Abstract.
A decade after reporting its last case of Guinea worm disease (GWD), a waterborne parasitic disease targeted for eradication, Chad reported 20 confirmed human cases from 17 villages—10 cases in 2010 and 10 cases in 2011. In 2012, the first GWD dog infections were diagnosed. We conducted a case-control study during April–May 2012 to identify human transmission risk factors and epidemiologic links. We recruited 19 cases and 45 controls matched by age, sex, time, and location of exposure based on the case patients’ periods of infection 10–14 months earlier. Data were analyzed with simple conditional logistic regression models using Firth penalized likelihood methods. Unusually, GWD did not appear to be associated with household primary water sources. Instead, secondary water sources, used outside the village or other nonprimary sources used at home, were risk factors (matched odds ratio = 38.1, 95% confidence interval = 1.6–728.2). This study highlights the changing epidemiology of GWD in Chad—household primary water sources were not identified as risk factors and few epidemiologic links were identified between the handfuls of sporadic cases per year, a trend that continues. Since this investigation, annual dog infections have increased, far surpassing human cases. An aquatic paratenic host is a postulated mode of transmission for both dogs and humans, although fish could not be assessed in this case-control study due to their near-universal consumption. GWD’s evolving nature in Chad underscores the continued need for interventions to prevent both waterborne and potential foodborne transmission until the true mechanism is established.
INTRODUCTION
Dracunculiasis or Guinea worm disease (GWD) is caused by the roundworm Dracunculus medinensis. Transmission is waterborne and occurs through drinking contaminated stagnant water, typically from lakes, lagoons, ponds, or unprotected wells. A person becomes infected after drinking water containing copepods (small crustaceans) that carry the infective Guinea worm larvae. After a 10- to 14-month incubation period, the female Guinea worm creates a painful blister on the skin. Because of the burning pain associated with the blister, victims often immerse the affected body part in water. On contact with water, the worm emerges through the blister and releases hundreds of thousands of larvae into the water, some of which are consumed intact by copepods and the cycle begins again.1 In 1986, the World Health Assembly adopted a formal resolution calling for the global eradication of GWD.2
In 2000, Chad reported its last cases of GWD, after which the Chad Guinea Worm Eradication Program (CGWEP) transitioned from active to passive surveillance.3 Surveys for GWD were conducted in a small number of limited geographic areas in Chad in 2004, 2005, and 2006 and found no GWD.4–6 In 2008, the World Health Organization (WHO) organized an international certification team to evaluate the national GWD surveillance system as part of the process to certify the interruption of GWD transmission in Chad. The team reported its findings to the International Commission for the Certification of Dracunculiasis Eradication, which concluded that it could not certify Chad as GWD-free and requested additional GWD surveillance be provided through the Integrated Disease Surveillance and Response System.3 In 2008, WHO assisted the CGWEP with the implementation of a US $100 cash reward for confirmed cases of GWD to further enhance surveillance.3 In 2009, Chad reported two rumors of possible GWD but no cases were confirmed on investigation.3 Then, a decade after the reported interruption of GWD transmission in Chad, two suspect cases of GWD were reported to the CGWEP in April and June 2010.7 These two case reports were brought to the attention of a WHO team during a field mission in July 2010, which subsequently carried out further investigations and took samples of the suspect worms from the two patients that were then sent to the U.S. Centers for Disease Control and Prevention (CDC) where they were confirmed as D. medinensis by laboratory testing.7 In total, 10 confirmed GWD cases were identified in 2010 in eight villages, and an additional 10 cases were identified in 2011 in nine villages.8 None of the 2011 cases occurred in villages reporting cases in 2010, and few epidemiologic links were identified between the 2010 and 2011 case patients.8 This was unlike GWD transmission in other countries, where many cases were typically clustered in a single village, and cases occurring one year could be linked with cases from the preceding year through a single identified common water source. Adding to the unusual nature of this newly detected GWD transmission in Chad, a few rumors of worms emerging from dogs had begun to surface in 2011, the descriptions of which sounded like emerging Guinea worms, although CGWEP staff had not yet confirmed D. medinensis infections in dogs at that time.9 In April 2011, the Chadian Ministry of Public Health requested The Carter Center (TCC) to assist the CGWEP. The following year, the CGWEP active village-based surveillance system was launched in more than 600 villages. In March 2012, the CDC was invited by the CGWEP to conduct a GWD investigation in collaboration with TCC. Between April and May 2012, we collected data to determine the risk factors for and the mode of transmission of GWD in humans in Chad and to identify possible epidemiologic links between cases.
METHODS
Case-control study.
We conducted a matched case-control study during April and May 2012. Case patients were defined as persons with visual confirmation of worm emergence by a CGWEP supervisory staff member during 2010 or 2011. The period of infection (POI) for each case patient was defined as the 10–14 months before the emergence of the first worm, corresponding to the incubation period of GWD. The possible village of transmission for each case patient was defined as the self-identified single location/village where the case patient spent the most time during the POI.
Controls were selected from among persons without a history of GWD, and were matched to case patients by age group (0–5, 6–14, 15–25, 26–35, 36–49, and 50 years and older), sex, time of exposure (case patient’s POI), and location at the time of exposure (case patient’s possible location/village of transmission). We attempted to recruit up to three controls per case patient. To ensure random selection of controls, we began in the center of the location/village, spun a bottle, and headed in the direction of the bottle to the first household encountered, where we inquired about potential controls. Subsequent households were then selected by spinning the bottle again. To avoid clustering of households, the interval between visited households was determined locally and was based on the size of the village. For small villages, the next household in the direction of the bottle was visited; for larger villages, the second or third household was selected. Only one control per household was recruited. If multiple persons in one household met the selection criteria, the person closest in age to the case patient was selected.
We interviewed all participating case patients and controls using a standardized questionnaire and collected a detailed travel history during the POI as well as information on demographics and water sources used during the POI. We evaluated both the drinking water source type (i.e., river, lake, lagoon, pond, well, borehole, spring, cistern, canal, rain water, bottled water, tanker, or cart water) as well as the context in which the different water types were used for drinking (i.e., primary source, secondary sources, and travel-related water sources). The primary drinking water source was the single main source of drinking water used at home by the respondent on a daily basis. Secondary drinking water sources were any sources used on a regular basis in addition to the primary source; multiple sources could have been used as secondary sources. These were typically sources used outside the village of residence (such as those used during farming and fishing) or other nonprimary sources used at home, but did not include sources used while traveling or at school, or when in the market. Travel-related drinking water sources were all sources used when traveling far away from home, even if used only once, and were not mutually exclusive. Water sources used at school were included in the travel-related drinking water source category, unless respondents brought water with them from home (e.g., from their primary water sources). In addition to drinking water, we also asked about recreational and bathing exposure to water. Finally, we also asked food-related questions focused on the procurement, preparation, and consumption of aquatic animals, including but not limited to fish, frogs, and lizards. We asked about food consumption and food preparation practices related to aquatic animals to assess the possibility of a novel paratenic transmission pathway for humans because of the unusual epidemiology of the cases to date.
Statistical analyses.
Data were collected using Epi Info™ 7 (CDC, Atlanta, GA) and analyzed in SAS, version 9.3 (SAS Institute, Cary, NC). To identify risk factors for infection, we performed simple unadjusted conditional logistic regression models using Firth penalized likelihood methods to account for the small sample size. Because of the small sample size, we were unable to perform multivariable modeling and assess confounding and effect modification.
Ethical aspects.
The Minister of Public Health in Chad granted permission for this investigation. Because the data for the case-control study were collected to identify, characterize, and control disease in response to an ongoing public health threat in Chad, this investigation and the evaluation protocol were determined to be a nonresearch public health emergency response and were exempt from CDC Institutional Review Board review by the CDC Center for Global Health Human Subjects Team. However, this investigation was conducted in accordance with the Declaration of Helsinki and complied with U.S. government regulations for protecting patient privacy.10
Informed consent was obtained from all respondents before enrollment. For children younger than 18 years of age, permission to participate in the investigation was provided by a parent or guardian and assent was obtained from the child. A parent or guardian was present during the interview of all children younger than 15 years of age to assist with answering questions.
RESULTS
Epidemiology and demographics.
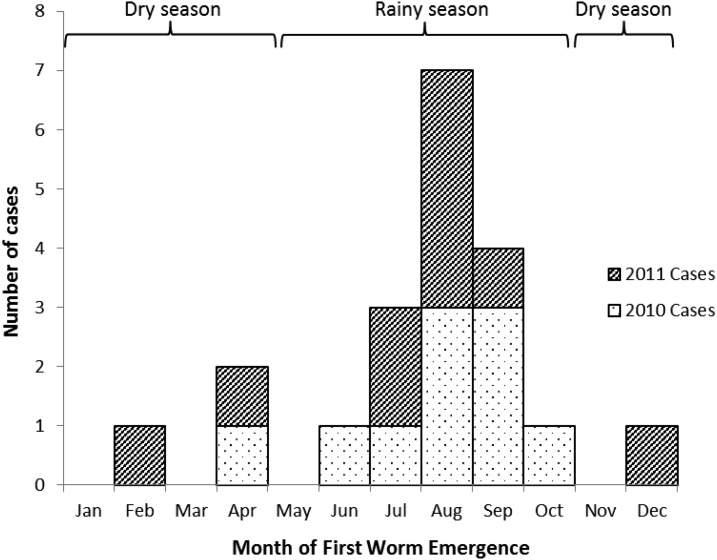
Of the 20 GWD cases identified during 2010–2011, 19 case patients were enrolled in the case-control study, as one case patient died in 2012 from unrelated causes before this investigation took place. Based on the dates of worm detection provided by the CGWEP, the peak of first worm emergence occurred during August in both years, corresponding with the rainy season in the southern part of Chad (from May to October) (Figure 1). In total, eight case patients reported the emergence of more than one worm. A total of 45 controls were enrolled and matched to case patients by sex and age group so these characteristics were similar between the two groups. The most common occupation among both case patients and controls was farming (68% and 60%, respectively). The next most common occupation was student; 26% of case patients and 22% of controls went to school during the POI. Both case patients and controls could have multiple occupations; no significant differences between case patients and controls were found with regard to occupation (Table 1).
Figure 1.
Epidemiologic curve showing Guinea worm disease cases by month of first worm emergence—Chad, 2010–2011.
Table 1.
Demographic characteristics of case patients and controls in a case-control study of Guinea worm disease transmission—Chad, 2010–2011
| Characteristic | Case patients (N = 19) n (%) | Controls (N = 45) n (%) | mOR | 95% CI |
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 10 (53) | 27 (60) | N/A | N/A |
| Age | ||||
| Median age in years (range) | 17 (4–72) | 20 (5–75) | N/A | N/A |
| Occupation | ||||
| Farmer | 13 (68) | 27 (60) | 1.4 | 0.3–6.4 |
| Student | 5 (26) | 10 (22) | 6.3 | 0.3–156.6 |
| Fisher | 4 (21) | 8 (18) | 1.0 | 0.2–5.4 |
| Housewife | 2 (11) | 5 (11) | 1.7 | 0.1–30.8 |
| Herder | 2 (11) | 1 (2) | 3.8 | 0.1–165.8 |
| Nomad | 1 (5) | 0 (0) | 5.9 | 0.1–535.4 |
mOR = matched odds ratio; 95% CI = 95% confidence interval; N/A = not applicable. Case patients and controls were matched by sex and age.
Water sources.
All case patients and 78% (35/45) of controls reported using multiple drinking water sources during the POI. The most common type of drinking water used by both case patients and controls was a lagoon (a body of water marginal to the Chari River) or a pond. We combined lagoons and ponds for analysis purposes as most people did not differentiate between these two types of stagnant water bodies. All other water source types are reported in Table 2. No one reported using protected dug wells, protected springs, unprotected springs, rain water, bottled water, tanker, or cart water for any use (i.e., primary source, secondary sources, or travel-related sources). Drinking water from an unprotected dug well at any time during the POI, even if it was just once (at home, while traveling, or at school) was significantly associated with illness (95% confidence interval [CI] = 1.3–693.3). No significant differences existed between case patients and controls with regard to the use of a specific primary drinking water source. All case patients, and all but one control, used the same primary drinking water source as the other members of their households. Differences were noted, however, with regard to the use of secondary drinking water sources. Overall, 89% (17/19) of case patients used a secondary water source, as opposed to only 44% (20/45) of controls; this exposure was significantly associated with GWD (matched odds ratio [mOR] = 38.1, 95% CI = 1.6–728.2). In particular, the development of GWD was associated with the use of a lagoon, pond, or unprotected dug well as a secondary source. In contrast, sources used while traveling were not found to be associated with illness. No case patients and only one control reported treating (in this case, boiling) his or her primary source of drinking water during the POI.
Table 2.
Drinking water sources used during the period of infection* among case patients and controls in a case-control study of Guinea worm disease transmission—Chad, 2010–2011
| Drinking water sources | Case patients (N = 19) n (%) | Controls (N = 45) n (%) | mOR | 95% CI |
|---|---|---|---|---|
| Source type (any use) | ||||
| Lagoon or pond | 18 (95) | 35 (78) | 11.9 | 0.4–372.5 |
| Unprotected dug well | 9 (47) | 9 (20) | 29.6 | 1.3–693.3 |
| Cistern | 3 (16) | 6 (13) | 9.1 | 0.1–851.2 |
| Borehole | 12 (63) | 22 (49) | 2.0 | 0.5–8.1 |
| River | 5 (26) | 13 (29) | 0.9 | 0.2–4.1 |
| Canal | 1 (5) | 2 (4) | 1.0 | 1.0–1.0 |
| Primary source† | ||||
| Lagoon or pond | 11 (58) | 24 (53) | 1.0 | 0.2–6.0 |
| Unprotected dug well | 3 (16) | 6 (13) | 2.2 | 0.2–26.7 |
| Cistern | 1 (5) | 5 (11) | 0.3 | 0.0–30.8 |
| Borehole | 4 (21) | 5 (11) | 4.0 | 0.4–38.9 |
| River | 0 (0) | 4 (9) | 0.1 | 0.0–6.2 |
| Canal | 0 (0) | 1 (2) | 0.6 | 0.01-62.1 |
| Secondary sources‡ | ||||
| Any secondary source | 17 (89) | 20 (44) | 38.1 | 1.6–728.2 |
| Lagoon or pond | 12 (63) | 15 (33) | 3.6 | 1.1–12.5 |
| Unprotected dug well | 7 (37) | 3 (7) | 11.1 | 1.7–74.6 |
| Cistern | 2 (11) | 2 (4) | 9.1 | 0.1–585.8 |
| Borehole | 2 (11) | 3 (7) | 3.2 | 0.2–40.8 |
| River | 1 (5) | 4 (9) | 0.7 | 0.07–6.2 |
| Canal | 1 (5) | 1 (2) | 1.6 | 0.02–152.8 |
| Travel-related sources§ | ||||
| Any travel-related source | 14 (74) | 29 (64) | 1.9 | 0.4–9.2 |
| Lagoon or pond | 10 (53) | 12 (27) | 3.6 | 0.9–14.5 |
| Borehole | 10 (53) | 15 (33) | 2.6 | 0.7–10.1 |
| Unprotected dug well | 3 (16) | 6 (13) | 2.6 | 0.2–33.3 |
| River | 5 (26) | 9 (20) | 1.5 | 0.4–6.1 |
| Cistern | 1 (5) | 1 (2) | 3.0 | 0.2–48.0 |
| Canal | 0 (0) | 0 (0) | — | — |
mOR = matched odds ratio; 95% CI = 95% confidence interval. Bolded values are statistically significant.
The period of infection (POI) for the case patient was the 10- to 14-month time period before the emergence of the first worm, corresponding to the incubation period of Guinea worm disease. Controls were questioned about their drinking water sources during the same POI as the case patients to whom they were matched.
The primary drinking water source was the single main source of drinking water used at home by the respondent on a daily basis
Secondary drinking water sources were any sources used on a regular basis in addition to the primary source and were not mutually exclusive. These were typically sources used outside the village of residence (such as those used during farming and fishing) or other nonprimary sources used at home, but did not include sources used while traveling or at school.
Travel-related drinking water sources were all sources used when traveling far away from home, even if only once, and were not mutually exclusive. Water sources used at school were included in the travel-related drinking water source category, unless respondents brought water with them from home (i.e., from their primary water sources).
In addition to water for drinking, we also asked about water for recreation and hygiene; 89% (17/19) of case patients reported bathing or swimming in a lagoon or pond in comparison to 60% (27/45) of controls, but this difference was not statistically significant (mOR = 95% CI = 0.8–411.4). Similarly, there were no significant differences between case patients and controls with respect to swimming in a river or bathing with water from a river or taken from an unprotected dug well, cistern, or borehole. At the time of the investigation, five (31%) of the 16 villages where case-patients were residing had safe water sources, defined by the CGWEP as drinking water sources free of infected copepods, such as water from boreholes, protected hand-dug wells, and rivers; similarly, five (31%) of 16 villages where case-patients reported their worms emerged had safe water sources at the time of worm emergence.
Other exposures.
All case patients and all but one control reported consuming aquatic animals during the POI. No significant differences were found with respect to food exposures (Table 3). Fish, the most commonly consumed aquatic animal, was reportedly never eaten raw by case patients or controls. Common preparation methods of fish included grilling, smoking, frying, boiling, and drying but no methods were found to be significant for GWD (Table 4). Other aquatic animals reportedly consumed included lizards, snakes, turtles, and frogs. Almost all case patients and controls reported drinking prepared beverages such as tea, alcohol, and juice during the POI (Table 3). A minority of both case patients (1/19, 5%) and controls (5/45, 11%) reportedly obtained food and/or beverages somewhere other than their usual places but there was no statistically significant difference between the groups (mOR = 0.5, CI = 0.1–3.6). All respondents used their primary drinking water sources for cooking and preparing food and beverages. In total, 79% (15/19) of case patients and 64% (29/45) of controls traveled outside their villages during the POI (e.g., for school, work, recreation, visiting friends or relatives, shopping at a market, attending a special event such as a funeral, or a wedding); again, this difference was not statistically significant (mOR = 2.9, CI = 0.5–30.2).
Table 3.
Foods and beverages consumed during the period of infection* among case patients and controls in a case-control study of Guinea worm disease transmission—Chad, 2010–2011
| Food and beverage types | Case patients (N = 19) n (%) | Controls (N = 45) n (%) | mOR | 95% CI |
|---|---|---|---|---|
| Fish | 19 (100) | 44 (98) | 1.0 | 0.01–92.4 |
| Other aquatic animals (apart from fish)† | 6 (32) | 17 (38) | 0.4 | 0.1–2.6 |
| Lizards | 5 (26) | 13 (29) | 0.4 | 0.04–4.4 |
| Frogs | 3 (16) | 10 (22) | 0.6 | 0.1–2.8 |
| Turtles | 2 (11) | 3 (7) | 1.2 | 0.1–13.5 |
| Snakes | 2 (11) | 4 (9) | 0.6 | 0.03–10.3 |
| Dogs | 0 (0) | 0 (0) | – | – |
| Specialties/delicacies‡ | 2 (11) | 12 (27) | 0.4 | 0.1–1.9 |
| Prepared beverages§ | 17 (89) | 41 (91) | 1.0 | 0.1–10.5 |
| Tea | 9 (47) | 27 (60) | 0.5 | 0.1–2.2 |
| Juices | 7 (37) | 23 (51) | 0.3 | 0.1–1.6 |
| Alcohol | 5 (26) | 11 (24) | 1.3 | 0.3–6.2 |
| Herbal/homemade remedies¶ | 4 (21) | 14 (31) | 0.3 | 0.1–1.8 |
| Other beverages‖ |
mOR = matched odds ratio; 95% CI = 95% confidence interval.
The period of infection (POI) for the case patient was the 10- to 14-month period before the emergence of the first worm, corresponding to the incubation period of Guinea worm disease. Controls were questioned about the foods and beverages consumed during the same POI as the case patients to whom they were matched.
Other aquatic animals consumed during the POIs included lizards, frogs, turtles, and snakes. These may have been eaten raw or prepared in some way (e.g., boiled, dried, marinated, smoked, salted). Two controls also indicated they ate wild duck. Subcategories of aquatic animals were not mutually exclusive.
Respondents were asked if they ate any special foods or delicacies during their POIs. Nine (64%) people reported various types of meat (chicken, beef, mutton, goat, and bush meat—monkey, pig, and porcupine). The remaining five responses referred to various plant-based products.
Prepared beverages consumed during the POI could be purchased or homemade, and included teas, juices, flavored drinks, home-brewed and commercial alcoholic beverages, and local drinks including drinks made from fruits, vegetables, plants, leaves, and flowers. They also included herbal remedies, supplements, and medicines, as well as refreshments. The subcategories of prepared beverages were not mutually exclusive.
Herbal/homemade remedies included neem drink, quincaliba, kaisedra, and others. None were statistically significant.
Other beverages includes soda, milk, sorghum, porridge, and thorn beverage. Soda, milk, and porridge were the most popular but none were statistically significant.
Table 4.
Acquisition and processing of aquatic foods consumed during the period of infection* among case patients and controls in a case-control study of Guinea worm disease transmission—Chad, 2010–2011
| Animal type | Obtained/prepared | Case patients (N = 19) n (%) | Controls (N = 45) n (%) | mOR | 95% CI | |
|---|---|---|---|---|---|---|
| Fish | How fish were obtained† | Purchased | 6 (32) | 14 (31) | 1.5 | 0.2–12.6 |
| Given | 0 (0) | 4 (9) | 0.3 | 0.01–7.9 | ||
| Caught | 14 (74) | 27 (60) | 2.4 | 0.3–18.0 | ||
| How fish were prepared† | Raw | 0 (0) | 0 (0) | – | – | |
| Prepared/cooked | 19 (100) | 44 (98) | 1.0 | 0.01–92.4 | ||
| Fried | 2 (11) | 7 (16) | 0.8 | 0.1–4.6 | ||
| Dried | 9 (47) | 19 (42) | 1.2 | 0.3–4.8 | ||
| Boiled | 15 (79) | 37 (82) | 0.9 | 0.2–3.5 | ||
| Grilled/roasted | 8 (42) | 17 (38) | 1.1 | 0.3–4.1 | ||
| Smoked | 10 (53) | 23 (51) | 0.9 | 0.2–3.8 | ||
| Salted | 0 (0) | 0 (0) | – | – | ||
| Other aquatic animals‡ | How other aquatic animals were obtained† | Purchased | 1 (5) | 0 (0) | 5.9 | 0.1–535.4 |
| Given | 0 (0) | 3 (7) | 0.4 | 0.01–13.1 | ||
| Caught | 6 (32) | 15 (33) | 0.7 | 0.1–3.7 | ||
| How other aquatic animals were prepared† | Raw | 0 (0) | 0 (0) | – | – | |
| Prepared/cooked | 6 (32) | 17 (38) | 0.4 | 0.1–2.6 | ||
| Fried | 1 (5) | 4 (9) | 0.7 | 0.1–6.5 | ||
| Dried | 0 (0) | 0 (0) | – | – | ||
| Boiled | 6 (32) | 17 (38) | 0.4 | 0.1–2.6 | ||
| Grilled/roasted | 2 (11) | 7 (16) | 0.6 | 0.1–3.3 | ||
| Smoked | 0 (0) | 3 (7) | 0.3 | 0.01–8.8 | ||
| Salted | 0 (0) | 0 (0) | – | – | ||
mOR = matched odds ratio; 95% CI = 95% confidence interval.
The period of infection (POI) for the case patient was the 10- to 14-month time period before the emergence of the first worm, corresponding to the incubation period of Guinea worm disease. Controls were questioned about the acquisition and processing of aquatic foods consumed during the same POI as the case patients to whom they were matched.
Subcategories were not mutually exclusive.
Other aquatic animals include lizards (N = 5, case patients; N = 13, controls), frogs (N = 3, case patients; N = 10, controls), turtles (N = 2, case patients; N = 3, controls), and snakes (N = 2, case patients; N = 4, controls).
DISCUSSION
The investigation conducted in Chad in 2012 about cases in 2010 and 2011 highlights the unusual and changing epidemiology of dracunculiasis. Our investigation did not identify a contaminated primary water source serving villages with geographically linked clusters of cases as seen in GWD outbreaks in other countries. Instead, the only risk factor we found associated with GWD was drinking water from a secondary water source. Secondary sources (lagoon, ponds, or unprotected dug wells) were generally sources used outside the village of residence or other nonprimary sources used at home. In 2010–2011, peak GW emergence leading to GWD transmission occurred during July–September during the rainy season in Chad when flooding of the Chari River resulted in the formation of many stagnant bodies of water that could have be used as secondary drinking water sources. Because of this abundance, a person had ample choices of water bodies in which to submerge affected body parts as Guinea worms emerged from painful blisters, thereby contaminating the water. To be viable, these first-stage larvae must be ingested by copepods within 5 days, after which there is a 10- to 14-day period when they mature into their infectious third-stage forms.11 Because there is a finite window in which a contaminated water supply is infectious, few people might have used the same contaminated secondary water source or the same contaminated location within a large water body, or returned to use the contaminated water source multiple times to receive an adequate infectious dose of infected copepods. Accordingly, contaminated secondary water sources may have resulted in sporadic single cases occurring in persons living in different villages.
There are other factors in Chad that may also help explain the sporadic single cases in different villages from year to year. The population in the part of Chad where the case patients are concentrated is very mobile with people attending markets, fishing, and farming outside their village of residence. This, combined with nomadic populations across the country, may result in single cases spread out across many villages. Infection may occur in one place but worm emergence and water contamination 10–14 months later may occur a great distance away in a seemingly unrelated location. Also, many water sources observed by the investigation team were large, even during the peak of the dry season. Dilution of larvae in a large water source may result in a lower risk of infection associated with that particular source. It is possible that the concentration of Guinea worm-infected copepods in large water sources is low, thereby reducing the likelihood that many people in one village will contract GWD. Additionally, it is possible that the association between GWD and secondary water sources may be a confounder for other risk factors that accompany the use of such water sources. For example, perhaps those who use secondary sources away from their village of residence are more likely to consume improperly prepared food (e.g., aquatic animals) caught in the immediate area.
The current working hypothesis for the CGWEP is the presence of an aquatic paratenic host (i.e., an intermediate host in which no development of the D. medinensis larva occurs) because of the finding of D. medinensis in dogs and the seasonal and geographic association with the large domestic and commercial fishing industry along the Chari River.11,12 The first laboratory-confirmation of a D. medinensis infection in a dog occurred in April 2012 during the time of this investigation (CDC unpublished data). The D. medinensis infecting dogs has been found to be genetically indistinguishable from the D. medinensis infecting humans.12 In 2012, 27 dog infections were identified and laboratory confirmed.13 Since then, the number of Guinea worm infections in dogs has risen each year to 54 infections in 2013, 113 infections in 2014, 503 in 2015, and 1,011 in 2016, whereas the number of GWD cases in humans has remained small in number and has been relatively stable at 9–16 cases per year from 2010 to 2016, specifically 10 cases in 2010, 10 in 2011, 10 in 2012, 14 in 2013, 13 in 2014, nine in 2015, and 16 in 2016.12–16 Since 2010, when the first GWD cases in humans were discovered in Chad after a 10-year absence, human cases have remained sporadic showing a dispersed pattern with reports from multiple different villages each year and few epidemiologic links between human cases. Now, with the increasing number of dog infections, both human cases and dog infections are occurring in the same villages. For example, in 2014, two of the 11 villages that reported human cases also reported dog infections; in 2015, five of the nine villages that reported human cases also reported dog infections.17,18 GW infections in both humans and dogs tend to cluster around the Chari River. The early seasonal pattern in human GWD cases observed in 2010–2011 has now disappeared and human GWD cases are now scattered throughout the year, but there remains an annual peak in dog infections in May and June that appears to correspond with the mass fish harvesting that occurs at the end of the dry season in the large lagoons and ponds that form along the Chari and Logone Rivers.9,19 Because the peak in annual GWD cases corresponds with the fish harvest and because human cases and dog infections tend to cluster around the Chari River, the prevailing theory is that dogs are being infected by eating an aquatic animal that is itself infected with D. medinensis (e.g., raw, dried, or smoked fish that dogs are able to steal; fish entrails that are discarded during fish processing; or perhaps other aquatic animals such as frogs that dogs might catch themselves). With this theory, the occasional human would get infected by eating the same raw or undercooked aquatic paratenic host.12,13 We asked respondents about food consumption practices, but there were no differences in consumption of aquatic animals between cases and controls. Since fish were eaten by nearly all respondents, including both cases and controls, we were unable to identify the risk of fish consumption. The risks of consuming of other aquatic animals, such as frogs, were not statistically significant. None of the respondents reported eating raw fish, but it is possible that infection could occur by eating undercooked fish. Common methods of preparation like smoking, drying, grilling, and frying, are frequently inadequate to kill parasites.20,21
Our investigation was subject to several limitations. First, recall bias and misclassification bias are likely, as case patients and controls may remember their potential exposures differently and respondents may have had limited recollection of events that occurred as far back as 3 years before the investigation. However, the proportion of case patients and controls reportedly using particular source water types as primary drinking water sources or as travel-related water sources were similar, as were food consumption and preparation practices, so it appears unlikely that bias played a major role between the two groups. Second, interviews were translated from French into one or more local dialects leaving them vulnerable to translation inaccuracies. Third, our total sample size was small, which limited our power to detect risk factors, particularly less common risk factors, including certain aquatic animals or particular food preparation methods; or to adjust for potential confounders for the association with the disease. Finally, the first D. medinensis infections in dogs had only recently been identified when this study took place and the paratenic host concept developed more fully after completion of this study. Therefore, the survey instrument was not designed to address the paratenic host research question. Consequently, questions about consumption of aquatic animals were limited and we were unable to evaluate whether particular aspects of fish or aquatic animal consumption (e.g., a certain fish species or a certain fishing location) were associated with an increased risk for GWD.
Chad presents a unique situation in the history of the Guinea Worm Eradication Program. The 10-year absence of reported cases between 2000 and 2010 followed by the reestablishment of indigenous transmission could have resulted from an imported case that had gone undetected. However, the observed weakness of GWD surveillance during this period, which prevented the International Commission for the Certification of Dracunculiasis Eradication from conferring certification of transmission interruption in spite of the lack of reported cases, lends weight to the theory that low-level indigenous transmission may have been occurring undetected throughout this time.22 This was not a point-source outbreak that infected multiple case patients and could be linked to a primary water source. Results from this study, carried out early in the outbreak, indicated that secondary drinking water sources were associated with GWD. However, transmission in Chad still presents an unusual epidemiological pattern with an unclear mode of transmission. The presence of GWD infections in dogs could be the result of a unique mode of transmission, possibly including a paratenic aquatic host. However, identifying and linking contaminated secondary water sources in the flood plain of the Chari River, which still play a role with a paratenic host, has proven to be an elusive task as has teasing out a potential risk due to aquatic animal consumption, particularly fish which is almost universally consumed. Since this study was conducted, there has been an increasing proportion of cases reported upriver—over time a greater proportion of cases has been reported from the Moyen–Chari Region, where the Chari River first enters Chad, and more recently from the Salamat Region, through which runs a tributary of the Chari River. Both of these regions share southern borders with the Central African Republic and together they reported 46% (6/13), 44% (4/9), and 75% (12/16) of the total reported human cases in 2014, 2015, and 2016, respectively.14,16,19 In contrast, these two regions reported 10% (1/10), 0% (0/10), 30% (3/10), and 36% (5/14) of the reported human cases in 2010–2013, respectively.23 The continued sporadic nature of around a dozen human cases per year, the increasing number of infections in dogs potentially associated with a paratenic host without a corresponding increase in human cases, the apparent spread of cases upriver along the Chari River, and a statistically significant association between human cases and secondary water sources highlight the unusual and evolving nature of dracunculiasis in Chad and underscore the continued need for both traditional Guinea Worm Eradication Program efforts to prevent waterborne transmission of GWD as well as more novel approaches to reduce the likelihood of possible foodborne transmission until the true mode or modes of transmission can be established.
To help prevent the potential recurrence of traditional, waterborne GWD transmission, the program should consider a wider distribution of pipe filters for use away from primary household water supplies, particularly in at-risk villages with human cases or dog infections, villages identified by travel histories of case-patients, and villages sharing water sources with other villages with confirmed cases. GWD education sessions currently provide information about cooking fish well and burying fish entrails so dogs cannot eat them, and tethering dogs with suspect or confirmed GWD infections to prevent water contamination.14 These sessions should also emphasize the use of pipe filters for secondary drinking water sources if the person is away from the village of residence. An expanded use of temephos larvicide to kill copepods in potentially contaminated drinking water supplies would also help interrupt transmission, whether transmission is occurring via secondary water sources or paratenic hosts or a combination of both, since infected copepods are common to both mechanisms. Further research is currently underway to explore possible transmission via paratenic hosts. Such research includes implementing a protocol for detecting Dracunculus DNA in fish and/or copepods and trying to experimentally infect fish and tadpoles with D. medinensis using infected copepods.
Acknowledgmenets:
We thank The Carter Center technical advisors Corey Farrell, Kristen Grenon, Melinda Denson and their drivers, field supervisors, and village volunteers who helped us carry out our investigation. We also thank Neloumta Lucienne, data manager, for the GWEP for her assistance with the investigation.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Ruiz-Tiben E, Hopkins DR, 2006. Dracunculiasis (Guinea worm disease) eradication. Adv Parasitol 61: 275–309. [DOI] [PubMed] [Google Scholar]
- 2.World Health Assembly, 1986. Elimination of Dracunculiasis: Resolution WHA 39.21 of the 39th World Health Assembly Available at: http://www.who.int/neglected_diseases/mediacentre/WHA_39.21_Eng.pdf. Accessed July 5, 2016.
- 3.World Health Organization, 2010. Dracunculiasis eradication: global surveillance summary, 2009. Wkly Epidemiol Rec 85: 166–176. Available at: http://www.who.int/wer/2010/wer8519.pdf?ua=1. Accessed February 17, 2017. [PubMed] [Google Scholar]
- 4.World Health Organization, 2005. Dracunculiasis eradication: global surveillance summary, 2004. Wkly Epidemiol Rec 80: 165–176. Available at: http://www.who.int/wer/2005/wer8019.pdf?ua=1. Accessed March 10, 2017. [PubMed] [Google Scholar]
- 5.World Health Organization, 2007. Dracunculiasis eradication: global surveillance summary, 2006. Wkly Epidemiol Rec 82: 133–140. Available at: http://www.who.int/wer/2007/wer8216.pdf?ua=1. Accessed March 10, 2017. [PubMed] [Google Scholar]
- 6.Department of Health and Human Services, 2006. Guinea Worm Wrap-Up No. 159 Available at: https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/159.pdf. Accessed March 10, 2017.
- 7.Djidina MR, Guialoungou H, Dono BB, Ngarhor N, Padjaina M, Biswas G, Sankara D, Maiga A, Djimrassengar H, Roy SL, El Bcheraoui C, Walldorf JA, 2011. Renewed transmission of dracunculiasis: Chad, 2010. Morb Mortal Wkly Rep 60: 744–748. [PubMed] [Google Scholar]
- 8.Ruiz-Tiben E, Eberhard ML, Roy SL, 2012. Progress toward global eradication of dracunculiasis: January 2011–June 2012. Morb Mortal Wkly Rep 61: 854–857. [PubMed] [Google Scholar]
- 9.Eberhard ML, Ruiz-Tiben E, Hopkins DR, Farrell C, Toe F, Weiss A, Withers PC, Jr, Jenks MH, Thiele EA, Cotton JA, Hance Z, Holroyd N, Cama VA, Tahir MA, Mounda T, 2014. The peculiar epidemiology of dracunculiasis in Chad. Am J Trop Med Hyg 90: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association, 2015. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects Available at: http://www.wma.net/en/30publications/10policies/b3/. Accessed February 8, 2016.
- 11.Muller R, 1971. Dracunculus and dracunculiasis. Dawes B, ed. Advances in Parasitology, volume 9. London, United Kingdom: Academic Press, 73–151. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins DR, Ruiz-Tiben E, Eberhard ML, Roy SL, 2015. Progress toward global eradication of dracunculiasis: January 2014–June 2015. Morb Mortal Wkly Rep 64: 1161–1165. [DOI] [PubMed] [Google Scholar]
- 13.Department of Health and Human Services, 2015. Guinea Worm Wrap-Up No. 234 Available at: http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/234.pdf. Accessed July 5, 2016.
- 14.Department of Health and Human Services, 2016. Guinea Worm Wrap-Up No. 238 Available at: http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/238.pdf. Accessed July 5, 2016.
- 15.Department of Health and Human Services, 2016. Guinea Worm Wrap-Up No. 239 Available at: https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/239.pdf. Accessed July 5, 2016.
- 16.Department of Health and Human Services, 2017. Guinea Worm Wrap-Up No. 246 Available at: https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/245.pdf. Accessed February 17, 2017.
- 17.World Health Organization, 2015. Dracunculiasis eradication: global surveillance summary, 2014. Wkly Epidemiol Rec 90: 201–216. Available at: http://www.who.int/wer/2015/wer9019.pdf?ua=1. Accessed February 17, 2017.25958417 [Google Scholar]
- 18.World Health Organization, 2016. Dracunculiasis eradication: global surveillance summary, 2015. Wkly Epidemiol Rec 91: 219–236. Available at: http://www.who.int/wer/2016/wer9117.pdf?ua=1. Accessed February 17, 2017. [PubMed] [Google Scholar]
- 19.Department of Health and Human Services, 2015. Guinea Worm Wrap-Up Issue No. 231 Available at: http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/231.pdf. Accessed July 5, 2016.
- 20.U.S. Food and Drug Administration, 2014. Fish and Fishery Products Hazards and Controls Guidance, 4th edition. Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Seafood/ucm2018426.htm. Accessed July 5, 2016.
- 21.U.S. Food and Drug Administration, 2015. Food Code 2013 Available at: http://www.fda.gov/Food/GuidanceRegulation/RetailFoodProtection/FoodCode/ucm374275.htm. Accessed July 5, 2016.
- 22.World Health Organization, 2011. Dracunculiasis eradication: global surveillance summary, 2010. Wkly Epidemiol Rec 86: 189–198. Available at http://www.who.int/wer/2011/wer8620.pdf?ua=1. Accessed March 10, 2017. [Google Scholar]
- 23.Department of Health and Human Services, 2015. Guinea Worm Wrap-Up Issue No. 232 Available at: https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/232.pdf. Accessed July 5, 2016.