Abstract.
Zika virus (ZIKV) has a wide clinical spectrum of associated neurologic disease including microcephaly and Guillain–Barre syndrome but, despite its known neurotropism, ZIKV meningoencephalitis and myelitis have been rare complications. We describe a case of ZIKV meningoencephalitis and probable myelitis and its associated magnetic resonance imaging findings that rapidly resolved during recovery in a previously healthy adult.
INTRODUCTION
The geographical distribution of Zika virus (ZIKV) has steadily broadened since the virus was first detected in Uganda in 1947 and now includes parts of the United States where Aedes aegypti exists. This flavivirus has a wide clinical spectrum of associated neurologic disease including microcephaly and Guillain–Barre syndrome but, ZIKV meningoencephalitis and myelitis have been rare complications.1,2 We describe a case of ZIKV-associated meningoencephalitis and probable myelitis and associated rapidly resolving magnetic resonance imaging (MRI) findings in a previously healthy adult.
CASE REPORT
A 36-year-old woman presented with fever to 102°F, fatigue, headaches, and rash 4 days after returning to the United States from Bonao, Dominican Republic. Five days after symptom onset, she was admitted to another hospital for acute onset of confusion and neck stiffness. A computed tomographic scan of the head was normal. Cerebrospinal fluid (CSF) obtained on the day of presentation revealed: leukocyte count 72 cells per microliter (normal < 5) with 81% lymphocytes, 16% mononuclear cells, and 3% polymorphonuclear leukocytes, glucose concentration of 57 mg/dL (normal 40–85mg/dL) with simultaneous serum glucose of 91 mg/dL, and protein concentration of 105 mg/dL (normal 15–45 mg/dL).
That evening, the patient had generalized convulsive seizures that did not respond to lorazepam. She was intubated and started on propofol and levetiracetam with cessation of her convulsions. Ampicillin, acyclovir, vancomycin, ceftriaxone, and dexamethasone were administered and the patient was transferred to our intensive care unit where on day 1, she was normotensive, tachycardic, and febrile (103.1°F). No rash was present, and her neck was supple. She had bilateral conjunctival erythematous suffusion. She did not open her eyes spontaneously or to verbal or nociceptive stimuli. Pupils were 4 mm, equal, and reactive to light. Tone was mildly spastic in the lower extremities. She withdrew all limbs from pain symmetrically. The muscle stretch reflexes were 3+ diffusely with clonus at the ankles, and plantar reflexes were extensor bilaterally. Pregnancy testing was negative. Doxycycline was added to her regimen. Ampicillin and dexamethasone were discontinued.
A repeat lumbar puncture was performed on day 2. CSF was sent for ZIKV, dengue (DENV), chikungunya (CHIK), varicella zoster, herpes simplex, cytomegalovirus, Powassan, West Nile virus (WNV), and eastern equine encephalitis polymerase chain reactions (PCRs). CSF was additionally sent to check for syphilis venereal disease research laboratory (VDRL), lyme antibodies, and cryptococcal antigen. Serum was sent for ZIKV, DENV, CHIK, and WNV PCR. Antibody tests for human immunodeficiency virus (HIV), ZIKV, DENV, CHIK, Borrelia burgdorferi, and Rickettsia rickettsii were sent.
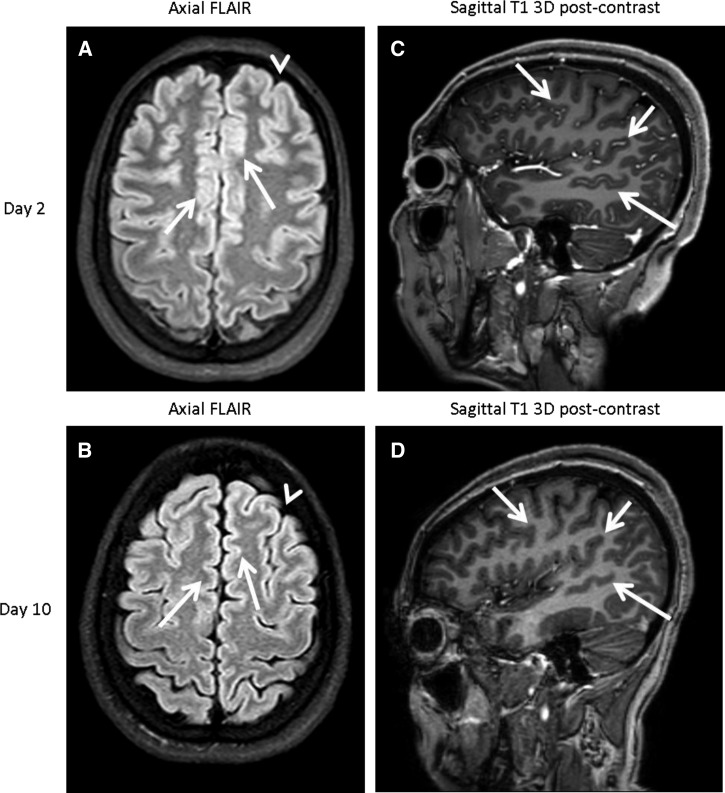
An electroencephalogram showed no epileptiform activity. MRI of the brain with gadolinium enhancement conducted on day 2 was diagnostic of meningoencephalitis (Figure 1).
Figure 1.
Zika virus cortical edema and leptomeningeal enhancement (meningoencephalitis) that resolved seen on 3 tesla magnetic resonance imaging. Axial FLAIR image of the brain performed on day 2 (A) demonstrates extensive superficial gray matter thickening consistent with cortical edema (white arrows), with associated effacement of sulci (arrow head). Axial FLAIR image conducted on day 10 (B) demonstrates resolution of cortical edema. The findings are most evident when one compares the thickness of the gyri (arrows) and sulci (arrow heads) on day 2 with those of day 10. Sagittal T1 postcontrast image performed on day 2 (C) demonstrates extensive sulcal leptomeningeal enhancement as evidenced by abnormal enhancement (white arrows), a finding which also resolved by day 10 (D; white arrows).
A urine reverse-transcriptase PCR (RT-PCR) for Zika performed at the Massachusetts Department of Public Health was reported to be positive on day 3. CSF ZIKV PCR was not detected in either CSF sample. Serum ZIKV PCR was also negative. Serum and CSF ZIKV IgM were both positive. Other serologies performed at the Centers for Disease Control and Prevention are delineated in Table 1. Blood and CSF bacterial and fungal cultures were negative and antibiotics were stopped. No further fevers were noted after day 2 of hospitalization. All other studies were negative.
Table 1.
Flavivirus serology summary
| IgM | PCR | PRNT | |
|---|---|---|---|
| Serum (day 2 of hospitalization) | |||
| DENV | Negative | Negative | > 1,280 |
| CHIK | Negative | Negative | N/A |
| POW | Negative | Negative | N/A |
| ZIKV | Positive | Negative | > 1,280 |
| CSF (days 1 and 2 of hospitalization) | |||
| POW | Negative | Negative | N/A |
| ZIKV | Positive | Negative | > 256 |
| DENV | N/A | Negative | > 256 |
| Urine (day 2 of hospitalization) | |||
| CHIK | N/A | Negative | N/A |
| DENV | N/A | Negative | N/A |
| ZIKV | N/A | Positive | N/A |
CHIK = chikungunya; CDC = Centers for Disease Control and Prevention; CSF = cerebrospinal fluid; DENV = dengue; PCR = polymerase chain reaction; PRNT = plaque-reduction neutralization test; POW = Powassan; ZIKV = Zika virus. LRN Trioplex Real-time reverse-transcriptase-PCR Assays were performed at the CDC. IgM Capture enzyme-linked immunosorbent assays and PRNT assays were performed at the CDC. PRNT assay performed at the CDC with 90% inhibition endpoint. PRNT is negative (normal) in serum when < 10, and negative in CSF when < 2.
Early in the admission, the patient required continuous sedation and analgesia due to severe headaches. The lower limb hyperreflexia and spasticity became more pronounced. Her confusion improved, facilitating extubation on day 7, after which the severe headache and photophobia persisted. After extubation, her neurological examination was notable for mild encephalopathy without localizing features, normal strength in all limbs, and diffuse hyperreflexia most prominent in the lower extremities with bilateral ankle clonus and persistent extensor plantar responses.
Due to persistent signs of upper motor neuron dysfunction in the lower extremities, MRI of the cervical and the thoracic spine was conducted on day 10 and showed no significant abnormalities. A repeat brain MRI (Figure 1) showed resolution of the prior leptomeningeal enhancement and cortical edema. By the time of discharge on day 12, her headache and lower extremity spasticity and hyperreflexia were improving and the encephalopathy had resolved. No recurrent seizures were noted during hospitalization. On follow-up examination 10 days after discharge she no longer had any lower extremity upper motor neuron signs, but continued to have mild headache, myalgia, and arthralgia.
DISCUSSION
This patient’s clinical syndrome of severe meningoencephalitis and probable concomitant mild myelitis, in combination with the detection of ZIKV RNA in the urine and IgM antibodies to ZIKV in the serum and CSF are consistent with acute ZIKV infection. Autochthonous transmission of ZIKV has been confirmed in 31 out of 32 provinces of the Dominican Republic (including the city of Bonao) as of epidemiological week 48 in 2016.3 Using data from the Pan American Health Organization, the approximate incidence rate of suspected ZIKV cases in the population between the ages of 35 and 39 was 75 per 100,0004 at the time the patient was in Bonao.
ZIKV detection by RT-PCR has been found to persist longer in urine samples as compared with serum samples, which correlates with our findings in Table 1. In a small cohort, Gourinat and others detected RT-PCR in urine for 36 days after disease onset.5 It is currently unclear how long RT-PCR can detect ZIKV in CSF in patients with neurological syndromes. There is substantial serological cross-reactivity among the flaviviruses and current IgM antibody assays cannot reliably distinguish between ZIKV and DENV infections, both of which are endemic in the Dominican Republic. Therefore, a positive IgM enzyme-linked immunosorbent assay result for DENV or ZIKV can only be considered indicative of a recent flavivirus infection.6 Plaque-reduction neutralization tests (PRNTs) are usually more specific, and can be performed to measure virus-specific neutralizing antibodies and may be able to discriminate between cross-reacting antibodies in primary flavivirus infections7; but PRNT assays have not been found to be as specific in acute ZIKV infections. In our case, her CSF was ZIKV IgM positive and both CSF and serum PRNT were positive for ZIKV and DENV. In this case, we believe that the assays demonstrate a cross-reactivity between ZIKV and DENV rather than a coinfection. With serum assays obtained 7–8 days after symptom onset, DENV IgM would be expected to be positive in an acute infection. Having a detectable DENV PRNT more likely demonstrates a cross-reaction given the decreased specificity of the test in acute flavivirus infections. As well, DENV PCR was negative in serum, urine, and CSF. Though coinfections have been described8 and may be relatively common, we believe our case does not appear consistent with an acute coinfection of the two viruses.
In patients, who have been immunized against yellow fever or Japanese encephalitis viruses or who were previously infected with another flavivirus (e.g., WNV or St. Louis encephalitis virus), cross-reactive antibodies in both the IgM and neutralizing antibody assays may make it difficult to identify which flavivirus is causing a patient’s illness.6 Convalescent-phase serum specimens collected 3 weeks apart may be used to confirm recent infection.
This case highlights the fact that ZIKV can cause severe meningoencephalitis even in previously healthy adults and adds to the growing number of cases of adult cases with severe central nervous system (CNS) involvement with ZIKV infection, which have sometimes been fatal.9 Although the rapidity of resolution of an initially severe clinical presentation was unexpected, similar cases of rapidly improving meningoencephalitis have been described in a subset of patients with meningoencephalitis due to infection with other flaviviruses, including WNV,10 and Murray Valley encephalitis virus.11 Given the increasing prevalence and geographic distribution of ZIKV infection, ZIKV should be considered in patients with acute meningoencephalitis and appropriate environmental exposures.
The case also furthers knowledge about pathophysiology and radiographic manifestations of ZIKV meningoencephalitis. Although there are a few recent adult case reports noting ZIKV-associated imaging abnormalities such as scattered subcortical white matter and sulcal fluid attenuation inversion recovery (FLAIR) hyperintesity1 as well as cranial nerve enhancement,12 this is to our knowledge the first adult report in the literature with imaging evidence of diffuse cortical edema as well as sulcal leptomeningeal enhancement. The purely cortical pattern of encephalitis in this case differs from the typical locations of other viral infections such as multifocal or diffuse (Epstein–Barr virus, Japanese encephalitis, varicella zoster virus), limbic system (herpes simplex virus type 1), basal ganglia and/or thalamic (eastern equine encephalitis, Japanese encephalitis, HIV-1, Murray Valley encephalitis, WNV), cerebral white matter (cytomegalovirus, HIV-1, Nipah viral encephalitis, WNV), cerebral peduncles (Murray Valley encephalitis), or substantia nigra (St. Louis encephalitis, WNV).11,13–15 Furthermore, this is also the first demonstration of the rapid resolution of MRI findings coincident with the patient’s clinical recovery from her encephalitis. In addition, based on signs of upper motor neuron dysfunction most prominent in the lower extremities that lasted longer than her encephalitis, our patient probably also had mild myelitis, another as yet rarely described adult complication.2 Finally, establishing the diagnosis may be difficult, with a lag between systemic and CNS manifestations and nonspecific clinical and CSF abnormalities. The serologic cross-reactivity with other flaviviruses and the need to test for ZIKV in multiple body fluids, especially urine pose additional problems. Physicians should be aware of these issues and test for ZIKV in patients with meningoencephalitis or myelitis who have visited endemic areas. Ongoing research to better understand the neurotropism of ZIKV, including the molecular basis of microcephaly is being pursued.16
Acknowledgments:
We acknowledge the members of both the CDC and the Massachusetts Department of Public Health for their assistance—specifically: Catherine M. Brown (Deputy State Epidemiologist, Massachusetts Department of Public Health, Bureau of Infectious Disease and Laboratory Sciences); Sandra Smole (Virology Lab Director, Massachusetts Department of Public Health, Bureau of Infectious Disease and Laboratory Sciences); Nicole Lindsey (Epidemiologist, Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC); Daniel Pastula (Neurologist, Medical Epidemiologist, CDC).
REFERENCES
- 1.Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, Mekontso Dessap A, 2016. Zika virus associated with meningoencephalitis. N Engl J Med 374: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 2.Mécharles S, Herrmann C, Poullain P, Tran TH, Deschamps N, Mathon G, Landais A, Breurec S, Lannuzel A, 2016. Acute myelitis due to Zika virus infection. Lancet 387: 1481. [DOI] [PubMed] [Google Scholar]
- 3.Dominican Republic Ministry of Public Health, 2016. Weekly Epidemiological Bulletin. EW 48 of 2016. Available at: http://digepisalud.gob.do/docs/Boletines%20epidemiol%C3%B3gicos/Boletines%20semanales/2016/Bolet%C3%ADn%20Se manal%2048-2016.pdf. Accessed February 12, 2017.
- 4.Pan American Health Organization/World Health Organization, 2016. Zika—Epidemiological Report Dominican Republic. December 2016. Washington, DC: Pan American Health Organization/Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.Gourinat AC, O’connor O, Calvez E, Goarant C, Dupont-rouzeyrol M, 2015. Detection of Zika virus in urine. Emerg Infect Dis 21: 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, Hills S, Wasley A, Fischer M, Powers AM, 2016. Interim guidance for interpretation of zika virus antibody test results. MMWR Morb Mortal Wkly Rep 65: 543–546. [DOI] [PubMed] [Google Scholar]
- 7.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F, 2014. Zika virus infection complicated by Guillain-Barre syndrome–case report, French Polynesia, December 2013. Euro Surveill 19: 20720. [DOI] [PubMed] [Google Scholar]
- 8.Waggoner JJ, Gresh L, Vargas MJ, Ballesteros G, Tellez Y, Soda KJ, Sahoo MK, Nuñez A, Balmaseda A, Harris E, Pinsky BA, 2016. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 63: 1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarmiento-Ospina A, Vásquez-Serna H, Jimenez-Canizales CE, Villamil-Gómez WE, Rodriguez-Morales AJ, 2016. Zika virus associated deaths in Colombia. Lancet Infect Dis 16: 523–524. [DOI] [PubMed] [Google Scholar]
- 10.Sejvar JJ, 2007. The long-term outcomes of human West Nile virus infection. Clin Infect Dis 44: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 11.Speers DJ, Flexman J, Blyth CC, Rooban N, Raby E, Ramaseshan G, Benson S, Smith DW, 2013. Clinical and radiological predictors of outcome for Murray Valley encephalitis. Am J Trop Med Hyg 88: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontes CA, Dos Santos AA, Marchiori E, 2016. Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection. Neuroradiology 58: 837–838. [DOI] [PubMed] [Google Scholar]
- 13.Mahan M, Karl M, Gordon S, 2014. Neuroimaging of viral infections of the central nervous system. Handb Clin Neurol 123: 149–173. [DOI] [PubMed] [Google Scholar]
- 14.Handique SK, 2011. Viral infections of the central nervous system. Neuroimaging Clin N Am 21: 777–794, vii. [DOI] [PubMed] [Google Scholar]
- 15.Vachha B, Rojas R, Prabhu SP, Bhadelia R, Moonis G, 2014. Magnetic resonance imaging in viral and prion diseases of the central nervous system. Top Magn Reson Imaging 23: 293–302. [DOI] [PubMed] [Google Scholar]
- 16.Ganguly B, Ganguly E, 2016. Disruption of human astn2 function by ZIKV ns4b gene as a molecular basis for Zika viral microcephaly. bioRxiv 054486. [Google Scholar]