Abstract
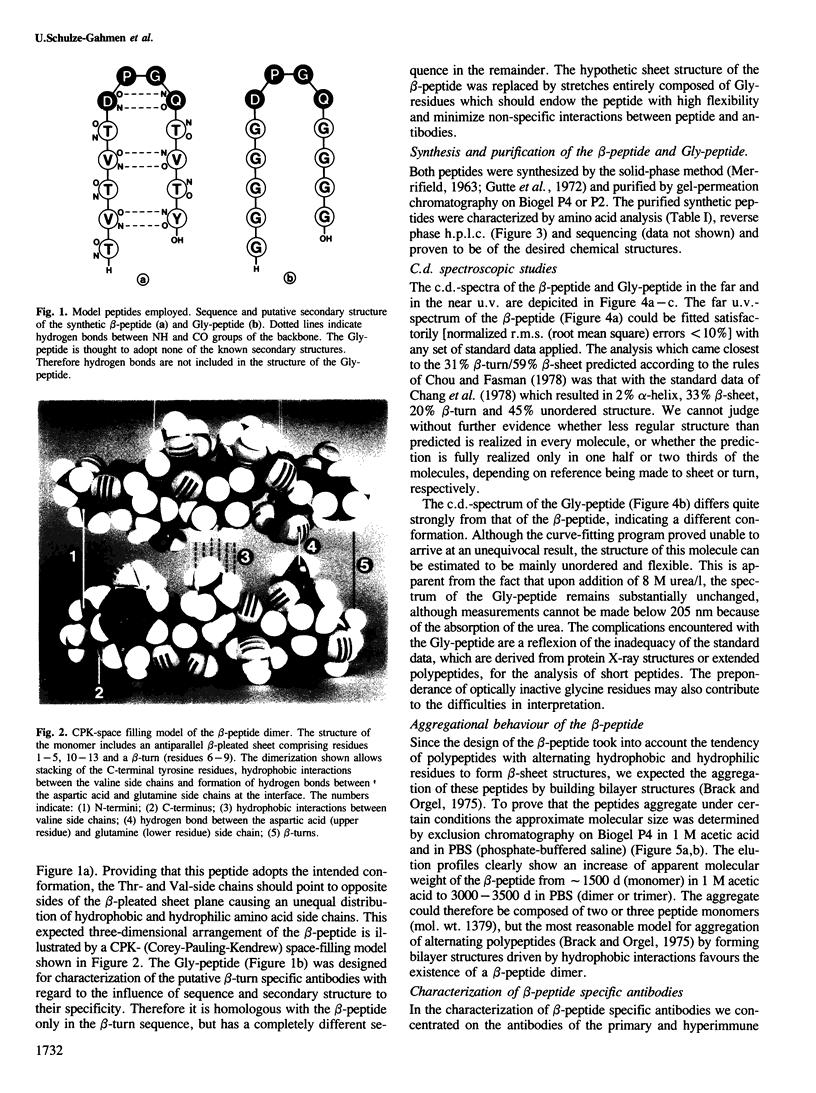
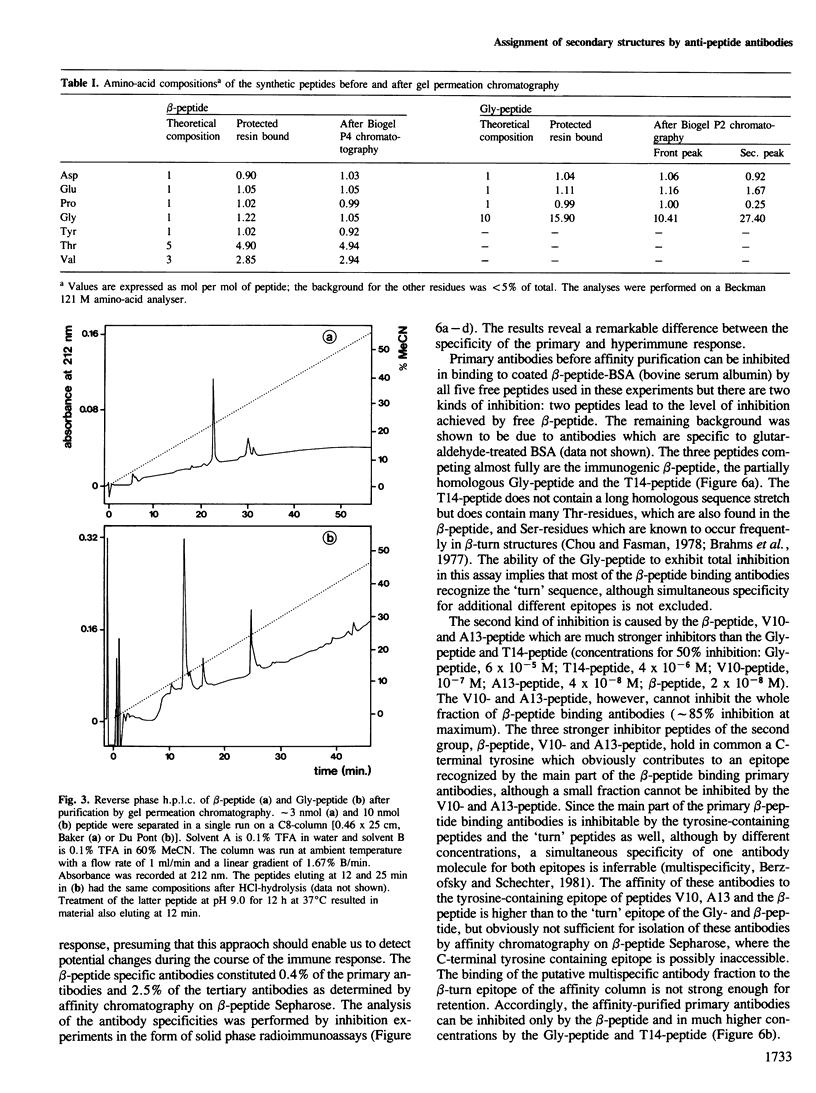
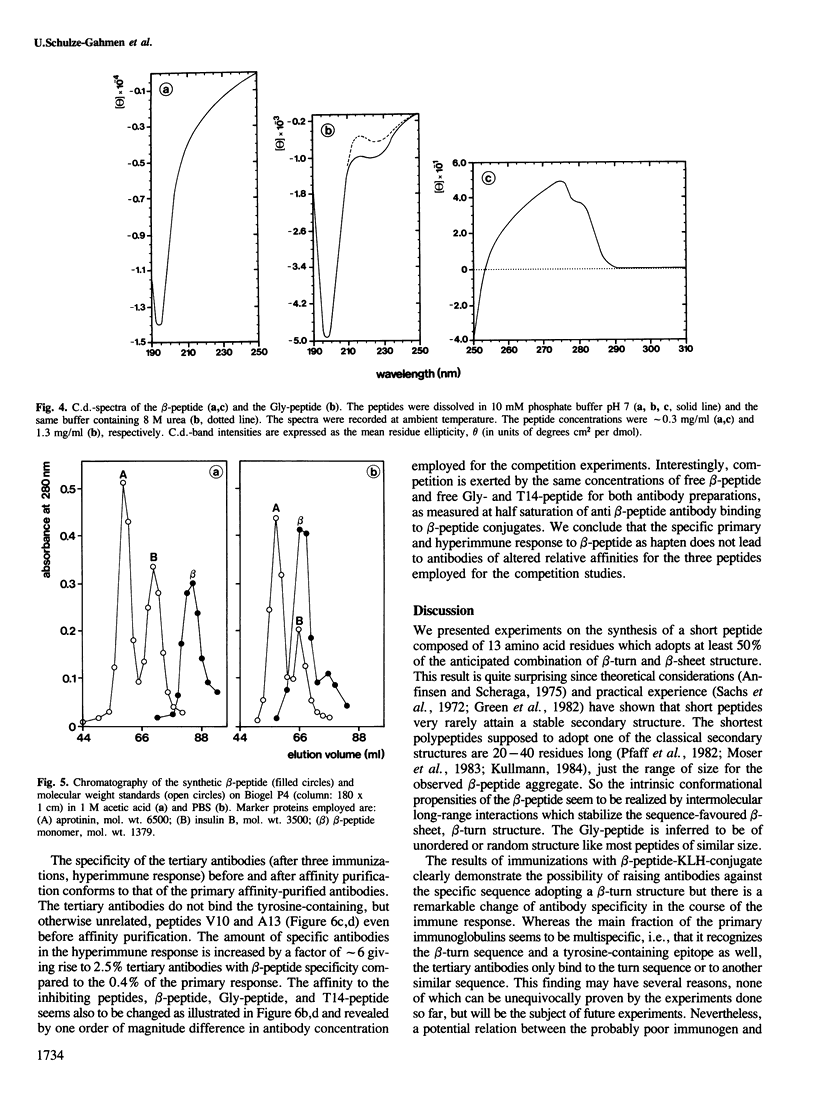
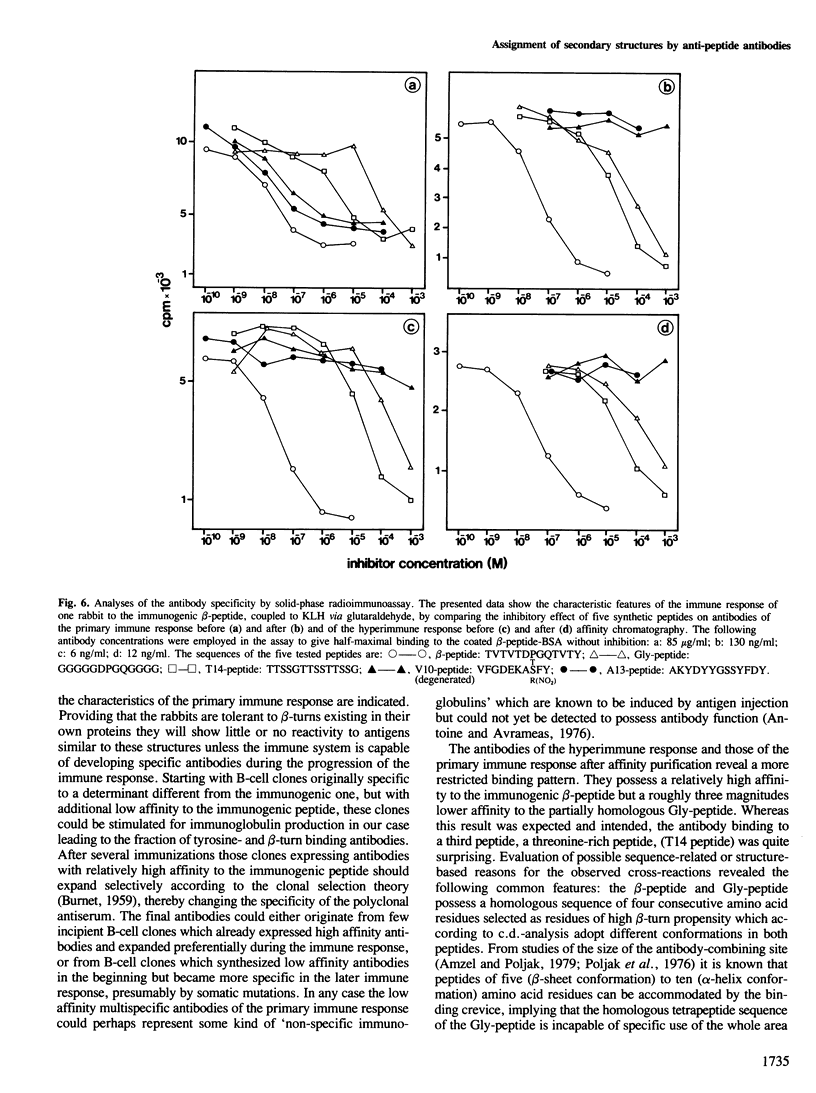
In an attempt to assign secondary structure elements to protein primary structures with antibodies, we synthesized a model peptide (beta-peptide: TVTVTDPGQTVTY) with a putative beta-turn structure and analysed the anti-peptide antibodies for their specificity towards the turn sequence. At least 50% of the peptide fraction adopts the intended conformation of a beta-turn (DPGQ) inserted between the two segments of an antiparallel beta-sheet structure. The specific anti-beta-peptide antibodies of the hyperimmune response bind the beta-turn containing epitope of the immunogenic beta-peptide with a three orders of magnitude higher affinity than the synthetic control peptide (Gly-peptide: GGGGGDPGQGGGG). The affinity of the antibodies with specificity for the beta-turn region increases from the primary to the hyperimmune response. Therefore, probing of secondary structure elements, i.e., of individual beta-turn regions, by anti-peptide antibodies now seems feasible for proteins of known sequence and may result in sequence assignments of secondary structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine J. C., Avrameas S. Correlations between immunoglobulin- and antibody-synthesizing cells during primary and secondary immune responses of rats immunized with peroxidase. Immunology. 1976 Apr;30(4):537–547. [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A., Schechter A. N. The concepts of crossreactivity and specificity in immunology. Mol Immunol. 1981 Aug;18(8):751–763. doi: 10.1016/0161-5890(81)90067-5. [DOI] [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Brack A., Orgel L. E. Beta structures of alternating polypeptides and their possible prebiotic significance. Nature. 1975 Jul 31;256(5516):383–387. doi: 10.1038/256383a0. [DOI] [PubMed] [Google Scholar]
- Brahms S., Brahms J. Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism. J Mol Biol. 1980 Apr;138(2):149–178. doi: 10.1016/0022-2836(80)90282-x. [DOI] [PubMed] [Google Scholar]
- Brahms S., Brahms J., Spach G., Brack A. Identification of beta,beta-turns and unordered conformations in polypeptide chains by vacuum ultraviolet circular dichroism. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3208–3212. doi: 10.1073/pnas.74.8.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Gutte B., Lin M. C., Caldi D. G., Merrifield R. B. Reactivation of des(119-, 120-, or 121-124) ribonuclease A by mixture with synthetic COOH-terminal peptides of varying lengths. J Biol Chem. 1972 Aug 10;247(15):4763–4767. [PubMed] [Google Scholar]
- Gutte B., Merrifield R. B. The synthesis of ribonuclease A. J Biol Chem. 1971 Mar 25;246(6):1922–1941. [PubMed] [Google Scholar]
- Kullmann W. Design, synthesis, and binding characteristics of an opiate receptor mimetic peptide. J Med Chem. 1984 Feb;27(2):106–115. doi: 10.1021/jm00368a002. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLEY A., SAHA A., HALLIDAY W. J. IMMUNOCHEMICAL STUDIES OF HEMOCYANIN FROM THE GIANT KEYHOLE LIMPET (MEGATHURA CRENULATA) AND THE HORSESHOE CRAB (LIMULUS POLYHEMUS). J Immunol. 1965 Jul;95:141–147. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Chen B. L., Chiu Y. Y., Phizackerley R. P., Saul F., Ysern X. Three-dimensional structure and diversity of immunoglobulins. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):639–645. doi: 10.1101/sqb.1977.041.01.073. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Wetlaufer D. B. The number of turns in globular proteins. Nature. 1977 Aug 25;268(5622):769–770. doi: 10.1038/268769a0. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Sequential resonance assignments in protein 1H nuclear magnetic resonance spectra. Basic pancreatic trypsin inhibitor. J Mol Biol. 1982 Mar 5;155(3):347–366. doi: 10.1016/0022-2836(82)90009-2. [DOI] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]



