Abstract.
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel phlebovirus that was identified to be the etiological pathogen of the emerging infectious disease, severe fever with thrombocytopenia syndrome (SFTS). SFTSV could be transmitted through tick bite. Transmission of SFTSV among humans has also been reported mainly through direct blood contact. In July 2014, a cluster of six suspected SFTS cases occurred in Shandong Province, China. In this cluster, both symptomatic and asymptomatic persons were included. By analyzing the clinical data and results of laboratory tests, and conducting the epidemiological interviews with the cases and their families, risk factors responsible for the transmission were evaluated. The findings suggested that SFTSV transmission among humans may cause asymptomatic infection via personal contact without blood exposure.
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) was firstly described in central China in 2009, which posed threat to public health as the fatality rate was initially reported to be up to 30%.1 Severe fever with thrombocytopenia syndrome virus (SFTSV) was identified to be the etiological agent of the disease.1 It has three segments of single-stranded negative sense RNA (the S, M, and L segments), and is classified into the genus Phlebovirus, the family Phenuiviridae.1 SFTSV could be transmitted to humans by ticks via blood feeding. Some patients reported a history of tick bite before illness onset,1,2 and SFTSV RNA was detected in ticks like Haemaphysalis longicornis and Rhipicephalus microplus.3,4 Additionally, clusters caused by the transmission of SFTSV among humans has been described from 2007 to 2015 in the epidemic provinces in China, including Henan,5 Jiangsu,6 Liaoning,7 Shandong,2,8,9 Anhui,10 and Hubei,11 as well as in South Korea.12,13 It is important to investigate the symptomatic SFTSV infections of both fatal and nonfatal patients in the clusters which provided better understanding of the clinical course of SFTS disease and thus guide the treatment of SFTS. Furthermore, the potential contacts responsible for person-to-person transmission of SFTSV among the patients were evaluated. The major risk factor was direct blood exposure without personal protection equipment.2,5–11,13 Other possible contacts such as urine, feces, and respiratory secretions were evaluated to be not associated significantly with person-to-person transmission.2,5 In recent years, asymptomatic infections have been noted in a few cluster reports of SFTSV infection,10,12,13 suggesting that SFTSV infection could be asymptomatic via person-to-person transmission, which, however, needs additional attention.
In this study, we report a cluster of six cases of SFTSV infection occurred in Shandong Province, China, in July 2014. The cluster included the deceased index patient, two secondary patients reported direct blood exposure, and three asymptomatic persons who had close contact with secondary patients but had no history of blood exposure or tick bite. By analyzing the clinical and laboratory evidence, and performing epidemiological interviews, we confirmed that SFTSV was transmitted from the index patient to the secondary patients via direct blood exposure, and found that the asymptomatic persons had recent SFTSV infection most likely due to the close contact with the body fluids and mucosa of the secondary patients. Therefore, the results showed that SFTSV could be transmitted among humans through routes besides direct blood contact and that SFTSV could induce asymptomatic infection through person-to-person transmission.
MATERIALS AND METHODS
Patients and clinical samples.
In July 2014, a cluster of six suspected SFTS cases occurred in Shandong Province. The index patient (case A) was a 65-year-old woman who experienced a sudden onset of fever (39.5°C), fatigue, diarrhea, vomiting and nausea on July 6, 2014, and died on July 14 in the evening after she was back home. Two secondary patients were A’s son (case B) and son-in-law (case C). They had illness onset 6–7 days after A’s death. Case B died on July 31, and case C survived and was discharged on August 3. Three persons reported close contact with the secondary patients, including C’s daughter (case D), B’s neighbor (case E), and a male doctor who had contact with both cases B and C (case F). None of them developed disease.
Due to the lack of a serum sample from case A, the blood smear was used for reverse transcription polymerase chain reaction (RT-PCR) detection. For the other five cases, the sera samples were collected on July 30.
Epidemiological investigations.
Epidemiological investigations were conducted to inquire information including the living surroundings, history of tick bites and animal contacts, the routes of possible exposure to the risk factors, and the use of protective devices as well as protective behaviors.
Laboratory tests and virus isolation.
Total RNAs were purified from the serum smear from case A and the sera samples of other five cases using TRIzol (Invitrogen, Carlsbad, CA), and were subjected to reverse transcription using a Moloney murine leukemia virus reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Nested PCR was carried out with cDNA from each sample and primers specific for the SFTSV S, M, and L segments (Supplemental Table 1) using a Tsingke master mix (Tsingke biological technology, Beijing, China) in the conditions of one cycle of 95°C for 5 minutes, followed by 30 cycles of 95°C for 30 seconds, 59°C annealing for 30 seconds, and 72°C extension for 40 seconds, and then a final 72°C for 5 minutes. PCR products were sequenced by Sanger sequencing.
Sera RT-PCR positive for SFTSV RNA were incubated with DH82 cells (from American Type Culture Collection, CRL-10389) for virus isolation as previously described.1,14 Isolated virus was identified by indirect immunofluorescences assay (IFA) and RT-PCR tests. The complete genome of the isolated virus was sequenced and deposited in GenBank (accession numbers: KR706565, KR706566, and KR706567 for S, M, and L segments, respectively). SFTSV-specific serological tests were performed to detect anti-SFTSV IgG and IgM antibodies. IFA was performed as described with modifications.15 The fixed and permeabilized infected cells were blocked and then incubated with serum samples from cases of the cluster (1:100 dilution), anti-SFTSV nucleoprotein (NP) (see Supplemental methods) or nonstructural protein (NSs) polyclonal antibodies (1:500 dilution).16 The fluorescein isothiocyanate–conjugated anti-human IgM or IgG antibodies (Abcam, Cambridge, United Kingdom), or anti-rabbit IgG antibodies (Abcam) were added as the secondary antibody. Cellular enzyme-linked immunosorbent assay (cELISA) using the infected cells as antigens was performed to determine the antibody titers from each serum sample. Vero cells seeded in 96-well plates (Cell-nest, Wuxi, China) were infected with SFTSV at a multicity of infection of 5 TCID50 unites per cell. On 4 days postinfection, cells were fixed, permeabilized, and blocked as described.17 The infected cells were then incubated with the 2-fold serially diluted primary antibodies at 37°C for 1 hour. Anti-human IgM or IgG conjugates (Abcam) were added and incubated at 37°C for 1 hour. The optical density (OD) was measured at both 450 nm and 630 nm. Results are shown as the reciprocal of the dilution resulting in an OD equal to two times the mean of the background of the assay. Microneutralization assay (MNA) was performed as described.8
Bio-information analysis.
The complete RNA genome of the isolated virus was sequenced. The genomic segments of the isolated virus were aligned with the sequences of RT-PCR products from the sera of other cases using MegAlign (version 7.1.0) in DNAStar (Madison, WI) and edited with Genedoc software. Phylogenetic trees were constructed by Mega 5.0 using the maximum likehood method and tested by Bootstrap method with a value of 1,000.
Ethics statement.
All participants provided written consent for anonymous use of their specimens and clinical information for research. The ethics review boards of all participating institutions approved this study.
RESULTS
Clinical characterization of the index patient and secondary patients.
The clinical data of cases A, B, and C in the admission days were summarized in Table 1 and Supplemental Table 2. Case A had severe leukopenia and thrombocytopenia on the first day of hospitalization. Routine urine tests revealed high levels of proteinuria (2+) and hematuria (3+). On the next day before her death, case A had significantly elevated liver-associated enzyme levels and myocardial enzyme levels, indicating severe multiple organism damage (MOD) according to the definition of this syndrome.18,19 Case B also had leukopenia and thrombocytopenia. With supportive therapy and infusion with blood platelets (PLTs) and human granulocyte colony-stimulating factor (G-CSF), the white blood cells (WBCs) and neutrophil counts increased; the lymphocyte and monocyte counts remained at a normal level; and the eosinophil and PLT counts were at an extremely low level (Supplemental Table 2). His condition worsened as the biochemistry parameters continued to deviate significantly from the normal range (Supplemental Table 2). On the 10 day after disease onset, case B had proteinuria (Table 1) and a high level of liver-associated enzymes and myocardial enzymes (Supplemental Table 2). And he died in the afternoon. Case C had a normal WBC count, but had thrombocytopenia when transferred to the hospital on the 6 day postonset of illness (Table 1). Case C’s condition improved with the infusion of PLT and G-CSF, and other supportive care measures. The blood cell counts returned to almost within the normal range on the 9 day, except that the PLT count was still lower than normal (Supplemental Table 2). Blood chemistry testing showed that he had normal hepatic function after therapy (Supplemental Table 2), and had neither proteinuria nor hematuria (Table 1).
Table 1.
Personal information, potential contacts, and protective measures, clinical features, and laboratory tests of cases involved in the cluster
| Case A | Case B* | Case C | Case D | Case E | Case F | ||
|---|---|---|---|---|---|---|---|
| Personal information and relations | |||||||
| Age and gender | 65, female | 45, male | 40, male | 20, female | 40, female | 33, male | |
| Occupation | Farmer | Flower grower | Street cleaner | Office worker | Farmer | Doctor | |
| Relationship | N/A | A’s son | A’s son-in-law | C’s daughter | Caring for B | Doctor treating B and C | |
| Date of onset | July 6 | July 21 | July 22 | N/A | N/A | N/A | |
| Outcome | Fatal | Fatal | Survived | Asymptomatic | Asymptomatic | Asymptomatic | |
| Risk factors | |||||||
| Direct exposure | |||||||
| Blood | N/A | Yes | Yes | No | No | No | |
| Soiled coat | N/A | Yes | Yes | No | No | No | |
| Mucosa | N/A | Yes | Yes | Yes | Yes | Yes | |
| Body fluids | N/A | Yes | Yes | Yes | Yes | Yes | |
| Protective measures | |||||||
| Washing hands | N/A | No | Yes | No | No | No | |
| Glove | N/A | No | No | No | No | No | |
| Mask | N/A | No | No | No | No | Yes | |
| Clinical tests† | |||||||
| Blood counts | |||||||
| WBC (×109/L) | 1.83↓ | 3.91↓ | 4.51 | N/A | N/A | N/A | |
| PLT (×109/L) | 6↓ | 21↓ | 46↓ | N/A | N/A | N/A | |
| Urine routine test | |||||||
| Proteinuria | ++ | ++ | − | N/A | N/A | N/A | |
| Hematuria | +++ | − | − | N/A | N/A | N/A | |
| Serum biochemistry | |||||||
| ALT (U/L) | 1,018↑ | 290↑ | 28 | N/A | N/A | N/A | |
| AST (U/L) | 2,091↑ | 1,847↑ | 26 | N/A | N/A | N/A | |
| LDH (U/L) | 4,602↑ | 4,309↑ | 192 | N/A | N/A | N/A | |
| CK (U/L) | 15,268↑ | 9,912↑ | 36 | N/A | N/A | N/A | |
| CK-MB (U/L) | 360↑ | 110↑ | 10 | N/A | N/A | N/A | |
| Laboratory tests | |||||||
| RT-PCR | S | + | + | − | − | − | − |
| M | − | − | − | − | − | − | |
| L | + | + | − | − | + | + | |
| IgM | cELISA‡ | N/A | 512 | 256 | 512 | 2,048 | 1,024 |
| IFA | N/A | − | − | − | + | + | |
| IgG | cELISA‡ | N/A | 320 | 160 | − | − | − |
| IFA | N/A | + | − | − | − | − | |
| MNA | N/A | N/A | − | − | 80 | 40 | |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; cELISA = Cellular enzyme-linked immunosorbent assayCK = creatine kinase; CK-MB = creatine kinase-MB; IFA = Immunofluorescence assay; LDH = lactate dehydrogenase; MNA = microneutralization assay; N/A = not applicable PLT = platelet count; WBC = white blood cells. + = positive; − = negative. Clinical parameters below the normal range are shown in bold numbers with down arrows; and those over the normal range are shown in bold with up arrows.
Virus was isolated from the serum sample of case B collected on July 30.
Listed are the blood counts on the first day of admission and the serum biochemistry on the last day of admission.
The positive antibody titer was expressed as the reciprocal of the dilution at which the optical density value was three times higher than the negative controls.
Epidemiological investigation of the cases in the cluster.
The personal information and relationship of each case to others in the cluster are presented in Table 1.
The index patient (case A) is a female farmer 65 years of age. She had a sudden fever and gastrointestinal symptoms on July 6 and visited the village clinic on July 8. On July 13, her condition worsened, was transferred to municipal hospital A in the morning, and then to the Emergency Department of municipal hospital B in the afternoon. She went into shock and coma, and developed disseminated intravascular coagulation and MOD syndrome on July 14. Her relatives insisted on returning her back home. She had nasal and oral bleeding on the way back and died in the evening. She lived in a small village and mostly worked in the fields and hills near her house. Cattles and goats were grazing there, and ticks could be found on the animals. But she denied a history of tick bite within 2 weeks before illness onset.
The deceased secondary patient (case B) is a flower grower who resided in the same village as case A. The survived secondary patient (case C) is a street cleaner who lived in another village about 300 km away. Both patients had no history of tick bite and animal contact within 2 weeks before illness onset. On the way sending case A back home, their coats and faces were contaminated when case A was vomiting blood. Case B wiped blood off A’s mouth with his bare hands. Case C threw the bloody coat away, washed his hands, and took a shower. Case B was still wearing the bloody coat when taking care of case A. The two cases successively fell sick about 1 week after A’s death.
Three persons who reported very close contact with the secondary patients were interviewed. Case D is an office worker who lived in the same village with her father (case C) and cared for him for days when case C had illness onset. She had exposure to C’s skin, respiratory secretions, saliva, and urine. Case E visited case B in hospital to offer bedside care on July 25 and contacted B’s skin, saliva, and mucosa. Neither of them had protective precautions. Case F is the physician in the Infectious Department in Hospital B who touched cases B and C’s skin, oral cavity, and lymph node without wearing gloves during the physical examinations. None of them had direct blood contact with cases A, B, and C.
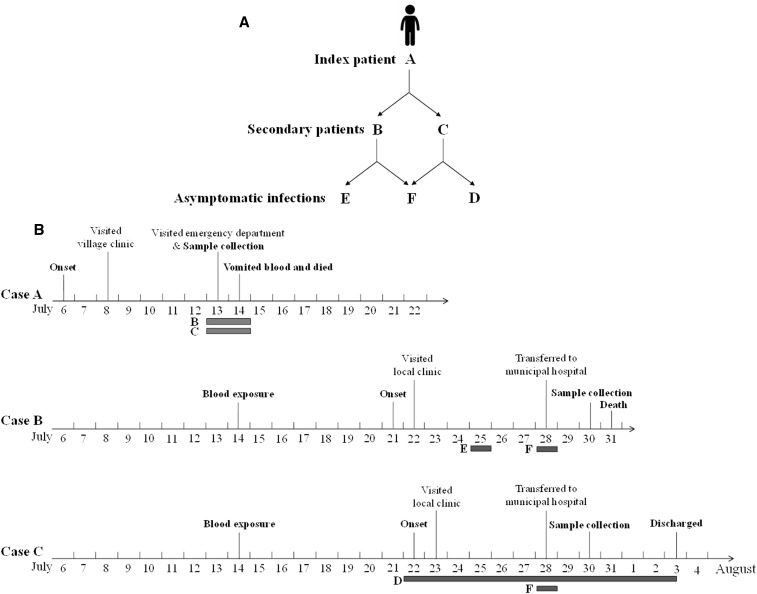
So the potential transmission routes among the cases in the cluster were from the index patient (case A) to the secondary patients (cases B and C), then from case B to cases E and F, and from case C to cases D and F (Figure 1A). The timeline with important events is presented in Figure 1B.
Figure 1.
Presentation of the epidemiological relationship of cases in the cluster and a timeline of important events. (A) The epidemiological relations and possible transmission routes between the index patient (case A), secondary patients (cases B and C), and asymptomatic infections (cases D, E, and F). (B) Timeline of important events describing the progress of illness in cases A, B, and C. The days of possible contacts between cases are presented in grey boxes along with the timeline of each case.
Laboratory tests confirmed the cases of the cluster had SFTSV infection.
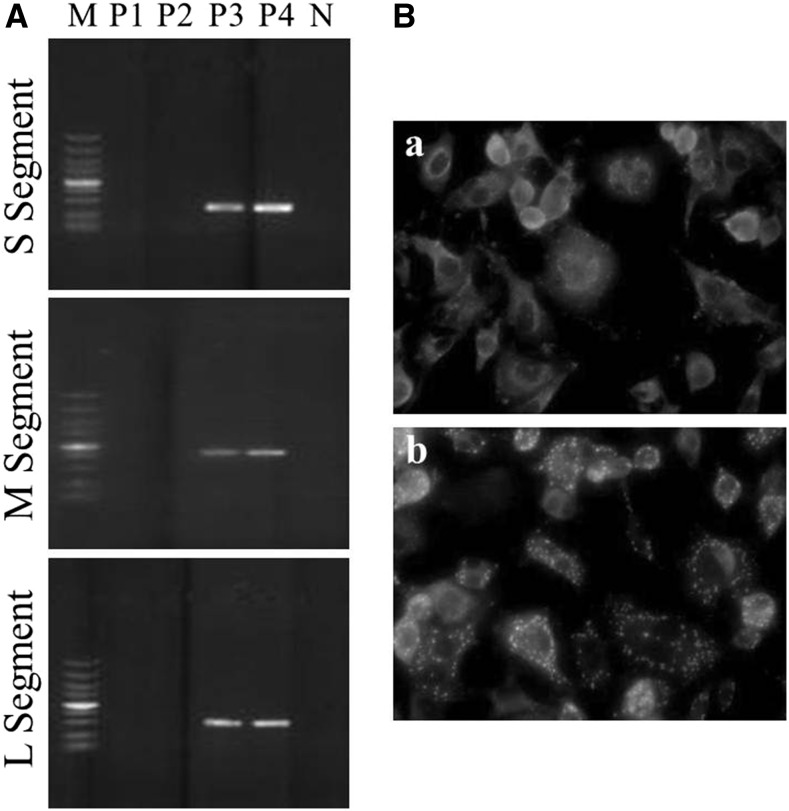
The infection by Anaplasma and Ehrlichia were excluded from all the collected samples through PCR tests according to the technical guidelines of Prevention and Control of Human Granulocytic Anaplasmosis issued by the Chinese Ministry of Health in 2008 (http://www.nhfpc.gov.cn) (data not shown). RNA fragments of the SFTSV S (1,160–1,513 nt) and L segments (589–806 nt) were detected in the blood smear of case A and the serum sample of case B but not of case C by RT-PCR (Table 1). Case D was found negative for SFTSV RNA by her serum sample, whereas the SFTSV L segment was detected in the serum samples of cases E and F (Table 1). Therefore, cases A, B, E, and F had SFTSV infection based on the results of viral RNA detection. In addition, a strain of SFTSV was isolated from case B’s serum sample. SFTSV RNA segments were detected in supernatants of the third and fourth passages (Figure 2A, lanes P3 and P4). NP and NSs expression could be visualized in infected cells (Figure 2B). The results further confirmed that case B had SFTSV infection.
Figure 2.
Virus isolation from the serum sample of case B. (A) reverse transcriptase polymerase chain reaction detection of severe fever with thrombocytopenia syndrome virus S, M, and L segments in supernatants from the first to fourth passage. M, DNA ladder; P1-P4, supernatant from the first to fourth passage; N, negative control; (B) IFA detection of the viral protein expression of the isolated virus in infected cells. (a) immunofluorescences assay (IFA) detection of nucleoprotein expression in infected cells and (b) IFA detection of nonstructural proteins expression in infected cells.
IFA and cELISA were performed to detect virus-specific IgM and IgG antibodies using SFTSV infected cells (Table 1 and Supplemental Figure 1). Different levels of IgM response were detected in the sera samples of cases B, C, D, E, and F by cELISA, whereas the sera of cases E and F were detected positive for IgM by IFA. Cases B and C had low levels of IgG response detected by cELISA, whereas IFA showed that the serum sample of case B was positive for IgG. Further, MNA showed that the isolated virus from case B could be neutralized by the sera samples of cases E and F (Table 1). These results suggested that cases D, E, and F might have a recent infection of SFTSV. According to the results of serological detection for SFTSV-specific antibodies, cases B, C, D, E, and F had SFTSV infections.
According to the guideline issued by the Chinese Ministry of Health (http://www.moh.gov.cn), a patient would be confirmed as the SFTSV infected case as he or she is found to meet any one of the three conditions that 1) viral RNAs are detected in the sera; 2) specific antibodies are detected; and 3) the virus is isolated from the patient’s sera. Hence, the six cases in the cluster all had SFTSV infection according to the guideline. Cases A, B, and C were symptomatic patients, whereas cases D, E, and F were asymptomatic infections.
Viral sequence analysis provided genetic evidence for possible transmission in the cluster.
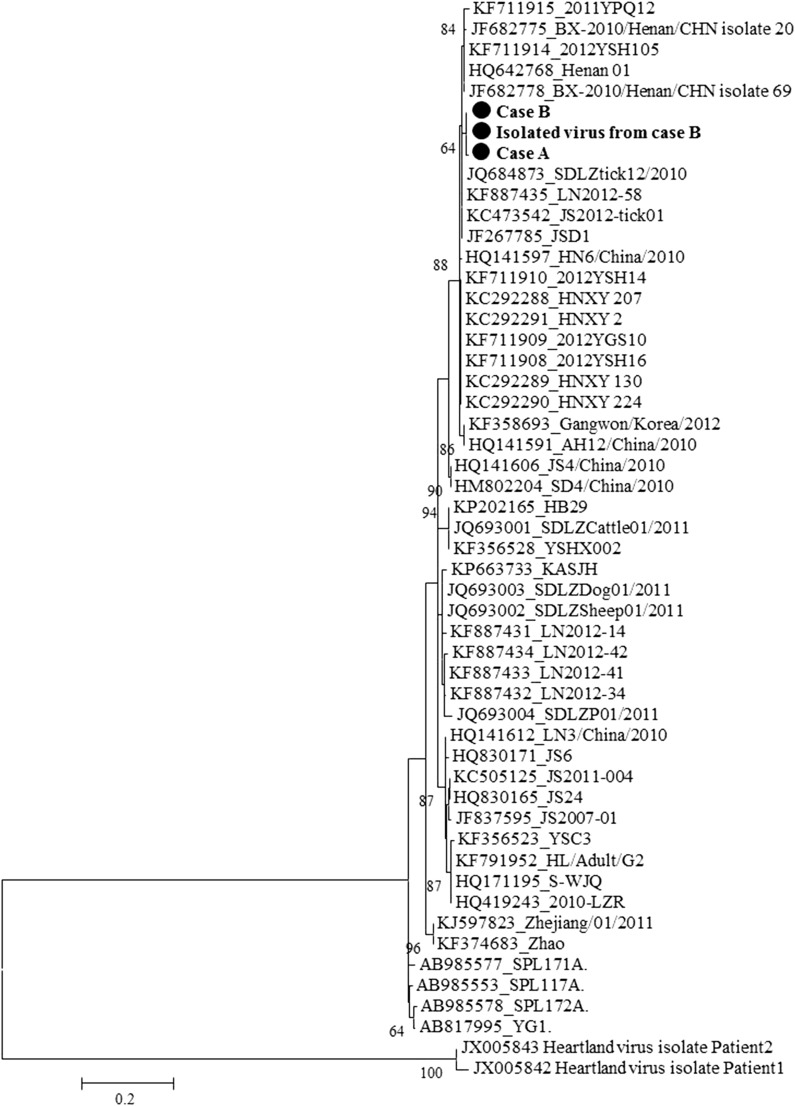
PCR products from cases A, B, E, and F were sequenced. The sequences of PCR products of S and L segments from case B (354 base pairs [bp] and 218 bp, respectively) were identical to the isolated virus. The sequence of PCR products of S segment from case A (199 bp) shared 99.5% nucleotide identity to case B, whereas the sequence of PCR products of L segment from case A (218 bp) was identical to case B. Phylogenetic analysis showed that the sequences of S segment from the isolated virus clustered with sequences of the PCR products from cases A and B (Figure 3). Furthermore, the sequence of PCR products of L segment from cases E and F (218 bp) were identical to cases A and B as well as the isolated virus. Thus, the results suggested that the viral sequences detected from cases A, B, E, and F and the isolated virus might share the same origins, which provided genetic evidence for the possible transmission routes of SFTSV in the cluster.
Figure 3.
Phylogenetic tree constructed based on partial sequences of S segment. The phylogenetic tree was constructed by Mega 5.0 and tested by Bootstrap method with a value of 1,000. The sequences from the isolated virus, cases A and B were labeled by black solid circles (●) and shown in bold. Other sequences of S segment of severe fever with thrombocytopenia syndrome virus were obtained from GenBank. Two strains of Heartland viruses were included as outgroup control.
DISCUSSION
Here we presented a cluster of SFTSV infections of three symptomatic patients and three asymptomatic persons due to person-to-person transmission occurred in 2014 in Shandong Province, China. All six cases were confirmed of SFTSV infection according to the results of laboratory tests. And SFTSV transmission from the index patient to secondary patients and from secondary patients to asymptomatic persons was confirmed by epidemiological investigations and viral sequence analysis. In the cluster, cases B and C had direct blood contact with the index patient, but they had different outcomes. Reports showed that a fatal outcome in SFTS patients is associated with high viral RNA load and serum liver transaminase level.19,20 Case B in this cluster had much higher serum liver transaminase levels even after therapy. And most of the clinical parameters of case C returned to normal after admission. We subsequently investigated the possible transmission routes from case A to cases B and C. The risk factors that they might expose to were summarized according to the epidemiological investigations (Table 1). Both cases B and C had exposures to all the potential infectious sources including blood, soiled coat, mucosa, and body fluids without any protective devices, but they had different protective behaviors that case C washed his hands and body, whereas case B did not, which might be associated with more severe disease of case B.
In recent years, a few cases of asymptomatic infections have been noted in the clusters of person-to-person transmission.10,12,13 They had close contact with SFTS patients and presented no symptoms, however, viral RNA or IgG could be detected in their sera samples. The reasons and mechanisms that SFTSV caused asymptomatic infections remained unclear. Asymptomatic infections were also included in this current cluster. Concerning the risk factors of contacts in inducing asymptomatic infection, the three asymptomatic persons, D, E, and F, did not exposed to blood of SFTS patients or any soiled coats, but they had chance to touch mucosa and body fluids of SFTS patients (Table 1). Neither cases D and E used protective devices, except the doctor (case F) was wearing a mask (Table 1). So the three asymptomatic persons had SFTSV infection due to nonblood contacts without wearing proper protective devices.
Previous studies assessed risks of the nonblood exposure in SFTSV transmission, suggesting that nonblood contacts were much less associated with developing illness than direct blood contact.5,9 However, SFTSV RNA was detected in nonblood samples such as throat, urine, and fecal specimens in fatal cases and in a substantial proportion of SFTS patients,20 indicating the potential existence of infective virus particles in the patients’ mucosa and secretions. A recent study reported the nosocomial transmission of SFTSV occurred in Korea mentioned that exposure to respiratory secretions or gowns soiled by body fluids were significantly associated with SFTSV infections.12 So the mucosal secretions and body fluids of SFTS patients may also be infectious resources that the direct contact should be avoided. Similar to the asymptomatic infections in this study, a recent family cluster of SFTSV infection in Korea included an asymptomatic infection due to nonblood exposure.13 Therefore, nonblood contacts with SFTS patients may also be associated with SFTSV transmission among humans, and the asymptomatic infection caused by nonblood contacts should have attracted much attention as for the disease control and prevention.
Supplementary Material
Acknowledgments:
We thank Min Zhou and Yanfang Zhang in Wuhan Institute of virology, CAS for cell culture assistance, and we acknowledge Tao Zhang to guide the IgG and IgM detection technology.
Note: Supplemental methods, tables, and figures appear at www.ajtmh.org.
REFERENCES
- 1.Yu XJ, et al. , 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang XL, et al. , 2014. A cluster of person-to-person transmission cases caused by SFTS virus in Penglai, China. Clin Microbiol Infect 21: 274–279. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YZ, et al. , 2011. Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi 32: 209–220. [PubMed] [Google Scholar]
- 4.Zhang YZ, et al. , 2012. The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. J Virol 86: 2864–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X, et al. , 2013. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis 207: 736–739. [DOI] [PubMed] [Google Scholar]
- 6.Bao CJ, et al. , 2011. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis 53: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Deng B, Zhang J, Cui W, Yao W, Liu P, 2014. Person-to-person asymptomatic infection of severe fever with thrombocytopenia syndrome virus through blood contact. Intern Med 53: 903–906. [DOI] [PubMed] [Google Scholar]
- 8.Gai Z, et al. , 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis 54: 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang XL, et al. , 2015. A cluster of person-to-person transmission cases caused by SFTS virus in Penglai, China. Clin Microbiol Infect 21: 274–279. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ, 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis 12: 156–160. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Hu K, Zou J, Xiao J, 2013. A cluster of cases of human-to-human transmission caused by severe fever with thrombocytopenia syndrome bunyavirus. Int J Infect Dis 17: e206–e208. [DOI] [PubMed] [Google Scholar]
- 12.Kim WY, et al. , 2015. Nosocomial transmission of severe fever with thrombocytopenia syndrome in Korea. Clin Infect Dis 60: 1681–1683. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JR, Heo ST, Park D, Kim H, Fukuma A, Fukushi S, Shimojima M, Lee KH, 2016. Family cluster analysis of severe fever with thrombocytopenia syndrome virus infection in Korea. Am J Trop Med Hyg 95: 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowen MD, Trappier SG, Sanchez AJ, Meyer RF, Goldsmith CS, Zaki SR, Dunster LM, Peters CJ, Ksiazek TG, Nichol ST, Force RVFT, 2001. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology 291: 185–190. [DOI] [PubMed] [Google Scholar]
- 15.Xu BL, et al. , 2011. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new Bunyavirus. PLoS Pathog 7: e1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning YJ, Wang M, Deng M, Shen S, Liu W, Cao WC, Deng F, Wang YY, Hu Z, Wang H, 2014. Viral suppression of innate immunity via spatial isolation of TBK1/IKKepsilon from mitochondrial antiviral platform. J Mol Cell Biol 6: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen S, Wang M, Li X, Li S, van Oers MM, Vlak JM, Braakman I, Hu Z, Deng F, Wang H, 2016. Mutational and functional analysis of N-linked glycosylation of envelope fusion protein F of Helicoverpa armigera nucleopolyhedrovirus. J Gen Virol 97: 988–999. [DOI] [PubMed] [Google Scholar]
- 18.Deitch EA, 1992. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gai ZT, et al. , 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YZ, et al. , 2012. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis 54: 527–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.