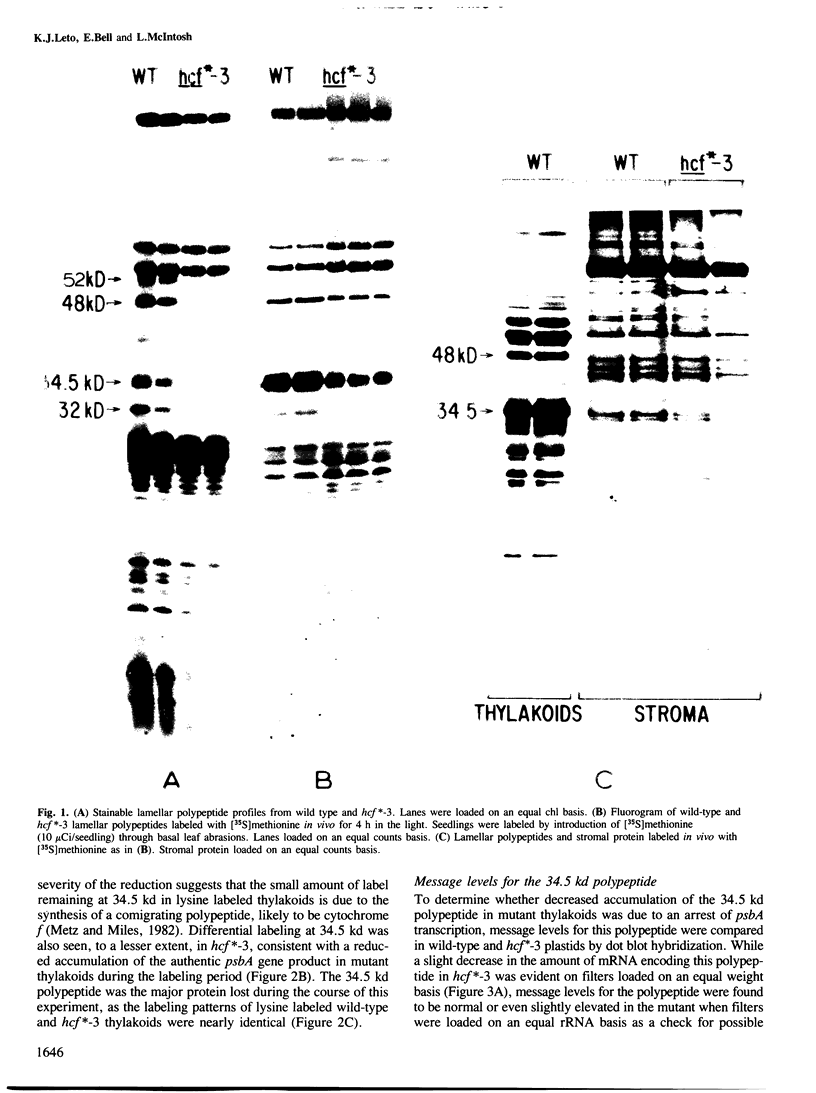
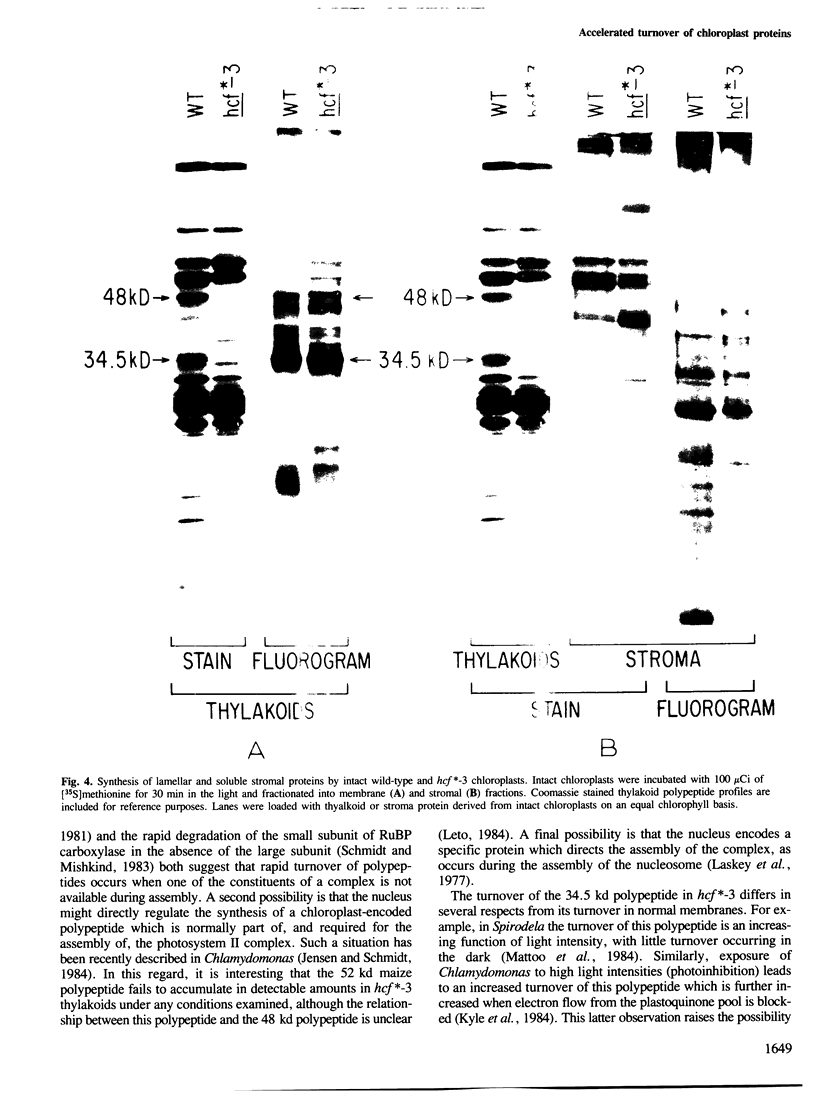
Abstract
We have studied the synthesis and accumulation of a chloroplast-encoded 48 kd chla-reaction center protein and the 34.5 kd `atrazine binding' protein in a nuclear maize mutant which fails to assemble photosystem II reaction centers. The failure of these polypeptides to accumulation in mutant thylakoids is not due to direct nuclear control over their synthesis but is rather due to their specific, accelerated turnover from the thylakoid membrane. The accelerated turnover of these polypeptides in mutant thylakoids is largely independent of illumination conditions, as accelerated turnover occurs in the dark as well as in the light. In contrast to wild type, the 48 kd and 34.5 kd polypeptides are preferentially associated with stroma, rather than grana, lamellae in mutant membranes, suggesting that turnover occurs before these polypeptides become enriched in the grana. The nucleus thus plays a role in the stabilization of these chloroplast-encoded photosystem II reaction center polypeptides.
Keywords: photosystem II polypeptides, thylakoid protein synthesis, thylakoid protein turnover, thylakoid membrane, 34.5 kd polypeptide
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Cuming A. C., Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem. 1981 Aug;118(1):71–80. doi: 10.1111/j.1432-1033.1981.tb05487.x. [DOI] [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J Biol Chem. 1982 Oct 10;257(19):11673–11679. [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Liver mitochondria contain an ATP-dependent, vanadate-sensitive pathway for the degradation of proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1869–1873. doi: 10.1073/pnas.79.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H., Boynton J. E., Gillham N. W. Chloroplast ribosome biogenesis in Chlamydomonas. Selection and characterization of mutants blocked in ribosome formation. J Cell Biol. 1974 Oct;63(1):160–179. doi: 10.1083/jcb.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Morris N. R., Wyllie A. H., Mertz J. E., De Roberts E. M., Gurdon J. B. Chromatin assembly and transcription in eggs and oocytes of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):171–178. doi: 10.1101/sqb.1978.042.01.019. [DOI] [PubMed] [Google Scholar]
- Leto K. J., Keresztes A., Arntzen C. J. Nuclear Involvement in the Appearance of a Chloroplast-Encoded 32,000 Dalton Thylakoid Membrane Polypeptide Integral to the Photosystem II Complex. Plant Physiol. 1982 Jun;69(6):1450–1458. doi: 10.1104/pp.69.6.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K. J., Miles D. Characterization of Three Photosystem II Mutants in Zea mays L. Lacking a 32,000 Dalton Lamellar Polypeptide. Plant Physiol. 1980 Jul;66(1):18–24. doi: 10.1104/pp.66.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J. G., Krueger R. W., Miles D. Chlorophyll-Protein Complexes of a Photosystem II Mutant of Maize : Evidence that Chlorophyll-Protein a-2 and a Chlorophyll-Protein Complex Derived from a Photosystem I Antennae System Comigrate on Polyacrylamide Gels. Plant Physiol. 1984 May;75(1):238–241. doi: 10.1104/pp.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. D., Daniel D. J. Chloroplast Reactions of Photosynthetic Mutants in Zea mays. Plant Physiol. 1974 Apr;53(4):589–595. doi: 10.1104/pp.53.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami E., Watanabe A. Thylakoid membranes: the translational site of chloroplast DNA-regulated thylakoid polypeptides. Arch Biochem Biophys. 1984 Dec;235(2):562–570. doi: 10.1016/0003-9861(84)90230-3. [DOI] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld A., Mattoo A. K., Edelman M. Processing of a chloroplast-translated membrane protein in vivo. Analysis of the rapidly synthesized 32 000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur J Biochem. 1982 May;124(1):125–129. doi: 10.1111/j.1432-1033.1982.tb05914.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V., Coe E. H. Nuclear gene iojap conditions a programmed change to ribosome-less plastids in Zea mays. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2760–2764. doi: 10.1073/pnas.76.6.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Alt J., Herrmann R. G. Localization of the genes for the two chlorophyll a-conjugated polypeptides (mol. wt. 51 and 44 kd) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J. 1983;2(12):2229–2237. doi: 10.1002/j.1460-2075.1983.tb01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Burke J., Autz G., Jagendorf A. T. Bound Ribosomes of Pea Chloroplast Thylakoid Membranes: Location and Release in Vitro by High Salt, Puromycin, and RNase. Plant Physiol. 1981 May;67(5):940–949. doi: 10.1104/pp.67.5.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]