Abstract.
Hymenolepis nana, the dwarf tapeworm, is a common intestinal infection of children worldwide. We evaluated infection and risk factor data that were previously collected from 14,761 children aged 2–15 years during a large-scale program in northern Peru. We found that 1,124 of 14,761 children (7.61%) had H. nana infection, a likely underestimate given that only a single stool sample was examined by microscopy for diagnosis. The strongest association with infection was lack of adequate water (adjusted prevalence ratio [aPR] 2.22, 95% confidence interval [CI] 1.82–2.48) and sanitation infrastructure in the house (aPR 1.94, 95% CI 1.64–2.29). One quarter of those tested did not have a bathroom or latrine at home, which doubled their likelihood of infection. Similarly, one quarter did not have piped public water to the house, which also increased the likelihood of infection. Continued efforts to improve access to basic water and sanitation services will likely reduce the burden of infection in children for this and other intestinal infections.
INTRODUCTION
Hymenolepis nana, the dwarf tapeworm, is one of the most prevalent parasitic diseases worldwide, mainly affecting children.1 The reported prevalence varies by region, including Europe (0.5–5%),2,3 Asia (0.2–28.4%),4,5 Africa (1.8–2.9%),6,7 and the Americas (0.9–23%).8,9 The adult egg-laying tapeworm resides in the small intestine and releases eggs excreted in the stool. Others become infected via fecal–oral transmission when they ingest H. nana eggs excreted in fecal contamination, or via consumption of infected arthropods. The parasite therefore primarily affects populations with limited basic sanitation services.10,11 Although most infections are asymptomatic, clinical presentations can range from mild nonspecific symptoms1,12 to rare but severe disease.13 In spite of the increased basic service availability in Peru (59% at the 2007 national census), there is still a large segment of the population without basic services that are exposed.14 Prior studies in Peru have reported prevalence as high as 37.5% in studies conducted in school children.8,9 The goal of this study was to describe the prevalence of and risk factors for H. nana infection in children aged 2–15 years in a large population-based sample in northern Peru.
MATERIALS AND METHODS
We conducted a cross-sectional secondary analysis of data collected during a large-scale elimination demonstration program for cysticercosis in Tumbes, Peru, which included 107 peri-urban and rural communities between 2009 and 2010.15 Tumbes is a northern coastal region in Peru with a land area of 4,669 km2 and elevation ranging from 5 to 380 m above sea level. Residents are mostly of “mestizo” heritage (“a mix of Spanish and Amerindian”) who subsists primarily through small-scale agricultural and fishing. During the elimination program, field teams visited each household in the 107 villages to apply a demographic survey to the head of household, followed by treatment with niclosamide for taeniasis for all consenting household members over the age of 2 years, and collection of a single posttreatment stool sample.
Whole stool samples were collected by field personnel trained in biosafety. Aliquots of 10 g of feces were placed in 40 mL of 5% phosphate-buffered saline formal for preservation and stored upright in falcon tubes. Samples were allowed to settle by gravity for a period of 24 hours before the fecal sediment was analyzed by light microscopy for the presence of parasites. The spontaneous settlement technique was used given its high sensitivity for parasitological diagnosis.16 Testing was performed at the Center for Global Health in Tumbes, quality control of all positive samples, as well as of the samples immediately before and after the positive, was performed at the Universidad Peruana Cayetano Heredia (UPCH) Infectious Disease Laboratory in Lima.
Statistical analyses were conducted using Stata V14.0 (College Station, TX). We used binomial family generalized lineal models with logarithmic link function to estimate the prevalence ratio of selected variables while controlling for other factors including age and sex of the participant, as well as urban city, water supply, and basic hygienic services at the home. Robust sandwich-type standard errors were used to account for household clustering. The log likelihood ratio was used to evaluate each variable for inclusion in the final model. We report 95% confidence intervals (CIs) and set statistical significance at P < 0.05. This study was approved by the UPCH Ethical Review Committee (SIDISI 60361). Children were provided the opportunity to assent; however, written informed consent by the parent or guardian was required for participation of minors. Participants also provided written permission to use samples and data for future studies.
RESULTS
A total of 20,249 children between 2 and 15 years of age were included in the elimination program; 14,761 (72.90%) provided a fecal sample and were therefore included in this secondary analysis. Girls and residents of peri-urban villages were slightly more likely to participate than their counterparts (Table 1). The mean age of participants was 8 years (± 3.8). Just over half of samples were collected from children in peri-urban villages. Lack of basic sanitation infrastructure in the household was common, with over one quarter living in homes that lacked a bathroom or latrine, and a similar proportion with no public water source to their homes (Table 1).
Table 1.
Demographic characteristics of children who did and did not provide a stool sample for this study, Tumbes, Peru, 2009
| Non participants (N = 5,488) |
Participants (N = 14,761) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Female | 2,576 | 46.9 | 7,388 | 50.1 |
| Male | 2,912 | 53.1 | 7,373 | 49.9 |
| Age (years) | ||||
| Mean ± standard deviation | 8.0 | 4.6 | 8.4 | 3.8 |
| Location of homes | ||||
| Urban | 2,784 | 50.7 | 8,171 | 55.4 |
| Rural | 2,704 | 49.3 | 6,590 | 44.6 |
| Housing material | ||||
| Brick and concrete | 1,607 | 29.3 | 4,080 | 27.6 |
| Locally available | 3,881 | 70.7 | 10,681 | 72.4 |
| Water supply | ||||
| Public network | 4,141 | 75.4 | 11,284 | 76.4 |
| Surface water | 904 | 16.5 | 2,084 | 14.1 |
| Cistern trucks | 443 | 8.1 | 1,393 | 9.5 |
| Basic hygienic services | ||||
| Bathroom | 1,984 | 36.2 | 5,165 | 34.9 |
| Latrine | 1,988 | 36.2 | 5,442 | 36.9 |
| Neither | 1,516 | 27.6 | 4,154 | 28.2 |
| Electricity | ||||
| Public service | 4,695 | 85.5 | 12,431 | 84.2 |
| No public service | 793 | 14.5 | 2,330 | 15.8 |
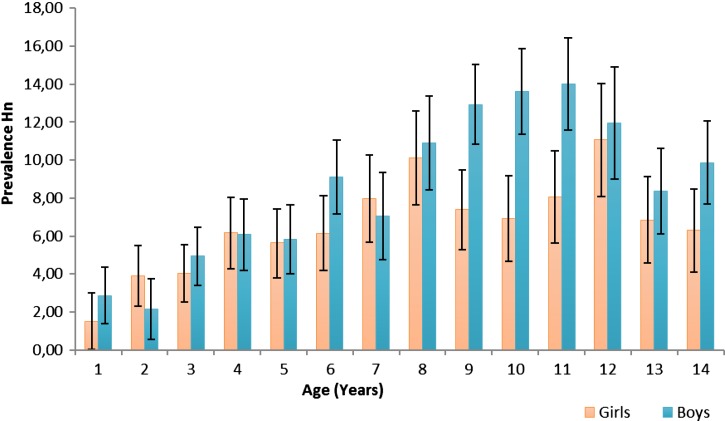
Microscopy showed that 1,124 of 14,761 children (7.61%; 95% CI 7.19–8.04%) had H. nana infection. Polyparasitism was common (6,718/14,761; 45.51%) as was infection with any pathogenic parasite (5,132/14,761; 34.77%). In the multiple variable analysis, the prevalence was 38% lower among residents of rural areas compared with peri-urban areas, increased 8% per year of age and was 30% higher among boys than girls (Table 2). The difference by gender appeared to be primarily driven by higher prevalence among boys aged 10–12 years compared with their female counterparts (Figure 1). The prevalence was nearly double in children whose homes lacked a bathroom or latrine, and was also higher among those whose homes did not have public piped water; 26% higher for homes that collected surface water and more than double for homes that received water deliveries by truck.
Table 2.
Risk factors for Hymenolepis nana infection in children aged 2–15 years, Tumbes, Peru
| Without Hymenolepis |
With Hymenolepis |
||||||
|---|---|---|---|---|---|---|---|
|
N = 13,637 |
N = 1,124 |
PR* | P value | aPR* | |||
| n | % | n | % | ||||
| Sex | |||||||
| Female | 6,897 | 93.4 | 491 | 6.6 | Reference | Reference | |
| Male | 6,740 | 91.4 | 633 | 8.6 | 1.29 | < 0.001 | 1.30 (1.16–1.45) |
| Age (years) | |||||||
| Mean ± standard deviation | 8.3 | 3.8 | 9.4 | 3.4 | 1.08 | < 0.001 | 1.08 (1.07–1.10) |
| Urbanity | |||||||
| Peri-urban | 7,467 | 91.4 | 704 | 8.6 | Reference | Reference | |
| Rural | 6,170 | 93.6 | 420 | 6.4 | 0.74 | < 0.001 | 0.62 (0.54–0.72) |
| Water supply | |||||||
| Public network | 10,546 | 93.5 | 738 | 6.5 | Reference | Reference | |
| Surface water | 1,927 | 92.5 | 157 | 7.5 | 1.15 | = 0.10 | 1.26 (1.02–1.55) |
| Cistern tanks | 1,164 | 83.6 | 229 | 16.4 | 2.51 | < 0.001 | 2.22 (1.82–2.48) |
| Basic hygienic services | |||||||
| Bathroom | 4,903 | 94.9 | 262 | 5.1 | Reference | Reference | |
| Latrine | 5,003 | 91.9 | 439 | 8.1 | 1.59 | < 0.001 | 1.68 (1.41–2.00) |
| Neither | 3,731 | 89.8 | 423 | 10.2 | 2.01 | < 0.001 | 1.94 (1.64–2.29) |
Apr = adjusted prevalence ratio; PR = prevalence ratio.
Unadjusted PR and aPR estimated using binomial family generalized linear models with log link function and robust standard error to control for household clustering.
Figure 1.
Prevalence of Hymenolepis nana infection by age and sex.
DISCUSSION
Results from this large population-based study show that H. nana is a common infection of childhood in the Tumbes region of northern Peru. Despite the fact that diagnosis was based on analysis of a single stool sample, about one in 13 children were found to harbor this tapeworm infection. This is a likely underestimate of the true prevalence given the low sensitivity of microscopy on a single sample even with the best methods.17 The large size of this study and the representative nature of the sample taken allow evaluation of potential risk factors with a high degree of precision.
Hymenolepis nana is transmitted through ingestion of fecal contamination. This study verifies that existence of basic sanitary services in the home, including both clean water and adequate disposal of sewage, are associated with protection against infection in children. Other large studies have had similar findings, with one study showing that three of every four children with H. nana in Mexico have substandard water and sanitation at the home.18 Another study in Mexico reported that lack of basic services at the home doubled the odds of infection.19 These household factors were much more strongly associated with infection than were individual characteristics such as sex or age.20 Increasing efforts to provide adequate household sanitation are therefore probably important to reduce the burden of this and other diseases caused by fecal–oral transmission.
Interestingly, children who lived in homes where the primary source of water was delivery through trucks that filled onsite cisterns were more than twice as likely to be infected, compared with children living in households with water piped directly to the home. This was independent of whether a bathroom or latrine was present, or whether the family practiced outdoor defecation. Further investigation is needed to understand whether this risk is attributed to the source of the water, to fecal contamination of storage tanks, or to another factor. Because of the cross-sectional retrospective nature of this study, our analysis is limited to those data collected during the elimination program. Some important factors, such as type of water storage containers used, hand-washing practices or evidence of rodent infestation in the home, could not be evaluated.
Acknowledgments:
We thank the villagers of all 107 communities involved in the studies and the Regional Directorate of Health, Tumbes. We are grateful to field and laboratory team of the CWGP. Percy M. Vilchez-Barreto thanks the support and guidance received from the faculty and fellow students of the program Masters in Epidemiological Research from Universidad Peruana Cayetano Heredia and the U.S. Naval Medical Research Unit Six, Lima, Peru, and thanks the support and guidance received from Hector H. Garcia (Executive Director of Center for Global Health) and Andrés (Willy) Lescano (Associate Professor, Universidad Peruana Cayetano Heredia), and also appreciates his lovely family for their spiritual support and advice.
Disclaimer: The statements contained in this document are those of the authors and should not be construed as official points of view of the Universidad Peruana Cayetano Heredia or other organizations mentioned.
REFERENCES
- 1.Botero D, Restrepo M, 1998. Parasitosis Intestinales por Cestodos y Trematodos. Parasitosis Humana, 3rd edition, 144–150. [Google Scholar]
- 2.Dąbrowiecki Z, Korzeniewski K, Morawiec B, Dąbrowiecka M, Olszański R, 2009. Intestinal helminthes and protozoan infections among children of Chechen refugees in Poland. Exp Med 1: 14–19. [Google Scholar]
- 3.Soriano JM, Domenech G, Martinez MC, Manes J, Soriano F, 2011. Intestinal parasitic infections in hosted Saharawi children. Trop Biomed 28: 557–562. [PubMed] [Google Scholar]
- 4.Akhlaghi L, Shamseddin J, Meamar AR, Razmjou E, Oormazdi H, 2009. Frequency of intestinal parasites in Tehran. Iran J Parasitol 4: 44–47. [Google Scholar]
- 5.Mohd Zain SN, Behnke JM, Lewis JW, 2012. Helminth communities from two urban rat populations in Kuala Lumpur, Malaysia. Parasit Vectors 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakr IM, Arafa NA, Ahmed MA, Mostafa Mel H, Mohamed MK, 2009. Prevalence of intestinal parasitosis in a rural population in Egypt, and its relation to socio-demographic characteristics. J Egypt Soc Parasitol 39: 371–381. [PubMed] [Google Scholar]
- 7.Houmsou RS, Amuta EU, Olusi TA, 2010. Prevalence of intestinal parasites among primary school children in Makurdi, Benue State-Nigeria. Int J Infect Dis 8: 1–7. [Google Scholar]
- 8.Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, Morales G, Díaz-Camacho SP, 2006. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health 6: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Guzmán LM, Hernández-Jerónimo EJ, Rodríguez-García R, 2000. Parasitosis intestinal en niños seleccionados en una consulta ambulatoria de un hospital. Rev Mex Pediatr 67: 117–122. [Google Scholar]
- 10.Gomez M, Orihuela JL, Orihuela ME, 1999. Parasitismo intestinal en círculos infantiles. Rev Cubana Med Gen Integr 15: 266–269. [Google Scholar]
- 11.Goncalves AL, Belizario TL, Pimentel Jde B, Penatti MP, Pedroso Rdos S, 2011. Prevalence of intestinal parasites in preschool children in the region of Uberlandia, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop 44: 191–193. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Frias MA, Fuentenebro-Yubero MJ, Jimenez-Martinez J, Gil-Veguillas S, Adrados-Razola I, Jiménez-Bustos JM, 1998. Dolor abdominal inespecífico por Hymenolepis nana. An Esp Pediatr 49: 105–107. [PubMed] [Google Scholar]
- 13.Muehlenbachs A, et al. , 2015. Malignant transformation of Hymenolepis nana in a human host. N Engl J Med 373: 1845–1852. [DOI] [PubMed] [Google Scholar]
- 14.INEI, 2009. Mapa del Déficit Habitacional a Nivel Distrital, ed. 14. [Google Scholar]
- 15.Garcia HH, Gonzalez AE, Tsang VC, O’Neal SE, Llanos-Zavalaga F, Gonzalvez G, Romero J, Rodriguez S, Moyano LM, Ayvar V, Diaz A, Hightower A, Craig PS, Lightowlers MW, Gauci CG, Leontsini E, Gilman RH, 2016. Elimination of Taenia solium transmission in northern Peru. N Engl J Med 374: 2335–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajuelo G, Lujan D, Paredes B, Tello R, 2006. Aplicación de la técnica de sedimentación espontánea en tubo en el diagnóstico de parásitos intestinales. Rev Biomed 17: 96–101. [Google Scholar]
- 17.Tello R, Terashima A, Marcos LA, Machicado J, Canales M, Gotuzzo E, 2012. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis 16: e414–e416. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero MT, Hernandez Y, Rada ME, Aranda A, Hernandez MI, 2008. Parasitosis intestinal y alternativas de disposición de excreta en municipios de alta marginalidad. Revista Cubana de Salud Pública 34: 1–5. [Google Scholar]
- 19.Martínez-Barbabosa I, Gutiérrez-Cárdenas EM, Gaona E, Shea M, 2010. The prevalence of Hymenolepis nana in schoolchildren in a bicultural community. Rev Biomed 21: 21–27. [Google Scholar]
- 20.Fuentes M, Galindez L, García D, Gonzalez N, Goyanes J, Herrera E, Sanchez J, 2011. Frequency of intestinal parasitism and epidemiological characteristics of the 1- to 12-year-old child population treated at the Cerro Gordo Type II Urban Outpatient Clinic. Barquisimeto, State of Lara. January-June 2007 [in Spanish]. Kasmera 39: 31–42. [Google Scholar]