Abstract
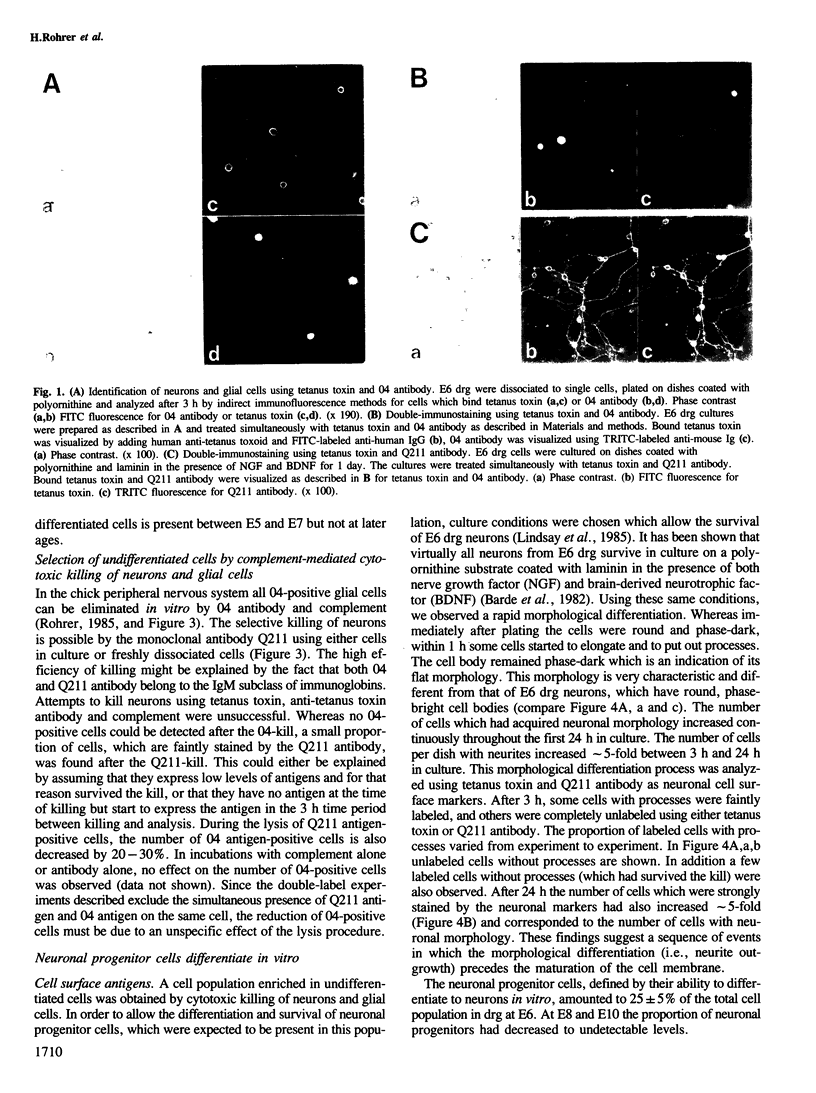
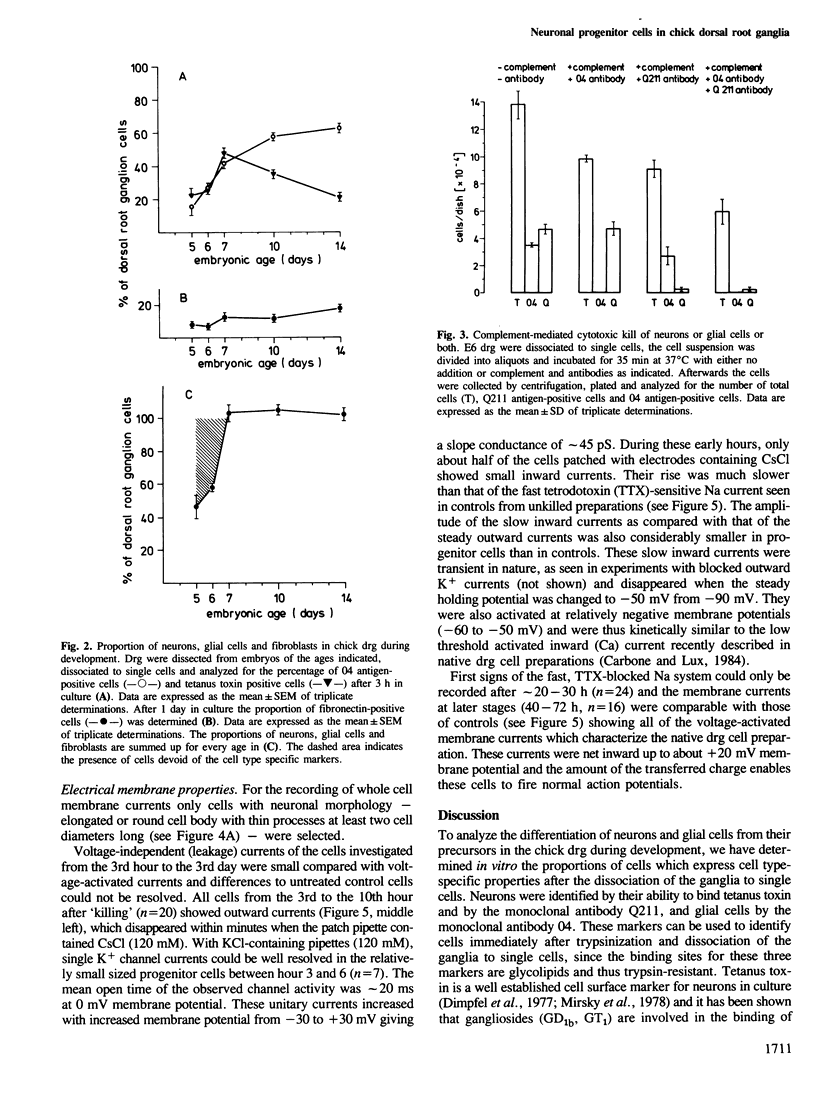
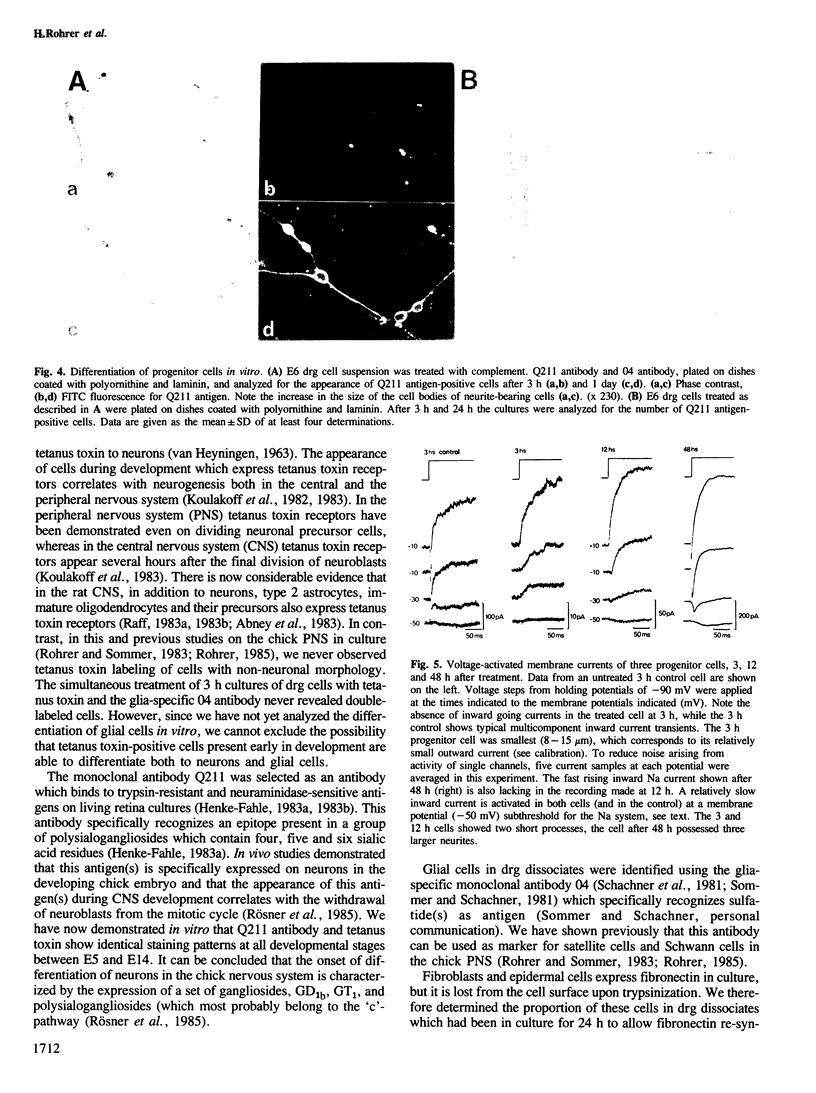
We have analyzed the appearance of neurons and glial cells in chick dorsal root ganglia during development. Neurons were identified by the presence of polysialogangliosides recognized by tetanus toxin (GD1b, GT1) or by the monoclonal antibody Q211 directed against polysialogangliosides containing four, five and six sialic acid residues. Glial cells were identified by the presence of 04 antigen. A population of undifferentiated cells, i.e., cells which express neither neuronal nor glial cell surface antigens, present in dorsal root ganglia until embryonic day 7, was separated from the neuronal and glial population. This cell population contains neuronal progenitor cells which differentiate to neurons within 1 day in culture. This differentiation process is characterized by the appearance of neuronal morphology, of neuron-specific gangliosides and by the appearance of voltage-dependent sodium and calcium channels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Williams B. P., Raff M. C. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting, and cell culture. Dev Biol. 1983 Nov;100(1):166–171. doi: 10.1016/0012-1606(83)90207-5. [DOI] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Dupin E., Kato A. C. Development of electrical membrane properties in cultured avian neural crest. 1983 Oct 27-Nov 2Nature. 305(5937):808–810. doi: 10.1038/305808a0. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Sensory neurons in culture: changing requirements for survival factors during embryonic development. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1199–1203. doi: 10.1073/pnas.77.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr V. M., Simpson S. B., Jr Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol. 1978 Dec 15;182(4):727–739. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Collins F. Axon initiation by ciliary neurons in culture. Dev Biol. 1978 Jul;65(1):50–57. doi: 10.1016/0012-1606(78)90178-1. [DOI] [PubMed] [Google Scholar]
- Dimpfel W., Huang R. T., Habermann E. Gangliosides in nervous tissue cultures and binding of 125I-labelled tetanus toxin, a neuronal marker. J Neurochem. 1977 Aug;29(2):329–334. doi: 10.1111/j.1471-4159.1977.tb09626.x. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakoff A., Bizzini B., Berwald-Netter Y. A correlation between the appearance and the evolution of tetanus toxin binding cells and neurogenesis. Brain Res. 1982 Oct;281(2):139–147. doi: 10.1016/0165-3806(82)90152-3. [DOI] [PubMed] [Google Scholar]
- Koulakoff A., Bizzini B., Berwald-Netter Y. Neuronal acquisition of tetanus toxin binding sites: relationship with the last mitotic cycle. Dev Biol. 1983 Dec;100(2):350–357. doi: 10.1016/0012-1606(83)90229-4. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Williams B. P., Miller R. H. The in vitro differentiation of a bipotential glial progenitor cell. EMBO J. 1984 Aug;3(8):1857–1864. doi: 10.1002/j.1460-2075.1984.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer H., Sommer I. Simultaneous expression of neuronal and glial properties by chick ciliary ganglion cells during development. J Neurosci. 1983 Aug;3(8):1683–1693. doi: 10.1523/JNEUROSCI.03-08-01683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösner H., Al-Aqtum M., Henke-Fahle S. Developmental expression of GD3 and polysialogangliosides in embryonic chicken nervous tissue reacting with monoclonal antiganglioside antibodies. Brain Res. 1985 Feb;350(1-2):85–95. doi: 10.1016/0165-3806(85)90252-4. [DOI] [PubMed] [Google Scholar]
- Schachner M., Kim S. K., Zehnle R. Developmental expression in central and peripheral nervous system of oligodendrocyte cell surface antigens (O antigens) recognized by monoclonal antibodies. Dev Biol. 1981 Apr 30;83(2):328–338. doi: 10.1016/0012-1606(81)90478-4. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Cell that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neurosci Lett. 1982 Apr 16;29(2):183–188. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Bennett G. S., Holtzer H. Neuronal precursor cells in the chick neural tube express neurofilament proteins. Nature. 1981 Aug 27;292(5826):836–838. doi: 10.1038/292836a0. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E. The fixation of tetanus toxin, strychnine, serotonin and other substances by ganglioside. J Gen Microbiol. 1963 Jun;31:375–387. doi: 10.1099/00221287-31-3-375. [DOI] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]




