Abstract.
People in southern Taiwan experienced two major dengue outbreaks in 2014 and 2015. The mortality and clinical features were very different between these 2 years. Dengue virus serotype 1 (DENV-1) caused epidemic outbreak in 2014 and DENV-2 was predominant in 2015. The characteristics of dengue hemorrhagic fever (DHF) cases in the 2 years was analyzed. We conducted a retrospective chart review to analyze the clinical and laboratory features of 206 adult patients with DHF in southern Taiwan in 2014 and 2015. The mortality rate of DHF cases in 2015 was higher than that of cases in 2014 (38.7% versus 12.4%, P < 0.0001). Compared with cases in 2014, DHF cases in 2015 had more complications, such as gastrointestinal bleeding (78.5% versus 61.9%, P = 0.01), severe hepatitis (30.1% versus 8%, P < 0.0001), and myocarditis (14% versus 0.9%, P < 0.0001). Among the mortality cases, diabetes, chronic renal failure, proton-pump inhibitors using, platelet transfusion, and Charlson comorbidity index score (Charlson score) were also higher in 2015. Multivariate analysis for the mortality cases revealed that the risk factors were Charlson score ≥ 5 (P = 0.02, odds ratio [OR] = 4.07, 95% confidence interval [CI] = 1.244–13.307), severe hepatitis (P < 0.0001, OR = 11.97, 95% CI = 3.831–37.396), and acute renal failure (P < 0.0001, OR = 98.76, 95% CI = 10.847–899.22). DHF cases in 2015 had higher mortality and more complications, such as gastrointestinal bleeding, severe hepatitis, and myocarditis, than in 2014 in southern Taiwan. In the 2-year DHF case series, Charlson score ≥ 5, severe hepatitis, and acute renal failure were independent significant variables for mortality.
INTRODUCTION
Dengue virus (DENV) infection is a global health threat affecting at least 3.6 billion people living in more than 125 countries in the tropics and subtropics.1–3 In Taiwan, a dengue virus serotype 1 (DENV-1) outbreak occurred in 1987–1988 and a dengue virus serotype 2 (DENV-2) outbreak in 2002. Since 2006, southern Taiwan has faced dengue outbreaks of different scales every year concentrated.4,5 Taiwan experienced a large dengue outbreak in 2014, with 15,492 indigenous cases, including 136 cases (0.88%) of dengue hemorrhagic fever (DHF) that caused 21 (15.4%) deaths.6 The affected areas were primarily in Kaohsiung City, with a population around 2,779,000 in an area of 2,947 km2.6 This epidemic was caused by DENV-1.6,7 In 2015, a dengue epidemic was caused mostly by DENV-2 and there were 43,784 cases in Taiwan (including 22,760 in Tainan and 19,723 in Kaohsiung) that resulted in 218 deaths.7 In contrast to findings in other endemic areas around the world, all the fatal cases were adults.
In this study, 2014 is referred to as the period of predominance of DENV-1, whereas 2015 (from September to December) corresponds to the period when DENV-2 dominated.6,7 A question has been raised whether different clinical manifestations of DHF can be associated with distinct DENV serotypes. However, few reports have demonstrated a link between distinct serotypes and specific manifestations, especially in adults. Furthermore, most studies have not focused on fatality among adults or older age groups. The characteristic of dengue circulation in Taiwan, with a predominant serotype in each epidemic, allowed us to address the issue of the association of particular DENV serotypes with specific clinical manifestations in DHF. This study of fatal cases of dengue infection was undertaken in light of dengue in this region, Kaohsiung, Taiwan, in 2014–2015 and intended to find demographic, clinical, and laboratory features of fatal and nonfatal DHF.
MATERIALS AND METHODS
Study subjects and ethics statement.
The 2014 Taiwan dengue outbreak occurred mainly in Kaohsiung and the 2015 dengue outbreak occurred in Tainan and Kaohsiung. There were 136 DHF or dengue shock syndrome (DHF/DSS) cases in Taiwan in 2014, while 132/136 cases were in Kaohsiung. There were 674 DHF/DSS cases in Taiwan in 2015, but only 211/674 cases were in Kaohsiung.
According to the Center for Disease Control (CDC) in Kaohsiung data, a total of 343 DHF patients with age distribution in Kaohsiung city were found during 2014–2015 (Supplemental Figure 1). Age < 20 was excluded. A total of 206 DHF patients in Kaohsiung city were enrolled during 2014–2015. One hundred and thirteen DHF patients in the Kaohsiung city in the 2014 outbreak were enrolled in the study, including 14 fatal and 99 nonfatal DHF patients from seven Hospitals. In addition, 93 DHF patients in Kaohsiung City in the 2015 (September to December) outbreak were enrolled, including 36 fatal and 57 nonfatal from three Hospitals (Supplemental Table 1). Because of lack of further cooperation with four hospitals, the participant hospital number in 2015 is lower than 2014. In the study, we included 113 (113/132, 85.6%) DHF cases in Kaohsiung City in 2014 and 93 (93/211, 44.1%) DHF cases in 2015.
Clinical information was collected retrospectively on these patients, and then reported to the CDC, Taiwan.
This work was approved by the Institutional Ethics Committee of Kaohsiung Medical University Hospital, KMUHIRB-E(II)-20150222. The Institutional Ethics Committee of Kaohsiung Medical University Hospital waived the need for written informed consent from the participants because of the nature of the study and the retrospective collection of routine medical practice data.
Definitions.
Clinical case definition: The medical records of 206 patients at Kaohsiung City with DHF were reviewed and notes were transcribed into standardized data entry forms. All the laboratory diagnostic methods were performed at the laboratories of the CDC, Taiwan. The diagnosis of dengue fever was confirmed by meeting one of the following criteria: (1) virus isolation; (2) positive result of real-time polymerase chain reaction; (3) positive result of higher titer dengue-specific IgM and IgG antibody in a single serum specimen, in which cross-reaction to Japanese encephalitis had been excluded; (4) positive seroconversion or ≥ 4-fold rise in dengue-specific IgM or IgG antibody from the acute phase compared with convalescent phase; or (5) a positive test for Dengue Nonstructural protein 1 Ag STRIP (Bio-Rad Laboratories, Marnes-la-Coquette, France). DHF was defined as per the World Health Organization (WHO) 1997 criteria.8 The definition of chronic kidney disease (CKD) stages 3, 4, and 5 includes patients who have an estimated glomerular filtration rate (eGFR) of < 60 mL/minutes per 1.73 m2 at admission and a concomitant history of chronic renal failure or eGFR < 60 mL/minutes per 1.73 m2 before this episode of infection.9,10 Acute renal failure (ARF) was defined as a rapid increase in serum creatinine (Cr) level ≥ 0.5 mg/dL compared with that found at the patient’s hospital presentation.11
The diagnosis of clinical myocarditis was established after clinical evaluation of the patient by a cardiologist on the basis of history, physical examination, and investigation results (electrocardiographic findings, high serum cardiac-specific troponin I level, and cardiac echo) in the absence of an endomyocardial biopsy.12 Severe hepatitis was defined as serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥ 1,000 IU/L. Gastrointestinal bleeding was defined as the passage of tarry or bloody stool. Intracranial hemorrhage (ICH) or infarction was defined by brain computed tomography or magnetic resonance imaging study while the patient was admitted to a hospital. Pancreatitis was defined by a lipase level 3-fold greater than the upper limit of normal and ultrasound (image) findings.13 Charlson comorbidity index (Charlson score), potentially scored from 0 to 37, was used to assess the impact of comorbidity on the disease outcome.14
Statistical analysis.
Statistical analyses were performed using a χ2 test or Fisher’s exact test for categorical variables, and the t test for continuous variables. Clinical and laboratory findings were compared using the Statistical Package for Social Sciences version 12.0 for Windows (SPSS, Chicago, IL). Selected variables in univariate analysis were examined with the receiver operating characteristic (ROC) curve for choosing the best cut-point value. ROC curves were generated using MedCalc version 9.3.2.0 (MedCalc Software, Mariakerke, Belgium) with the level of significance set at a two-tailed P value of < 0.05 assessed by conditional logistic regression modeling. After finding the significant variables by single variate analysis, we used logistic regression model for multivariate analysis. The degree of linear relationship between age group, Charlson comorbidity index and mortality rate was calculated using Spearman’s rank correlation, and the correlation was considered significant at the level of 0.05 (two tailed).
RESULTS
Demographic data.
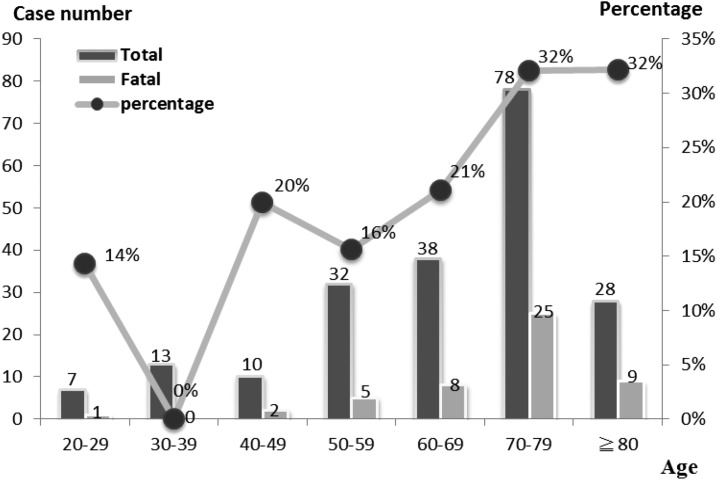
There were 50 deaths (24.3%) among the 206 DHF cases. The demographics and underlying diseases are presented in Table 1. The majority of cases of DHF were in the sixth to eighth decades (144 cases, 69.9%). A significant correlation was noted between age groups and mortality rate [correlation coefficient (Rs) = 0.929, P = 0.003] (Figure 1). A significant correlation was also noticed between age and length of stay (correlation coefficient = 0.14, P = 0.044).
Table 1.
Comparison of clinical characteristics between fatal and survival dengue hemorrhage fever cases in Kaohsiung, 2014–2015
| Characteristics | Fatal (N = 50) | Survival (N = 156) | P values |
|---|---|---|---|
| 2014/2015 | 14/36 | 99/57 | < 0.0001 |
| Demographics and underlying diseases | |||
| Male (%) | 24/50 (48.0%) | 80/156 (51.3%) | 0.686 |
| Age (year) | 70.9 ± 11 (50) | 63.3 ± 16.2 (156) | 0.002 |
| Age ≥ 65 years | 39/50 (78.0%) | 89/156 (57.1%) | 0.006 |
| Diabetes mellitus | 27/50 (54.0%) | 42/156 (26.9%) | < 0.0001 |
| Hypertension | 33/50 (66.0%) | 85/156 (54.5%) | 0.152 |
| Congestive heart failure | 4/50 (8.0%) | 9/156 (5.8%) | 0.521 |
| COPD | 2/50 (4.0%) | 7/156 (4.5%) | 1* |
| CVA | 11/50 (22.0%) | 14/156 (9.0%) | 0.014 |
| ESRD | 1/50 (2.0%) | 6/156 (3.8%) | 1* |
| CKD stage 3, 4, or 5 | 39/50 (78.0%) | 72/156 (46.2%) | < 0.0001 |
| Charlson comorbidity index score | 6.48 ± 1.9 | 4.4 ± 2.59 | < 0.0001 |
| Clinical manifestations and management | |||
| ARF (acute renal failure) | 16/50 (32.0%) | 1/156 (0.6%) | < 0.0001 |
| Hemodialysis after admission | 10/50 (20.0%) | 1/156 (0.6%) | < 0.0001* |
| Bacterial coinfection | 8/50 (16.0%) | 15/156 (9.6%) | 0.212 |
| Nosocomial infection | 3/50 (6.0%) | 8/156 (5.1%) | 0.730* |
| Bacteremia | 3/50 (6%) | 2/156 (1.3%) | 0.093* |
| Performance of EGD | 1/50 (2.0%) | 17/156 (10.9%) | 0.08* |
| PPI use | 26/50 (52.0%) | 82/156 (52.6%) | 0.945 |
| NSAID use in emergency room | 11/50 (22.0%) | 52/156 (33.3%) | 0.13 |
| Platelet transfusion | 36/50 (72.0%) | 69/156 (44.2%) | 0.001 |
| PRBC transfusion | 23/50 (46.0%) | 28/156 (17.9%) | < 0.0001 |
| Admission duration (days) | 6.8 ± 7.2 (50) | 11.4 ± 11.8 (156) | < 0.0001 |
CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CVA = cerebral vascular accident; EGD = esophageal gastroduodenoscopy; ESRD = end-stage renal disease; NSAID = nonsteroidal anti-inflammatory drugs; PPI = proton pump inhibitor; PRBC = packed red blood cell.
Fisher test.
Figure 1.
Age-specific groups of dengue hemorrhage fever cases with fatal and survival outcomes in 2014 and 2015. A significant correlation was noted between age group and mortality rate [correlation coefficient (Rs) = 0.929, P = 0.003].
Clinical features on admission.
When comparing the clinical characteristics of fatal and surviving cases, and differences between 2014 and 2015, age above 65 years old, diabetes, cerebral vascular accident (CVA), CKD stages 3, 4, or 5, Charlson comorbidity index score (Charlson score), receiving H/D after hospital admission, ARF, platelet infusion, packed red blood cell infusion, and admission duration were significantly associated with mortality (Table 1).
Most clinical characteristics were similar between fatal and nonfatal DHF patients (data not shown), but the fatal group had a higher rate of altered consciousness and hematuria than the nonfatal group (22% versus 4.5%, P < 0.0001; 50% versus 32.1%, P = 0.022). Patients in the fatal group had significantly higher mean values than the nonfatal group for initial AST, maximum AST, initial ALT, maximum ALT, initial serum blood urea nitrogen (BUN), initial serum Cr, initial C-reactive protein (CRP), initial white blood cells (WBC), and initial neutrophils, and lower mean values than nonfatal group for initial albumin, initial lymphocytes, initial monocytes, and initial hemoglobin (Table 2).
Table 2.
Comparison of laboratory data between fatal and surviving dengue hemorrhage fever cases in Kaohsiung, 2014–2015
| Characteristics | Fatal (N = 50) | Survival (N = 156) | P values |
|---|---|---|---|
| Initial AST (IU/L) | 764.1 ± 1,756.6 (50) | 306.9 ± 1,970.4 (150) | 0.007 |
| Maximum AST (IU/L) | 6,151 ± 8,207.8 (50) | 596.9 ± 2,799.2 (150) | < 0.0001 |
| Initial ALT (IU/L) | 233.2 ± 462.3 (49) | 104.8 ± 369.2 (154) | 0.014 |
| Maximum ALT (IU/L) | 1,549.8 ± 1,665.1 (49) | 188.8 ± 511.8 (153) | < 0.0001 |
| Serum blood urea nitrogen (mg/dL) | 39.7 ± 26.1 (49) | 21.1 ± 16.2 (139) | < 0.0001 |
| Serum creatinine (mg/dL) | 2.5 ± 1.9 (50) | 1.5 ± 1.6 (156) | < 0.0001 |
| Initial C-reactive protein (mg/dL) | 52.4 ± 67.1 (46) | 22.2 ± 27.9 (149) | 0.001 |
| Albumin (g/dL) | 3 ± 0.7 (37) | 3.3 ± 0.6 (102) | 0.01 |
| Initial white blood cells (/mm3) | 8,220.7 ± 4,731.9 (50) | 5,446.2 ± 2,914.9 (156) | < 0.0001 |
| Initial neutrophils (%) | 76.3 ± 18.5 (41) | 72.4 ± 14.6 (138) | 0.037 |
| Initial lymphocytes (%) | 12.2 ± 10.6 (44) | 16.1 ± 12 (143) | 0.023 |
| Initial monocytes (%) | 7.4 ± 7.9 (44) | 9.5 ± 8.5 (142) | 0.003 |
| Initial hemoglobin (g/L) | 12.7 ± 2.7 (50) | 13.3 ± 2.2 (156) | 0.04 |
| Initial hematocrit (%) | 37.6 ± 8.1 (50) | 39.3 ± 6.1 (156) | 0.05 |
| Initial platelets (/mm3) | 92.9 ± 73.1 (50) | 102.7 ± 71.3 (156) | 0.303 |
| Prolongation of PT* n/N (%) | 17/45 (37.8) | 12/99 (12.1) | < 0.0001 |
| Prolongation of APTT† n/N‡ (%) | 41/45 (91) | 86/98 (87.8) | 0.554 |
ALT = alanine aminotransferase; APTT = activated prothrombin time; AST = aspartate aminotransferase; PT = prothrombin time.
Prolongation of PT is defined as a PT > 3 seconds than that of control.
Prolongation of APTT is defined as APTT > 20% than that of control.
No. of patients/no. of patients with data available.
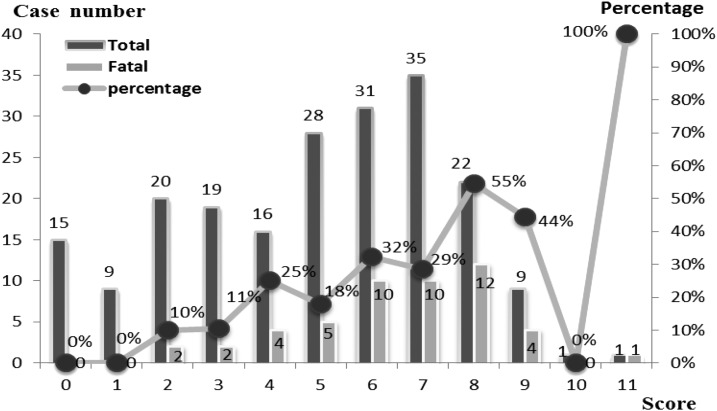
The patients in the fatal group tended to have a higher Charlson score than the survival group (6.48 versus 4.4, P < 0.0001) (Table 1). A significant correlation was noted between the Charlson score and the mortality rate [correlation coefficient (Rs) = 0.662, P = 0.019] (Figure 2). Based on ROC curve analysis, the sensitivity and specificity were 66% and 77.6%, respectively, when a Charlson score level of > 5 was used as a criterion to differentiate fatal and nonfatal (AUC = 0.756, 95% confidence interval [CI] = 0.691–0.813, P = 0.0001).
Figure 2.
Mortality rates of dengue hemorrhage fever cases with different Charlson comorbidity index scores. A significant correlation was noted between Charlson comorbidity index score and mortality rate [correlation coefficient (Rs) = 0.662, P = 0.019].
Among demographic factors, the mean age of cases in 2015 was 66.8 years old, which is older than the mean age of cases in 2014 (63.8 years old). DHF patients in the year 2015 had significantly higher percentages of diabetes, CVA, stage 3, 4, or 5 CKD, proton pump inhibitor use, platelet infusion rates, mortality rates, and higher Charlson score (Table 3).
Table 3.
Comparison of clinical characteristics between dengue hemorrhage fever cases in 2014 and in 2015
| Characteristics | 2014 (N = 113) | 2015 (N = 93) | P values |
|---|---|---|---|
| Demographic and underlying diseases | |||
| Male (%) | 58/113 (51.3%) | 46/93 (49.5%) | 0.79 |
| Age (year) | 63.8 ± 16.4 (113) | 66.8 ± 14.1 (93) | 0.234 |
| Age ≥ 65 years | 67/113 (59.3%) | 61/93 (65.6%) | 0.354 |
| Diabetes mellitus | 29/113 (25.7%) | 40/93 (43.0%) | 0.009 |
| Hypertension | 58/113 51.3%) | 60/93 (64.5%) | 0.057 |
| Congestive heart failure | 4/113 (3.5%) | 9/93 (9.7%) | 0.071 |
| COPD | 3/113 (2.7%) | 6/93 (6.5%) | 0.305* |
| CVA (Cerebral vascular accident) | 7/113 (6.2%) | 18/93 (19.4%) | 0.004 |
| ESRD (End stage renal disease) | 4/113 (3.5%) | 3/93 (3.2%) | 1* |
| CKD stage 3, 4, or 5 | 53/113 (46.9%) | 58/93 (62.4%) | 0.027 |
| Charlson comorbidity index score | 4.37 ± 2.56 | 5.65 ± 2.44 | < 0.0001 |
| Clinical manifestations and management | |||
| ARF | 8/113 (7.1%) | 9/93 (9.7%) | 0.5 |
| Received hemodialysis after admission | 5/113 (4.4%) | 6/93 (6.5%) | 0.52 |
| Bacterial coinfection | 12/113 (11%) | 11/93 (11.8%) | 0.784 |
| Nosocomial infection | 4/113 (3.5%) | 7/93 (7.5%) | 0.229* |
| Bacteremia | 3/113 (2.65%) | 2/93 (2.2%) | 1* |
| EGD perform | 13/113 (11.5%) | 5/93 (5.4%) | 0.121 |
| PPI use | 44/113 (38.9%) | 64/93 (68.8%) | < 0.0001 |
| NSAID use in emergency room | 32/113 (28.3%) | 31/93 (33.3%) | 0.437 |
| Platelet transfusion | 47/113 (41.6%) | 58/93 (62.4%) | 0.003 |
| PRBC transfusion | 23/113 (20.4%) | 28/93 (30.1%) | 0.107 |
| Mortality rate | 14/113 (12.4%) | 36/93 (38.7%) | < 0.0001 |
| Admission duration (days) | 10.2 ± 11.3 (113) | 10.4 ± 10.7 (93) | 0.386 |
ARF = acute renal failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CVA = cerebral vascular accident; EGD = esophageal gastroduodenoscopy; ESRD = end-stage renal disease; NSAID = nonsteroidal anti-inflammatory drugs; PPI = proton pump inhibitor; PRBC = packed red blood cell.
Fisher’s exact test.
Laboratory parameters of the DHF within 2014–2015 are presented in Table 4. DHF patients in 2015 had significantly higher mean values than in 2014 for initial ALT, maximum ALT, initial AST, maximum AST, initial serum BUN, initial serum Cr, initial CRP, initial WBC, initial neutrophils, and percentage of prothrombin time prolonged. DHF Patients in 2014 had significantly higher mean values for albumin, initial lymphocytes, and initial monocytes.
Table 4.
Comparison of laboratory data between dengue hemorrhage fever cases in 2014 and cases in 2015
| Characteristics | 2014 (N = 113) | 2015 (N = 93) | P values |
|---|---|---|---|
| Initial AST (IU/L) | 118.2 ± 135.2 (107) | 769.7 ± 2,785.8 (93) | 0.001 |
| Maximum AST (IU/L) | 979.9 ± 3,543.2 (107) | 3,142.3 ± 6,644.6 (93) | < 0.0001 |
| Initial ALT (IU/L) | 72.5 ± 91.1 (110) | 210.6 ± 569.8 (93) | 0.04 |
| Maximum ALT (IU/L) | 256.2 ± 673.5 (109) | 826.9 ± 1,384.4 (93) | < 0.0001 |
| Initial serum blood urea nitrogen (mg/dL) | 21.7 ± 17.7 (96) | 30.3 ± 23 (92) | 0.004 |
| Initial serum creatinine (mg/dL) | 1.5 ± 1.47 (113) | 2.1 ± 1.9 (93) | 0.001 |
| Initial C-reactive protein (mg/dL) | 19.6 ± 25.6 (106) | 41 ± 54.2 (89) | < 0.0001 |
| Albumin (g/dL) | 3.3 ± 0.607 (88) | 3 ± 0.6 (51) | 0.002 |
| Initial white blood cells (/mm3) | 4,884.5 ± 2,594.7 (113) | 7,620.4 ± 4,130.6 (93) | < 0.0001 |
| Initial neutrophils (%) | 71.1 ± 15.7 (99) | 76 ± 15.2 (80) | 0.015 |
| Initial lymphocytes (%) | 16.3 ± 10.6 (103) | 13.7 ± 13 (84) | 0.008 |
| Initial monocytes (%) | 10.4 ± 10.4 (102) | 7.3 ± 4.3 (84) | 0.005 |
| Initial hemoglobin (g/L) | 13.1 ± 2.05 (113) | 13.2 ± 2.7 (93) | 0.901 |
| Initial hematocrit (%) | 38.5 ± 5.7 (113) | 39.4 ± 7.7 (93) | 0.38 |
| Initial platelets (/mm3) | 98.7 ± 66.4 (113) | 102.3 ± 78 (93) | 0.957 |
| Prolongation of PT n/N (%) | 8/69 (11.6) | 21/75 (28) | 0.014 |
| Prolongation of APTT n/N (%) | 62/68 (91.2) | 65/75 (86.7) | 0.437 |
ALT = alanine aminotransferase; APTT = activated prothrombin time; AST = aspartate aminotransferase; PT = prothrombin time.
DHF patients in 2015 had significantly higher rates of chills, gastrointestinal bleeding, hematuria, and vaginal bleeding (signs of bleeding). In contrast, DHF patients in 2014 had higher rates of retro-orbital pain, joint pain, arthralgia, myalgia (the classic dengue fever symptom and signs), vomiting, and pleural effusion.
Complications occurring with the 206 DHF cases.
Regarding severe complications seen with the 206 DHF cases (Table 5), 143 (69.4%) cases had gastrointestinal bleeding, 37 cases (18%) had increased severe hepatitis (AST or ALT ≥ 1,000 IU/L), 17 cases (8.25%) showed development of ARF, 14 patients (6.8%) had myocarditis, 11 cases (5.3%) had ICH or infarction, six cases (2.9%) had ICH, and five cases (2.4%) had pancreatitis. The fatal group had a higher rate of severe hepatitis (fatal versus nonfatal; 58% versus 5.1%, P < 0.0001), ARF (32% versus 0.6%, P < 0.0001), myocarditis (20% versus 2.6%, P < 0.0001), and of intracerebral hemorrhage (ICH) or infarction (12% versus 3.2%, P = 0.026) than the nonfatal group (Table 6).
Table 5.
Complications of dengue hemorrhage fever cases in Kaohsiung in 2014 and 2015
| Characteristics | 2014 (N = 113) (%) | 2015 (N = 93) (%) | P values |
|---|---|---|---|
| Gastrointestinal bleeding | 70/113 (61.9) | 73/93 (78.5) | 0.01 |
| Severe hepatitis* | 9/113 (8.0) | 28/93 (30.1) | < 0.0001 |
| Acute renal failure | 8/113 (7.1) | 9/93 (9.7) | 0.5 |
| Myocarditis† | 1/113 (0.9) | 13/93 (14.0) | < 0.001 |
| ICH or infarction | 5/113 (4.4) | 6/93 (6.45) | 0.549‡ |
| ICH | 2/113 (1.77) | 4/93 (4.3) | 0.413‡ |
| Pancreatitis | 2/113 (1.8) | 3/93 (3.2) | 0.66‡ |
| Bacteremia | 3/113 (2.7) | 2/93 (2.2) | 1‡ |
ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Severe hepatitis: AST or ALT > 1,000 mg/dL
Clinical myocarditis was established after clinical evaluation of the patient by a cardiologist on the basis of history, physical examination, and investigation results (electrocardiographic findings, high serum cardiac-specific troponin I level, cardiac echo) in the absence of an endomyocardial biopsy.
Fisher’s exact test.
Table 6.
Comparison of complications between fatal and survival dengue hemorrhage fever cases
| Characteristics | Fatal (N = 50) (%) | Survival (N = 156) (%) | P values |
|---|---|---|---|
| Gastrointestinal bleeding | 37/50 (74.0) | 106/156 (67.9) | 0.419 |
| Severe hepatitis | 29/50 (58.0) | 8/156 (5.1) | < 0.0001 |
| Acute renal failure | 16/50 (32.0) | 1/156 (0.6) | < 0.0001* |
| Myocarditis | 10/50 (20.0) | 4/156 (2.6) | < 0.0001* |
| ICH or cerebral infarction | 6/50 (12.0) | 5/156 (3.2) | 0.026* |
| ICH | 3/50 (6) | 3/156 (1.92) | 0.155* |
| Pancreatitis | 0/50 (0) | 5/156 (0.64) | 0.339* |
| Bacteremia | 3/50 (6.0) | 2/156 (1.3) | 0.093* |
Fisher’s exact test.
The DHF cases in 2015 had more complications of gastrointestinal bleeding (78.5% versus 61.9%, P = 0.01), severe hepatitis (30.1% versus 8%, P < 0.0001), and myocarditis (14% versus 0.9%, P < 0.0001) than in 2014 (Table 5). These three fatal cases had bacteremia including Aeromonas hydrophila, Escherichia coli, oxacillin-sensitive Staphylococcus aureus among the 50 deaths, and two nonfatal cases had Acinetobacter baumannii or Salmonella group B bacteremia among the 156 survivors.
The significant variables in univariate analysis, including differences between 2014 and 2015, Charlson score, altered consciousness, hematuria, severe hepatitis, ARF, myocarditis, and ICH or infarction, were put into multivariate analysis. Because age and comorbidity are factors of the Charlson comorbidity index, we did not put age categories or specific comorbidities into the multivariate analysis when the Charlson comorbidity index score was included for the analysis. This revealed that the risk factors for mortality were Charlson score ≥ 5 (P = 0.02, odds ratio [OR] = 4.07, 95% CI = 1.24–13.31), severe hepatitis (P < 0.01, OR = 11.97, 95% CI = 3.83–37.4), and ARF (P < 0.01, OR = 98.76, 95% CI = 10.85–899.2) (Table 7).
Table 7.
Multivariate logistic regression analysis of risk factors associated with mortality for dengue hemorrhage fever cases
| Variables* | Odds ratio | 95% CI | P values |
|---|---|---|---|
| Different year 2014 vs. 2015 | 2.71 | 0.95–7.79 | 0.06 |
| Hematuria | 2.21 | 0.82–5.96 | 0.12 |
| Severe hepatitis | 11.97 | 3.83–37.4 | < 0.01 |
| Acute renal failure | 98.76 | 10.85–899.2 | < 0.01 |
| Altered consciousness | 2.47 | 0.65–9.33 | 0.18 |
| Myocarditis | 2.45 | 0.55–10.93 | 0.24 |
| ICH or cerebral infarction | 2.45 | 0.44–13.77 | 0.31 |
| Charlson score ≥ 5* | 4.07 | 1.24–13.31 | 0.02 |
CI = confidence interval.
Charlson score (Charlson comorbidity index score) was divided into two groups (score 0–4 vs. score ≥ 5).
DISCUSSION
Multiple factors have been suggested to contribute to DHF such as secondary infections, age, comorbidities of the host, viral load, and infecting serotype and genotype.15–17 In this DHF case series study where DENV-1 was predominant in 2014 and DENV-2 was predominant in 2015,6,7 we have a chance to compare the characteristics of cases in different years. We observed that the mortality rate of DHF cases in 2015 (38.7%) was three times higher than that of cases in 2014 (12.4%). In addition, cases in 2015 had higher complication rates, including 78.5% with gastrointestinal bleeding, 30.1% with severe hepatitis, and 14% with myocarditis.
Regarding clinical symptoms, more chills and signs of bleeding (gastrointestinal bleeding, hematuria, and vaginal bleeding) were observed in cases in 2015 (when DENV-2 predominated), although this is not statistically significant for mortality in multivariate analysis (Table 7). However, more classic dengue fever symptoms and signs (retro-orbital pain, joint pain, arthralgia, and myalgia) were found in cases in 2014 (when DENV-1 predominated) in DHF patients. This reveals the difference in clinical manifestations among different DENV serotypes/strain infections. It could be possible that cases without classic dengue fever symptoms had delayed diagnosis and care for the dengue infection or the interaction with the DENV-1 season before.
It has been reported that Asian DENV-2 viruses are more frequently associated with severe disease if a DENV-2 epidemic is after a DENV-1 epidemic, because the Asian DENV-2 strain is less sensitive than American genotype DENV-2 viruses to antibody-mediated neutralization by sera from individuals previously infected with DENV-1.16 This could be the reason that cases in 2015 suffered more severe disease manifestations.
The age of cases in 2015 was higher but not significantly different from cases in 2014 (63.8 ± 16.4 versus 66.8 ± 14.1). However, there are significant differences in comorbidity, including diabetes mellitus, old CVA, and CKD. The Charlson score is significantly different between cases in 2014 and 2015 (Table 3). The significant difference in mortality rates between cases in 2014 and 2015 in univariate analysis suggested the effect of DENV serotype and strains (Table 1). However, in multivariate analysis, cases in 2015 are not a significant risk factor for mortality in DHF, which suggests the DENV factor plays a marginal but not significant role for mortality. Multivariate analysis reveals that the risk factors for mortality were Charlson score ≥ 5 (P = 0.02, OR = 4.07, 95% CI = 1.24–13.31), severe hepatitis (P < 0.01, OR = 11.97, 95% CI = 3.83–37.4), and ARF (P < 0.01, OR = 98.76, 95% CI = 10.85–899.2). It indicates that age and comorbidities are more important than DENV serotype/strain in terms of the mortality of adult DHF cases, other than with severe complications of severe hepatitis and ARF.
In southeast Asia, most of the dengue fever cases are children, but dengue patients in Taiwan are adults. The median age of dengue patients admitted to ICU was 20–42.6 years old and the mortality rate is 6.1–29.6%,18 but in Singapore data showed the higher median age of 44 years old.19 However, in this series, the majority of cases of DHF were in the sixth to eighth decades (59 cases, 49.6%) and more elderly than in previous studies.4,5,11,20 Among demographic factors, the mean age of fatal cases was 70.9 years old, and that of survival cases was 63.4 years old. To the best of our knowledge, this DHF group is the oldest in the literature, and the case fatality rate of DHF was 24.3%, which is significantly higher than the < 1% average figure for southeast Asia or Latin America.3,5,21–23 It is important to note that the DHF cases in southeast Asia or Latin America were less than 18 years old, whereas most of the DHF cases in Taiwan were adults.
For adults DHF cases, comorbidities have been reported to have an impact on the outcome of DHF. A Singapore study showed only 3.8% of a DF nonfatal group had Charlson score > 3.24 In contrast, 28.6% of DF fatal cases had Charlson score > 3, P = 0.033 in a younger age dengue fever group. However, Charlson score was not used in DHF cases before that study. In this case series study, these DHF patients who fulfilled the criteria for Charlson score ≥ 5 points on hospital admission were found to be significantly associated with mortality. We also see a significant trend of increasing mortality rates associated with the increase of this score. Our results indicated that Charlson score can be practically used to predict death in DHF cases. Therefore, physicians may apply Charlson score prospectively to assess the DHF cases in clinical practice and for the future studies.
In a previous study, the mean length of stay (LOS) in dengue fever/DHF patients was approximately 3.50–5.6 days for cases aged 31.87 ± 13.55 years.25 The mean LOS was 10.2 days in 2014 and 10.4 days in 2015 in this study. We observed a significant correlation between age and LOS. Besides, the mean LOS in our series was 6.8 days in the DHF mortality group, which is shorter than the survivor group’s LOS of 11.4 days. This indicates that DHF mortality group deteriorated rapidly, and the aggressive intensive care in the first 7 days is very important for adult DHF cases.
It is rare to see such high rates of central nervous system (CNS) and myocarditis complications among DHF cases in this study, when compared with other case series.26,27 CNS complications associated with dengue are very rare, with a reported frequency of around 1%, and less than 15 cases documented in the English literature.26 In a reported 5,400 patients with dengue fever, 27 of whom (0.5%) presented with CNS involvement, but only one had an intracranial hemorrhage.27 Interestingly, six (2.91%) showed development of intracranial hemorrhage in our series. Previous proposed mechanisms that lead to ICH in dengue infections are mostly related to impaired hemostasis, encephalitis, and inflammatory vasculopathy.27 Our results highlight the incidence and impact of ICH and cerebral infarct for adult DHF patients.
Regarding cardiac involvement in DF, Wali and others showed that 70% of 17 patients with DHF/DSS who underwent a myocardial scintigraphic study suffered diffuse left ventricular hypokinesis with a mean ejection fraction of 40%28 and Kabra and others reported that 16.7% of 54 children with dengue illness had a decreased left ventricular ejection fraction of < 50%.29 In this case series, 14 of 206 patients (6.8%) presented with myocarditis. Cases of myocarditis were mainly seen in 2015 (13/14, 92.9%), suggesting the role of DENV-2. Further studies are required in this field to learn the incidence, pathogenesis, and management of myocardial involvement in DHF.
The limitations of our study are the following: first, the small number of fatal cases in 2014 makes the statistical power limited. Some risk factors might be underestimated for their rare occurrence. Second, being a retrospective study, it was unavoidable that there was missing data for some included patients. Third, we do not know whether the cases had primary or secondary dengue infection, as that is not regularly checked clinically in Taiwan. Fourth, whether a delayed acquisition of medical service and critical service is a factor associated with mortality was not investigated in this study. Fifth, DENV serotype identification is not routinely done in our medical service; this prevents us from identifying the specific DENV serotype of each case in the study.
In conclusion, DHF cases in 2015 had more complications of gastrointestinal bleeding, severe hepatitis, and cardiac involvement than in 2014 in southern Taiwan. More comorbidities and older age are associated with the mortality of DHF cases. Charlson score ≥ 5, severe hepatitis, and ARF are independent variables for mortality.
Supplementary Material
Supplemental Tables and Figure.
Note: Supplemental tables and figure appear at www.ajtmh.org.
REFERENCES
- 1.Gibbons RV, Vaughn DW, 2002. Dengue: an escalating problem. BMJ 324: 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinheiro FP, Corber SJ, 1997. Global situation of dengue and dengue hemorrhagic fever, and its emergence in the Americas. World Health Stat Q 50: 161–169. [PubMed] [Google Scholar]
- 3.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Nguyen MD, Nguyen TH, Tran TH, Farrar JJ, 2006. The WHO dengue classification and case definitions: Time for a reassessment. Lancet 368: 170–173. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Hwang KP, Chen TC, Lu PL, Chen TP, 2006. Clinical characteristics of dengue and dengue hemorrhagic fever in a medical center of southern Taiwan during the 2002 epidemic. J Microbiol Immunol Infect 39: 121–129. [PubMed] [Google Scholar]
- 5.Liu CC, et al., 2008. High case-fatality rate of adults with dengue hemorrhagic fever during an outbreak in non-endemic Taiwan: risk factors for dengue-infected elders. Am J Infect Dis 4: 10–17. [Google Scholar]
- 6.Wang SF, Wang WH, Chang K, Chen YH, Tseng SP, Yen CH, Wu DC, Chen YM, 2016. Severe dengue fever outbreak in Taiwan. Am J Trop Med Hyg 94: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SF, Chang K, Loh EW, Wang WH, Tseng SP, Lu PL, Chen YH, Chen YM, 2016. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg Microbes Infect 5: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO), 1997. Dengue Hemorrhagic Fever: Diagnosis, Treatment, and Control. Geneva, Switzerland: WHO. [Google Scholar]
- 9.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 10.Kuo MC, Lu PL, Chang JM, Lin MY, Tsai JJ, Chen YH, Chang K, Chen HC, Hwang SJ, 2008. Impact of renal failure on the outcome of dengue viral, infection. Clin J Am Soc Nephrol 3: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee IK, Liu JW, Yang KD, 2012. Fatal dengue hemorrhagic fever in adults: emphasizing the evolutionary pre-fatal clinical and laboratory manifestations. PLoS Negl Trop Dis 6: e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salgado DM, et al. , 2010. Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J 29: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SSAcute Pancreatitis Classification Working Group, 2013. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 62: 102–111. [DOI] [PubMed] [Google Scholar]
- 14.Charlson Score Available at: http://www.fpnotebook.com/prevent/Exam/ChrlsnCmrbdtyIndx.htm. Accessed June 6, 2017.
- 15.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL, 2004. Dengue viral infections. Postgrad Med J 80: 588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochel TJ, et al. , 2002. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet 360: 310–312. [DOI] [PubMed] [Google Scholar]
- 17.Balmaseda A, et al. , 2006. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg 74: 449–456. [PubMed] [Google Scholar]
- 18.Pang J, Leo YS, Lye DC, 2016. Critical care for dengue in adult patients: an overview of current knowledge and future challenges. Curr Opin Crit Care 22: 485–490. [DOI] [PubMed] [Google Scholar]
- 19.Pang J, Thein TL, Leo YS, Lye DC, 2014. Early clinical and laboratory risk factors of intensive care unit requirement during 2004–2008 dengue epidemics in Singapore: a matched case–control study. BMC Infect Dis 14: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CC, Huang YH, Shu PY, Wu HS, Lin YS, Yeh TM, Liu HS, Liu CC, Lei HY, 2010. Characteristic of dengue disease in Taiwan: 2002–2007. Am J Trop Med Hyg 82: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA, 2002. Epidemiology, of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol 156: 40–51. [DOI] [PubMed] [Google Scholar]
- 22.Pang J, Salim A, Lee VJ, Hibberd ML, Chia KS, Leo YS, Lye DC, 2012. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS Negl Trop Dis 6: e1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juneja D, Nasa P, Singh O, Javeri Y, Uniyal B, Dang R, 2011. Clinical profile, intensive care unit course, and outcome of patients, admitted in intensive care unit with dengue. J Crit Care 26: 449–452. [DOI] [PubMed] [Google Scholar]
- 24.Thein TL, Leo YS, Fisher DA, Low JG, Oh HML, Gan VC, Wong JG, Lye DC, 2013. Risk factors for fatality among confirmed adult dengue inpatients in Singapore: a matched case-control study. PLoS One 8: e81060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkash O, Almas A, Jafri SM, Hamid S, Akhtar J, Alishah H, 2010. Severity of acute hepatitis and its outcome in patients with dengue fever in a tertiary care hospital Karachi, Pakistan (south Asia). BMC Gastroenterol 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas-Sánchez A, Chiquete E, Gutiérrez-Plascencia P, Castañeda-Moreno V, Alfaro-Castellanos D, Paredes-Casillas P, Ruiz-Sandovala JL, 2014. Cerebellar hemorrhage in a patient during the convalescent phase of dengue fever. J Stroke 16: 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED, 2001. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 65: 848–851. [DOI] [PubMed] [Google Scholar]
- 28.Wali JP, Biswas A, Chandra S, Malhotra A, Aggarwal P, Handa R, Wig N, Bahl VK, 1998. Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol 64: 31–36. [DOI] [PubMed] [Google Scholar]
- 29.Kabra SK, Juneja R, Madhulika, Jain Y, Singhal T, Dar L, Kothari SS, Broor S, 1998. Myocardial dysfunction in children with dengue haemorrhagic fever. Natl Med J India 11: 59–61. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables and Figure.