Abstract.
Human granulocytic anaplasmosis (HGA) is a tick-borne infectious disease caused by Anaplasma phagocytophilum, an obligate intracellular bacterium. Until now, the utility of tick-bite site samples for HGA diagnosis has not been reported. Using a patient's buffy coat and tick-bite site crust samples, we performed polymerase chain reaction (PCR) testing using Ehrlichia- or Anaplasma-specific primers. PCR with buffy coat and crust samples obtained before doxycycline administration was positive. Six days after doxycycline administration, PCR with the buffy coat sample was negative but PCR with a crust tissue sample from the tick-bite site remained positive. This is the first case to suggest that crust tissue at the tick-bite site may be useful for early HGA diagnosis in patients who have already been treated with antibiotics such as doxycycline.
Introduction
Human granulocytic anaplasmosis (HGA) is a tick-borne infectious disease caused by Anaplasma phagocytophilum, an obligate intracellular bacterium that was first identified as a human pathogen in 1994.1 In Korea, the first case of HGA was reported in 2013 as an emerging infectious disease.2 HGA is mainly transmitted by ticks of the Ixodes species, which are common vectors of Lyme disease and babesiosis.3 As patients with HGA develop nonspecific symptoms such as fever, malaise, headache, and chills, the diagnosis may be challenging. As such, differential diagnoses typically include rickettsial diseases, infectious mononucleosis, Lyme disease, and West Nile virus infection.4
Isolation of A. phagocytophilum in culture is difficult and usually takes longer than 30 days.5 Therefore, the use of polymerase chain reaction (PCR) testing for the rapid diagnosis of HGA is important. Herein, we aimed to evaluate the usefulness of a crust tissue at the tick-bite site for the diagnosis of HGA via PCR testing.
Case Report
A 79-year-old man was admitted for fever, which started 6 days prior to his admission. Four days prior to admission, the patient experienced myalgia accompanied by chills. The patient was monitored for continuous symptoms and eventually admitted to hospital due to worsening of his condition. He was a farmer who had worked in rice and pepper fields, and, aside from right ureter stones, he had no other medical conditions.
The physical examination conducted on admission revealed that the patient had a fever of 37.8°C, a pulse rate of 102 beats/minute, and a respiratory rate of 22 breaths/minute. On admission, he had hyperemic conjunctiva and a lesion that resembled a tick-bite site was found on his left flank area (Figure 1).
Figure 1.
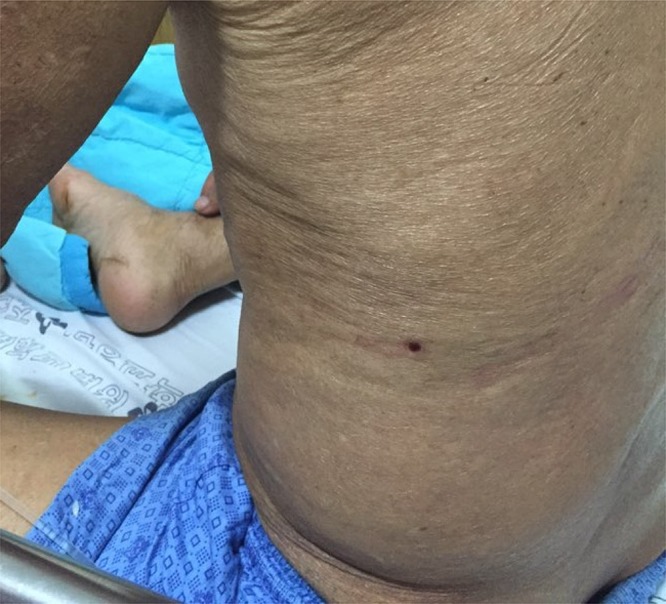
A crust lesion suspected to be a tick-bite site on the left flank area of a 79-year-old man. This figure appears in color at www.ajtmh.org.
The patient's white blood cell count was 3,310/mm3 (91.9% polymorphonuclear cells and 2.2% lymphocytes), his hemoglobin level was 13.2 g/dL (reference values: 11–17 g/dL), and his platelet count was 24,000/mm3 (reference values: 150,000–400,000/μL). Other laboratory values were as follows: aspartate aminotransferase level, 93.5 U/L (reference values: 0–35 U/L); alanine aminotransferase level, 36.3 U/L (reference values: 0–35 U/L); lactate dehydrogenase level, 619 U/L (reference values: 100–190 U/L); and creatine phosphokinase level, 309 U/L (reference values: 0–150 U/L).
For a febrile illness, 2 g ceftriaxone was administered at admission. On the second day of admission (seventh day since the onset of symptoms), a crust lesion and blood samples were collected and sent to the laboratory for testing (Table 1); we collected the tick-bite site crust sample by simply detaching the crust with a scalpel (no. 11 blade) and tweezers.
Table 1.
Diagnostic test results of a 79-year-old patient with human granulocytic anaplasmosis
| Collection date | Time from symptom onset to specimen collection (days) | IFA | Specimen used | groEL nested PCR result |
|---|---|---|---|---|
| April 24, 2015 | 7 | IgM (−) | Buffy coat | Positive |
| IgG (−) | Crust | Positive | ||
| April 27, 2015 | 10 | IgM (−) | Buffy coat | Positive |
| IgG 1:80 | ||||
| April 29, 2015 | 12 (doxycycline start) | IgM (−) | Buffy coat | Positive |
| IgG 1:80 | ||||
| May 5, 2015 | 18 | IgM (−) | Buffy coat | Negative |
| IgG 1:80 | Crust | Positive | ||
| May 20, 2015 | 33 | IgM (−) | Buffy coat | Negative |
| IgG 1:80 | Crust | Negative | ||
| June 22, 2015 | 66 | IgM (−) | Buffy coat | Negative |
| IgG 1:320 | ||||
| August 3, 2015 | 108 | IgM (−) | Buffy coat | Negative |
| IgG 1:160 |
groEL = GroEL heat-shock protein operon gene; IFA = immunofluorescent antibody assay; PCR = polymerase chain reaction.
HGA was suspected, but morulae were not observed on peripheral blood smear. PCR was performed with genomic DNA from the patient's buffy coat and crust samples. Conventional PCR or nested PCR were performed using Ehrlichia- or Anaplasma-specific primers targeting the GroEL heat-shock protein operon gene (groEL), 16S ribosomal RNA gene (16S rRNA), ankyrin-repeat protein AnkA gene (ankA), and major surface protein 2 (msp2) gene.6–9 The primer sequences and PCR conditions used are summarized in Table 2. The amplified PCR products were cloned into a PCR 2.1 vector from the TA cloning kit (Invitrogen, Carlsbad, CA) and positive recombinant plasmids were sequenced. The sequencing result was checked using BlastN from the National Center for Biotechnology Information (Bethesda MD) to identify the bacterium.
Table 2.
PCR conditions and oligonucleotide primers used in this study
| Target gene* | Primer name (sequences) | Annealing temperature (°C) | Product size (base pairs) | References |
|---|---|---|---|---|
| groEL nested PCR for Anaplasmataceae (external primers) | GR0607F (5′-GAAGATGCWGTWGGWTGTACKGC-3′) | 57 | 688 | 6 |
| GR01294R (5′-AGMGCTTCWCCTTCWACRTCYTC-3′) | ||||
| groEL nested PCR for Anaplasmataceae (internal primers) | GR0677F (5′-ATTACTCAGAGTGCTTCTCARTG-3′) | 57 | 445 | 6 |
| GR01121R (5′-TGCATACCRTCAGTYTTTTCAAC-3′) | ||||
| 16S rRNA nested PCR for Anaplasma and Ehrlichia species (external primers) | AE1-F (5′-AAGCTTAACACATGCAAGTCGAA-3′) | 59 | 1,406 | 7 |
| AE1-R (5′-AGTCACTGA CCCAACCTTAAATG-3′) | ||||
| 16S rRNA nested PCR for E. chaffeensis (internal primers) | EC-F (5′-CAATTGCTTATAACCTTTTGGTTATAAAT-3′) | 56 | 390 | 7 |
| EC-R (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′) | ||||
| 16S rRNA nested PCR for A. phagocytophilum (internal primers) | AP-F (5′-GTCGAACGGATTATTCTTTATAGCTTGC-3′) | 56 | 926 | 7 |
| AP-R (5′-CCCTTCCGTTAAGAAGGATCTAATCTCC-3′) | ||||
| ankA nested PCR for A. phagocytophilum (external primers) | ANK-F1 (5′-GAAGAAATTACAACTCCTGAAG-3′) | 53 | 705 | 8 |
| ANK-R1 (5′-CAGCCAGATGCAGTAACGTG-3′) | ||||
| ankA nested PCR for A. phagocytophilum (internal primers) | ANK-F2 (5′-TTGACCGCTGAAGCACTAAC-3′) | 54 | 664 | 8 |
| ANK-R2 (5′-ACCATTTGCTTCTTGAGGAG-3′) | ||||
| msp2 conventional PCR for A. phagocytophilum | MSP3F (5′-CCA GCG TTT AGC AAG ATA AGA G-3′) | 54 | 334 | 9 |
| MSP3R (5′-GCC CAG TAA CAT CAT AAG C-3′) |
ankA = ankyrin-repeat protein gene; groEL = GroEL heat-shock protein operon gene; msp2 = major surface protein 2 gene; 16S rRNA = 16S ribosomal RNA gene.
A serological test (IgG and IgM) for the detection of A. phagocytophilum was performed using an indirect immunofluorescent antibody assay (IFA) from a commercial kit (Fuller Laboratories, Fullerton, CA) according to the manufacturer's protocol.10 The serological positive cutoff value of the kit is 1:80 for the IgG titer and 1:16 for the IgM titer.
The PCR to detect A. phagocytophilum and sequencing were performed using the buffy coat and crust lesion samples obtained 7 days from symptom onset (Table 1). All of the sequencing results confirmed the presence of A. phagocytophilum.
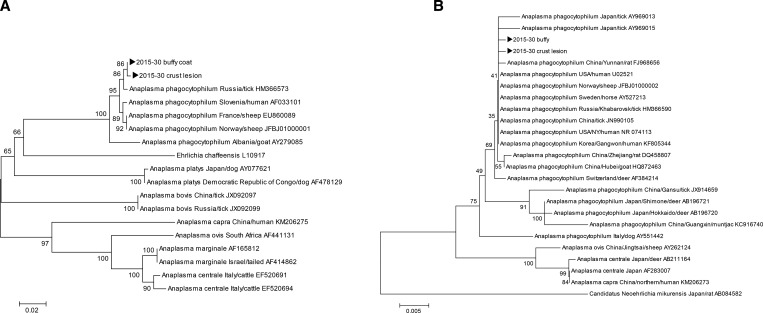
Twelve days from the onset of symptoms, ceftriaxone therapy was switched to 100 mg doxycycline BID administration. A nested PCR for groEL was performed using the buffy coat and crust lesion specimens at each time point after the symptoms had begun (Table 1). On day 6 of doxycycline administration, which was 18 days after the onset of symptoms, PCR for the buffy coat samples was negative. However, PCR for the crust lesion sample remained positive (Supplemental Figure 1). On admission, using the buffy coat and crust lesion samples, PCR for 16S rRNA and groEL genes were performed, and their respective 926 base pair (bp) and 362 bp amplicons were cloned. The sequencing results showed 2 bp differences for 16S rRNA and 1 bp difference for groEL.2,11 Phylogenetic trees were constructed based on sequences of 16S rRNA and groEL genes from GenBank using the neighbor-joining method on the ClustalX software program (Dublin, Ireland; Figure 2). In a homology test, A. phagocytophilum was 99.9% homologous to the A. phagocytophilum strain HZ detected in the United States. It was also 99.9% homologous to the A. phagocytophilum strain confirmed in Gwangwon, Korea (accession no. KF805344), as well as to the Russian strain (accession no. HM366590) and Chinese strain (accession no. JN990105).
Figure 2.
A phylogenetic tree based on (A) groEL (362 base pair [bp]) and (B) 16S rRNA (810 bp) sequences from GenBank and buffy coat and crust lesion specimens collected in this study (►). Scale bars indicate (A) 0.02 and (B) 0.005 base substitutions per site.
Seven days from the initiation of symptoms, the IFA results were negative for both IgM and IgG, whereas 10 days from the initiation of symptoms, the IgG titer increased to 1:80. However, IgM remained negative even at 108 days from the onset of symptoms.
Doxycycline administration continued for 10 days and the patient was discharged without complications.
Discussion
The laboratory diagnosis of HGA is based on Anaplasma culture, a PCR test, or antibody titer increase using specimens obtained from the acute phase of the disease and during recovery.12 However, serological tests are challenging and may take several weeks to detect a 4-fold increase in antibodies, which is required to confirm the diagnosis. In this case, which is the second reported HGA case from Korea, it took longer than a week to detect the antibodies. Even afterward, a relatively low IgG titer was observed and IgM was not detected on day 108 from symptom onset. In some cases, delays in the administration of effective antibiotics may cause fatal complications.13,14 In addition, it is technically challenging and lengthy process to isolate Anaplasma in culture. For these reasons, HGA may be difficult to diagnose without molecular technology. Therefore, a rapid and accurate diagnosis is necessary to ensure appropriate treatment. PCR is a useful tool for diagnosing infectious diseases caused by microbes that are difficult to culture or slow growing.15 For the diagnosis of rickettsial diseases, the usefulness of PCR testing using a buffy coat sample is well known.16 In addition, PCR conducted with eschar skin biopsy specimens detected Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in patients with rickettsial diseases such as scrub typhus or spotted fever.17 However, Anaplasma does not form typical eschars on the tick-bite site, and as such, a diagnosis using PCR testing of a tick-bite site crust sample has not been reported.
In a study that examined a tick-feeding site in sheep that were infected by human A. phagocytophilum isolates, biopsy of the feeding site showed signs of necrosis, and higher numbers of eosinophils and neutrophils were observed. Anaplasma phagocytophilum–infected granulocytes were also observed in the skin biopsy immunohistochemistry study.18 In another study, skin biopsies were collected from tick feeding lesions on 38 grazing lambs. PCR analysis of blood samples showed 33 lambs (86.8%) were positive for A. phagocytophilum. However, 37 lambs (97.4%) had one or more skin biopsies and 70 of 76 biopsies from tick attachment sites (92.1%) were PCR positive.19
Therefore, like eschars from the scrub typhus or spotted fever groups, in patients with HGA, tick-bite sites were hypothesized to have a large inoculum around the site in which the bacteria had multiplied and spread.20,21
From both blood and crust samples obtained before doxycycline administration, the Anaplasma PCR tests were positive. Six days after administering doxycycline, PCR for the buffy coat sample was negative; however, PCR for the tick-bite site crust sample remained positive. Thus, bacterial DNA from the crust appears to last longer than that from the blood. The positive PCR result of A. phagocytophilum in a crust after the onset of treatment with doxycycline might be the result of high inoculum or theoretically less than optimal diffusion (access) of doxycycline into the crust tissue. We did not perform immunohistochemical staining of crust tissue. Further study is required.
In conclusion, this study argues for the usefulness of PCR testing using a tick-bite site crust sample, similar to the use of the buffy coat sample, in the early and acute diagnosis of HGA. This study is the first to suggest that the crust tissue at the tick-bite site may be a useful specimen for the diagnosis of HGA, even in patients who received antibiotics such as doxycycline, which are active against HGA. More samples are required to verify whether PCR positivity on crust tissue at the tick-bite site is useful for diagnosing the disease even after antibiotic administration.
Supplementary Material
Note: Supplemental figure appears at www.ajtmh.org.
REFERENCES
- 1.Chen SM, Dumler JS, Bakken JS, Walker DH, 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 32: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KH, Yi J, Oh WS, Kim NH, Choi SJ, Choe PG, Kim NJ, Lee JK, Oh MD, 2014. Human granulocytic anaplasmosis, South Korea, 2013. Emerg Infect Dis 20: 1708–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadelman RB, Horowitz HW, Hsieh TC, Wu JM, Aguero-Rosenfeld ME, Schwartz I, Nowakowski J, Varde S, Wormser GP, 1997. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med 337: 27–30. [DOI] [PubMed] [Google Scholar]
- 4.Dumler JS, Bakken JS, 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis 20: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 5.Silaghi C, Kauffmann M, Passos LM, Pfister K, Zweygarth E, 2011. Isolation, propagation and preliminary characterization of Anaplasma phagocytophilum from roe deer (Capreolus capreolus) in the tick cell line IDE8. Ticks Tick Borne Dis 2: 204–208. [DOI] [PubMed] [Google Scholar]
- 6.Takano A, Ando S, Kishimoto T, Fujita H, Kadosaka T, Nitta Y, Kawabata H, Watanabe H, 2009. Presence of a novel Ehrlichia sp. in Ixodes granulatus found in Okinawa, Japan. Microbiol Immunol 53: 101–106. [DOI] [PubMed] [Google Scholar]
- 7.Oh JY, Moon BC, Bae BK, Shin EH, Ko YH, Kim YJ, Park YH, Chae JS, 2009. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. J Bacteriol Virol 39: 257–267. [Google Scholar]
- 8.Massung RF, Levin ML, Munderloh UG, Silverman DJ, Lynch MJ, Gaywee JK, Kurtti TJ, 2007. Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J Clin Microbiol 45: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin ML, Nicholson WL, Massung RF, Sumner JW, Fish D, 2002. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis 2: 125–136. [DOI] [PubMed] [Google Scholar]
- 10.Eremeeva ME, Balayeva NM, Raoult D, 1994. Serological response of patients suffering from primary and recrudescent typhus: comparison of complement fixation reaction, Weil-Felix test, microimmunofluorescence, and immunoblotting. Clin Diagn Lab Immunol 1: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabara K, Arai S, Kawabuchi T, Itagaki A, Ishihara C, Satoh H, Okabe N, Tsuji M, 2007. Molecular survey of Babesia microti, Ehrlichia species and Candidatus neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol Immunol 51: 359–367. [DOI] [PubMed] [Google Scholar]
- 12.Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoërsdorff A, Blanco JR, Caruso G, Cinco M, Fournier PE, Francavilla E, Jensenius M, Kazar J, Laferl H, Lakos A, Lotric Furlan S, Maurin M, Oteo JA, Parola P, Perez-Eid C, Peter O, Postic D, Raoult D, Tellez A, Tselentis Y, Wilske B, 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect 10: 1108–1132. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Wei F, Liu Q, Qian J, 2012. Epidemiology and control of human granulocytic anaplasmosis: a systematic review. Vector Borne Zoonotic Dis 12: 269–274. [DOI] [PubMed] [Google Scholar]
- 14.Hamburg BJ, Storch GA, Micek ST, Kollef MH, 2008. The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore) 87: 53–60. [DOI] [PubMed] [Google Scholar]
- 15.Eiscenstein BI, 1990. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med 323: 178–183. [DOI] [PubMed] [Google Scholar]
- 16.Watthanaworawit W, Turner P, Turner C, Tanganuchitcharnchai A, Richards AL, Bourzac KM, Blacksell SD, Nosten F, 2013. A prospective evaluation of real-time PCR assays for the detection of Orientia tsutsugamushi and Rickettsia spp. for early diagnosis of rickettsial infections during the acute phase of undifferentiated febrile illness. Am J Trop Med Hyg 89: 308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denison AM, Amin BD, Nicholson WL, Paddock CD, 2014. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis 59: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reppert E, Galindo RC, Ayllón N, Breshears MA, Kocan KM, Blouin EF, de la Fuente J, 2014. Studies of Anaplasma phagocytophilum in sheep experimentally infected with the human NY-18 isolate: characterization of tick feeding sites. Ticks Tick Borne Dis 5: 744–752. [DOI] [PubMed] [Google Scholar]
- 19.Granquist EG, Aleksandersen M, Bergström K, Dumler SJ, Torsteinbø WO, Stuen S, 2010. A morphological and molecular study of Anaplasma phagocytophilum transmission events at the time of Ixodes ricinus tick bite. Acta Vet Scand 52: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DM, Kim HL, Park CY, Yang TY, Lee JH, Yang JT, Shim SK, Lee SH, 2006. Clinical usefulness of eschar polymerase chain reaction for the diagnosis of scrub typhus: a prospective study. Clin Infect Dis 43: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 21.Reppert E, Galindo RC, Ayllón N, Breshears MA, Kocan KM, Blouin EF, de la Fuente J, 2014. Studies of Anaplasma phagocytophilum in sheep experimentally infected with the human NY-18 isolate: characterization of tick feeding sites. Ticks Tick Borne Dis 5: 744–752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.