Abstract
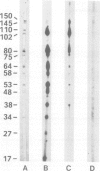
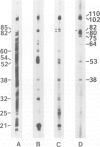
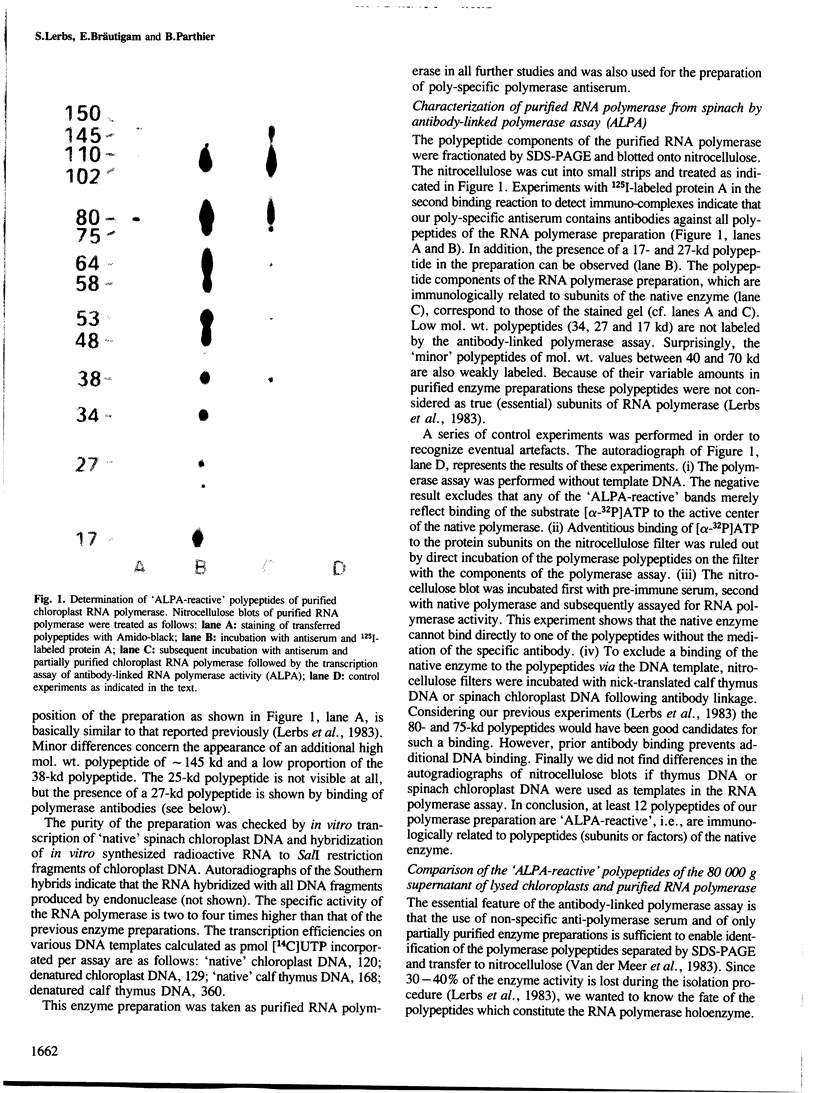
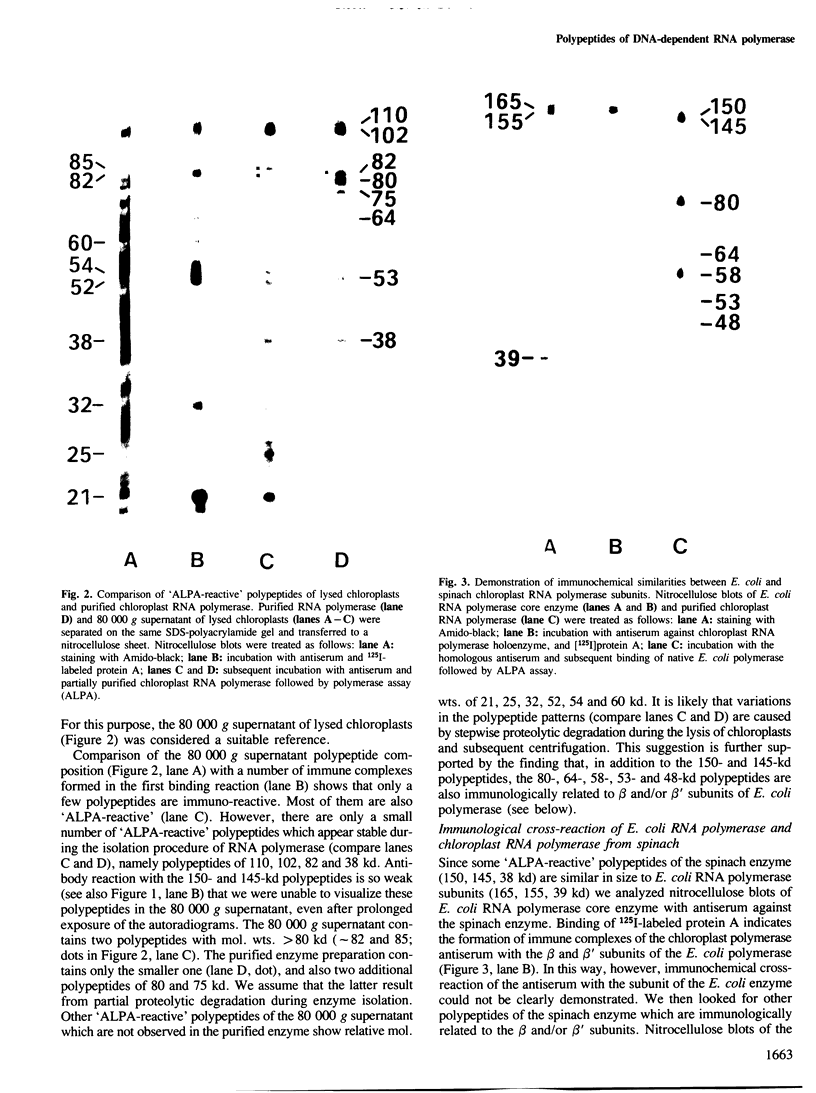
Using solid-phase `Sandwich' immunoassays we studied DNA-dependent RNA polymerase of spinach chloroplasts with regard to (i) polypeptide composition of the multimeric enzyme; (ii) immunological cross-reaction with Escherichia coli RNA polymerase; (iii) sites of synthesis of polymerase polypeptides. Our main results are as follows. (i) All polypeptides of isolated chloroplast RNA polymerase (150, 145, 110, 102, 80, 75 and 38 kd) are labeled by an antibody-linked polymerase assay (ALPA), i.e., they are immunologically related to subunits of the holoenzyme. On the other hand differences in the patterns of `ALPA-reactive' polypeptides of a crude RNA polymerase fraction and of the purified enzyme preparation indicate partial proteolytic degradation of polymerase polypeptides during purification. Thus the 80- and 75-kd polypeptides, which had been previously considered as true RNA polymerase polypeptides, probably result from partial proteolytic degradation. (ii) The 150- and 145-kd polypeptides show immunochemical similarities with the β and/orβ' subunits of E. coli RNA polymerase. (iii) Results from solidphase immunoassay of in vitro translated products of both chloroplast RNA and poly(A)+ (nuclear) RNA suggest that all chloroplast RNA polymerase polypeptides are coded for by the nucleus.
Keywords: chloroplasts, DNA-dependent RNA polymerase, E. coli, immunological cross-reaction, sites of synthesis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Bogorad L. Light-induced increase in the activity of maize plastid DNA-dependent RNA polymerase. Eur J Biochem. 1976 Aug 16;67(2):615–620. doi: 10.1111/j.1432-1033.1976.tb10727.x. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Whitfeld P. R. Cell-free transcription and translation of total spinach chloroplast DNA. Eur J Biochem. 1979 Jan 2;93(1):31–39. doi: 10.1111/j.1432-1033.1979.tb12791.x. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Dron M., Loiseaux S., Mache R. Structure and transcription of the spinach chloroplast rDNA leader region. Nucleic Acids Res. 1982 Nov 11;10(21):6865–6878. doi: 10.1093/nar/10.21.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Hartley M. R. Sites of synthesis of chloroplast proteins. Nature. 1971 Oct 13;233(5320):193–196. [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. High rates of protein synthesis by isolated chloroplasts. Plant Physiol. 1982 Oct;70(4):1107–1114. doi: 10.1104/pp.70.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Jolly S. O., Bogorad L. Preferential transcription of cloned maize chloroplast DNA sequences by maize chloroplast RNA polymerase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):822–826. doi: 10.1073/pnas.77.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., McIntosh L., Link G., Bogorad L. Differential transcription in vivo and in vitro of two adjacent maize chloroplast genes: The large subunit of ribulosebisphosphate carboxylase and the 2.2-kilobase gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6821–6825. doi: 10.1073/pnas.78.11.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. H., Bogorad L. A facile procedure for purifying maize chloroplast RNA polymerase from whole cell homogenates. Biochim Biophys Acta. 1980 Aug 26;609(1):14–30. doi: 10.1016/0005-2787(80)90197-5. [DOI] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muilerman H. G., ter Hart H. G., Van Dijk W. Specific detection of inactive enzyme protein after polyacrylamide gel electrophoresis by a new enzyme-immunoassay method using unspecific antiserum and partially purified active enzyme: application to rat liver phosphodiesterase I. Anal Biochem. 1982 Feb;120(1):46–51. doi: 10.1016/0003-2697(82)90315-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Smith H. J., Bogorad L. The polypeptide subunit structure of the DNA-dependent RNA polymerase of Zea mays chloroplasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4839–4842. doi: 10.1073/pnas.71.12.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J., Dorssers L., Zabel P. Antibody-linked polymerase assay on protein blots: a novel method for identifying polymerases following SDS-polyacrylamide gel electrophoresis. EMBO J. 1983;2(2):233–237. doi: 10.1002/j.1460-2075.1983.tb01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]