Abstract.
Schistosomiasis remains one of the most prevalent parasitic diseases worldwide and the infection is frequently found in travelers and migrants. The European Network for Tropical Medicine and Travel Health conducted a sentinel surveillance study on imported schistosomiasis between 1997 and 2010. This report summarizes epidemiological and clinical data from 1,465 cases of imported schistosomiasis. Direct pathogen detection and serology were the main diagnostic tools applied. Of these, 486 (33%) cases were identified among European travelers, 231 (16%) among long-term expatriates, and 748 (51%) among non-European immigrants. Overall, only 18.6% of travelers had received pretravel advice; 95% of infections were acquired in the African region. On species level, Schistosoma mansoni was identified in 570 (39%) and Schistosoma haematobium in 318 (22%) cases; 57.5% of patients were symptomatic. Acute symptoms were reported in 27% of patients leading to earlier presentation within 3 months. Praziquantel was used in all patients to treat schistosomiasis. Many infections were detected in asymptomatic patients. In 47.4% of asymptomatic patients infection was detected by microscopy and in 39% by serology or antigen testing. Schistosomiasis remains a frequent infection in travelers and migrants to Europe. Travelers should be made aware of the risk of schistosomiasis infection when traveling to sub-Saharan Africa. Posttravel consultations particularly for returning expatriates are useful given the high potential for detecting asymptomatic infections.
INTRODUCTION
Schistosomiasis is one of the most prevalent parasitic diseases worldwide, affecting > 200 million people in 78 countries. It is estimated that around 120 million individuals are symptomatic and 20 million have severe disease.1,2 According to World Health Organization estimates, over 260 million people required treatment of schistosomiasis in the year 2013 of whom 92% live in the African region.3
Patients born in endemic countries acquire the infection during childhood and may develop a chronic disease because of continuous reinfection through freshwater contact. However, schistosomiasis is also an important travel-associated infection and frequently seen among returnees from endemic areas.4 Symptoms in travelers differ from symptoms in chronically infected individuals. Travelers may present with symptoms of the acute phase of infection which is also known as Katayama syndrome, a nonspecific febrile illness with typically high eosinophilia and a skin rash, usually occurring in the first 3 months after infection. Swimmer’s itch may also be reported by travelers several days after freshwater contact. Initially, serology can still be negative and no eggs are found in urine or stool. In this acute phase of infection, diagnosis is very difficult5 despite recent advances in diagnostic methods such as polymerase chain reaction (PCR) or antigen testing.6,7 Symptoms of chronic disease occur several months or years after travel or leaving endemic areas and can be mistaken for other diseases, especially outside endemic countries.
Schistosomiasis is considered a neglected tropical disease. In Europe, it is diagnosed and treated in referral centers for tropical medicine. International travel and migration is increasing8,9 and there is also a potential for reemergence in the Mediterranean areas of Europe.10,11 The European Network for Tropical Medicine and Travel Health (TropNet, http://www.tropnet.eu), a network of clinical referral centers for tropical and travel medicine Europe, conducted a sentinel surveillance study of imported tropical diseases from 1997 to 2010. We present demographic, clinical, and geographic features of imported schistosomiasis from this large dataset.
METHODS
TropNet collected demographic and clinical data from patients with travel or migration history seen at one of the 38 member sites in 16 countries across Europe between the years 1997 and 2010. Patients who presented to one of these participating centers and in whom a diagnosis of schistosomiasis was established were reported using a standardized questionnaire in either paper-based or electronic format. Data were anonymized before transfer to the central database.
The primary objective of this analysis was to provide clinical, epidemiological, and geographic information on schistosomiasis imported to Europe with regard to patient origin and type of travel as well as an analysis of possible risk factors associated with infection.
The specific final diagnosis of schistosomiasis was reported by the individual TropNet center via the respective International Classification of Diseases (ICD)-10 code for schistosomiasis (ICD-10 B65 + subgroup). Furthermore, centers were required to classify their final diagnosis as “confirmed,” “probable,” or “suspected” on the basis of the following definitions: cases were reported as “confirmed” in case of clinical symptoms with positive microscopic egg detection in urine, semen, stool, or positive biopsy material and PCR. A “probable” definition was declared in case of typical clinical symptoms together with a positive serology for IgG or IgM or antigen detection (via indirect hemagglutination, fluorescence antibody test or enzyme-linked immunosorbent assay). Cases classified as “suspected” (evident clinical signs of schistosomiasis together with a typical travel history as well as cases with any concomitant acute infection) were also reported but excluded from further analysis. In addition, the questionnaire allowed to specify one diagnostic method used to establish the diagnosis.
Cases were classified according to permanent residence/origin, reason for travel, and symptoms of acute or chronic schistosomiasis. Data on permanent residence and origin were used to classify travelers as 1) immigrants or refugees from endemic countries living in Europe and visitors to Europe from endemic areas (termed as “non-Europeans”), 2) individuals from Europe visiting endemic areas for a defined period of time (termed as “Europeans”), and 3) individuals from Europe living and working in endemic areas for a prolonged period of time of over 1 year (termed as “expatriates”).
Reasons for travel included 1) business trip, 2) migration, 3) military service, 4) missionary/humanitarian, 5) scientific, 6) tourism, and 7) visiting friends and relatives (VFR). The dates of return from travel or migration were recorded as well as the time point of presentation; presentation within 3 months was classified as early presentation.
Symptoms were reported as 1) fever, 2) fatigue, 3) skin symptoms, 4) respiratory symptoms, 5) headache, 6) lymphadenopathy, 7) musculoskeletal symptoms, 8) diarrhea, 9) vomiting, 10) otolaryngologic symptoms (eyes, nose, throat), and 11) neurological symptoms. Multiple entries per patient were allowed. Further symptoms or conditions were reportable by text. To differentiate between acute or chronic presentation, we assigned the reported symptoms to two groups: 1) acute/subacute schistosomiasis was assumed if patients presented with fever, fatigue, skin symptoms, respiratory symptoms, headache, musculoskeletal symptoms, otolaryngologic symptoms, night sweats, vomiting, diarrhea, neurological symptoms, and eosinophilia together with fever or respiratory symptoms. 2) Chronic schistosomiasis was assumed for cases presenting with weight loss, signs of hepatosplenic disease, liver disease, melena, hematuria, hematospermia, or hematochezia.
Statistical analysis was carried out using JMP (JMP 11.0, SAS Institute Inc, NC). Categorical data were analyzed using the χ2 test and Fisher’s exact test, as appropriate, at an adjusted two-sided significance level of α < 0.01 due to multiple comparisons. Continuous data were analyzed using the Mann–Whitney U test.
The study was approved by the ethics committee of Charité University Hospital Berlin. Ethical clearance for transfer of anonymized patient data was sought at participating TropNet centers according to local regulations.
RESULTS
Patient classification and demographic data.
The database contained 1,587 cases with a diagnosis of schistosomiasis over 14 years. After exclusion of suspected cases (N = 35), cases with inconsistent data (N = 72) and cases with more than one diagnosis of acute infection (N = 15), 1,465 cases remained available for analysis. Of these, 61.3% of cases (899/1,465) were reported as confirmed and 38.7% of cases (566/1,465) were reported as probable. Demographic data are given in Table 1. Of these, 486 (33%) cases were diagnosed among Europeans, 231 (16%) among expatriates, and 748 (51%) among non-Europeans.
Table 1.
Demographic data by patient type
| Total N = 1,465 | European N = 486 (33%) | Expatriate N = 231 (16%) | Non-European N = 748 (51%) | Pearson χ2 test | |
|---|---|---|---|---|---|
| Sex | P = 0.0014 | ||||
| Male | 983 (67.1%) | 299 (61.5%) | 153 (66.2%) | 531 (71.0%) | |
| Female | 478 (32.6%) | 187 (38.5%) | 78 (33.8%) | 213 (28.5%) | |
| No data | 4 (0.3%) | 0 (0.0%) | 0 (0.0%) | 4 (0.5%) | |
| Age | P = 0.0001 | ||||
| IQR 25 | 22 | 24 | 14.25 | 20.75 | |
| Median | 29 | 30 | 35 | 28 | |
| IQR 75 | 38 | 39 | 45.75 | 36 | |
| Pretravel advice | P < 0.0001 | ||||
| Yes | 273 (18.6%) | 201 (41.4%) | 65 (28.1%) | 7 (0.9%) | |
| No | 298 (20.4%) | 60 (12.3%) | 15 (6.5%) | 223 (29.8%) | |
| Unknown | 894 (61.0%) | 225 (46.3%) | 151 (65.4%) | 518 (69.3%) | |
| Purpose of travel* | P < 0.0001 | ||||
| Migration | 486 (33.2%) | 1 (0.2%) | 1 (0.4%) | 484 (64.7%) | |
| Missionary/humanitarian | 171 (11.7%) | 49 (10.1%) | 106 (45.9%) | 16 (2.1%) | |
| Tourist | 343 (23.4%) | 326 (67.1%) | 4 (1.7%) | 13 (1.7%) | |
| Visiting friends/relatives | 191 (13.0%) | 11 (2.3%) | 3 (1.3%) | 177 (23.7%) | |
| Symptoms | P = 0.0001 | ||||
| Yes | 842 (57.5%) | 294 (60.5%) | 87 (37.7%) | 461 (61.6%) | |
| Asymptomatic/not reported | 623 (42.5%) | 192 (39.5%) | 144 (62.3%) | 287 (38.4%) | |
IQR = interquartile range.
Not all categories listed.
Time of presentation.
During the study period of 14 years, a median of 120.5 patients (IQR25 68.25, IQR75 134.5 patients) presented each year. The overall number of presenting patients was stable without any significant trend. Data on the time to presentation to a health-care center after leaving schistosomiasis endemic areas are given in Table 2: 53.2% of patients presented within 3 months and 46.8% in > 3 months after leaving endemic areas. Patients who had obtained pretravel advice presented more likely within 3 months (P < 0.0001). Europeans and expatriates presented earlier than non-Europeans.
Table 2.
Time to presentation for all patients
| Time to presentation < 3 months | Time to presentation > 3 months | Pearson χ2 test | |
|---|---|---|---|
| N = 713 (53.2%)* | N = 628 (46.8%)* | ||
| Patient origin | |||
| European | 255 (57%) | 192 (43%) | P < 0.0001 |
| Expatriate | 177 (81.9%) | 39 (18.1%) | |
| Non-European | 281 (41.5%) | 397 (58.5%) | |
| Purpose of travel† | |||
| Tourist | 183 (59.8%) | 123 (40.2%) | P < 0.0001 |
| VFR | 83 (52.2%) | 76 (47.8%) | |
| Migration | 162 (35.2%) | 298 (64.8%) | |
| Missionary/humanitarian | 110 (69.2%) | 49 (30.2%) | |
| Pretravel advice obtained | |||
| Yes | 173 (68.4%) | 80 (31.6%) | P < 0.0001 |
VFR = visiting friends and relatives.
Information on 246 cases was not given.
Not all categories listed.
Species of schistosomiasis.
Species information was available in 898/1,465 (61%) of cases. Urogenital schistosomiasis due to Schistosoma haematobium was diagnosed in 22% of cases (318/1,465). Among these, 63.8% (203/318) were found in non-Europeans, 4.7% (15/318) among expatriates, and 31.5% (100/318) among Europeans. Intestinal schistosomiasis due to Schistosoma mansoni was diagnosed in 39% of cases (570/1,465). Among those, 61% (348/570) were found among non-Europeans, 23% (131/570) among expatriates, and 16% (91/570) among Europeans. Five cases of Schistosoma japonicum were identified (two non-Europeans, two expatriates, and one European), one confirmed infection with Schistosoma intercalatum (an immigrant from Cameroon), and four cases of Schistosoma mekongi (all among European tourists returning from travels from southeast Asia). Double infections were reported in seven cases; these patients were all infected with S. mansoni and S. haematobium, both species were detected via microscopy. Of 1,465 cases, 567 were not further classified or reported on a species level (39%).
Place of infection and Schistosoma species.
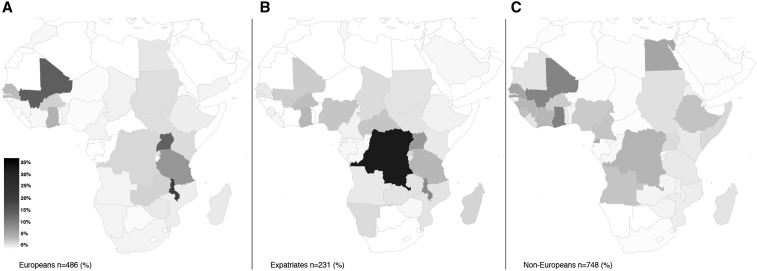
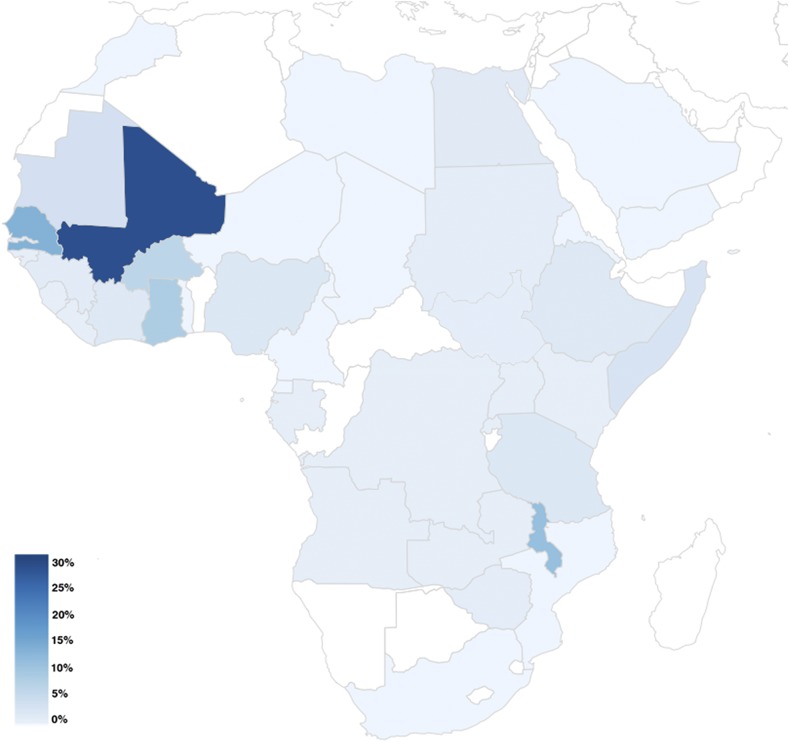
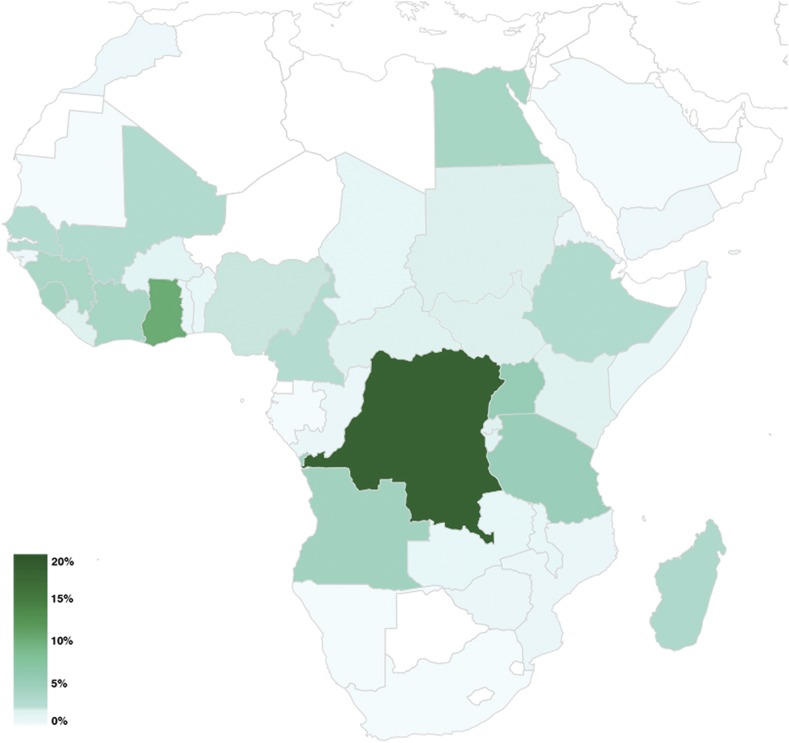
Most infections were acquired in Africa (N = 1,405, 95%) followed by South America (N = 21, 1.4%) and southeast Asia (N = 21, 1.4%). Table 3 shows the regions of infection. Figure 1 illustrates the geographical distribution of the origin of all schistosomiasis infections by patient type: 99% of infections with S. haematobium were acquired in Africa (315/318 cases) (Figure 2); 67% (N = 216) of cases were imported from west Africa with most cases originating from Mali (N = 86), Senegal (N = 41), and Ghana (N = 26); and 23% (N = 74) of cases were acquired in east Africa, where most cases were imported from Malawi (N = 34) mainly by tourist travelers. Intestinal schistosomiasis due to S. mansoni was primarily imported from Africa in 96% of cases (547/570), followed by South America with 2.6% of cases (15/570) and the Caribbean/Central America with 0.7% of cases (4/570). On the African continent, most infections occurred in west Africa (36%) followed by Central Africa (28%) and east Africa (27%) (Figure 3).
Table 3.
Region of infection by patient type
| Region of infection | Total | European | Expatriate | Non-European |
|---|---|---|---|---|
| Africa, not further definition | 15 (1%) | 9 (0.6%) | 4 (0.3%) | 2 (0.1%) |
| Africa, Central | 245 (16.7%) | 21 (1.4%) | 92 (6.3%) | 132 (9%) |
| Africa, East | 490 (33.4%) | 261 (17.8%) | 82 (5.6%) | 147 (10%) |
| Africa, Northern | 59 (4%) | 10 (0.7%) | 1 (< 0.1%) | 48 (3.3%) |
| Africa, Southern | 15 (1%) | 9 (0.6%) | 5 (0.3%) | 1 (< 0.1%) |
| Africa, Western | 581 (40%) | 144 (10%) | 41 (3%) | 396 (27%) |
| America, Central* | 2 (0.1%) | 2 (0.1%) | 0 | 0 |
| America, South | 21 (1.4%) | 8 (0.6%) | 1 (< 0.1%) | 12 (0.8%) |
| Asia, East | 2 (0.1%) | 1 (< 0.1%) | 1 (< 0.1%) | 0 |
| Asia, South | 4 (0.2%) | 2 (0.1%) | 0 | 2 (0.1%) |
| Asia, Southeast | 21 (1.4%) | 13 (0.9%) | 3 (0.2%) | 5 (0.3%) |
| Asia, West | 7 (0.5%) | 3 (0.2%) | 1 (< 0.1%) | 3 (0.2%) |
| Caribbean | 2 (0.1%) | 2 (0.1%) | 0 | 0 |
| Unknown | 1 (< 0.1%) | 1 (< 0.1%) | 0 | 0 |
Information as given by travellers. Since Schistosomiasis is not endemic in Central America, the infection may have been acquired in other countries visited before.
Figure 1.
Geographical distribution of all reported schistosomiasis infections by patient type: (A) European patients, (B) expatriate patients, and (C) non-European patients; gray scale in percent of all infections on the African continent among each patient group.
Figure 2.
Infections due to Schistosoma haematobium on the African continent. Geographical distribution of all S. haematobium infections; color scale in percent of N = 318 infections. This figure appears in color at www.ajtmh.org.
Figure 3.
Infections due to Schistosoma mansoni on the African continent. Geographical distribution of all S. mansoni infections; color scale in percent of N = 570 infections. This figure appears in color at www.ajtmh.org.
In 188 cases (all species), information on the exact places of infection was given: the five most frequently named were Lake Victoria (N = 38), Lake Malawi (N = 37), and lakes near Lubumbashi (Democratic Republic of the Congo) (N = 23), river Nile (N = 19), and Lake Kivu (N = 19).
Symptoms of schistosomiasis.
Table 4 gives an overview of reported symptoms: 57.5% of patients presented with clinical symptoms. Acute/subacute presentation of schistosomiasis, as defined earlier, was significantly more frequent in European travelers (N = 167, 34%) than in expatriates (N = 53, 23%; P = 0.0019) or non-Europeans (N = 171, 23%; P < 0.0001). Fever and acute/subacute symptoms in general were associated with early presentation within 3 months (P < 0.0001 and P = 0.008, respectively).
Table 4.
Symptoms by patient type
| Acute symptoms among all cases of schistosomiasis (all species N = 1,465) | |||||
|---|---|---|---|---|---|
| Acute symptoms (not all listed) | Total (%) N = 1,465 | European (%) N = 486 | Expatriates (%) N = 231 | Non-Europeans (%) N = 748 | Pearson χ2 test |
| Fever | 175 (12%) | 101 (21%) | 22 (10%) | 52 (7%) | P < 0.0001 |
| Skin symptoms | 99 (7%) | 46 (9%) | 7 (3%) | 46 (6%) | P = 0.0037 |
| Respiratory symptoms | 61 (4%) | 37 (8%) | 10 (4%) | 14 (2%) | P < 0.0001 |
| Musculoskeletal symptoms | 77 (5%) | 46 (9%) | 6 (3%) | 25 (3%) | P < 0.0001 |
| Headache | 79 (5%) | 34 (7%) | 10 (4%) | 35 (5%) | P = 0.15 |
| Fatigue | 129 (9%) | 51 (10%) | 29 (13%) | 49 (7%) | P = 0.005 |
| Vomiting | 32 (2%) | 9 (2%) | 1 (< 1%) | 22 (3%) | P = 0.024†, P = 0.26‡, P = 0.18§ |
| Diarrhea | 148 (10%) | 67 (14%) | 18 (8%) | 63 (8%) | P = 0.0042 |
| Abdominal pain | 131 (9%) | 27 (6%) | 9 (4%) | 95 (13%) | P < 0.0001 |
| Neurological symptoms | 20 (1%) | 5 (1%) | 5 (2%) | 10 (1%) | P = 0.4 |
| All acute/subacute symptoms (as defined earlier) | 391 (27%) | 167 (34%) | 53 (23%) | 171 (23%) | P < 0.0001 |
| Chronic symptoms in cases of schistosomiasis urogenitalis (Schistosoma haematobium, N = 318) | |||||
| Specific chronic symptoms | Total N = 318 | European (%) N = 100 | Expatriates (%) N = 15 | Non-Europeans (%) N = 203 | Pearson χ2 test |
| Chronic/urogenital symptoms | 166 (52%) | 40 (40%) | 3 (20%) | 123 (61%) | *P = 0.0026†, P = 0.0009‡, P = 0.16§ |
| Chronic symptoms in cases of hepato-intestinal schistosomiasis (Schistosoma mansoni, N = 570) | |||||
| Specific chronic symptoms | Total N = 570 | European (%) N = 91 | Expatriates (%) N = 131 | Non-Europeans (%) N = 348 | Pearson χ2 test |
| Overall chronic symptoms | 66 (12%) | 8 (9%) | 9 (7%) | 49 (14%) | P = 0.059 |
| Hepato-/splenomegaly | 14 (2%) | 2 (2%) | 4 (3%) | 8 (2%) | P = 0.74†, P = 1‡, P = 1§ |
| Hematochezia, melena | 9 (1.6%) | 2 (2%) | 0 | 7 (2%) | statistical testing not relevant/significant |
| Weight loss | 18 (3%) | 0 | 0 | 18 (5%) | statistical testing not relevant/significant |
Fisher’s exact test applied.
Expatriate versus non-European.
European versus non-European.
Expatriate versus European.
In the subgroup of patients with urogenital schistosomiasis, non-Europeans presented in 61% (N = 123) of cases with chronic or urogenital symptoms, which was significantly higher than that of among Europeans (N = 40, 40%; P = 0.0007). Patients with intestinal schistosomiasis (S. mansoni) presented with symptoms in 57% of cases (323/570). Chronic symptoms were seen among non-Europeans in 14% (49/348), among expatriates in 7% (9/131), and among Europeans in 9% of cases (8/91). Spleno- and/or hepatomegaly were reported in 14 patients, two of whom were Europeans traveling as tourists, four expatriates, and eight non-Europeans.
Diagnosis of schistosomiasis.
Direct pathogen detection was reported in 43.4% of cases (635/1,465). Rates among non-Europeans (55.5%) were higher than among Europeans (24.7%) and expatriates (43.3%). A positive serologic test was used for diagnosis in 29.3% of cases (430/1,465) and antigen testing was used in 5.4% of cases (79/1,465). Use of PCR (sample type not reported) for diagnosis was reported in one case.
Asymptomatic patients (N = 623) were diagnosed via microscopy in 47.4% of cases (295/623) and in 39% of cases (243/623) via serology or antigen testing. Asymptomatic non-Europeans were more often diagnosed via microscopy in 62.4% of cases (179/287) than with serology or antigen testing (29.3%, 84/287). Likewise, the majority of asymptomatic expatriates had positive microscopy in 52.1% (75/144). In contrast, asymptomatic Europeans received the diagnosis more often via serology or antigen testing in 57.8% of cases (111/192) than with microscopy, which was positive in 21.3% of cases (41/192).
Treatment and hospitalization of travelers with schistosomiasis.
Of 1,465 patients, 15.7% (230/1,465) were hospitalized due to schistosomiasis. Duration of hospitalization ranged from 1 to 90 days (median: 5 days). Of hospitalized cases, 63.4% (N = 146) were attributable to S. mansoni and 19.1% (N = 44) to S. haematobium. Among hospitalized S. mansoni patients, 65% presented with acute symptoms or abdominal pain. In contrast, hospitalized patients with S. haematobium infection presented with acute symptoms in only 34% of cases but in 59% with chronic symptoms such as hematuria.
Praziquantel was used in all patients for whom treatment data were available (N = 1385); glucocorticoids were used in 21 patients. No deaths due to schistosomiasis were reported.
DISCUSSION
This TropNet study examined patients presenting with schistosomiasis after leaving schistosomiasis-endemic countries either because of travel or migration to Europe. This study is the largest database of human schistosomiasis cases outside endemic areas to date, providing detailed data on epidemiology and clinical features of patients with schistosomiasis. This analysis includes and analyzes the complete dataset of schistosomiasis patients from 1997 to 2010 recorded by TropNet. Parts of this data were published earlier as sentinel data (1999–2001) and as a preliminary report only for the year 2007.12,13
Consistent with other studies,14,15 travelers and migrants in our study were young with an overall median age of 29. Interestingly, European travelers with schistosomiasis appear to be younger than the average short-term traveler population and travelers diagnosed with other infections. Median ages among other large traveler populations to Africa were found to range between 36 and 39 years.8,9 Younger patients may either be less informed or more willing to accept the risk of infection by swimming in freshwater. Another consistent observation in comparison to other studies14,15 is that men are almost twice as likely to be affected as women, regardless of the type of travel.
Not surprisingly, pretravel advice was obtained only by a minority of travelers. Rates among expatriates were however unexpectedly low, as these individuals usually receive a pretravel consultation. European travelers showed the highest rate of pretravel consultations, although it was obtained only in 41% of cases. This is below reported rates of over 50% among short-term travelers in the 2010 EuroTravNet Study, which included returning travelers and migrants in Europe.8 Interestingly, only 2% (4/177) of non-European VFR travelers diagnosed with schistosomiasis obtained pretravel advice. This rate is also lower than the reported rate of 25% among VFRs in the 2010 study.8 Furthermore, another study investigating returning VFRs showed a pretravel advice rate of 16% among non-European VFRs.16
Positive associations of pretravel advice were previously shown to improve presentation rates within 6 months regardless of symptoms in schistosomiasis patients.14 A finding which we confirm in our data, as pre-travel advice led to higher presentation rates within the first 3 months after return. It is evident that rates of pretravel consultation among all patient groups are unsatisfactory and efforts need to be intensified to counsel all travelers on risks and prevention of schistosomiasis infection, in particular among VFR travelers.
In line with previous reports,14,15,17–19 most cases (95%) of schistosomiasis were acquired on the African continent. Our study provides detailed information on regional distribution of schistosomiasis infections among travelers and migrants adding valuable information to previous studies,14,15 which provided epidemiological data with differentiation of the schistosomiasis species but less specification on the traveler type.
The high numbers of infections among Europeans in East Africa most likely reflects frequent travel to the developed tourist infrastructure in this region. Swimming in freshwaters of the East African great lakes area including Lake Malawi or Lake Victoria remains very attractive to tourists, especially since some local tour operators deny a risk of schistosomiasis transmission. The risk of acquiring schistosomiasis may also be taken deliberately.20 In West Africa, the Dogon county in Mali is a popular tourist destination and is another focus for schistosomiasis infections among travelers as reported by several other outbreak reports and case series. Furthermore, clusters of cases often are reported among groups of travelers to these regions.21–23 Visser and others described the type of schistosomiasis patient to Mali as “uninformed and inexperienced” or “the more adventurous traveller.”21 The west African tourist in our study obtained pretravel advice with lower probability contrast to tourists visiting East Africa (P = 0.009).
High numbers of infections among expatriates in our study imply that they take the risk of swimming in freshwater despite knowing the risk of infection. In line with our data, volunteers and aid workers were shown to have a 2-fold higher risk for schistosomiasis compared with other travellers.14 Expatriates tend to adopt local habits and have repeated exposures. Young children of expatriates are particularly at risk of acquiring continuous reinfection.1,24
Fifty seven and a half percent of patients presented with symptoms, whereas 42.5% of patients were asymptomatic or without reported symptoms. Previous studies in similar populations report rates of asymptomatic patients between 36% and 50%.14,15,19 Many asymptomatic patients are diagnosed when attending a posttravel medical checkup. Other individuals may be worried about an infection acquired in the tropics and present to travel clinics regardless of symptoms.19 Thus, the number of unreported cases or undiagnosed cases may even be higher. A recent small study for example suggests a large reservoir of untreated schistosomiasis among immigrants from endemic countries.25 We show high rates of asymptomatic presentation (62%) among expatriates indicating that medical consultations after returning from assignments in tropical countries are useful for detection of asymptomatic schistosomiasis. Whitty and others point out that targeted laboratory tests including blood count, serology plus microscopy yield good detection rates in asymptomatic travellers.19 Our data show high detection rates via microscopy among asymptomatic patients, especially in patients originating from endemic regions or long-term visitors. Among European short-term travellers, serology more often led to diagnosis, indicating a low parasitic burden in travelers after a single exposure.
Presentation of symptomatic patients differed between the patient groups. Europeans presented more likely because of fever and musculoskeletal symptoms. Previous studies likewise describe fever as the major symptom of acute schistosomiasis.14,17,18 Fatigue or general tiredness reported in earlier studies15,19 as a major symptom in up to 26% of cases was however less frequent in our study population (9%). Clinical presentation of non-Europeans occurred more often because of symptoms indicative for chronic schistosomiasis such as hematuria, bloody diarrhea, or weight loss.
Microscopy and serology remain the central diagnostic methods in schistosomiasis.1,26 Forty-three percent were diagnosed via microscopy, which is in line with other reports (22–45%).15,17–19 Five percent of cases were diagnosed via antigen testing and PCR was only used in one patient. Recently published studies demonstrate high sensitivity and specificity for PCR.26 Furthermore, this method can be used for pathogen detection in serum, feces and urine.27,28 This method may be highly suitable for diagnosing patients with acute as well as chronic infections.26 Other approaches such as high sensitive antigen assays have equally shown promising results.6,26 If PCR as a routine method is not available, a combination of full blood count, serology, and microscopy of stool and urine yields best diagnostic results.19
The retrospective study design has inherent limitations. First, bias could have occurred by the method of passive case finding, resulting in a relevant number of cases with no schistosomiasis species information. Second, a bias toward symptomatic individuals as well as patients with severe symptoms presenting to referral centers for tropical medicine must be assumed. Likewise, a bias due to different reporting practices and a potential preference in reporting of confirmed versus suspected cases could have occurred. Only one diagnostic method was reportable in the questionnaire, although several methods may have been used.
In conclusion, schistosomiasis remains a relevant infection in travelers and migrants to Europe. Most infections in Europeans occur in travelers visiting a small number of countries in west and east Africa. These travelers should be counseled intensively on the risk of schistosomiasis infection. Given the generally low rate of pretravel advice in all patient groups and its effectiveness in prevention of infection, the coverage of pretravel advice should be extended. Well-informed patients present faster to a travel clinic in case of symptoms. Swimming in freshwater was however not prevented by pretravel advice. Posttravel consultations particularly for returning expatriates are useful, given the high potential of microscopy and serology for detecting asymptomatic infections demonstrated in this report.
REFERENCES
- 1.Colley DG, Bustinduy AL, Secor WE, King CH, 2014. Human schistosomiasis. Lancet 383: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitsulo L, Engels D, Montresor A, Savioli L, 2000. The global status of schistosomiasis and its control. Acta Trop 77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, 2015. Weekly epidemiological record No. 5, Vol. 90. Geneva, Swizerland: World Health Organization, 25–32. [Google Scholar]
- 4.Clerinx J, Van Gompel A, 2011. Schistosomiasis in travellers and migrants. Travel Med Infect Dis 9: 6–24. [DOI] [PubMed] [Google Scholar]
- 5.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP, 2007. Katayama syndrome. Lancet Infect Dis 7: 218–224. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhao J, Wang X, Xu X, Pan W, 2015. Evaluation of six novel antigens as potential biomarkers for the early immunodiagnosis of schistosomiasis. Parasit Vectors 8: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DMS, Secor WE, Polman K, van Lieshout L, 2015. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl Trop Dis 9: e0003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, et al., 2012. Infectious diseases among travellers and migrants in Europe, EuroTravNet 2010. Euro Surveill 17: pii: 20205. [PubMed] [Google Scholar]
- 9.Mendelson M, et al., 2014. Regional variation in travel-related illness acquired in Africa, March 1997–May 2011. Emerg Infect Dis 20: 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtfreter MC, Moné H, Müller-Stöver I, Mouahid G, Richter J, 2014. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Euro Surveill 19: pii: 20821. [DOI] [PubMed] [Google Scholar]
- 11.Berry A, et al., 2014. Schistosomiasis haematobium, Corsica, France. Emerg Infect Dis 20: 1595–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobusch MP, et al., 2003. Imported schistosomiasis in Europe: sentinel surveillance data from TropNetEurop. J Travel Med 10: 164–169. [DOI] [PubMed] [Google Scholar]
- 13.Jelinek TEuropean Network on Imported Infectious Disease Surveillance, 2008. Imported schistosomiasis in Europe: preliminary data for 2007 from TropNetEurop. Euro Surveill 13: pii: 8038. [DOI] [PubMed] [Google Scholar]
- 14.Nicolls DJ, Weld LH, Schwartz E, Reed C, von Sonnenburg F, Freedman DO, Kozarsky PE; GeoSentinel Surveillance Network, 2008. Characteristics of schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am J Trop Med Hyg 79: 729–734. [PubMed] [Google Scholar]
- 15.Coltart CEM, Chew A, Storrar N, Armstrong M, Suff N, Morris L, Chiodini PL, Whitty CJM, 2015. Schistosomiasis presenting in travellers: a 15 year observational study at the Hospital for Tropical Diseases, London. Trans R Soc Trop Med Hyg 109: 214–220. [DOI] [PubMed] [Google Scholar]
- 16.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J; GeoSentinel Surveillance Network, 2006. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis 43: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 17.Bierman WFW, Wetsteyn JCFM, van Gool T, 2005. Presentation and diagnosis of imported schistosomiasis: relevance of eosinophilia, microscopy for ova, and serology. J Travel Med 12: 9–13. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer E, Artom G, Marva E, Assous MV, Rahav G, Schwartzt E, 2006. Schistosomiasis among travelers: new aspects of an old disease. Emerg Infect Dis 12: 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitty CJ, Mabey DC, Armstrong M, Wright SG, Chiodini PL, 2000. Presentation and outcome of 1107 cases of schistosomiasis from Africa diagnosed in a non-endemic country. Trans R Soc Trop Med Hyg 94: 531–534. [DOI] [PubMed] [Google Scholar]
- 20.Rochat L, Bizzini A, Senn N, Bochud P-Y, Genton B, de Vallière S, 2015. Acute schistosomiasis: a risk underestimated by travelers and a diagnosis frequently missed by general practitioners-a cluster analysis of 42 travelers. J Travel Med 22: 168–173. [DOI] [PubMed] [Google Scholar]
- 21.Visser LG, Polderman AM, Stuiver PC, 1995. Outbreak of schistosomiasis among travelers returning from Mali, West Africa. Clin Infect Dis 20: 280–285. [DOI] [PubMed] [Google Scholar]
- 22.Corachán M, Ruiz L, Valls ME, Gascon J, 1992. Schistosomiasis and the Dogon country (Mali). Am J Trop Med Hyg 47: 6–9. [DOI] [PubMed] [Google Scholar]
- 23.Colebunders R, Verstraeten T, Van Gompel A, Van den Ende J, De Roo A, Polderman A, Visser L, 1995. Acute schistosomiasis in travelers returning from Mali. J Travel Med 2: 235–238. [DOI] [PubMed] [Google Scholar]
- 24.Cetron MS, Chitsulo L, Sullivan JJ, Pilcher J, Wilson M, Noh J, Tsang VC, Hightower AW, Addiss DG, 1996. Schistosomiasis in Lake Malawi. Lancet 348: 1274–1278. [DOI] [PubMed] [Google Scholar]
- 25.Rapoport AB, McCormick D, Cohen PA, 2015. Screening for Schistosoma mansoni and Strongyloides stercoralis infection among Brazilian immigrants in the United States. Open Forum Infect Dis 2: ofv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S, 2015. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 21: 529–542. [DOI] [PubMed] [Google Scholar]
- 27.Cnops L, Soentjens P, Clerinx J, Van Esbroeck M, 2013. A Schistosoma haematobium-specific real-time PCR for diagnosis of urogenital schistosomiasis in serum samples of international travelers and migrants. PLoS Negl Trop Dis 7: e2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cnops L, Tannich E, Polman K, Clerinx J, Van Esbroeck M, 2012. Schistosoma real-time PCR as diagnostic tool for international travellers and migrants. Trop Med Int Health 17: 1208–1216. [DOI] [PubMed] [Google Scholar]