Abstract.
Artemisinin-based combination therapies are recommended as first-line agents for treating uncomplicated Plasmodium falciparum malaria. Ferroquine, a 4-aminoquinolone, is a novel long-acting combination partner for fast-acting drugs like artesunate (AS). We did a small phase 2a, multicenter, open-label, safety-focused dose-ranging randomized study of ferroquine at three African hospitals: two Gabonese and one Kenyan. We recruited adult men with symptomatic uncomplicated P. falciparum monoinfection. Four escalating doses of ferroquine (100, 200, 400, and 600 mg) were assessed in sequence, versus an amodiaquine comparator. After a 2:1 randomization (block size three, equating to N = 12 for each ferroquine dose and N = 6 for each of four amodiaquine comparator groups) patients received daily for three consecutive days, either ferroquine + AS (200 mg/day) or amodiaquine (612 mg/day) + AS (200 mg/day). Safety, electrocardiograms, parasite clearance times, efficacy, and pharmacokinetics were assessed to day 28. Seventy-two patients were randomized. Ferroquine + AS showed generally mild increases (Grade 1 toxicity) in alanine aminotransferase (ALT) levels with a dose trend starting at 400 mg. There were two Grade 2 ALT events: one patient receiving 200 mg (3.8 upper limit of normal [ULN], day 7) and one receiving 600 mg (3.3 ULN, day 14), both without increased bilirubin. One ferroquine 100 mg + AS patient after one dose was withdrawn after developing a QTcF interval prolongation > 60 milliseconds over baseline. Parasitemias in all patients cleared quickly, with no recurrence through day 28. Hepatic, as well as cardiac, profiles should be monitored closely in future trials. (ClinicalTrials.gov: NCT00563914)
INTRODUCTION
Short-acting artemisinin derivatives combined with medium- or long-acting partner drugs with different modes of action from artemisinin are currently the gold standard treatment of uncomplicated malaria.1 A relatively new long-acting potential combination drug is ferroquine, a 4-aminoquinolone analogue containing a ferrocenyl group.2 Ferroquine is active in vitro against chloroquine (CQ)-sensitive and CQ-resistant Plasmodium falciparum and Plasmodium vivax isolates.3–6 The main circulating metabolite of ferroquine, N-monodesmethyl ferroquine, has similar activities as its parent compound on CQ-sensitive strains. Now, ferroquine, in partnership with other drugs, is being developed for treating uncomplicated P. falciparum malaria in adults and children.7–9
The clinical program for ferroquine consists of four Phase 1 studies and one Phase 2 study. The first Phase 1 study, a first-in-humans single-dose administration of ferroquine, up to 800 mg in healthy German subjects (TDU5419), had a favorable safety profile and was well tolerated (unpublished data). The remaining three Phase 1 studies were done in asymptomatic Gabonese adults infected with P. falciparum. The second Phase 1 study (TDU5967), assessing a single dose of 400–1,600 mg ferroquine in 40 adults, and the third Phase 1 study (TDR5969) assessing a 3-day regimen of 400–1,000 mg/day of ferroquine in 26 adults, published together, had generally favorable safety profiles and was well tolerated.10 However, at the 1,000 mg 3-day dose, moderate changes in T-wave morphology and QTcF interval prolongations occurred in some patients, the latter none > 60 milliseconds. The fourth Phase 1 study (FED5968) in 16 adults showed food had no effect on ferroquine exposure after a single dose of 100 mg.11 In 2009, following the 2008 completion of the Phase 2a study described here, a phase 2 (DRI10382) noninferiority study assessing ferroquine (2, 4, and 6 mg/kg over 3 days) + artesunate (AS) in African adults and children, with symptomatic P. falciparum malaria, showed high cure rates and a generally favorable safety profile.7 Nonetheless, there were eight patients with prolonged QTcF interval > 60 milliseconds over baseline, seven in children. This was regarded as possibly due to the higher baseline temperature and heart rate of children. No QTcF of > 500 milliseconds was noted, changes in the QTcF interval in the ferroquine groups were not dose dependent, and intervals returned to normal shortly after the end of study treatment and disease.
Like established 4-aminoquinolones used to treat malaria, ferroquine treatment of malaria is associated with sporadic, but transient increases of hepatic-specific alanine transferase (ALT), sometimes less than three times the upper limit of normal (ULN), and on at least one occasion with significantly increased bilirubin.10 A combination of increased ALT and bilirubin carries a higher risk of drug-related liver failure, known as Hy’s law.12 Ferroquine may also affect certain electrocardiogram (ECG) parameters, QT intervals in particular, but daily doses up to 600 mg have generally been the highest dose not associated with QTcF (Fridericia’s correction) interval prolongation > 60 milliseconds from baseline, or inverted T waves.10 Therefore, this small safety study focused on the hepatic safety of escalating ferroquine doses of up to 600 mg daily + AS, along with ECG monitoring and blood concentrations of ferroquine. Since non-drug-related increases in ALT are common at the study locations, amodiaquine (AQ) + AS was used as a comparator.
In 2011, after our study and later the 2009–2010 Phase 2 study were completed, Sanofi-Aventis (SA) changed its development strategy, intending to develop ferroquine in combination with a different drug class, currently artefenomel (OZ439) (NCT02497612).
METHODS
Study design and participants.
We did a Phase 2a multicenter, parallel-group, open-label, randomized, dose-escalating study of ferroquine at three hospitals in Africa: two in Gabon (Lambaréné and Libreville) and one in Kenya (Kisumu). We recruited 72 adult African men ≥ 18 years of age (≥ 21 years of age for Gabon) and < 65 years of age, with body weight from 50 to 90 kg, a body mass index value > 18 kg/m2, a body temperature ≥ 37.5°C or history of fever within the last 24 hours, and monoinfection with P. falciparum parasitemia, between 100 and 200,000/μL. As certain baseline ECG abnormalities were common in patients at the study sites, an amendment to the protocol deemed baseline ECG abnormalities in heart rate, PR and QRS values not exclusionary. A QTcF > 480 milliseconds was exclusionary to err on the side of caution. We obtained written informed consent from all patients. Men only were recruited to avoid hormonal influences on hepatic enzymes.
Exclusion criteria were evidence of active hepatitis B or C, defined by HBsAg detection and hepatitis C serum positivity tests, respectively, human immunodeficiency virus (HIV) 1 or 2 serum positivity, clinically significant abnormal laboratory values, clinically significant disease, splenectomy, past treatment with an antimalarial drug, or a drug metabolized via cytochrome P450-2D6, within five times the elimination half-life of the drug or within the past 14 days, and any malaria vaccines. Hepatitis B and C viral loads were not done if serology was positive, nor were tests for hepatitis E.
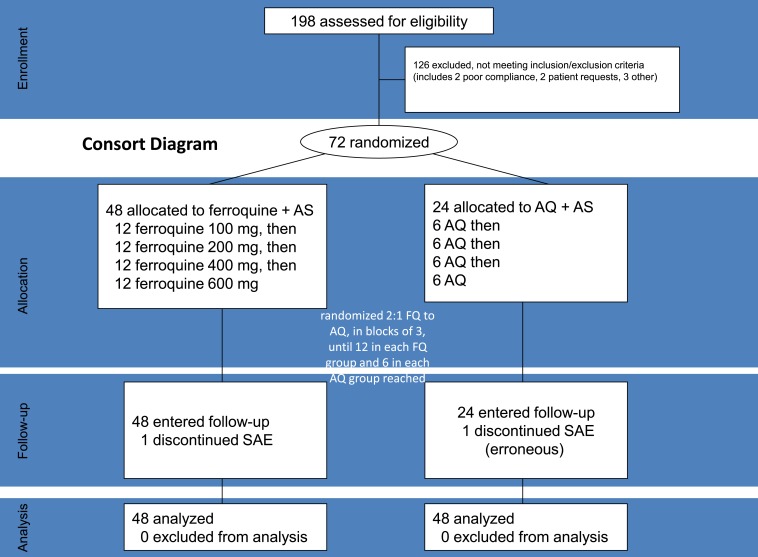
The study duration was 29–30 days, with 12 visits: screening (day −2/−1), three daily treatments (days 0–2; patients hospitalized for 96 hours) and eight follow-up assessments on days 4, 5, 6, 7, 9, 14, 21, and 28. Four escalating doses of ferroquine (100, 200, 400, and 600 mg) were assessed in sequence (Figure 1) in fasted patients, under direct observation. After a 2:1 randomization (block size three, equating to N = 12 for each ferroquine dose and N = 6 for each of four AQ comparator groups), patients received daily for three consecutive days, either ferroquine + AS (200 mg/day) or AQ (612 mg/day) + AS (200 mg/day).
Figure 1.
Consort diagram.
Ferroquine was provided as 100- and 300-mg capsules (batch number: CL-10166 for cohorts 1 and 2 and batch number: CL-11967 for cohorts 3 and 4), and AS was supplied as Arsumax® (Guilin Pharmaceuticals Co., Ltd, Shanghai, China) 50-mg tablets (batch number: AR070522 for cohorts 1 and 2 and batch number: AR071572 for cohorts 3 and 4). AQ (Flavoquine®, Sanofi, Compiegne, France) was supplied as 153-mg tablets (batch number: AR069147 for cohort 1 and batch number: AR071244 for cohorts 2–4). Paracetamol, at nonhepatotoxic doses, was allowed during the trial.
The study was approved by the local ethics committees of the trial sites: in Gabon by the Comité d´Éthique Regional Indépendent de Lambaréné for the two Gabonese sites, Libreville and Lambaréné; in Kenya by the Kenya Medical Research Institute Ethical Review Committee and the Walter Reed Army Institute of Research Institutional Review Board. This study was performed in compliance with Good Clinical Practices and 18th World Health Congress (the declaration of Helsinki, 1964).
Randomization.
We randomized patients at Visit 2 (day 1) using a centralized randomization procedure with an interactive web response system. Randomization was stratified by dose level, but not by site. Within each ferroquine dose cohort, patients were randomly allocated to either ferroquine + AS or AQ + AS using a 2:1 randomization ratio, as described earlier (Figure 1) starting with the 100-mg ferroquine dose. Three populations were analyzed: 1) intention to treat (ITT), used for demographic description; 2) modified-ITT (m-ITT), defined as a subset of the ITT population composed of patients having a post-randomization parasitemia evaluation, used for efficacy; and 3) treated population, defined as patients who received at least one dose of study medication used for baseline and on treatment analyses, as well as pharmacokinetic (PK) analysis, regardless of vomiting.
Outcomes.
Laboratory safety evaluations were based on values defined as potentially clinically significant abnormalities (PCSAs; U.S. Food and Drug Administration), as in previous studies.7,10,13 For this report, PCSAs can generally be considered akin to a ≥ Grade 2 adverse event, moderate or worse (Common Toxicity Criteria for Adverse Events, version 4).13 Treatment emergent adverse events (TEAEs) were coded using Medical Dictionary for Regulated Activities (MedDRA, version 11.1). Proceeding from one cohort to the next was guided by a six-person data monitoring committee.
The primary safety outcome was to assess the laboratory safety of escalating doses of ferroquine + AS, in comparison with AQ + AS. Normal ranges were specified at each local laboratory, thus results were expressed as a multiple of ULN wherever appropriate. Hepatic parameters included ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and gamma glutamyl transferase (GGT) at least at screening, and days 3, 5, 6, 7, 9, 14, 21, and 28.
An ALT ≥ 3 ULN if during dosing was a PCSA (≥ Grade 2), and was to stop treatment. After dosing, ALT ≥ 3 ULN was a PCSA, whereas a serious adverse event (SAE) if > 10 ULN (Grade 4), or if ALT ≥ 3 ULN was associated with total bilirubin ≥ 2 ULN, jaundice, a coagulation disorder, or hepatic encephalopathy. A combination of increased ALT and bilirubin carries an increased risk of drug-induced death, known as “Hy’s law.”12
Other blood tests included white and red blood cell count, renal function, creatine phosphokinase, electrolytes, calcium, glucose, albumin, protein, lipids, and lipase.
The secondary safety outcome was ECG parameters, to include heart rate, PR interval, QRS interval, and QT interval, the latter to include QTcF and QTcB (Bazett’s correction). QTcF were reported here. We did a 12-lead ECG at screening, baseline, twice daily during treatment, and weekly during follow-up until day 28. ECGs were centrally read (MDS Pharma Services, France). A QTcF > 60 milliseconds versus baseline was an SAE, whether during or after dosing.
Heart rate and blood pressure (BP) were measured most often using an automated sphygmomanometer. In the supine position for at least 10 minutes: slow heart rate was ≤ 50 bpm and decrease from baseline ≥ 20 bpm, and rapid heart rate was ≥ 120 bpm and increase from baseline ≥ 20 bpm; systolic hypotension was ≤ 95 mmHg and decrease from baseline ≥ 20 mmHg, and hypertension was ≥ 160 mmHg and increase from baseline ≥ 20 mmHg; diastolic hypotension was ≤ 45 mmHg and decrease from baseline ≥ 10 mmHg, and hypertension ≥ 110 mmHg and increase from baseline ≥ 10 mmHg. Postural hypotension, for pressures taken after 10 minutes supine, then 2 minutes standing, was defined as a decrease in systolic BP of ≥ 20 mmHg or a decrease in diastolic BP of ≥ 10 mmHg.
The efficacy outcome, parasite clearance time (PCT), was assessed at day 1 (T6 and T12 hours), day 2 (T0 and T6 hours), day 3 (T0, T6, and T12 hours), day 4 (T24 and T36 hours), and days 7, 14, 21, and 28, at variable times. Parasitemia data as a continuous endpoint (number of parasites/µL) were also analyzed as the secondary efficacy variable until study end. Asexual parasite counts were done on Giemsa-stained thick blood smears by two trained microscopists, blinded to patient identities and to each other’s results. We measured parasitemia by counting parasites versus 200 white blood cells, with an assumed white blood cell count of 8,000 cells/μL. At least 100 high-power fields were read before a slide was declared negative.
Blood samples were collected on filter paper for PK analysis from days 1 to 28. The blood concentrations of ferroquine and its metabolite N-monodesmethyl ferroquine were determined using a validated liquid chromatography with tandem mass spectrometry method with a lower limit of quantification of 5 ng/mL. PK analysis was performed using a noncompartmental approach using validated software (WinNonLin Professional, version 4.0.1).11 The assays and PK analysis were done at SA (Chilly-Mazarin and Longjumeau, France).
Statistical analysis and data quality.
Sample size calculations were based on empiric considerations by SA to observe safety trends, primarily hepatic, in a small-dose escalation study versus an established 4-aminoquinolone comparator, AQ. SA did an initiation visit at each site to assure a common understanding of the protocol. Regular site monitoring ensured the quality of trial conduct. Data entry, verification, and validation were done using standard computer software, and were stored in an Oracle database on a Digital VMS computer. A double-entry method was used to ensure data accuracy.
RESULTS
Among 198 patients screened, we randomized 72 beginning on October 16, 2007, with the last follow-up visit November 9, 2008. Randomization exclusions were 126, nearly all for not meeting less than or equal to one inclusion/exclusion criteria, typically a laboratory abnormality, or HIV positivity (Figure 1). All randomized patients had symptomatic uncomplicated P. falciparum malaria with a history of fever, and most had headache and fatigue. Two patients with SAEs during treatment were withdrawn, one erroneously in the AQ group, leaving 70 (97%) in the m-ITT population. For this report, the erroneously withdrawn AQ patient was not considered an SAE. Demographics were similar among study groups (Table 1).
Table 1.
Summary of randomized patient characteristics at baseline
| FQ + AS | ||||||
|---|---|---|---|---|---|---|
| 100 mg (N = 12) | 200 mg (N = 12) | 400 mg (N = 12) | 600 mg (N = 12) | AQ + AS (N = 24) | All (N = 72) | |
| Race, n (%) | ||||||
| Black | 12 (100) | 12 (100) | 12 (100) | 12 (100) | 24 (100) | 72 (100) |
| Age (years) | ||||||
| Mean (SD) | 35 (13) | 30 (8) | 36 (12) | 25 (3) | 33 (11) | 32(11) |
| Age group, n (%) | ||||||
| 18–44 | 8 (67) | 11 (92) | 8 (67) | 12 (100) | 18 (75) | 57 (79) |
| 45–64 | 4 (33) | 1 (8) | 4 (33) | 0 | 6 (25) | 15 (21) |
| Weight (kg) | ||||||
| Mean (SD) | 61.53 (8.29) | 58.84 (6.32) | 61.81 (7.36) | 60.17 (7.55) | 65.64 (9.48) | 62.27 (8.36) |
| BMI (kg/m2) | ||||||
| Mean (SD) | 21 (2.17) | 21 (2.13) | 21 (2.86) | 21 (1.97) | 23 (2.97) | 21 (2.57) |
| Parasitemia (parasites/uL) | ||||||
| Mean (SD) | 6,827 (9,663) | 21,301 (25,213) | 13,157 (25,431) | 12,432 (14,330) | 6,305 (14,370) | 11,055 (18,474) |
| Geometric mean | 1,925.4 | 6,561.4 | 1,490.1 | 5,824.1 | 1,719.8 | 2,621.1 |
| Range | 115–27,685 | 268–66,240 | 102–81,300 | 108–48,500 | 108–67,505 | 102–81,300 |
| Median | 1,325.5 | 11,411.5 | 865.0 | 7,746.0 | 1,969.5 | 2,395.0 |
| Minimum:Maximum | 115:27,685 | 268:66,240 | 102:81,300 | 108:48,500 | 108:67,505 | 102:81,300 |
AQ = amodiaquine; AS = artesunate; BMI = body mass index; SD = standard deviation.
Safety.
There were no deaths during the study (Table 2). Of 72 randomized patients (12 in each ferroquine dose group [N = 48], and 24 total in the AQ group), 70 completed their assigned treatment, and all had a post-randomization parasitemia evaluation. The safety evaluation was based on patients who took at least one dose of an investigational product (N = 72).
Table 2.
Safety profile (top) and common or relevant TEAEs (bottom)
| FQ + AS | |||||
|---|---|---|---|---|---|
| Top | 100 mg (N = 12) | 200 mg (N = 12) | 400 mg (N = 12) | 600 mg (N = 12) | AQ + AS (N = 24) |
| Any TEAE (including SAE) | 3 | 9 | 6 | 5 | 16 |
| Any SAE* | 1 | 0 | 0 | 0 | 2 |
| Deaths | 0 | 0 | 0 | 0 | 0 |
| Withdrawn from treatment | 1 | 0 | 0 | 0 | 1‡ |
| Primary system organ class | |||||
| Gastrointestinal disorders | 0 | 5 | 4 | 4 | 9 |
| Vomiting | 0 | 1 | 1 | 2 | 1 |
| Abdominal pain | 0 | 0 | 1 | 2 | 2 |
| Nausea | 0 | 3 | 2 | 1 | 6 |
| Dyspepsia | 0 | 0 | 0 | 1 | 0 |
| Diarrhea | 0 | 3 | 1 | 0 | 3 |
| General disorders | 0 | 0 | 2 | 1 | 4 |
| Pyrexia | 0 | 0 | 1 | 1 | 1 |
| Chills | 0 | 0 | 1 | 0 | 1 |
| Fatigue | 0 | 0 | 0 | 0 | 2 |
| Nervous system disorders | 2 | 7 | 3 | 0 | 14 |
| Headache | 1 | 5 | 2 | 0 | 8 |
| Dizziness | 1 | 2 | 1 | 0 | 6 |
| Cardiac disorders | 1 | 0 | 0 | 0 | 2 |
| QTcF interval > 500 milliseconds | 1† | 0 | 0 | 0 | 0 |
| Delta QTcF interval > 60 milliseconds vs. baseline | 1† | 0 | 0 | 0 | 2 |
| Blood disorders | |||||
| Eosinophilia (> ULN, 0.5 giga/L) | 2 | 2 | 2 | 4 | 4 |
AQ = amodiaquine; AS = artesunate; SAE = serious adverse event; TEAE = treatment emergent adverse events; ULN = upper limit of normal.
Four SAEs in three patients; one AQ + AS patient had two SAEs.
Same patient.
Study error.
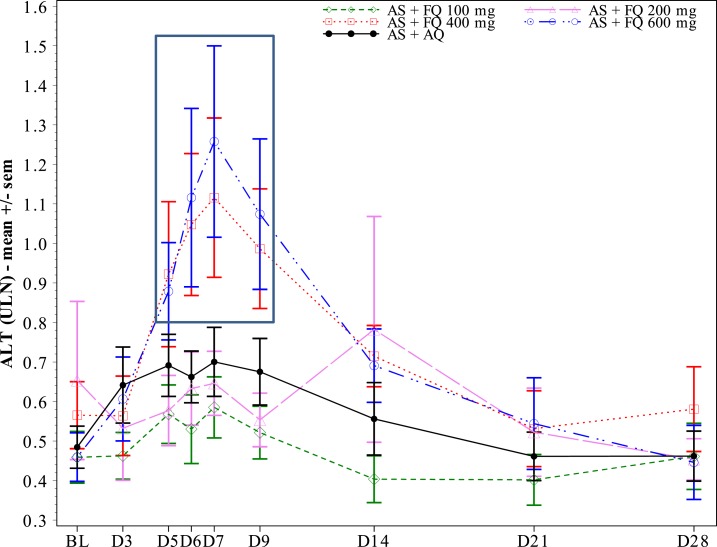
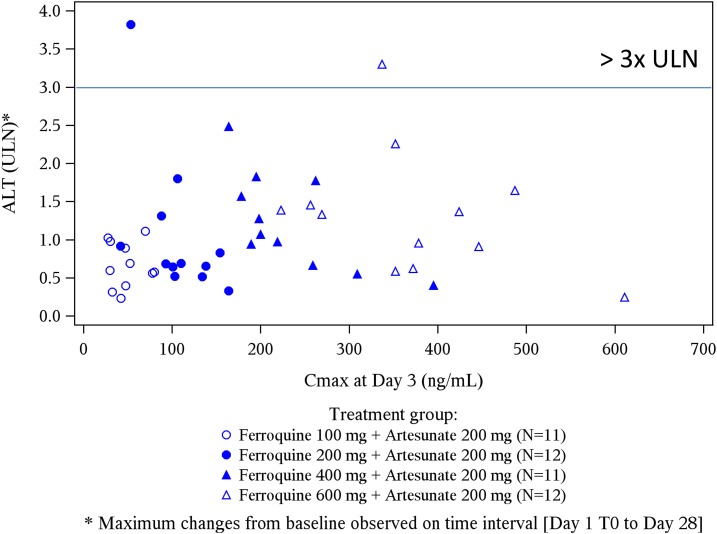
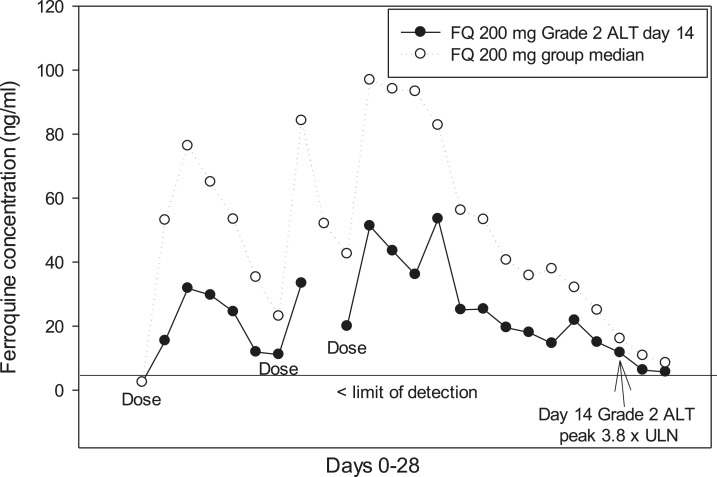
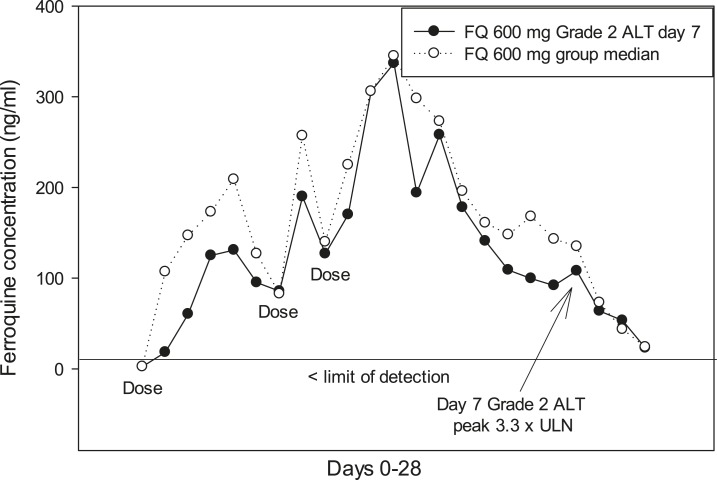
For liver function tests, there were no SAEs, or treatment withdrawals. Most ferroquine and AQ patients had ALT ≤ 1 ULN, whereas a minority developed transient ALT > 1 and < 2 ULN (Table 3). A ferroquine dose-related effect on ALT levels started at 400 mg, being most evident on day 5–9 (Figure 2). There was no correlation between ferroquine Cmax and maximum changes from baseline for ULN of ALT (Figure 3). There were two PCSAs (Grade 2) for ALT ≥ 3 ULN ≤ 10 in ferroquine patients, after dosing completed: one 200-mg ferroquine patient (maximum value: 3.8× ULN on day 14; ferroquine blood concentration 13 ng/mL) and one 600-mg ferroquine patient (maximum value: 3.3 × ULN on day 7; ferroquine blood concentration 89 ng/mL), both without concurrent bilirubin increases ≥ 1.5 ULN (Figures 4 and 5; Table 3). The median blood concentration of ferroquine for the 200- and 600-mg patient groups as a whole over the 28-day study period trended above or similarly to the two Grade 2 ALT patients, respectively (Figures 4 and 5).
Table 3.
Liver function tests, showing PCSAs vs. baseline status
| AS + FQ | |||||
|---|---|---|---|---|---|
| Parameter | 100 mg (N = 12) | 200 mg (N = 12) | 400 mg (N = 12) | 600 mg (N = 12) | AS + AQ (N = 24) |
| ALT (IU/L) | |||||
| ≤ 1 ULN (within normal limits) | 9 | 9 | 6 | 5 | 18 |
| > 1 and < 2 ULN (mild) | 3 | 2 | 5 | 5 | 4 |
| ≥ 2 and < 3 ULN (mild) | 0 | 0 | 1* | 1* | 2* |
| ≥ 3 and ≤ 10 ULN (PCSA*; moderate) | 0 | 1 | 0 | 1 | 0 |
| > 10 ULN (SAE) | 0 | 0 | 0 | 0 | 0 |
| AST (IU/L) | |||||
| ≤ 1 ULN | 10 | 9 | 6 | 7 | 18 |
| > 1 and < 2 ULN (mild) | 2 | 2 | 4 | 4 | 6 |
| ≥ 2 and < 3 ULN (mild) | 0 | 1 | 2 | 1 | 0 |
| ≥ 3 ULN (PCSA; moderate) | 0 | 0 | 0 | 0 | 0 |
| Total bilirubin (umol/L) ≥ 1.5 ULN (PCSA) | 0 | 0 | 0 | 0 | 0 |
| ALT ≥ 3 ULN and total bilirubin ≥ 2 ULN (SAE) | 0 | 0 | 0 | 0 | 0 |
| Alkaline phosphatase (IU/L) ≥ 1.5 ULN (PCSA) | 0 | 1 | 0 | 0 | 1 |
| Gamma GT (IU/L) ≥ 3 ULN (PCSA) | 0 | 0 | 0 | 0 | 0 |
AQ = amodiaquine; AS = artesunate; GT = glutamyl transferase; PCSA = potentially clinically significant affect; SAE = serious adverse event; ULN = upper limit of normal.
Occurred after dosing was completed.
Figure 2.
Mean alanine aminotransferase (ALT) raw values expressed by upper limit of normal (ULN), by visit and by treatment. A dose–response trend occurred in doses of 400 and 600 mg ferroquine, approximately days 5–9 (box). There was no relation between ferroquine Cmax and maximum ALT elevations (Figure 3).
Figure 3.
Ferroquine Cmax (drug exposure) and maximum changes from baseline for upper limit of normal (ULN) of alanine aminotransferase (ALT). There was no trend between the two factors.
Figure 4.
A ferroquine 200 mg patient had a Grade 2 alanine aminotransferase (ALT) adverse event, maximum level was 3.8 × upper limit of normal (ULN) on day 14. The median ferroquine blood concentration in the 200-mg ferroquine group trended higher than the Grade 2 ALT patient.
Figure 5.
A ferroquine 600 mg patient had a Grade 2 alanine aminotransferase (ALT) adverse event, maximum level was 3.3 × upper limit of normal (ULN) on day 7. The median ferroquine blood concentration in the 600-mg ferroquine group trended closely with the Grade 2 ALT patient.
There were no PCSAs in the ferroquine groups for AST, bilirubin, or ALT + bilirubin (Table 3). Four ferroquine patients, all in the 200 mg group, and one AQ patient, developed PCSAs (Grade 2) for ALP ≥ 1.5 ULN (Table 3). The 200-mg ferroquine group had the highest ALP baseline values of all the groups (Supplemental Figure 1); in three (of four) ALP PCSAs, the patients were above ULN at baseline, ranging from 1.7 to 2.4 × ULN.
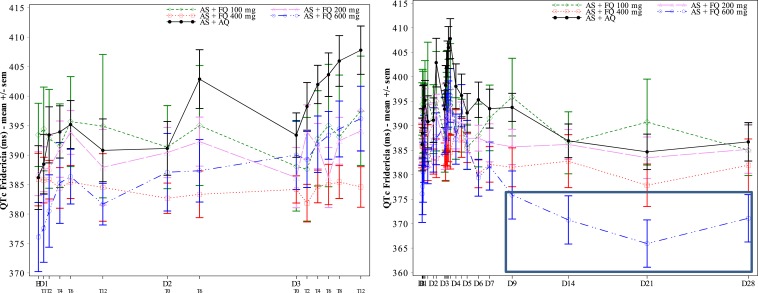
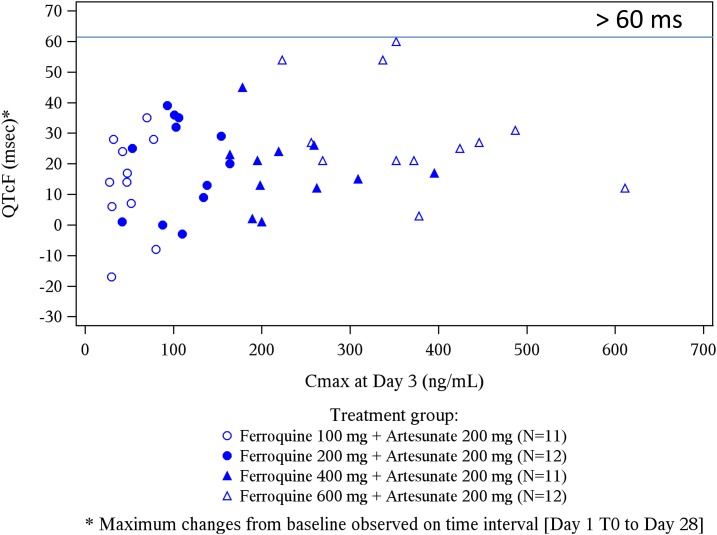
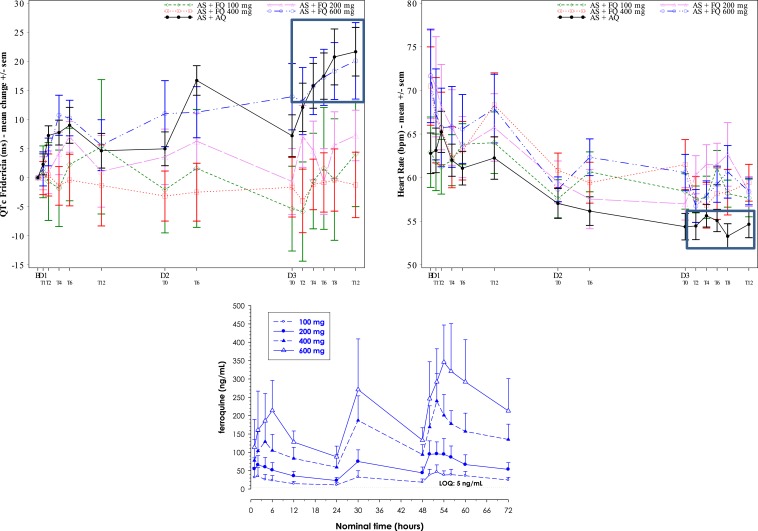
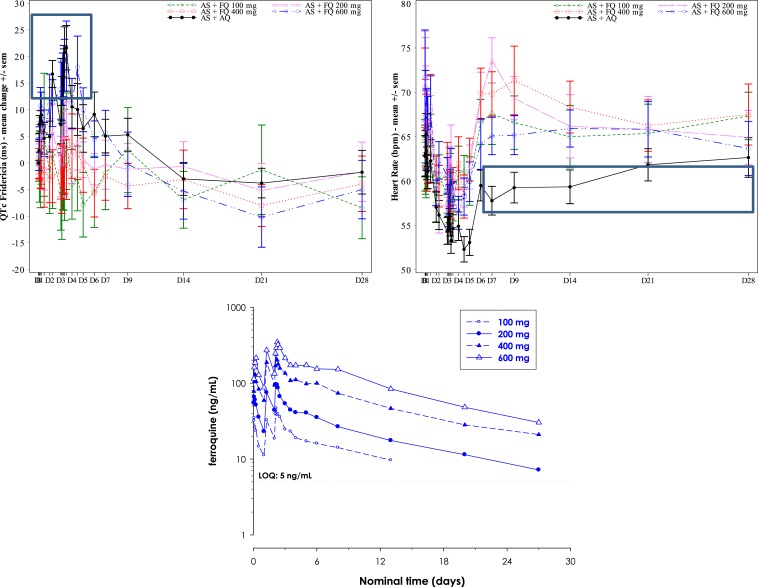
For most 100 (11/12 patients), 200-, 400-, and 600-mg ferroquine patients, mild or moderate changes in QTcF interval prolongation (< 60 milliseconds) from baseline were observed. During the 3-day dosing period, especially at day 3, the 600-mg ferroquine and AQ groups showed a trend toward mildly higher mean QTcF interval increases than the other three ferroquine groups (Figures 6 and 7). This was less obvious for raw QTcF interval plots, which showed a trend toward lower QTcF intervals by day 9 in the 600-mg ferroquine group (Figure 8). Otherwise, there was no apparent ferroquine dose relation to day 28, and there was no dose trend between ferroquine Cmax and maximum changes from baseline for QTcF prolongations (Figure 9).
Figure 8.
Raw QTcF values, days 0–3, left, and days 0–28, right. By day 9, note trend for 600 mg ferroquine, right, toward lower QTcF values (box), supporting the notion of lack of a dose response with changes in QTcF.
Figure 9.
Ferroquine Cmax (drug exposure) and maximum changes from baseline for QTcF. There was no trend between the two factors. One 100-mg ferroquine patient developed > 60 milliseconds QTcF interval, day 1, and was withdrawn.
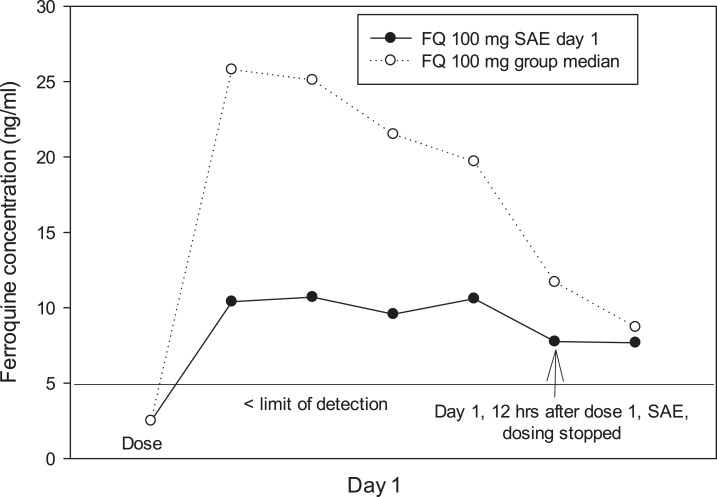
Among four SAEs, all related to QTcF prolongations > 60 milliseconds, one was in a ferroquine 100 mg patient and three were in two AQ patients (Table 2). Two of four SAEs led to stopping treatment, one erroneously. Withdrawn patient 1 (Gabon; 100 mg ferroquine + AS), 12 hours after dose one, developed a QTcF interval prolongation > 60 milliseconds (+104 milliseconds, from 397 milliseconds at baseline), also increasing the QTcF interval to 501 milliseconds. The ECG was not available for review. At screening, the patient’s ECG showed inverted T-wave morphology. The patient’s ferroquine blood concentration at withdrawal was 8 ng/mL. The median ferroquine concentration for the 100 mg group as a whole trended higher than the SAE patient after the single ferroquine dose (Figure 10). AQ + AS was given for 2 days as rescue therapy, where the patient again developed increases in QTcF prolongations (+100 and +103 milliseconds, respectively); the ECG returned to normal during follow-up.
Figure 10.
A ferroquine 100 mg patient had a serious adverse event (SAE), > 60 milliseconds QTcF change from baseline, day 1, 12 hours after dose one. The median ferroquine blood concentration in the 100-mg ferroquine group trended higher than the SAE patient.
Withdrawn patient 2 (Kenya; AQ + AS), 1 hour after dose two, developed palmar and plantar itching and an urticarial rash that resolved with chlorpheniramine. This was a study error and the patient was not recorded an SAE for this report.
The remaining three SAEs were reported in two AQ patients, after dosing was completed. Patient 3 (Gabon), with one SAE, developed a QTcF interval prolongation > 60 milliseconds (+68 milliseconds, from 356 milliseconds at baseline). The interval remained > 60 milliseconds into day 4, but returned to normal during follow-up. Patient 4 (Kenya), with two SAEs, developed a QTcF interval prolongation > 60 milliseconds (+84 milliseconds, from 348 milliseconds at baseline), which normalized in 4 days. On day 28, the patient presented with a second QTcF interval prolongation > 60 milliseconds (+63 milliseconds), the outcome of which was unknown at study end.
There was one transient PCSA for creatinine in the 200-mg ferroquine group on day 14, and one urea PCSA in another 200-mg ferroquine patient on day 28, both judged not clinically relevant. Vital sign abnormalities, consisting of orthostatic hypotension, were all in the AQ + AS group (N = 3); these were attributed to malaria and not listed as TEAEs. Supine systolic and diastolic BPs are shown in Supplemental Figure 2.
Tolerability.
MedRA TEAE trends were generally similar between FQ and AQ patients (Table 2). The most common TEAEs in all groups were gastrointestinal (GI) upset, headache, and dizziness. For ferroquine 100 mg dose only, there were no GI complaints, whereas the three higher dose ferroquine groups showed similar GI side effect trends, with no dose trends. Eosinophilia was present in 16 ferroquine and eight AQ patients at baseline, and was treatment emergent in 10 ferroquine and four AQ patients (Table 2).
PCTs and efficacy.
Median PCTs were ≤ 24 hours for all ferroquine groups, and the AQ group, regardless of parasitemia, the former with no relationship to ferroquine dose (Table 4). No parasitemia recurrence was observed in any patient through day 28.
Table 4.
Summary of parasite clearance time in hours
| AS + FQ | |||||
|---|---|---|---|---|---|
| 100 mg (N = 12) | 200 mg (N = 12) | 400 mg (N = 12) | 600 mg (N = 12) | AS + AQ (N = 24) | |
| Number | 12 | 12 | 12 | 12 | 24 |
| Mean (SD) | 23.91 (9.84) | 23.00 (15.31) | 17.49 (13.66) | 19.95 (11.55) | 18.23 (7.78) |
| Median | 23.84 | 23.95 | 12.00 | 23.79 | 23.67 |
| Minimum:maximum | 12.0:47.8 | 6.0:54.0 | 5.9:48.0 | 5.8:48.0 | 6.0:30.0 |
AQ = amodiaquine; AS = artesunate; SD = standard deviation. There was no recurrence of parasitemia through day 28.
Pharmacokinetics.
PK analyses of ferroquine and its main metabolite N-monodemethyl ferroquine were done on subjects with available PK blood samples (ferroquine only shown: Table 5; Figures 6 and 7). Briefly, ferroquine was absorbed with a tmax between 1.15 and 5 hours on day 1 and between 4 and 6 hours on day 3, at the different dose levels. In the 100–600 mg ferroquine dose range, ferroquine exposure increased with the dose. Evidence of dose proportionality was observed between 100 and 400 mg for ferroquine exposure on each day, whereas in the 100–600 mg dose range, exposure (Cmax, area under the curve [AUC] 0–24, and cumulated AUCs) increased more than expected by dose proportionality. The metabolite-over-parent-compound exposure ratio (calculated from AUCcumlast) in blood ranged from 91% to 128% for ferroquine in the dose range of 200–600 mg.
Table 5.
Mean blood PK parameters of ferroquine following 3-day repeated ferroquine + AS
| PK parameter | Day | tmax (hours) | Cmax (ng/mL) | AUC0-24 (ng.h/mL) | AUCcum (ng.h/mL) |
|---|---|---|---|---|---|
| 100 mg* | 1 | 1.15 (0.75, 6.00) | 39.5 (59) | 441 (47) | – |
| 3 | 4.00 (1.77, 11.92) | 48.8 (40) | 828 (32) | 9,580† (29) | |
| 200 mg | 1 | 2.07 (0.98, 12.00) | 70.0 (38) | 943 (32) | – |
| 3 | 4.00 (1.99, 8.00) | 107 (34) | 1,730 (34) | 18,900 (36) | |
| 400 mg* | 1 | 4 (0.98, 12.00) | 139 (31) | 2,150 (34) | – |
| 3 | 4.04 (3.83, 6.00) | 233 (29) | 3,920 (27) | 45,700 (35) | |
| 600 mg | 1 | 5 (2.05, 12.00) | 247 (34) | 3,340 (26) | – |
| 3 | 6.00 (3.92, 12.00) | 376 (29) | 6,550 (36) | 77,600 (33) |
AS = artesunate. Tabulated values are mean (CV%) except for tmax where values are median (minimum, maximum).
N = 11.
N = 8.
Figure 6.
Day 0-3, mean changes from baseline in QTcF intervals (left), raw heart rates (right) and ferroquine blood levels (lower). By day 3, a dose–response trend for mean QTcF interval prolongation was noted in 600-mg ferroquine and amodiaquine (AQ) groups (box, left), and AQ heart rates trended lowest (box, right). One 100-mg ferroquine patient developed > 60 milliseconds QTcF elongation, day 1, and was withdrawn.
Figure 7.
Days 0–28, mean changes from baseline in QTcF intervals (left), raw heart rates (right), and ferroquine blood levels (lower). By day 3, a dose–response trend for QTcF interval elongation was noted in 600-mg ferroquine and amodiaquine (AQ) groups (box, left). By day 5, there was a trend of lower heart rates in AQ (box, right).
DISCUSSION
A small 3-day dose escalation study of ferroquine up to 600 mg (+AS 200 mg), in African men with symptomatic uncomplicated P. falciparum malaria, showed generally favorable safety and tolerability trends, similar to an AQ (+AS 200 mg) comparator. PCTs in all groups were rapid, attributed to AS, with no recurrence of parasitemia observed to day 28, similar to a Phase 2 efficacy study.7, 10 Nonetheless, this small a study underscores the need to focus on trends, with cautious interpretation.
A known adverse drug reaction of 4-aminoquinolones in the treatment of malaria patients is elevation of hepatic transaminases, especially ALT. Per the primary outcome, we saw nil or modest toxicity trends in most patients for hepatic profiles, notably though with dose trends for ALT toxicity beginning at 400 mg of ferroquine, most apparent days 5–9. Nonetheless, most ALT values for ferroquine patients remained within normal limits (≤ 1 ULN), whereas others were only mildly increased (> 1 ULN < 2; Grade 1). We observed PCSAs (Grade 2) for ALT ≥ 3 ULN in two ferroquine patients, and two AQ patients, the former both after dosing was finished. A 200-mg ferroquine patient (maximum value: 3.8 × ULN on day 14) and one 600-mg ferroquine patient (maximum value: 3.3 × ULN on day 7) had ferroquine levels of 13 and 89 ng/mL on those days, respectively. The median ferroquine concentration for the 200- and 600-mg ferroquine groups as a whole trended higher or similarly to the two Grade 2 ALT patients, respectively, supporting the notion of a lack of dose response. Indeed, ferroquine Cmax and maximum ALT elevations from baseline were unrelated.
A delay in reaching maximum ALT levels, relative to ferroquine dosing time, has been reported, and one could not exclude that it might be related to the long half-lives of ferroquine and its metabolite, approximately 20 days.7 Neither patient was symptomatic, nor had concomitant increases in bilirubin ≥ 2 ULN (Grade 2), the latter known as Hy’s law, which associates the combination of elevated ALT and bilirubin with a higher risk of drug-related liver failure12; this suggested limited Grade 2 toxicity involving ALT levels.13 Coupled with minimal effects on AST, bilirubin and GGT, and two ALT PCSAs (≥ 3 × ULN, Grade 2), this trend suggested at worst the potential for moderate, reversible liver toxicity. Though an ALT PCSA observed in the highest 600-mg ferroquine dose might be expected, that the only other ALT PCSA occurred in the second highest 200-mg ferroquine group, and with a maximum ALT value relatively late on day 14, and overall not associated with ferroquine Cmax, might raise the question of other factors involved in ALT elevation. GGT was not elevated in either suggesting it was not from drinking ethanol.
Excluding the 200-mg ferroquine patient with an ALT PCSA, we nonetheless observed a general dose–response trend between ferroquine dose and mean ALT elevations starting at 400 mg, most evident days 5–9, timing which also coincided with the 600-mg ferroquine patient’s ALT PCSA; in line with this, ferroquine blood concentrations mirrored dose.
Among ferroquine patients, there were four transient, asymptomatic ALP PCSAs (Grade 2), notably all in the 200 mg dose group, and one ALP PCSA in the AQ group. Among the four ALT PCSAs in ferroquine 200 mg, three patients had elevated ALP at screening, ranging from 1.7 to 2.4 × ULN, suggesting these were not TEAEs and making the elevations difficult to ascribe to a drug effect. Paralleling this, other studies have not reported clinically meaningful ALP levels in association with ferroquine.7 Nonetheless, hepatic profiles, not just ALT and bilirubin, should be carefully monitored in future studies.
ECG changes, particularly QTcF prolongation, are also a generally known adverse drug event of 4-aminoquiolones such as AQ and piperaquine,14 including ferroquine,7 in treating malaria patients. Here, there were four SAEs, all regarding QTcF prolongations: one in a 100-mg ferroquine patient and three in AQ patients. One AQ patient was erroneously graded an SAE for a hypersensitivity rash after two doses, and was not graded an SAE for this report. For the ferroquine 100 mg patient, 12 hours after dose one, there was a QTcF interval prolongation > 60 milliseconds from baseline, stopping treatment. The QTcF prolongation of +104 milliseconds from 397 milliseconds at baseline, also resulted in QTcF interval of 501 milliseconds. At screening, his ECG showed abnormal T-wave morphology, not uncommon in young men,15 and was not exclusionary. The single ECG SAE for ferroquine, in the lowest 100 mg dose, with a ferroquine blood level of just 8 ng/mL, far below the median concentrations for the 100-mg ferroquine group as a whole, underscores a possible lack of dose relationship with potentially important ECG changes, previously described. 7 Indeed, we also saw no relationship between ferroquine Cmax and maximum changes in QTcF intervals. Three other SAEs, in two AQ patients, occurred after dosing was completed and consisted of QTcF interval prolongations > 60 milliseconds from baseline. Vital sign PCSAs consisted of orthostatic hypotension, in three AQ patients, attributed to malaria and not listed as TEAEs. However, per study methods, only 2 minutes standing after being supine is too insensitive to measure this, weakening this outcome.
We assessed QT prolongation with QTcF, often considered optimal for correcting the QT interval for heart rate,16 and measured ferroquine blood concentrations. Here, we saw dose–response trends by day 3 for ferroquine 600 mg and AQ, both having the largest QTcF changes from baseline, accompanied by confirmatory blood concentrations of ferroquine 600 mg being the highest among all ferroquine groups. Similar to ALT elevations, however, ferroquine Cmax values were not associated with maximum QTcF prolongations. Nonetheless, that one ferroquine patient was withdrawn after one 100 mg dose for > 60 milliseconds increase in tandem with a QTcF interval of > 500 milliseconds, underscores the need for a better understanding of ferroquine’s effect on QTcF, and cardiac profiling in future studies.
The incidence of MedDRA TEAEs was generally similar in the ferroquine and AQ groups, with headache and GI complaints most common. All were mild and transient. For ferroquine, the trends suggested most TEAEs were unrelated to dose, with the exception of no GI complaints in the 100-mg ferroquine group only, versus the three higher ferroquine dose groups with similar rates. Like an earlier Phase 1 study suggesting a dose-dependent increase in GI events, we found that a relatively low dose of ferroquine, 100 mg daily, but no higher, showed trends of relatively fewer GI side effects.11 Other less common TEAEs, such as fatigue were transient, and similar in number across all groups. Some patients had eosinophilia at baseline, and fewer developed while on study, all attributed to nonmalarial parasites acquired before the study.
Our small study and others clearly indicate hepatic and cardiac profiles should be monitored carefully in future ferroquine studies, the latter especially in children per a completed Phase 2 study.7 Future studies should thus involve children, largely the intended population, as well as adults with hepatitis B or C, and those with HIV not on anti-retrovirals. For hepatitis C, especially, viral loads should be measured given the high rate of false-positive antibody tests, at least in America.17 Ideally, ferroquine would prove safe enough for licensing in all patients, adding a useful weapon in treating uncomplicated P. falciparum malaria.
Supplementary Material
Acknowledgments:
We thank the study clinical officers, laboratory technicians, and nurses, volunteers in Gabon and Kenya, and the Data Monitoring Committee (DMC). DMC members and affiliations (four experts in malaria [one clinical pharmacologist, one epidemiologist, two clinicians], a statistician and a hepatologist): Sanjeev Krishna, MD, Chairperson, Division of Cellular and Molecular Medicine Centre for Infection, St. George’s University of London, United Kingdom; Joris Cauquil, MS EFFI-STAT, Biostatistician, Paris, France; Jürgen May, MD, Bernhard-Nocht-Institute for Tropical Medicine Infection Epidemiology, Hamburg, Germany; Ogobara Doumbo, MD, Professor and Head of Department, Director, Malaria Research and Training Center DEAP/FMPOS University of Bamako, Bamako, Mali; Christophe Rogier, MD, Head of the Parasite Biology and Epidemiology Department, Institute for Tropical Medicine of the French Army, Marseille-Armées, France.
Note: Supplemental figures appear at www.ajtmh.org.
Disclaimer: The opinions or assertions contained herein are the private views of the Walter Reed Project authors and are not to be construed as official or reflecting views of the U.S. Department of the Army or the U.S. Department of Defense. Sanofi-Aventis, the study funder, participated in study design, data analysis, data interpretation, and writing of the clinical report. The funder had no role in data collection. The corresponding author had full access to all the data in the study. Ferroquine is licensed from l’Université de Lille 1 (France).
REFERENCES
- 1.Hawkes M, Conroy AL, Kain KC, 2014. Spread of artemisinin resistance in malaria. N Engl J Med 371: 1944–1945. [DOI] [PubMed] [Google Scholar]
- 2.Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D, 2011. The antimalarial ferroquine: from bench to clinic. Parasite 18: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atteke C, Ndong JM, Aubouy A, Maciejewski L, Brocard J, Lébibi J, Deloron P, 2003. In vitro susceptibility to a new antimalarial organometallic analogue, ferroquine, of Plasmodium falciparum isolates from the Haut-Ogooue region of Gabon. J Antimicrob Chemother 51: 1021–1024. [DOI] [PubMed] [Google Scholar]
- 4.Barends M, Jaidee A, Khaohirun N, Singhasivanon P, Nosten F, 2007. In vitro activity of ferroquine (SSR 97193) against Plasmodium falciparum isolates from the Thai-Burmese border. Malar J 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyase FL, Akala HM, Johnson JD, Walsh DS, 2011. Inhibitory activity of ferroquine, versus chloroquine, against western Kenya Plasmodium falciparum field isolates determined by a SYBR Green I in vitro assay. Am J Trop Med Hyg 85: 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreidenweiss A, Kremsner PG, Dietz K, Mordmuller B, 2006. In vitro activity of ferroquine (SAR97193) is independent of chloroquine resistance in Plasmodium falciparum. Am J Trop Med Hyg 75: 1178–1181. [PubMed] [Google Scholar]
- 7.Held J, Supan C, Salazar CL, Tinto H, Bonkian LN, Nahum A, Moulero B, Sié A, Coulibaly B, Sirima SB, Siribie M, Otsyula N, Otieno L, Abdallah AM, Kimutai R, Bouyou-Akotet M, Kombila M, Koiwai K, Cantalloube C, Din-Bell C, Djeriou E, Waitumbi J, Mordmüller B, Ter-Minassian D, Lell B, Kremsner PG, 2015. Ferroquine and artesunate in African adults and children with Plasmodium falciparum malaria: a phase 2, multicentre, randomised, double-blind, dose-ranging, non-inferiority study. Lancet Infect Dis 15: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 8.Wani WA, Jameel E, Baig U, Mumtazuddin S, Hun LT, 2015. Ferroquine and its derivatives: new generation of antimalarial agents. Eur J Med Chem 101: 534–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells TN, Hooft van Huijsduijnen R, 2015. Ferroquine: welcome to the next generation of antimalarials. Lancet Infect Dis 15: 1365–1366. [DOI] [PubMed] [Google Scholar]
- 10.Mombo-Ngoma G, Supan C, Dal-Bianco MP, Missinou MA, Matsiegui PB, Ospina Salazar CL, Issifou S, Ter-Minassian D, Ramharter M, Kombila M, Kremsner PG, Lell B, 2011. Phase I randomized dose-ascending placebo-controlled trials of ferroquine—a candidate anti-malarial drug—in adults with asymptomatic Plasmodium falciparum infection. Malar J 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supan C, Mombo-Ngoma G, Dal-Bianco MP, Ospina Salazar CL, Issifou S, Mazuir F, Filali-Ansary A, Biot C, Ter-Minassian D, Ramharter M, Kremsner PG, Lell B, 2012. Pharmacokinetics of ferroquine, a novel 4-aminoquinoline, in asymptomatic carriers of Plasmodium falciparum infections. Antimicrob Agents Chemother 56: 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro VJ, Senior JR, 2006. Drug-related hepatotoxicity. N Engl J Med 354: 731–739. [DOI] [PubMed] [Google Scholar]
- 13.Common Toxicity Criteria for Adverse Events, version 4.0 (CTCAEv4): National Cancer Institute, National Institutes of Health, Bethesda, MD.
- 14.Manning J, Vanachayangkul P, Lon C, Spring M, So M, Sea D, Se Y, Somethy S, Phann ST, Chann S, Sriwichai S, Buathong N, Kuntawunginn W, Mitprasat M, Siripokasupkul R, Teja-Isavadharm P, Soh E, Timmermans A, Lanteri C, Kaewkungwal J, Auayporn M, Tang D, Chour CM, Prom S, Haigney M, Cantilena L, Saunders D, 2014. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother 58: 6056–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atterhog JH, Malmberg P, 1981. Prevalence of primary T-wave changes in young men and their relationship to psychological and anthropometric data. Clin Cardiol 4: 91–97. [DOI] [PubMed] [Google Scholar]
- 16.Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, Ector J, Willems R, 2016. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 5: pii: e003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorman AC, Drobenuic J, Kamili S, 2017. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol 89: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.