SUMMARY
Ingestion is a highly regulated behavior that integrates taste and hunger cues to balance food intake with metabolic needs. To study the dynamics of ingestion in the vinegar fly Drosophila melanogaster, we developed Expresso, an automated feeding assay that measures individual meal-bouts with high temporal resolution at nanoliter scale. Flies showed discrete, temporally precise ingestion that was regulated by hunger state and sucrose concentration. We identify 12 cholinergic local interneurons (IN1) necessary for this behavior. Sucrose ingestion caused a rapid and persistent increase in IN1 interneuron activity in fasted flies that decreased proportionally in response to subsequent feeding bouts. Sucrose responses of IN1 interneurons in fed flies were significantly smaller and lacked persistent activity. We propose that IN1 neurons monitor ingestion by connecting sugar-sensitive taste neurons in the pharynx to neural circuits that control the drive to ingest. Similar mechanisms for monitoring and regulating ingestion may exist in vertebrates.
eTOC PARAGRAPH
A neural circuit that connects sweet taste neurons in the pharynx with local interneurons in the primary taste center allows flies to regulate their ingestion of food by integrating information about hunger state and food quality.
INTRODUCTION
All animals must simultaneously integrate external sensory stimuli with internal state to control behavioral decisions (Davis, 1979; Tinbergen, 1951). One behavior under strict neural and metabolic control is eating, which is regulated both by peripheral sensory detection of food, and internally generated states of hunger and satiety (Brobeck et al., 1943; Burton et al., 1976; Hoebel and Teitelbaum, 1962; Kennedy, 1953; Mayer, 1953; Raubenheimer and Simpson, 1997; Read et al., 1994). Perturbations in these homeostatic systems can lead to obesity, and associated health problems (Morton et al., 2014). In both vertebrates and insects, the optimization of food intake requires tight regulation of behaviors responsive to food quality and hunger state. Once food is ingested, it takes several minutes for enteric nutrient sensing to regulate subsequent eating behavior, which may be too slow to control the amount of food ingested (Dus et al., 2015; Miyamoto et al., 2012; Zukerman et al., 2011). The biology of ingestion is poorly understood.
Mechanisms of peripheral taste processing and the regulation of the hunger state have been studied intensively in vertebrates. Taste receptors in the mouth, and cortical regions in the brain that respond to taste qualities have been identified (de Araujo and Simon, 2009; Barretto et al., 2014; Chandrashekar et al., 2010; Chen et al., 2011; Huang et al., 2006; Mueller et al., 2005; Nelson et al., 2001; Zhao et al., 2003). Activation of sweet cells promote food acceptance in hungry animals, while activation of bitter cells stimulate food avoidance (Mueller et al., 2005; Zhao et al., 2003). Neurons in the hypothalamic neuroendocrine circuits express proopiomelanocortin (POMC), agouti-related peptide (AgRP) and melanocortin receptor (MC4R) and orchestrate ingestion in response to the hunger state of the animal (Aponte et al., 2011; Atasoy et al., 2012; Carter et al., 2013; Fan et al., 1997).
The mechanisms controlling taste and food intake in insects are remarkably similar to those in vertebrates. Drosophila flies detect and evaluate food using taste cells located in the periphery (Stocker, 1994). The insect equivalent of the vertebrate tongue is the labellum on the proboscis. This structure is decorated with taste sensilla that house gustatory neurons, which express gustatory receptors (GRs) that respond to sweet or bitter tastants (Chyb et al., 2003; Clyne et al., 2000; Dahanukar et al., 2001, 2007; Scott et al., 2001; Weiss et al., 2011). Stimulation of sweet taste neurons in the labellum and legs triggers extension of the proboscis in fasted flies, followed by initiation of food intake (Dethier, 1976; Dethier et al., 1956). Upon ingestion, food comes in contact with taste neurons located in the pharynx (Stocker, 1994). The function of these pharyngeal taste neurons is poorly understood, but a subset has been shown to regulate sugar ingestion (LeDue et al., 2015).
Taste neuron afferents from the mouthparts and pharynx target distinct regions of the subesophageal zone (Ito et al., 2014), the taste center of the fly brain (Marella et al., 2006). This is a densely innervated brain structure housing projection neurons, interneurons, and motor neurons that are required for taste acceptance, along with motor circuits that regulate ingestion (Flood et al., 2013; Gordon and Scott, 2009; Kain and Dahanukar, 2015; Manzo et al., 2012; Marella et al., 2012; Miyazaki et al., 2015; Pool et al., 2014). Additionally, neuromodulators including dopamine, serotonin, neuropeptide F, and short-neuropeptide F modulate food intake by altering the activity of sensory neurons that detect food stimuli, or by changing the activity of homeostatic neurons that regulate hunger (Albin et al., 2015; Inagaki et al., 2012, 2014; Root et al., 2011).
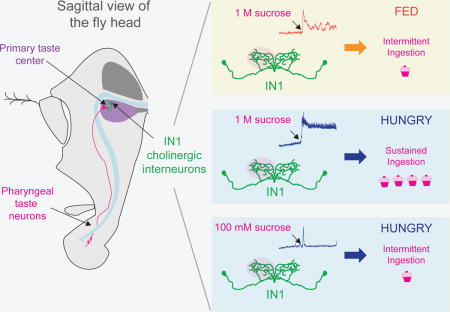
We hypothesized that there must be a rapid sensor of ingestion, interposed between peripheral taste detection and metabolic nutrient sensing. To identify such a sensor in Drosophila, we first developed a high resolution food ingestion assay, Expresso. This system measures ingestion of individual flies in real-time at nanoliter resolution. We used Expresso to show that flies make rapid feeding decisions based on hunger state and sucrose concentration. We identify 12 cholinergic local interneurons (IN1) in the taste center of the fly brain that regulate sucrose ingestion. IN1 neurons receive selective input from sweet taste neurons in the pharynx (LeDue et al., 2015). We used in vivo functional calcium imaging to show that IN1 activity increased rapidly in hungry flies after sucrose ingestion, switching these neurons into a state of sustained activation that lasted for minutes. As the fasted fly continued to ingest sucrose over the course of the experiment, the activity of IN1 neurons progressively decreased. Sustained IN1 activation was strongly attenuated in fed flies offered sucrose, or in fasted flies offered a lower concentration of sucrose. Furthermore, when IN1 neurons were optogenetically activated in fed flies, they ingested food as if they were fasted. Our work provides functional evidence for the existence of a taste circuit that senses food intake via the pharynx, and rapidly integrates taste information with hunger state to control the rate, volume, and timing of ingestion.
RESULTS
Expresso, a High Resolution Feeding Assay
No existing fly feeding assay measures real-time ingestion by single flies at high resolution (Cognigni et al., 2011; Deshpande et al., 2014; Edgecomb et al., 1994; Navawongse et al., 2015). The proboscis extension assay measures the likelihood that a fly will extend its proboscis in response to food (Shiraiwa and Carlson, 2007). Two groups recently automated this manual assay to quantify the interactions of a fly with a food source (Itskov et al., 2014; Ro et al., 2014). These assays provide a proxy for appetitive behavior, but they do not directly measure food ingestion. The capillary feeder (CAFE) assay (Ja et al., 2007) measures ingestion directly from groups of flies, and the the manual-feeding (MAFE) assay measures ingestion bouts of individual flies (Qi et al., 2015). Because both require manual measurements, their temporal resolution and/or throughput are limited.
To investigate factors regulating the temporal dynamics of food ingestion, we developed an automated version of the CAFE called “Expresso,” which allows us to capture nanoliter-volume meal-bouts of single flies simultaneously with high temporal resolution. Expresso consists of multiple single-fly feeding chambers each connected to a sensor bank (Figure 1A and 1B and Figure S1A–S1E). When a fly drinks from the capillary, Expresso analysis software automatically detects individual meal-bouts in real time by measuring the rapid decrease in liquid level (Figure 1C, Figure S1A–S1C, and Movie 1).
Figure 1. Fasting Tunes the Temporal Dynamics of Sucrose Ingestion.
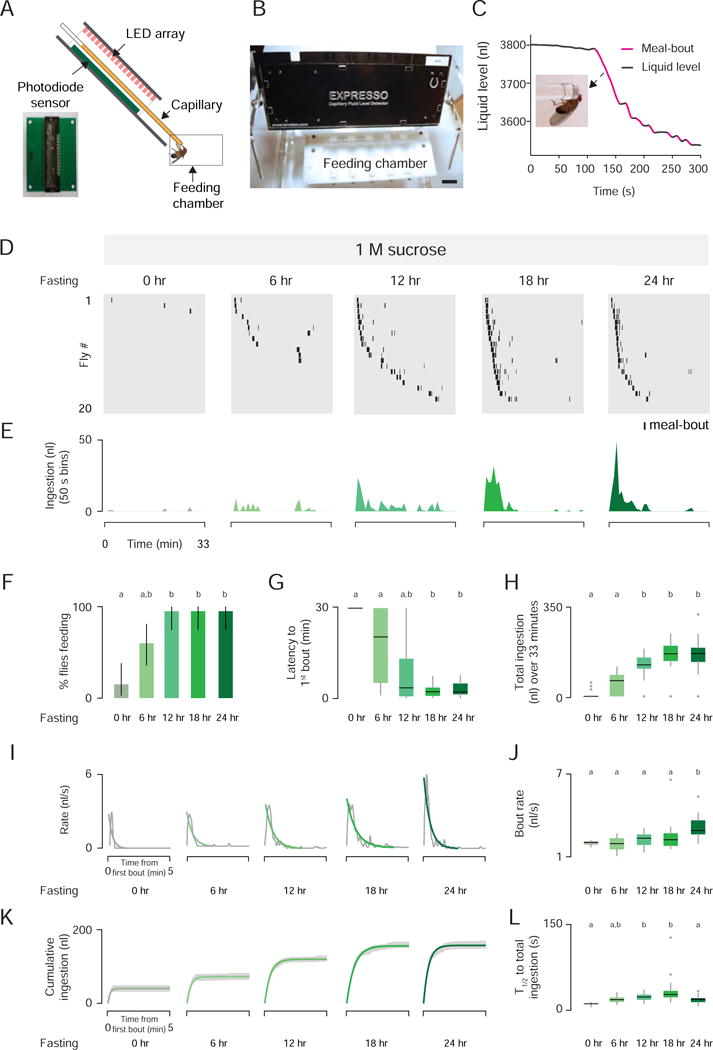
(A) Expresso photodiode sensor (left) and side view schematic of the Expresso system (right). (B) Front view of the Expresso system (Scale bar: 20 mm). (C) Real-time liquid food level measurements of a single 24 hr fasted male fly offered standard liquid food. Detected meal bouts shown in magenta (See also Figure S1 and Movie 1). Note that these data are reprinted in the lower panel in Figure S1C. (D) Meal-bout raster plots of n=20 male flies fasted for the indicated time before testing. (E) Total ingestion of all flies per 50 sec bin over 33 min experiment. (F) Feeding success (Chi-square test, pairwise post hoc comparisons with Fisher’s exact test with Bonferroni correction, p<0.005; population percentage and 95% confidence intervals). (G) Latency to first bout, and (H) total sucrose ingestion [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer (Nemenyi) test, p< 0.05; n=20] (I) Gray lines show raw data, green lines show the nonlinear regression. (J) Bout rate [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer (Nemenyi) test, p< 0.05]. (K) Green lines show the nonlinear regression; gray shades indicates ± SEM. (L) Time to half-total intake (T1/2) [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer and (Nemenyi) test, p< 0.05; n=20]. Here and throughout the paper, bar plots or box plots labeled with different letters in a given figure panel are significantly different.
Accuracy was verified by manually annotating videos of fly ingestion while ingestion volume was simultaneously monitored by the Expresso. The concordance between Expresso (hardware and software) and the human observer was 94% for all bouts and 100% for bouts larger than 10 nl (Figure S1D). Meal-bout volumes of fasted flies varied between 6 and 200 nl, and volume was highly correlated with duration (Figure S1E). We compared the feeding behavior of male and female flies fasted for 24 hr, and confirmed that flies of both sexes consumed liquid food robustly (Ja et al., 2007) (Figure S1F and S1G). We also quantified several measures that captured the dynamics of food ingestion (Figure S1H–S1N). Female flies showed a small increase in latency, but consumed food more rapidly than males (Figure S1K and S1L). Fasted flies concentrated their meal-bouts at the beginning of the experiment and rapidly reached half-total ingestion (median: 34.77 s in females, and 56.44 s in males) (Figure S1M and S1N).
Temporal Dynamics of Ingestion are Dramatically Altered by Hunger and Sucrose Concentration
Hunger state and the quality of food offered affect ingestion (Ostlund et al., 2013; Spector et al., 1998). We examined this by progressively fasting flies and monitoring sucrose ingestion. Most of the fed flies (0 hr fasted) did not consume 1 M sucrose. As fasting time increased, more flies initiated feeding with shorter latencies and higher rates, resulting in greater 1 M sucrose consumption (Figure 1D–1H). 24 hr fasted flies consumed 1 M sucrose at much higher rates than fed flies (Figure 1I and 1J). These hungry flies concentrated their meal-bouts within the first min (median half-total ingestion: 11.31 to 27.74 s) (Figure 1K and 1L), and their rate of ingestion decreased rapidly thereafter (Figure 1I and 1J).
Next, we tested the effects of sucrose concentration on ingestion dynamics. At all sucrose concentrations tested, the number of 24 hr fasted flies feeding was the same (Figure 2C), but feeding dynamics were strikingly different (Figure 2A–2J). Flies consumed greater volumes of 100 mM sucrose than 1 mM, 10 mM, and 1 M sucrose, indicating that both the taste, caloric content, and amount of sucrose determine the total amount of ingestion at longer time scales (Figure 2D–2F). At lower sucrose concentrations, flies consumed short meal-bouts spaced over the 33 min experiment, and the overall volume consumed was low (Figure 2D–2F). As sucrose concentration increased, the intensity of ingestion increased, leading to higher bout volumes and ingestion rates (Figure 2F–2H). This higher intensity feeding was also temporally concentrated, with animals offered 1 M sucrose reaching median half-total ingestion after 18.25 s (Figure 2I and 2J). In contrast, median half-total ingestion at lower sucrose concentrations ranged from 153 s to 520 s (Figure 2I and 2J).
Figure 2. Fasted Flies Adjust Ingestion Dynamics According to Sucrose Concentration.
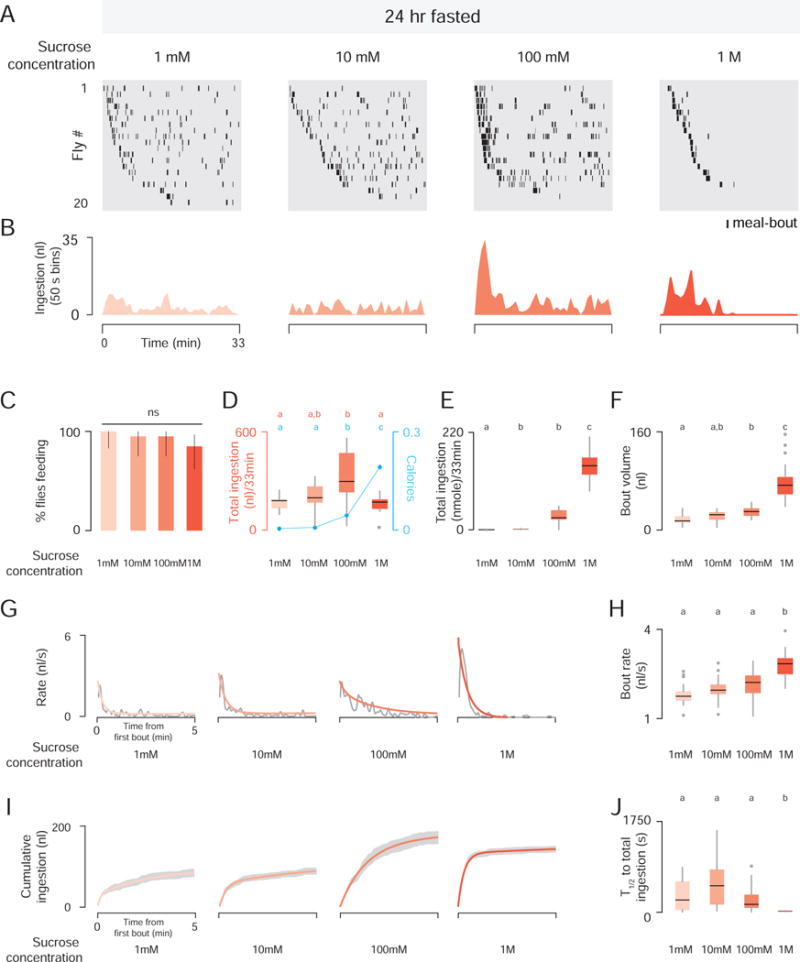
(A) Meal-bout raster plots of 24 hr fasted male flies (n=20) offered sucrose at the indicated concentration. (B) Total ingestion of all flies per 50 sec bin over 33 min experiment. (C) Feeding success (Chi-square test, ns; population percentage and 95% confidence intervals). (D) Total ingestion in volume and calories (cyan, mean ± SEM). (E) Total ingestion in nmoles. (F) Bout volume [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer and (Nemenyi) test, p< 0.05; n=20]. (G) Gray lines show the raw data, orange lines show the nonlinear regression. (H) Bout rate [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer and (Nemenyi) test, p< 0.05; n=20]. (I) Nonlinear regression (orange) ± SEM (gray). (J) Time to half-total intake (T1/2) [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer and (Nemenyi) test, p< 0.05; n=20].
A Genetic Screen for Neurons that Regulate Ingestion
Post-ingestive nutrient-sensing pathways compute the caloric content of ingested food and regulate subsequent feeding. But this response is relatively slow, developing over a period of minutes (de Araujo et al., 2008; Dus et al., 2011; Miyamoto et al., 2012; Zukerman et al., 2011). We hypothesized that flies have a mechanism to rapidly integrate hunger state with sucrose concentration to regulate moment-to-moment ingestion. This mechanism would operate between the initial sensing of sweet food and the post-prandial rise in circulating sugar levels.
To screen for neurons required for rapid modulation of ingestion, we genetically inactivated subpopulations of neurons and monitored ingestion dynamics. Using the Gal4/UAS system (Brand and Perrimon, 1993), we expressed tetanus toxin light chain, which blocks evoked synaptic transmission by cleaving neuronal synaptobrevin (Sweeney et al., 1995). Of 105 viable lines tested in the conventional CAFE assay (Ja et al., 2007), two lines reproducibly showed reduced food intake, and we focused on 57F03-Gal4 (hereafter 57F03>) (Figure 3A). 57F03> tetanus toxin flies ingested less liquid and solid food than genetic controls (Figure S2A–2C), but showed normal proboscis extension to food and normal locomotor/geotaxis behaviors (Figure S2D–S2F).
Figure 3. Genetic Silencing Screen Identifies Neurons that Regulate Ingestion Dynamics.
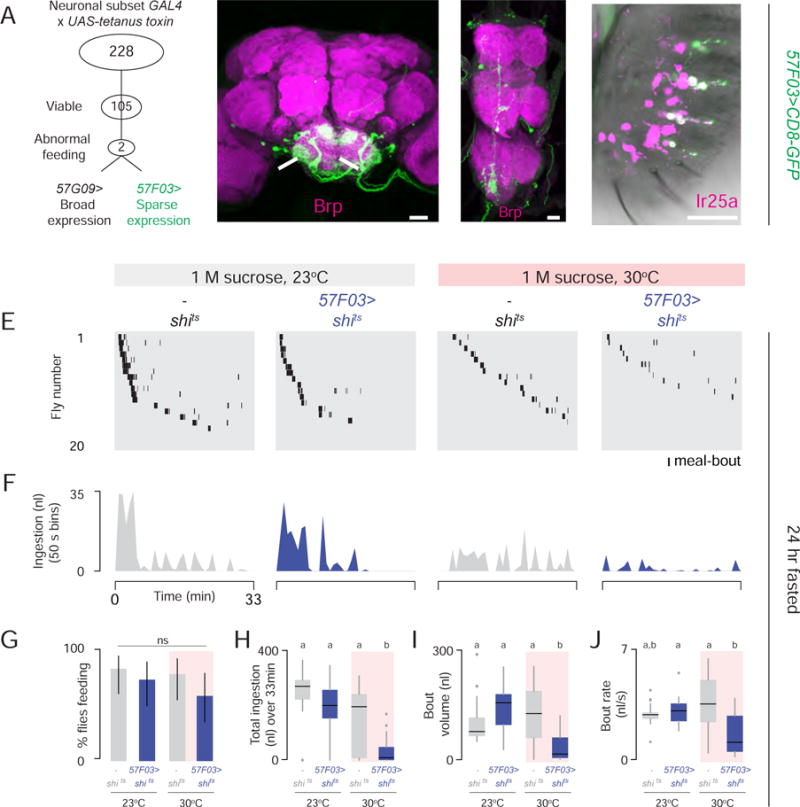
(A) Overview of neuronal silencing screen. (B–D) 57F03>CD8-GFP visualized by GFP (green) immunofluorescence in the brain (B), ventral nerve cord (C), and labellum (D). Magenta shows Brp staining of neuropil in B, C, and anti-Ir25a antibody in D (Scale bars: 20 μm) (See also Figure S2). (E–J) Expresso measurement of 24 hr fasted flies of indicated genotypes consuming 1 M sucrose (n= 20). (E) Meal-bout raster plots. (F) Total ingestion of all flies per 50 sec bin over 33 min experiment. (G) Feeding success (Chi-square test, pairwise post hoc comparisons with Fisher’s exact test with Bonferroni correction, ns; population percentage and 95% confidence interval). (H) Total sucrose ingestion volume, (I) bout volume, and (J) bout rate [in H–J, Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer (Nemenyi) test, p< 0.05; n=20].
Using a membrane-tethered green fluorescent protein (GFP), we detected 57F03> in small numbers of neurons in the brain (Figure 3B), ventral nerve cord (Figure 3C), and taste neurons in the labellum that express the chemosensory receptor Ir25a (Benton et al., 2009) (Figure 3D). 57F03> also labeled non-neuronal, non-glial cells in legs, maxillary palp, and antenna (data not shown). In the brain, 57F03> expression was limited to the subesophageal zone (Figure 3B).
Because the original screen used chronic synaptic silencing, we asked whether acute neuronal silencing also disrupted ingestion. We conditionally inactivated 57F03> neurons with the temperature-sensitive dominant-negative form of dynamin (shits). At temperatures above 29°C, shits reversibly inhibits synaptic transmission by blocking membrane recycling (Kitamoto, 2001). The percentage of 24 hr fasted 57F03>shits flies that consumed 1 M sucrose at the permissive temperature of 23°C was normal (Figure 3E–3G). However, silencing these neurons at the restrictive temperature of 30°C caused 57F03>shits flies to alter the temporal structure of feeding, resulting in a dramatic reduction in total ingestion, bout volume, and ingestion rate (Figure 3H–3J).
Cholinergic Local Taste Interneurons (IN1) Control the Dynamics of Sucrose Ingestion
To clarify which 57F03> neurons are functionally required for food ingestion, we inhibited the activity of Gal4 in selected tissues and cell types using the Gal80 repressor (Lee and Luo, 1999). 57F03> tetanus toxin flies expressing pan-neuronal Elav-Gal80 (Yang et al., 2009) restored normal feeding, confirming that the 57F03> effect on feeding is due to the silencing of neurons (Figure S2G and S2H). We ruled out a role for taste neurons in the labellum, interneurons in the ventral nerve cord and motor neurons in the brain using Ir25a-Gal80, Tsh-Gal80 (Clyne and Miesenbock, 2008), and Vglut-Gal80 (Bussell et al., 2014) respectively (Figure S2G and S2H). In these animals, 57F03> expression was limited to ~25 local interneurons in the brain (Figure S2H). To identify the minimum number of neurons responsible for the food ingestion defect, we screened the Janelia Farm FlyLight Gal4 Collection (Jenett et al., 2012) for expression patterns that overlap with 57F03> (Figure 4A) and identified 83F01>. We used FLP recombinase-mediated recombination to label the 57F03> and 83F01> intersection. FLP controls site-specific recombination between FRT sites (FLP recombination target sites) (Golic and Lindquist, 1989). We confirmed that this method recapitulated 57F03> expression (Figure S3A and S3B). In the brain, the 57F03> and 83F01> intersection selectively labeled ~12 interneurons (Figure 4A) with projections limited to the subesophageal zone (Figure 4B and 4C). IN1 (for “ingestion neurons”) processes cross the midline and arborize in both ipsilateral and contralateral sides of the subesophageal zone (Figure 4D). The IN1 intersection strain also labeled a pair of ascending neurons in the ventral nerve cord (Figure S3C), but the functional relevance of this structure for ingestion was previously excluded (Figure S2G and S2H). The percentage of 24 hr fasted IN1>tetanus toxin flies that consumed 1 M sucrose in Expresso did not differ from controls (Figure 4E–4G), but total ingestion, meal-bout volume, and ingestion rate were dramatically reduced (Figure 4H–4J).
Figure 4. IN1 Cholinergic Local Taste Interneurons Regulate Sucrose Ingestion.
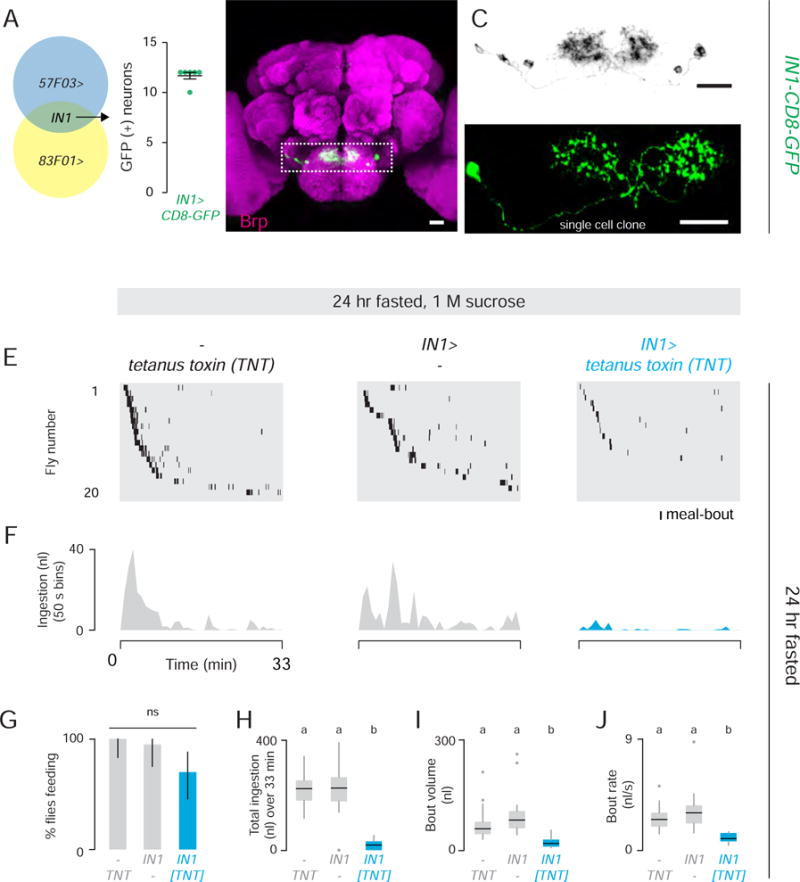
(A) Schematic of the 57F03> and 83F01> (IN1) intersection (left). Quantification of GFP-positive cells in the IN1 intersection (n=6 brains) (right). (B) IN1>CD8-GFP (GFP, green) Neuropil (Brp, magenta) (Scale bar: 20 μm) (See also Figure S3). (C) Gray scale image of the boxed region in B (Scale bar: 20 μm). (D) A single IN1 neuron labeled by GFP (Scale bar: 20 μm). (E–J) Expresso measurement of 24 hr fasted flies of the indicated genotypes consuming 1 M sucrose (n= 20). (E) Meal-bout raster plots of n=20 males. (F) Total ingestion of all flies per 50 sec bin over 33 min experiment. (G) Feeding success (Chi-square test, pairwise post hoc comparisons with Fisher’s exact test with Bonferroni correction, ns; population percentage and 95% confidence interval are shown). (H) Total ingestion, (I) bout volume, and (J) bout rate. [Kruskal-Wallis test, pairwise post hoc comparisons using Tukey and Kramer (Nemenyi) test, p< 0.05; n=20].
To characterize the polarity and neurochemistry of IN1 neurons, we labeled them with markers enriched in axons (GFP fused to synaptotagmin) or dendrites (GFP fused to Dscam) (Wang et al., 2004; Zhang et al., 2002). Synaptotagmin-GFP and Dscam-GFP labeled overlapping areas within the subesophageal zone (Figure S4A and S4B). IN1 neurons did not express dopaminergic, serotonergic, glutamatergic, or GABAergic markers (data not shown). However, IN1> expression was blocked by Gal80 expression in cholinergic cells, suggesting these neurons are cholinergic (Figure S4C and S4D). Acetylcholine is the major excitatory neurotransmitter in the Drosophila brain.
IN1 Interneurons Receive Presynaptic Input from Sugar-Sensitive Neurons in the Pharynx
Our behavioral and anatomical experiments suggest that IN1 neurons constitute a previously uncharacterized class of cholinergic local interneurons in the fly taste center required for sustained sucrose ingestion. We characterized sensory input to IN1 from the taste periphery (Figure 5A and 5B) with Gr-Gal4 strains to label neurons expressing sweet (Chyb et al., 2003; Dahanukar et al., 2007; Miyamoto et al., 2012) or bitter taste receptors (Weiss et al., 2011) The axons of bitter and sweet taste neurons with cell bodies in the labellum did not overlap with IN1 neurons (Figure 5C–5F, 5I–5L; right panels). In contrast, afferents from sugar-sensitive taste neurons that line the labral organ of the pharynx overlapped with IN1 interneuron arbors (Figure 5E–5H, 5K–5N; left panels).
Figure 5. IN1 Neurons Receive Presynaptic Input from Sugar-Sensitive Neurons in the Pharynx.
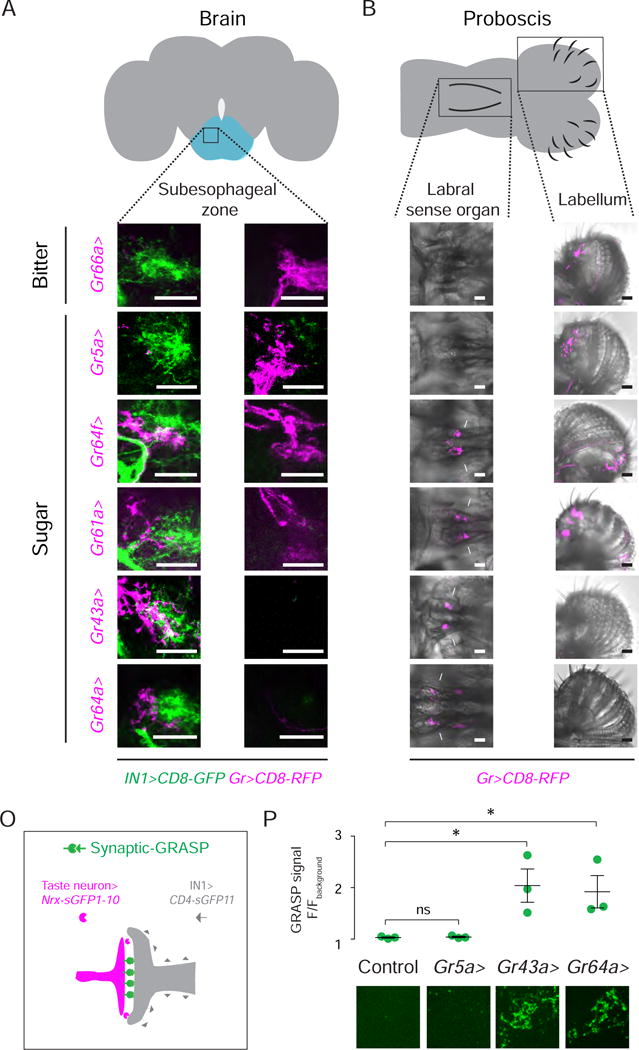
(A–B) Fly brain (grey) and proboscis (grey) schematics. Boxes indicate the taste center (subesophageal zone; blue), the labral sense organ, and the labellum. (C–H) Taste neuron afferents (magenta) and IN1 interneurons (green) in the anterior (left) and posterior (right) subesophageal zone (Scale bar: 20 μm) (See also Figure S4). (I–N) Taste neurons labeled by Gr-Gal4 lines in the labral sense organ (left panel, magenta) and in the labellum (right panel, magenta) (Scale bar: 20 μm). (O) Schematic of synaptic GRASP between IN1 interneurons (grey) and taste neurons (magenta). (P) Normalized GRASP signal (Scale bar: 1μm) (Unpaired t-test; mean ± SEM, n=3).
To determine whether IN1 neurons are synaptically connected to afferent sensory axons from the pharynx, we used a modification of the GFP Reconstitution Across Synaptic Partners (GRASP) method (Feinberg et al., 2008) to label synaptic sites selectively (Chen et al., 2014) (Figure 5O). There was significant GRASP signal between IN1 interneurons and sugar-sensitive pharyngeal taste neurons labeled by Gr43a and Gr64a (Figure 5P). We detected no GRASP signal between IN1 interneurons and Gr5a sweet taste neurons on the labellum (Figure 5P). We conclude that IN1 interneurons receive specific input from sweet taste neurons in the pharynx.
IN1 Activation by Sucrose Ingestion and its Modulation by Hunger State
We next asked whether IN1 neurons respond to sucrose ingestion, and if this activation is altered by satiation, as predicted by the behavioral and anatomical results presented above. We used in vivo two-photon imaging of head-fixed flies expressing the genetically-encoded calcium sensor GCaMP6s in IN1 neurons (Figure S5A and S5B). Rapid volumetric imaging of the GCaMP6s fluorescence in the IN1 neuronal arbor was monitored while 24 hour fasted flies were offered a drop of 1 M sucrose or water (Figure 6A and 6D; Movie 2). IN1 neurons did not respond when the proboscis touched the sucrose drop or when flies ingested water, but were strongly activated when 1 M sucrose was ingested (Figure 6A–6D). Because we did not image GCaMP6s activity in individual IN1 cell bodies, we cannot exclude the possibility that only some of these neurons responded to sucrose ingestion. In the example shown in Figure 6A and 6B, the fasted fly consumed the entire 20 nl drop in one continuous bout. As the fly drank, GCaMP6s activity rose rapidly and remained elevated throughout the ingestion bout, which lasted on average 10.4 s per fly. Remarkably, this activity persisted after the fly stopped ingesting 1 M sucrose (Figure 6A and 6B).
Figure 6. IN1 Activity Integrates Hunger State and Sucrose Concentration.
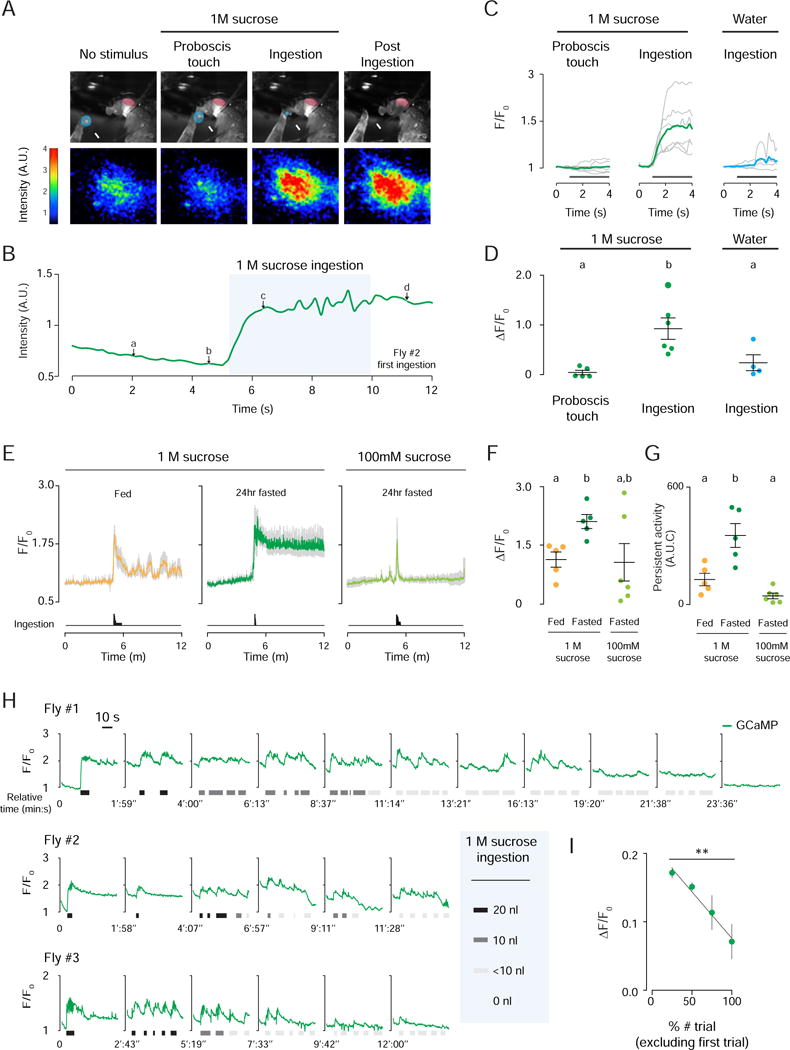
(A) Representative IN1>GCaMP6s responses recorded in the same 24 hr fasted female before, during, and after 1 M sucrose ingestion. Still images captured by the video camera (ad; see B for corresponding raw traces), with the eye pseudocolored in red and 1 M sucrose drop in blue (top). Heat map of IN1 neuron activity in response to indicated stimuli (bottom) (See also Figure S5A and S5B and Movie 2). (B) Trace of IN1 fluorescence in arbitrary units (A.U.), with letters a–d indicating the corresponding still image and activity heat maps in A. (C) Fluorescence traces are normalized using F0. The gray lines show data from individual flies; bold green and blue lines show average traces for the indicated stimuli. (D) Peak of stimulus-evoked IN1 neuron activity (One-way ANOVA with pairwise post hoc Bonferroni test; p<0.05; mean ± SEM, n=4–6). (E) Normalized IN1 neuron activity in fed or 24 hr fasted flies to 1 M or 100 mM sucrose. Mean traces for indicated stimuli and conditions (colored lines) ± SEM (gray). Summed histogram of ingestion duration (bottom). (F) Peak of stimulus- evoked IN1 neuron responses to indicated stimuli and conditions (Unpaired t-test with Bonferroni correction; p<0.05; mean ± SEM, n=5–6). (G) Area under the curve measurement of the GCaMP6s signal showing the persistent activity of IN1 neurons to indicated stimuli and conditions (Student’s t-test with Bonferroni correction; p<0.05; mean ± SEM, n=5–6). (H) Normalized IN1 neuron responses to repeated 1M sucrose stimulation. GCaMP6s fluorescence traces and episodes of 1 M sucrose ingestion are plotted per trial (I) Normalized IN1 responses binned by the percent number of trials, but excluding the first ingestion (F-test for linear regression, p<0.01; mean ± SEM, n=3).
To explore the effects of hunger state and sucrose concentration on IN1 responses and persistent activity, we measured GCaMP6s fluorescence in IN1 neuron processes in fed and fasted flies offered a single 20 nl drop of 1 M sucrose. 1 M sucrose ingestion elicited significantly higher stimulus-evoked activity in IN1 neurons in fasted compared to fed flies (Figure 6E–6G). In fasted flies, 1 M sucrose-evoked activity of IN1 neurons lasted for 7 minutes, decaying to 57% of the stimulus-evoked maximum F/F0 (SEM: ± 5.7). However in fed flies, this persistent activity was not sustained. When fasted flies ingested 100 mM sucrose, evoked GCaMP6s response was similar in amplitude to 1 M sucrose ingestion but the response was transient, with fluorescence quickly returning to baseline (Figure 6E). Thus, both hunger state and sucrose concentration modulate IN1 activity.
If IN1 neurons regulate the intensity of sucrose ingestion in hungry flies, IN1 activity should decrease steadily as cumulative sucrose ingestion satiates the fly. To test this hypothesis, we offered 24 hr fasted flies 20 nl drops of 1 M sucrose repeatedly. In these real-time sucrose ingestion/imaging experiments, the first ingestion event induced a large and persistent increase in GCaMP6s activity in IN1 neurons (Figure 6H). Subsequent ingestion events evoked neuronal activity on top of this elevated baseline. As the experiment progressed, the fly became less likely to consume the sucrose stimulus (Figure 6H). Initial full-volume ingestion events transitioned into half-volume events, and concluded with a series of minute-volume or failed ingestions. In parallel, evoked IN1 responses decreased across these trials (Figure 6H and 6I).
Activation of IN1 Suffices to Trigger Sucrose Ingestion
Fed flies ingest little or no food. If the activity of IN1 neurons regulates sucrose ingestion in hungry flies, artificial activation of these neurons should suffice to trigger food ingestion in satiated animals. We expressed the channelrhodopsin variant ReaChR (Inagaki et al., 2013) in sweet taste neurons or IN1 neurons (Figure S6A–S6C), and monitored behavior in response to photoactivation. Photoactivation of sweet taste neurons (Gr5a>ReaChr) reliably triggered robust proboscis extension that was temporally controlled by the light pulse (Figure 7A and 7B; Movie 3). In contrast, IN1> ReaChR flies did not show proboscis extension upon photoactivation when food was not present (Figure 7A and 7B). However, when flies were placed in close proximity to a drop of 1 M sucrose (Figure S6D), photoactivation increased the probability of food ingestion in IN1> ReaChR flies, but not in controls (Figure 7C–7H). Out of 15 IN1>ReaChr flies tested, 11 exhibited intermittent ingestion throughout the light stimulation period (Figure 7F). We estimated the ingested volume as the fly fed (Figure S6E and S6F). Photoactivation increased the volume and duration of 1 M sucrose ingestion in IN1> ReaChR flies but not in controls (Figure 7I and 7J). Light-stimulated ingestion events of IN1> ReaChR flies were not time-locked to photoactivation. Instead, IN1 activation increased the probability that flies ingest 1 M sucrose.
Figure 7. Photoactivation of IN1 Neurons Stimulates Sucrose Ingestion in Fed Flies.
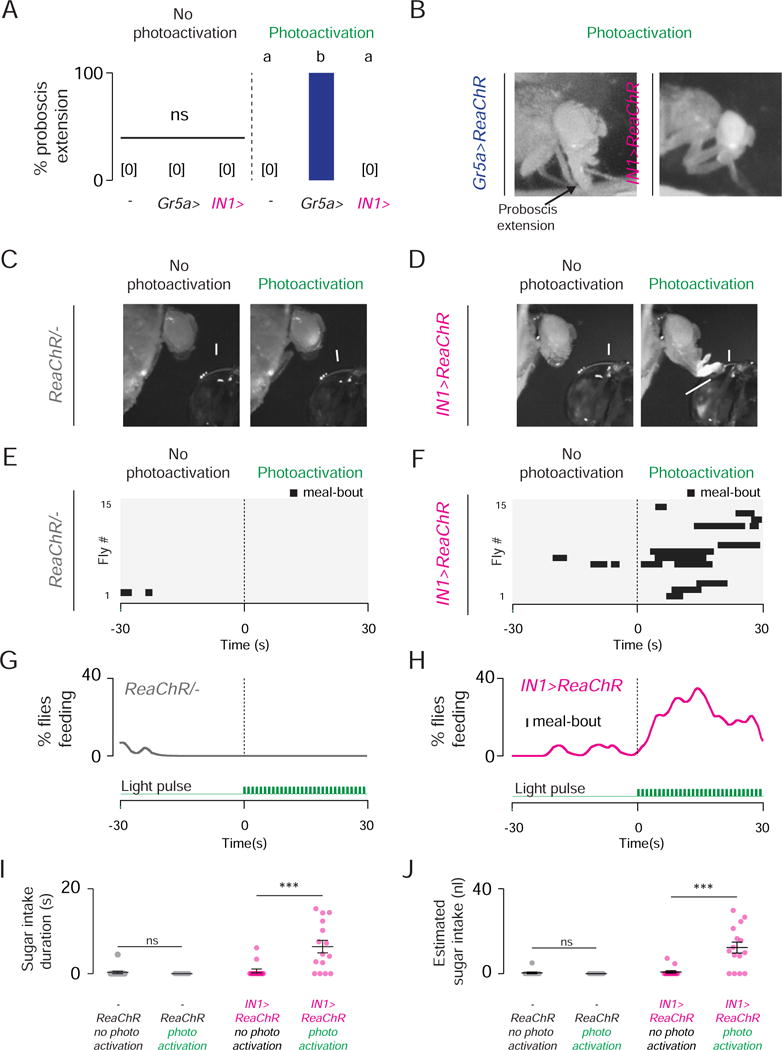
(A) Proboscis extension response of indicated genotypes in response to photoactivation in the absence of food (Chi-square test, ns; population percentage and 95% confidence intervals n=15). (B) Gr5a–ReaChR and IN1–ReaChR male flies during photoactivation, no food present. (C–D) A fed (0 hr fasted) control (C) and IN1–ReaChR (D) male flies before (left) and during (right) photoactivation (See also Figure S6A–S6C and Movie 3). (E–F) Raster plots of 1 M sucrose ingestion by control (E) and IN1–ReaChR (F) male flies before (left) and during (right) photoactivation (n=15 per genotype). The horizontal black bars show the time and duration of individual meal bouts ingested by single flies. (G–H) Time series data of 1 M sucrose ingestion by control (G) and IN1–ReaChR (H) male flies before (left) and during (right) photoactivation (n=15 for each genotype). Population averages are smoothened by the LOWESS method and plotted against time. The green traces represent the light pulses used for photoactivation (bottom). (I) Duration of 1 M sucrose ingestion of indicated genotypes and conditions (Wilcoxon signed-rank test, ns and p<0.001; mean ± SEM, n=15) (J) Estimated volume measurements of 1 M sucrose ingestion of indicated genotypes and conditions (Wilcoxon signed-rank test, ns and p<0.001; mean ± SEM, n=15) (See also Figure S6D–S6F).
DISCUSSION
The neural circuits that integrate taste, hunger, and metabolism to control food ingestion remain poorly understood (Dethier, 1976; Morton et al., 2006). We have discovered 12 cholinergic interneurons in the taste processing center of the fly brain that tune the dynamics of sucrose ingestion. Our data support a model in which sweet taste neurons in the pharynx project to the subesophageal zone, where they contact IN1 interneurons. When a hungry animal first drinks sucrose, pharyngeal sweet taste neurons are stimulated and activate IN1 neurons. In hungry flies, this leads to persistent IN1 activity, which in turn sustains ingestion. IN1 activity in fed flies or those offered a lower sucrose concentration is attenuated and these animals do not show sustained ingestion. As the fly becomes satiated, IN1 neurons become insensitive to sucrose, which decreases the drive to ingest, and results in shorter ingestion episodes. We propose that the IN1 circuit provides a fast feedback mechanism to regulate sucrose ingestion by integrating taste and hunger signals.
Functional Characterization of a Drosophila Ingestion Circuit
Although previous work investigating the dynamics of fly feeding used proboscis extension as a proxy for food intake (Inagaki et al., 2012, 2014; Kain and Dahanukar, 2015; Marella et al., 2012), this pre-ingestive response is not a reliable predictor of ingestion (Deshpande et al., 2014). In classic work on the blowfly, Vincent Dethier suggested that food consumption is controlled by factors that stimulate ingestion, rather than those that act on peripheral taste perception or post-ingestive nutrient-sensing (Dethier et al., 1956). Since IN1 neurons are activated when sucrose is ingested, and their activity is modulated by both hunger state and taste stimuli, they are uniquely situated to modulate ingestion. In support of this, our functional imaging experiments revealed that a single bout of sucrose ingestion by hungry flies induced activation of IN1 neurons that persisted for at least 7 min. Long-lasting changes in the activity of hypothalamic AgRP and POMC neurons have been previously linked to the regulation of state-dependent feeding decisions in mice (Betley et al., 2015; Chen et al., 2015). Likewise, persistent neural activity in IN1 neurons may prepare the nervous system in hungry flies for further food ingestion.
What are the origins of the persistent activity seen in IN1 neurons? Intrinsic or extrinsic factors may contribute. The area where IN1 processes arborize receives input from dopaminergic, serotonergic, and peptidergic afferents (data not shown). These neuromodulatory circuits may be sensitive to satiety state (Albin et al., 2015; Marella et al., 2012) and help to prolong IN1 activity elicited by sucrose ingestion in fasted animals. It is plausible that once the fly reaches satiety, neuromodulatory activity is tempered and IN1 neurons return to their basal state. Alternatively, IN1 neurons may have unique intrinsic biophysical features that sustain their activity after sucrose stimulation. A complete characterization of IN1 membrane excitability and circuitry is necessary to investigate these ideas.
Connectivity in Feeding Circuits
Recent studies have identified a number of functionally distinct populations of neurons in the fly taste circuit that regulate different aspects of food intake behavior. Neuropeptide F and dopamine signaling enhance the responsiveness of labellar taste sensory neurons in hungry flies, and increase the probability of initiating food intake (Inagaki et al., 2012, 2014; Marella et al., 2012). However, when labellar sweet taste perception is perturbed, ingestion is not affected (LeDue et al., 2015). Thus, labellar taste neuron circuitry likely regulates initial food evaluation, but not the later decision to ingest food. In contrast, IN1 activity is required specifically to sustain sucrose ingestion. This makes IN1 neurons functionally, and anatomically distinct from all previously described neural pathways that mediate taste responsiveness and feeding initiation. Recent work has identified interneurons that regulate the feeding motor program (Flood et al., 2013), GABAergic neurons that suppress nonselective ingestion (Pool et al., 2014), and motor neurons that regulate fluid ingestion (Manzo et al., 2012). How these neurons connect taste sensory input to the motor output of ingestion, as well as interpreting top-down information about hunger state is not known. We propose that IN1 neurons participate in this circuit as a key node that governs rapid food intake decisions.
A Conserved Mechanism for Ingestion-Sensing Via Pharyngeal Taste?
In vertebrates, taste cells are present not only in the tongue, but also in other organs including the pharynx (Henkin and Christiansen, 1967; Ishimaru et al., 2005; Travers and Nicklas, 1990). In zebrafish, the sweet taste receptors T1R2/T1R3 are expressed in the pharynx, as well as in the lip and gill raker (Ishimaru et al., 2005). Expression of T1R2/T1R3 in the pharynx has not been investigated in mammals, but it is known that the cranial nerve fibers innervating pharyngeal taste cells respond to sugar (Frank, 1991; Hanamori et al., 1988). These neurons project to the nucleus of solitary tract (NST) in the hindbrain (Carleton et al., 2010; Frank, 1991; Hanamori et al., 1988). The NST also receives peripheral satiety signals transmitted via vagal nerve fibers, indicating that this brain region is involved in many regulatory processes that regulate food intake including the decision to ingest (Carleton et al., 2010; Morton et al., 2006). Based on the similarities of insect and vertebrate taste processing systems, we speculate that interneuron populations in the NST with the physiological properties of IN1 neurons may exist. Taste perception by the pharynx would permit rapid assessment of food intake volume and quality, and provide real-time feedback to the central brain to regulate ingestion in vertebrates as well as in insects.
EXPERIMENTAL PROCEDURES
Detailed methods associated with all procedures below are available in the Supplemental Experimental Procedures.
Fly Strains
Simplified genotypes of fly strains are provided in the main text and figures for clarity.
Behavior
All behavioral assays were carried out in a 23–25°C incubator, under a 12 hr light: 12 hr dark cycle (lights on at 9 AM) and 50–60% relative humidity unless stated otherwise. All assays were performed at Zeitgeber time 5–10 with 3–10 day old males unless stated otherwise.
CAFE Food Intake Screen
This assay was modified from earlier studies (Ja et al., 2007). To measure post-fasting food intake, 24 hr fasted flies were given access to liquid food for 3 hr. For each genotype in the screen (Figure 3A), a group of 10 flies was tested.
Locomotion Assay
Fly locomotion was tracked in a previously described custom-made 70 mm circular arena (Simon and Dickinson, 2010) modified by (Bussell et al., 2014), with 10–15 flies per genotype tested.
Negative Geotaxis
This assay was modified from a previously described protocol (Stafford et al., 2012).
Proboscis extension
Procedures were modified from a previously described protocol (Shiraiwa and Carlson, 2007).
Solid Food Intake
10 flies were wet-fasted and then given access to solid food in a polystyrene vial (Fisher #AS-519) for 15 min. Flies were scored as fully fed only if their abdomen was completely filled with green food.
Expresso Feeding System
Assay hardware was designed and constructed in consultation with William Dickson at IO Rodeo Inc. The Expresso data analysis software was written in MATLAB 2013a (MathWorks).
Photoactivation
IN1>ReaChR-expressing flies were raised on standard fly food at 25°C and 50–60% relative humidity in an incubator. 3–5 day old male flies were collected and housed in groups of 10–20 on food containing 400 μM all-trans-retinal (Sigma-Aldrich #R2500) mixed into standard fly food. The flies were kept at 25°C and 50–60% relative humidity in an incubator in a light-protected box for 2–3 days before photoactivation experiments.
Immunostaining and Microscopy
Samples were prepared essentially as described (Yu et al., 2010). For details on procedures and antibodies used see the Supplemental Experimental Procedures.
Functional Imaging
Imaging experiments were performed as previously described (Cohn et al., 2015) on an Ultima two-photon laser-scanning microscope (Bruker Nanosystems). The stimuli (water or sucrose) were presented to 24 hr fasted or fed females that were head-fixed under the two photon microscope.
Statistical Analysis
Statistical analysis was performed with R software version R version 3.1.3 (2015-03-09) “Smooth Sidewalk” (http://www.r-project.org/) and GraphPad Prism Software version 6.0b (GraphPad Software).
Supplementary Material
HIGHLIGHTS.
Expresso system measures single fly ingestion in real time at nanoliter resolution
Flies regulate ingestion by integrating hunger state and food quality
IN1 interneurons receive input from pharyngeal taste neurons and regulate ingestion
IN1 neurons respond to sucrose ingestion in a hunger state-dependent manner
Acknowledgments
We thank Eleanor Clowney, Monica Dus, Gaby Maimon, Kevin Lee, and members of the Vosshall Lab for comments on the manuscript; Isabel Gutierrez for expert technical assistance; Kunal Shah of the Rockefeller Precision Fabrication Facility for building the LED photo activation system; IO Rodeo Inc. for developing the hardware for the Expresso; Larry Zipursky for sharing reagents prior to publication. N.Y. was supported by a Human Frontier Science Program Long-Term Postdoctoral Fellowship. This study was supported in part by a grant from Klarman Family Foundation Grants Program in Eating Disorders Research. Fly stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) and antibodies from the Developmental Studies Hybridoma Bank at the University of Iowa (NIH NICHD) were used in this study. L.B.V. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
N.Y. designed and carried out all the experiments except the functional imaging experiments in Figure 6. C.S. and N.Y. together designed the requirements for the Expresso data analysis software, which was coded by C.S., and used by N.Y. for data analysis. R.C., V.R., and N.Y. designed and analyzed the data from the functional imaging experiments in Figure 6, which were carried out by R.C. N.Y and L.B.V. together interpreted the results, designed the figures, and wrote the paper with feedback from all authors. The authors declare no competing financial interests.
References
- Albin SD, Kaun KR, Knapp JM, Chung P, Heberlein U, Simpson JH. A subset of serotonergic neurons evokes hunger in adult Drosophila. Curr Biol. 2015;25:2435–2440. doi: 10.1016/j.cub.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes. 2009;33:S34–S43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto RPJ, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJP, Zuker CS. The neural representation of taste quality at the periphery. Nature. 2014;517:373–376. doi: 10.1038/nature13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brobeck JR, Tepperman J, Long CN. Experimental Hypothalamic Hyperphagia in the Albino Rat. Yale J Biol Med. 1943;15:831–853. [PMC free article] [PubMed] [Google Scholar]
- Burton MJ, Rolls ET, Mora F. Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Exp Neurol. 1976;51:668–677. doi: 10.1016/0014-4886(76)90189-8. [DOI] [PubMed] [Google Scholar]
- Bussell JJ, Yapici N, Zhang SX, Dickson BJ, Vosshall LB. Abdominal-B neurons control Drosophila virgin female receptivity. Curr Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJP, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Akin O, Nern A, Tsui CY, Pecot MY, Zipursky SL. Cell-type-specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron. 2014;81:280–293. doi: 10.1016/j.neuron.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci U S A. 2003;100:14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V. Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell. 2015;163:1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WJ. Behavioural hierarchies. Trends Neurosci. 1979;2:5–7. [Google Scholar]
- Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, Ja WW. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 2014;11:535–540. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The hungry fly: A physiological study of the behavior associated with feeding. Cambridge, MA: Harvard University Press; 1976. pp. 4–67. [Google Scholar]
- Dethier VG, Evans DR, Rhoades MV. Some factors controlling the ingestion of carbohydrates by the blowfly. Biol Bull. 1956;111:204. [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GSB. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M, Lai JSY, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, Suh GSB. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, VanHoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–87. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamori T, Miller IJ, Smith DV. Gustatory responsiveness of fibers in the hamster glossopharyngeal nerve. J Neurophysiol. 1988;60:478–498. doi: 10.1152/jn.1988.60.2.478. [DOI] [PubMed] [Google Scholar]
- Henkin RI, Christiansen RL. Taste localization on the tongue, palate, and pharynx of normal man. J Appl Physiol. 1967;22:316–320. doi: 10.1152/jappl.1967.22.2.316. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJP, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods. 2013;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Okada S, Naito H, Nagai T, Yasuoka A, Matsumoto I, Abe K. Two families of candidate taste receptors in fishes. Mech Dev. 2005;122:1310–1321. doi: 10.1016/j.mod.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. A systematic nomenclature for the insect brain. Neuron. 2014;81:755–765. doi: 10.1016/j.neuron.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Itskov PM, Moreira JM, Vinnik E, Lopes G, Safarik S, Dickinson MH, Ribeiro C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun. 2014;5:4560. doi: 10.1038/ncomms5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain P, Dahanukar A. Secondary taste neurons that convey sweet taste and starvation in the Drosophila brain. Neuron. 2015;85:819–832. doi: 10.1016/j.neuron.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140:578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavio in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- LeDue EE, Chen YC, Jung AY, Dahanukar A, Gordon MD. Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat Commun. 2015;6:6667. doi: 10.1038/ncomms7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Manzo A, Silies M, Gohl DM, Scott K. Motor neurons controlling fluid ingestion in Drosophila. Proc Natl Acad Sci U S A. 2012;109:6307–6312. doi: 10.1073/pnas.1120305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Lin TY, Ito K, Lee CH, Stopfer M. A gustatory second-order neuron that connects sucrose-sensitive primary neurons and a distinct region of the gnathal ganglion in the Drosophila brain. J Neurogenet. 2015;29:144–155. doi: 10.3109/01677063.2015.1054993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJP. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Navawongse R, Choudhury D, Raczkowska M, Stewart JC, Lim T, Rahman M, Toh AGG, Wang Z, Claridge-Chang A. Drosophila learn efficient paths to a food source. bioRxiv. 2015 doi: 10.1016/j.nlm.2016.03.019. http://dx.doi.org/10.1101/033969. [DOI] [PubMed]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Kosheleff A, Maidment NT, Murphy NP. Decreased consumption of sweet fluids in μ opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology (Berl) 2013;229:105–113. doi: 10.1007/s00213-013-3077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool AH, Kvello P, Mann K, Cheung SK, Gordon MD, Wang L, Scott K. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83:164–177. doi: 10.1016/j.neuron.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Yang Z, Lin Z, Park JY, Suh GSB, Wang L. A quantitative feeding assay in adult Drosophila reveals rapid modulation of food ingestion by its nutritional value. Mol Brain. 2015;8:87. doi: 10.1186/s13041-015-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- Read N, French S, Cunningham K. The role of the gut in regulating food intake in man. Nutr Rev. 1994;52:1–10. doi: 10.1111/j.1753-4887.1994.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Ro J, Harvanek ZM, Pletcher SD. FLIC: High-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS ONE. 2014;9:e101107. doi: 10.1371/journal.pone.0101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp JoVE. 2007;193 doi: 10.3791/193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JC, Dickinson MH. A new chamber for studying the behavior of Drosophila. PloS One. 2010;5:e8793. doi: 10.1371/journal.pone.0008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci. 2012;32:14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory syste in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Oxford University Press; 1951. [Google Scholar]
- Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec. 1990;227:373–379. doi: 10.1002/ar.1092270313. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CHJ, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: Synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1635–R1647. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


