Abstract.
RNA interference caused by exogenous double-stranded RNA (dsRNA) is used to downregulate crucial genes to control insects. The reproductive success of all oviparous species depends on vitellogenin (Vg) biosynthesis and its accumulation in the developing oocytes. Adult females of Triatoma infestans were independently injected with two Vg dsRNAs (Vg1 dsRNA or Vg2 dsRNA) or nuclease-free water (control) 24 hours before feeding, and a group of adult females not injected was also analyzed (control). Vg1 and Vg2 messenger RNAs silencing was verified by reverse transcription quantitative polymerase chain reaction. The transcript levels of the Vg1 and Vg2 genes were significantly reduced after dsRNA treatment in fat body and ovary of T. infestans in relation to those detected in individuals injected with nuclease-free water and not injected (controls). Moreover, the present study demonstrated that the silencing of the Vg1 or Vg2 genes inhibits oviposition in the Chagas disease vector T. infestans. These findings may have important implications for the development of novel vector control strategies.
INTRODUCTION
Triatoma infestans (Hemiptera: Reduviidae) known as “vinchuca” is the main vector of Chagas disease in the southern Cone of Latin America between latitudes 10°S and 46°S, where it is primarily restricted to domestic and peridomestic environments. Pyrethroid insecticides have been the main means of controlling vector. However, pyrethroid resistance in the vector insect has been reported as one of the main explanations of the unsatisfactory control observed.1
RNA interference (RNAi) has been developed as an effective gene-silencing tool in a wide variety of organisms.2 Double-stranded RNA (dsRNA)–mediated RNAi has emerged as one of the most powerful strategies for the rapid analysis of gene function and has considerable potential for the development of applications for the control of insect vectors.3–5 For the last purpose, this posttranscriptional gene silencing technique requires as its target a gene involved in a vital process for the vector.
The reproductive success of all oviparous species depends on vitellogenin (Vg) biosynthesis and its accumulation in the developing oocytes. Recently, we have identified two Vg genes in T. infestans (Vg1 and Vg2, GeneBank accession numbers KF915268 and KF915267, respectively) and we have analyzed the expression of these genes at transcriptional and translational levels in fat body and ovaries of adult females at different times after ecdysis (previtellogenic phase) and after blood feeding and mating of the females (vitellogenic phase).6,7 Here we examine the role of the Vg genes in the oviposition of T. infestans using RNAi.
MATERIALS AND METHODS
Triatoma infestans was reared at 28 ± 1°C at a relative humidity of 60–70% with a 6-hour light/18-hour dark cycle and fed once every 2 weeks after molt on restrained chickens. Adult females and males were maintained segregated after emergence until they were able to have a blood meal (day 7 postecdysis).
Total RNA was isolated from pools of insect tissues using MasterPure RNA Purification Kit (Epicentre, Madison, WI). Synthesis of complementary DNA (cDNA) was performed from total RNA using Oligo-dT20 and SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA).
A fragment of 650 base pairs (bp) of the Vg1 gene and of 500 bp of the Vg2 gene were amplified by polymerase chain reaction (PCR) from fat body cDNA of females using specific primers containing T7 promoter sequences (Table 1). After the PCR products were electrophoresed, the bands corresponding to the expected sizes were excised from the agarose gel and purified using QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany). Then the PCR products were sequenced in an ABI 3130XL automated DNA sequencer (Applied Biosystems, Foster City, CA). Once the sequences of the Vg genes were verified, the products from the PCR reactions were used as templates for dsRNA synthesis using the T7 RiboMax Express RNAi System (Promega, Madison, WI) according to the manufacturer’s instruction. The dsRNA was isopropanol precipitated, resuspended in nuclease-free water, and quantified spectrophotometrically at 260 nm.
Table 1.
PCR primers containing T7 promoter sequences to amplify templates for dsRNA synthesis
| P6 | 5′-TAATACGACTCACTATAGGGGTAAAGTTAAGGTCAATGGC-3′ | Vg1-Forward |
| PARA | 5′-TAAACCAAGCGTTTGCCGGT-3′ | Vg1-Reverse |
| P8 | 5′-TAATACGACTCACTATAGGTAAACCAAGCGTTTGCCGGT-3′ | Vg1-Reverse |
| PARNAiF1 | 5′-GGTAAAGTTAAGGTCAATGGC-3′ | Vg1-Forward |
| P9 | 5′-TAATACGACTCACTATAGGGGTCTTGAAGCTTTTACTCCCG-3′ | Vg2-Forward |
| PBRNAiR | 5′-GGTACACAGTGAAACTCTACTCGC-3′ | Vg2-Reverse |
| P10 | 5′-TAATACGACTCACTATAGGGGTACACAGTGAAACTCTACTCGC-3′ | Vg2-Reverse |
| PBRNAiF | 5′-GGTCTTGAAGCTTTTACTCCCG-3′ | Vg2-Forward |
dsRNA = double-stranded RNA; PCR = polymerase chain reaction.
Females were injected with 5 μL of Vg1 dsRNA (0.5 µg, N = 27) or 5 μL of Vg2 dsRNA (0.5 µg, N = 27) or 5 μL of nuclease-free water (control, N = 22) 24 hours before feeding. Injections were made ventrally between the fifth and sixth abdominal segment with a Hamilton microsyringe (Hamilton, Reno, NV). To assess the effect of injection, a group of adult females in the same conditions that had not been injected were also analyzed (control, N = 22). After feeding, females were placed together with males in individual containers (each couple in one container). Successful mating was verified by observation of the spermatophore.
Vg1 and Vg2 messenger RNAs (mRNAs) silencing was verified by reverse transcription quantitative PCR (RT-qPCR). Total RNA was isolated from pools of fat bodies and of ovaries extracted of three adult females of each experimental group, 4 and 5 days after feeding, respectively. The transcript levels of Vg1 and Vg2 genes were measured by qPCR, using β-actin mRNA to normalize the expression levels.6,7 Gene-specific primers and Taqman probes were designed according to the corresponding cDNAs using Primer Express program (Applied Biosystems). RT-PCR analysis was used to verify that the PCR product, obtained with each specific primer pair, showed a single band of the expected size. The PCR products corresponding to Vg1, Vg2, and β-actin were cloned into the pCR4-TOPO TA cloning vector (Invitrogen) and sequenced to confirm the identity of the amplified fragments. Quantitative PCR was carried out using Mx3005P qPCR System with Brilliant qPCR Core Reagent Kit (STRATAGENE, La Jolla, CA). The reaction conditions were of 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 60 seconds at 58°C. The relative copy number of Vg mRNA was calculated according to 2−△△CT.8 The threshold cycle value difference △CT between Vg mRNA and β-actin mRNA of each reaction was used to normalize the level of total RNA. Two independent experiments were performed and data for each point were registered by triplicate to account for intraexperimental variation. Graphs and statistical tests were performed using GraphPad Prism, version 5.00 for Windows (GraphPad software, San Diego, CA). One-way analysis of variance with Bonferroni posttest were used for comparisons. The results were presented as mean ± standard deviation SD and a P value < 0.05 was considered statistically significant. On the other hand, to examine whether silencing of Vg1 and Vg2 genes influenced the oviposition, females of each experimental group were separated from males and placed in different containers and eggs deposited were counted daily.
RESULTS AND DISCUSSION
The use of RNAi for the management of insects holds considerable promise, as a system which can be used to reduce the fitness, and/or the fecundity as well as limit their capacity as vectors of diseases. However, the use of RNAi approaches to control vector insects is still in its infancy. Due to the limited number of transgenic insect species, the production of a systemic RNAi response in insects often requires delivery of dsRNA to cells and tissues via microinjection, which is used in most of these experiments to achieve high titer of dsRNA and expose more tissues to silencing factor.
In a previous work, the expression levels of Vg1 and Vg2 genes were analyzed by qPCR and western blot in fat body and ovaries of adult females throughout previtellogenesis and vitellogenesis.7 Vg1 and Vg2 start to be expressed slightly in the fat bodies and ovaries of adult females during previtellogenesis. After blood feeding, Vg1 and Vg2 were up regulated and significant levels of Vg transcripts as well as protein expression were observed in fat bodies sampled throughout vitellogenesis. These findings agreed with the studies that established that in most insect species the female fat body is the major site involved in Vg synthesis.9 However, in addition to the fat body, synthesis of Vgs has been observed in the ovaries of some insect species.10–13 The detection of Vg transcripts in ovaries of T. infestans sampled throughout vitellogenesis was in agreement with this observation.7 Based on the results of this study, adult females were injected after ecdysis (24 hours before feeding) with Vg1 dsRNA or Vg2 dsRNA or nuclease-free water (control) and a group of adult females that had not been injected were also analyzed (control). Subsequently, pools of fat bodies were extracted from adult females of each experimental group 4 days after feeding and pools of ovaries were extracted 5 days after feeding.
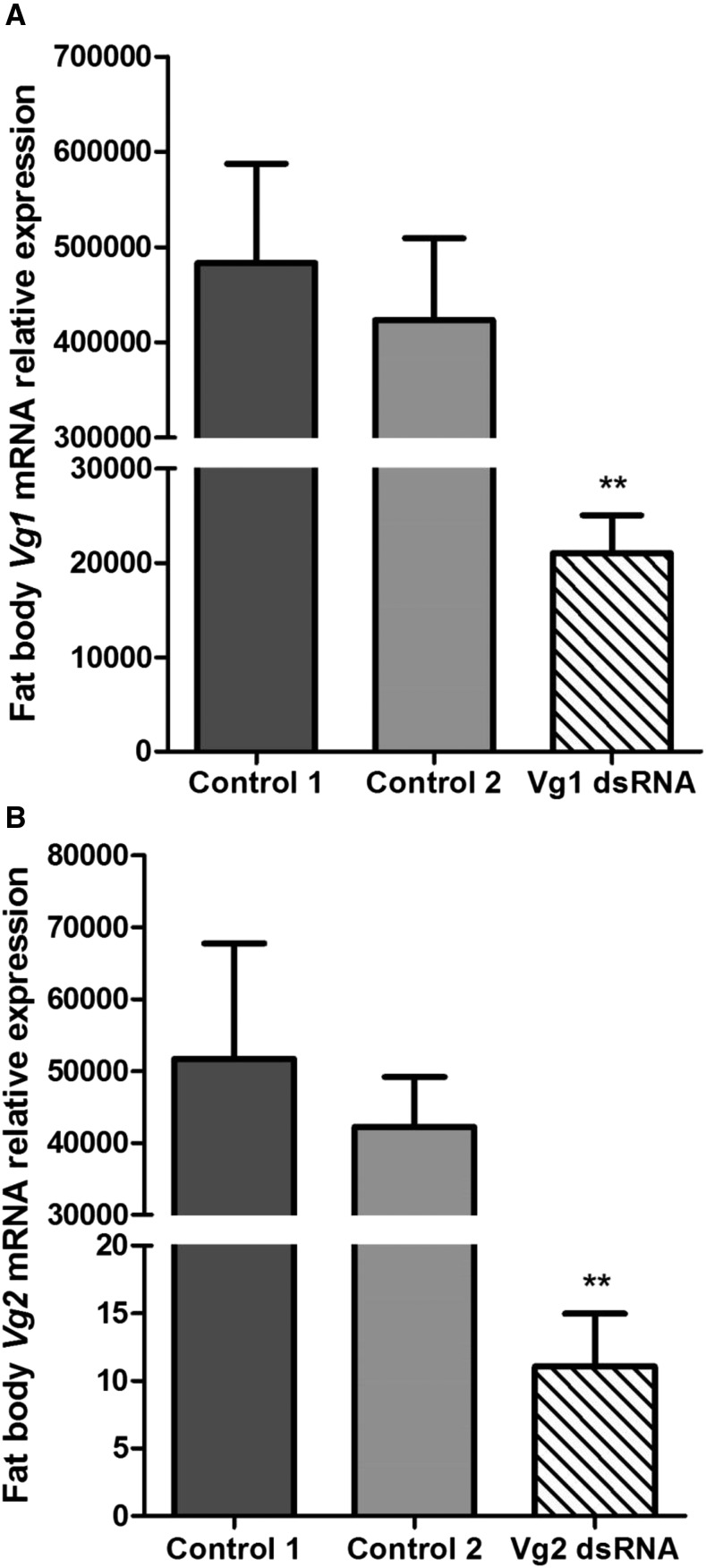
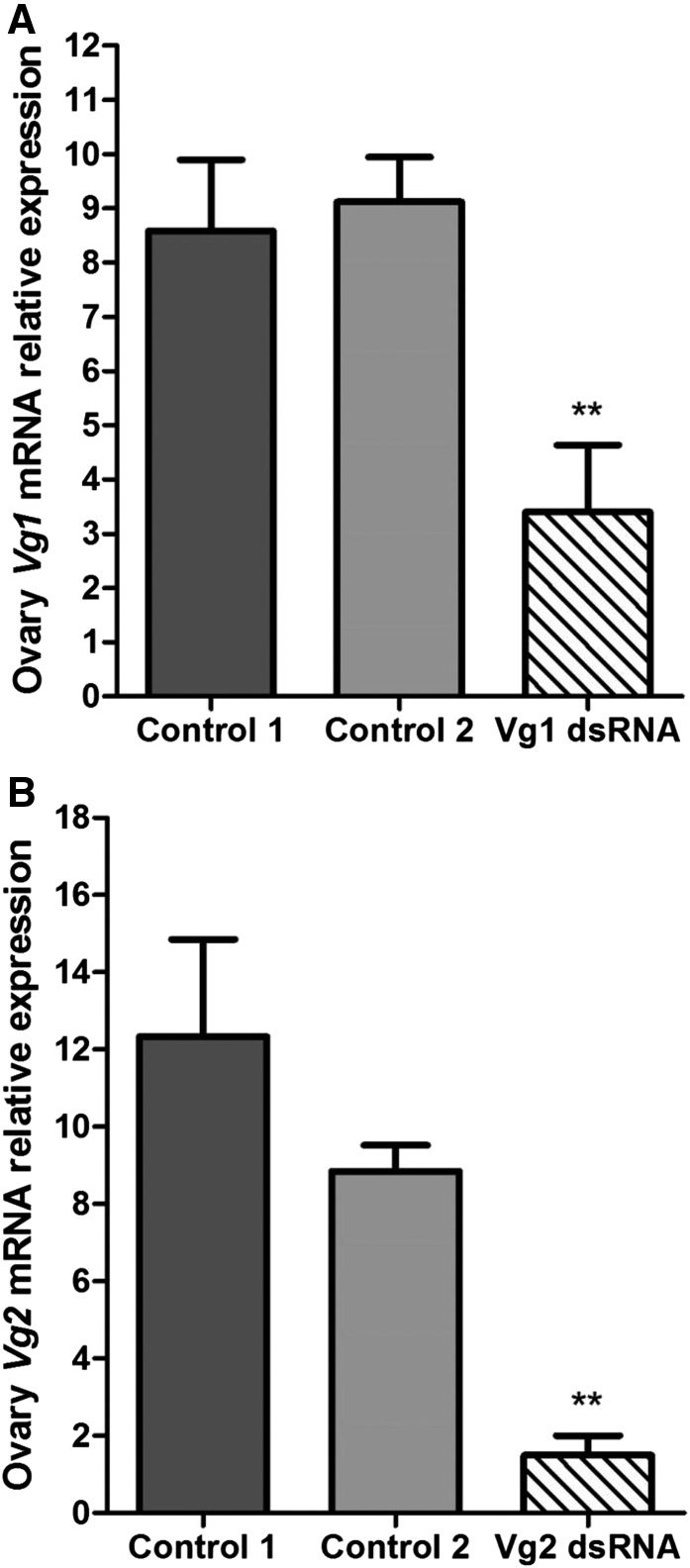
The relative expression levels of the Vg1 and Vg2 genes in fat body and ovary of adult females of T. infestans are shown in Figures 1 and 2. The transcript levels of the Vg1 and Vg2 genes were significantly reduced after dsRNA treatment in fat body (Figure 1A and B) and ovary (Figure 2A and B) in relation to those detected in individuals injected with nuclease-free water and not injected (controls). Besides, it is also important to point out that the expression levels either of Vg1 or Vg2 of the group of individuals injected with nuclease-free water and the group not injected did not present significant differences in both tissues and are congruent with those detected in our previous work.7
Figure 1.
Relative vitellogenin expression messenger RNA (mRNA) of (A) Vg1 and (B) Vg2 in the fat body of Triatoma infestans adult females after injecting Vg1 double-stranded RNA (dsRNA) and Vg2 dsRNA, respectively. The transcript levels of Vg genes were measured by quantitative polymerase chain reaction using β-actin mRNA to normalize the expression levels. The error bars represent the standard deviation of the mean. Two asterisks on the standard error bar indicate significant difference between the mean of the treatment with dsRNA and the mean of the controls at P < 0.01. Control 1: group injected with nuclease-free water; Control 2: group no injected.
Figure 2.
Relative vitellogenin expression messenger RNA (mRNA) of (A) Vg1 and (B) Vg2 in the ovary of Triatoma infestans adult females after injecting Vg1 double-stranded RNA (dsRNA) and Vg2 dsRNA, respectively. The transcript levels of Vg genes were measured by quantitative polymerase chain reaction (qPCR) using β-actin mRNA to normalize the expression levels. The error bars represent the standard deviation of the mean. Two asterisks on the standard error bar indicate significant difference between the mean of the treatment with dsRNA and the mean of the controls at P < 0.01. Control 1: group injected with nuclease-free water; Control 2: group no injected.
In insects, the vitellogenesis process is fundamental for egg development and therefore is a central event for their reproduction.14 To examine whether silencing of Vg1 and Vg2 genes influenced the oviposition, females of each experimental group were subjected to an oviposition bioassay. The Vg-silenced T. infestans adult females failed to lay eggs. The Vg1 dsRNA-treated group (N = 15) and the Vg2 dsRNA-treated group (N = 15) did not present egg production. Contrary, the females injected with nuclease-free water (N = 10) and not injected (N = 10) showed similar level of oviposition. Therefore, this study demonstrated that the silencing of Vg1 or Vg2 inhibits oviposition in the Chagas disease vector T. infestans. These findings are potentially helpful for the development of effective vector management strategies.
REFERENCES
- 1.Mougabure-Cueto G, Picollo MI, 2015. Insecticide resistance in vector Chagas disease: evolution, mechanisms and management. Acta Trop 149: 70–85. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ, 2002. RNA interference. Nature 418: 244–255. [DOI] [PubMed] [Google Scholar]
- 3.Tomoyasu Y, Denell RE, 2004. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214: 575–578. [DOI] [PubMed] [Google Scholar]
- 4.Huvenne H, Smagghe G, 2010. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56: 227–235. [DOI] [PubMed] [Google Scholar]
- 5.Gong L, Luo Q, Rizwan-UI-Haq M, Hu MY, 2012. Cloning and characterization of three chemosensory proteins from Spodoptera exigua and effects of gene silencing on female survival and reproduction. Bull Entomol 102: 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Blariza MJ, Soria NW, Torres AG, Grosso CG, García BA, 2014. cDNA isolation and characterization of two vitellogenin genes in the Chagas’ disease vector Triatoma infestans (Hemiptera, Reduviidae). Gene 543: 118–124. [DOI] [PubMed] [Google Scholar]
- 7.Blariza MJ, Leyria J, Canavoso LE, Soria NW, García BA, 2016. Dynamics of expression of two vitellogenin genes in the Chagas’ disease vector Triatoma infestans: analysis throughout pre-vitellogenesis and vitellogenesis. Acta Trop 156: 100–107. [DOI] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 9.Tufail M, Takeda M, 2008. Molecular characteristics of insect vitellogenins. J Insect Physiol 54: 1447–1458. [DOI] [PubMed] [Google Scholar]
- 10.Melo ACA, Valle D, Machado EA, Salerno AP, Paiva-Silva GO, Cunha E, Silva NL, de Souza W, Masuda H, 2000. Synthesis of vitellogenin by the follicle cells of Rhodnius prolixus. Insect Biochem Mol Biol 30: 549–557. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi F, Snigirevskaya ES, Raikel AS, 2005. The cell biology of yolk protein precursor synthesis and secretion. Raikel AS, ed. Reproductive Biology of Invertebrates. Progress in Vitellogenesis, Volume XII, Part B. Enfield, NH: Science Publishers Inc., 33–68. [Google Scholar]
- 12.Bellés X, 1998. Endocrine effectors in insect vitellogenesis. Coast GM, Webster SG, eds. Recent Advances in Arthropod Endocrinology. Cambridge, United Kingdom: Cambridge University Press, 71–90. [Google Scholar]
- 13.Bellés X, 2005. Vitellogenesis direct by juvenile hormone. Raikhel AS, ed. Reproductive Biology of Invertebrates. Progress in Vitellogenesis, Volume XII, Part B. Enfield, NH: Science Publishers Inc., 157–197. [Google Scholar]
- 14.Raikhel AS, 2005. Vitellogenesis of disease vectors, from physiology to genes. Marquardt WC, ed. Biology of Disease Vectors. London, United Kingdom: Elsevier Academic Press, 329–346. [Google Scholar]