Abstract
Background
Sickle cell disease (SCD) is one of the commonest severe monogenic disorders in the world, due to the inheritance of two abnormal haemoglobin (beta‐globin) genes. SCD can cause severe pain, significant end‐organ damage, pulmonary complications, and premature death. Kidney disease is a frequent and potentially severe complication in people with SCD.
Chronic kidney disease is defined as abnormalities of kidney structure or function, present for more than three months. Sickle cell nephropathy refers to the spectrum of kidney complications in SCD.
Glomerular damage is a cause of microalbuminuria and can develop at an early age in children with SCD, and increases in prevalence in adulthood. In people with sickle cell nephropathy, outcomes are poor as a result of the progression to proteinuria and chronic kidney insufficiency. Up to 12% of people who develop sickle cell nephropathy will develop end‐stage renal disease.
Objectives
To assess the effectiveness of any intervention in preventing or reducing kidney complications or chronic kidney disease in people with SCD (including red blood cell transfusions, hydroxyurea and angiotensin‐converting enzyme inhibitor (ACEI)), either alone or in combination with each other.
Search methods
We searched for relevant trials in the Cochrane Library, MEDLINE (from 1946), Embase (from 1974), the Transfusion Evidence Library (from 1980), and ongoing trial databases; all searches current to 05 April 2016. We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register: 13 April 2017.
Selection criteria
Randomised controlled trials comparing interventions to prevent or reduce kidney complications or chronic kidney disease in people with SCD. There were no restrictions by outcomes examined, language or publication status.
Data collection and analysis
Two authors independently assessed trial eligibility, extracted data and assessed the risk of bias.
Main results
We included two trials with 215 participants. One trial was published in 2011 and included 193 children aged 9 months to 18 months, and compared treatment with hydroxyurea to placebo. The second trial was published in 1998 and included 22 adults with normal blood pressure and microalbuminuria and compared ACEI to placebo.
We rated the quality of evidence as low to very low across different outcomes according to GRADE methodology. This was due to trials having: a high or unclear risk of bias including attrition and detection bias; indirectness (the available evidence was for children aged 9 months to 18 months in one trial and a small and select adult sample size in a second trial); and imprecise outcome effect estimates of significant benefit or harm.
Hydroxyurea versus placebo
We are very uncertain if hydroxyurea reduces or prevents progression of kidney disease (assessed by change in glomerular filtration rate), or reduces hyperfiltration in children aged 9 to 18 months, mean difference (MD) 0.58 (95% confidence interval (CI) ‐14.60 to 15.76 (mL/min per 1.73 m²)) (one study; 142 participants; very low‐quality evidence).
In children aged 9 to 18 months, hydroxyurea may improve the ability to concentrate urine, MD 42.23 (95% CI 12.14 to 72.32 (mOsm/kg)) (one study; 178 participants; low‐quality evidence).
Hydroxyurea may make little or no difference to SCD‐related serious adverse events including: incidence of acute chest syndrome, risk ratio (RR) 0.39 (99% CI 0.13 to 1.16); painful crisis, RR 0.68 (99% CI 0.45 to 1.02); and hospitalisations, RR 0.83 (99% CI 0.68 to 1.01) (one study, 193 participants; low‐quality evidence).
No deaths occurred in the trial. Quality of life was not reported.
ACEI versus placebo
We are very uncertain if ACEI reduces proteinuria in adults with SCD who have normal blood pressure and microalbuminuria, MD ‐49.00 (95% CI ‐124.10 to 26.10 (mg per day)) (one study; 22 participants; very low‐quality evidence). We are very uncertain if ACEI reduce or prevent kidney disease as measured by creatinine clearance. The authors state that creatinine clearance remained constant over six months in both groups, but no comparative data were provided (very low‐quality evidence).
All‐cause mortality, serious adverse events and quality of life were not reported.
Authors' conclusions
In young children aged 9 months to 18 months, we are very uncertain if hydroxyurea improves glomerular filtration rate or reduces hyperfiltration, but it may improve young children's ability to concentrate urine and may make little or no difference on the incidence of acute chest syndrome, painful crises and hospitalisations.
We are very uncertain if giving ACEI to adults with normal blood pressure and microalbuminuria has any effect on preventing or reducing kidney complications.
This review identified no trials that looked at red cell transfusions nor any combinations of interventions to prevent or reduce kidney complications.
Due to lack of evidence this review cannot comment on the management of either children aged over 18 months or adults with any known genotype of SCD.
We have identified a lack of adequately‐designed and powered studies, and no ongoing trials which address this critical question. Trials of hydroxyurea, ACEI or red blood cell transfusion in older children and adults are urgently needed to determine any effect on prevention or reduction kidney complications in people with SCD.
Plain language summary
Interventions to prevent or reduce kidney complications in people with sickle cell disease
Review question
We wanted to determine if there were any safe and effective interventions that prevent or reduce kidney complications in people with sickle cell disease (SCD).
Background
SCD is a serious inherited blood disorder where the red blood cells, which carry oxygen around the body, develop abnormally. Normal red blood cells are flexible and disc‐shaped, but in SCD they can become rigid and crescent shaped. Sickled cells are not only less flexible than healthy red blood cells, they are also stickier. This can lead to blockage of blood vessels, resulting in tissue and organ damage and episodes of severe pain. The abnormal blood cells are more fragile and break apart, which leads to a decreased number of red blood cells, known as anaemia.
Kidney complications can start at an early age in children with SCD and are common in adults with the condition. Kidney complications leading to kidney protein leak and chronic kidney disease can be severe with serious effects on health (such as the need for dialysis or a kidney transplant). Identifying therapies, which can prevent or slow down the decline in kidney function in people with SCD, will be critical in improving health outcomes.
Search date
The evidence is current to: 13 April 2017.
Study characteristics
We found two randomised controlled trials which enrolled a total of 215 participants. One trial, published in 2011, was conducted in 193 infants aged 9 months to 18 months and compared the drug hydroxyurea to placebo. The second trial, published in 1998, was conducted in 22 adults with normal blood pressure and microalbuminuria (an increase of protein in the urine) and compared captopril (a drug used to treat high blood pressure) to placebo.
Both trials received government funding.
Key Results
In infants aged 9 months to 18 months, hydroxyurea may increase the ability to produce normal urine, but we are very uncertain if it has any effect on the glomerular filtration rate (network of filters in the kidney that filter waste from the blood). Hydroxyurea may make little or no difference on the incidence of SCD‐related serious complications (including acute chest syndrome, painful crises and hospitalisations).
We are very uncertain if giving captopril to adults with SCD who have normal blood pressure and early signs of kidney damage (microalbuminuria) reduces progression of kidney damage.
Quality of life was not reported in either trial.
Quality of the evidence
The evidence for all outcomes was rated as low‐ to very low‐quality due to trials being at high risk of bias and because there were a small number of trials and a small number of participants included in the trials.
Summary of findings
Summary of findings for the main comparison. Hydroxyurea compared to placebo for preventing or reducing kidney complications in people with sickle cell disease.
| Hydroxyurea compared to placebo for preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease Setting: multiple centres Intervention: hydroxyurea Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (99% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with hydroxyurea | |||||
| Slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) | The mean slower progression or improvement in GFR mL per min per 1.73 m² (measured at 18 to 24 months) was 146.64 (43.7) | MD 0.58 higher (14.6 lower to 15.76 higher) | ‐ | 142 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2 | |
| Improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) | The mean improvement in ability to concentrate urine mOsm/kg (measured at 18 to 24 months) was 494.57 (110.07) | MD 42.23 higher (12.14 higher to 72.32 higher) | ‐ | 178 (1 RCT) | ⊕⊕⊝⊝ LOW 2,3 | |
| SAEs assessed with acute chest syndrome | Study population | RR 0.39 (0.13 to 1.16) | 193 (1 RCT) | ⊕⊕⊝⊝ LOW 2, 3 | ||
| 186 per 1000 | 72 per 1000 (24 to 215) | |||||
| SAEs assessed with painful crisis | Study population | RR 0.68 (0.45 to 1.02) | 193 (1 RCT) | ⊕⊕⊝⊝ LOW 2, 3 | ||
| 567 per 1000 | 386 per 1000 (255 to 578) | |||||
| SAEs assessed with hospitalisations | Study population | RR 0.83 (0.68 to 1.01) | 193 (1 RCT) | ⊕⊕⊝⊝ LOW 2, 3 | ||
| 866 per 1000 | 719 per 1000 (589 to 875) | |||||
| Mortality due to any cause | No deaths reported in either group | not estimable | 193 (1 RCT) | ⊕⊕⊝⊝ LOW 2, 4 | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GFR: glomerular filtration rate; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SAEs: serious adverse events. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by one due to unclear risk of attrition bias. 2 We downgraded the quality of evidence by one due to indirectness as the results apply only to small children aged 8 to 19 months. 3 We downgraded the quality of evidence by one due to imprecision as confidence intervals are wide indicating clinically significant harm or benefit. 4 We downgraded the quality of evidence by one due to imprecision; rare event no deaths occurred.
Summary of findings 2. ACEI compared to placebo in preventing or reducing kidney complications in people with sickle cell disease.
| ACEI compared to placebo in preventing or reducing kidney complications in people with sickle cell disease | ||||||
| Patient or population: people with sickle cell disease Setting: hospital outpatient Intervention: ACEI Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with ACEI | |||||
| Slower progression or reduction in proteinuria (mg/day 6 months follow‐up) | The mean slower progression or reduction in proteinuria (mg/day 6 months follow‐up) was 76 (45) | MD 49.00 lower (124.10 lower to 26.10 higher) | ‐ | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2 ,3 | |
| Improvement in ability to concentrate urine mOsm/kg | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with acute chest syndrome | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with painful crisis | Not reported | ‐ | ‐ | ‐ | ||
| SAEs assessed with hospitalisations | Not reported | ‐ | ‐ | ‐ | ||
| Mortality due to any cause | Not reported | ‐ | ‐ | ‐ | ||
| Quality of life | Not reported | ‐ | ‐ | ‐ | ||
| ACEI: angiotensin converting enzyme inhibitor; CI: confidence interval; MD: mean difference; SAEs: serious adverse events | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two due to unclear or high risk of bias in all domains. 2 We downgraded the quality of evidence by one due to indirectness because a small sample population of adults with normal blood pressure and microalbuminuria. 3 We downgraded the quality of evidence by one due to imprecision as very wide CIs including clinically significant harm or benefit.
Background
Please see an appendix for an explanation of some technical terms (Appendix 1).
Description of the condition
Sickle cell disease (SCD) is an inherited anaemia, which can lead to episodes of severe pain and life‐threatening acute complications such as chest crises, strokes and splenic sequestration in people with SCD (Pleasants 2014). Populations originating from sub‐Saharan Africa, Spanish‐speaking regions in the Western Hemisphere (South America, the Caribbean, and Central America), the Middle East, India and parts of the Mediterranean are predominantly affected. Reductions in infant and child mortality and increasing migration from highly affected countries have made this a worldwide problem (Piel 2012). Over 12,500 people in the UK and 100,000 in the USA suffer from the disease (NICE 2010; Pleasants 2014). A recent study estimated that approximately 305,800 babies were born with SCD worldwide in 2010, of which two thirds were born in Africa, and this could increase by 25% to approximately 404,200 by 2050 (Piel 2012).
The term 'sickle cell disease' refers to all genotypes that cause the clinical syndrome. There are three main types of SCD. Sickle cell anaemia is the most common form of the disease (up to 70% of cases of SCD in people of African origin) and is due to the inheritance of two beta‐globin S (βS) alleles (haemoglobin (Hb)SS). The second most common genotype (up to 30% of cases in people of African origin) is haemoglobin SC disease (HbSC disease), due to the co‐inheritance of the βS and βC alleles and tends to be a more moderate form of the disease. The third major type of SCD occurs when βS is inherited with a β‐thalassaemia allele, causing HbS/β‐thalassaemia (Rees 2010). People who have inherited a thalassaemia null mutation (HbSβº) have a disease that is clinically indistinguishable from sickle cell anaemia, whereas people with HbSβ⁺ thalassaemia have a milder disorder. In high‐income countries, people with SCD are expected to live into their 40's, 50's and beyond, whereas in low‐income countries, including some African nations, it is estimated that between 50% and 90% of children born with HbSS die before their fifth birthday (Gravitz 2014;Grosse 2011).
In SCD, under conditions of low oxygen levels, acidity and cellular dehydration, the HbS molecules polymerise and begin to distort the red blood cells which take on a sickled shape. The main determinant of disease severity is the rate and extent of this HbS polymerisation (Rees 2010). This is exemplified by the co‐inheritance of genetic factors that affect the intracellular HbS or fetal haemoglobin concentration, for example, the protective effects of co‐inherited α‐thalassaemia (Rumaney 2014; Steinberg 2012) or hereditary persistence of fetal haemoglobin (Akinsheye 2011; Steinberg 2012). Sickling of red blood cells results in both obstruction of blood flow leading to organ and tissue ischaemia, and haemolytic anaemia (Sparkenbaugh 2013). Both of these processes are thought to lead to increased inflammation and an increased tendency to develop a blood clot (Frenette 2007; Rees 2010). Reduced blood flow is mediated via a dynamic interaction between sticky HbS containing red blood cells, the vessel wall, and white cells (Rees 2010). Sickle red blood cells also have a shorter lifespan of 10 to 12 days versus 120 days for normal red blood cells, due to intravascular and extravascular haemolysis, leading to anaemia (Kato 2006a). Chronic intravascular haemolysis leads to a reduced nitric oxide level within the blood; nitric oxide is sequestered by free haemoglobin (Hb), which over time favours the development of pulmonary hypertension and ischaemic strokes (Kato 2006a; Kato 2006b).
Kidney dysfunction
Mechanisms of kidney dysfunction in SCD
1.
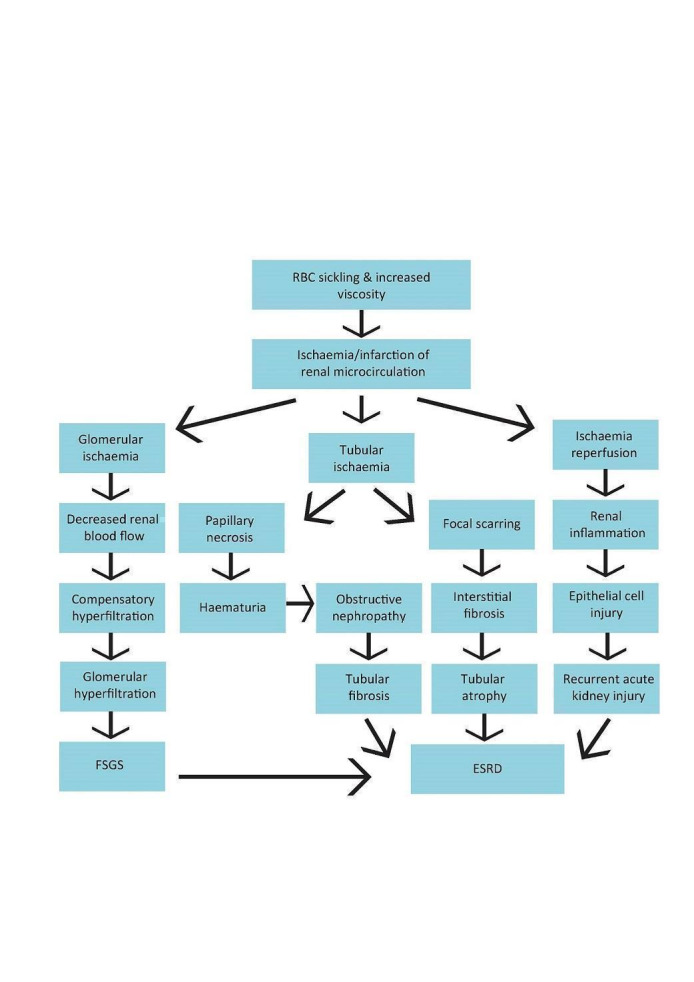
Sickle cell nephropathy pathophysiology in sickle cell disease: Adapted fromOkafor 2013andNath 2015
RBC: red blood cells; FSGS: focal segmental glomerulosclerosis; ESRD: end‐stage renal disease
2.
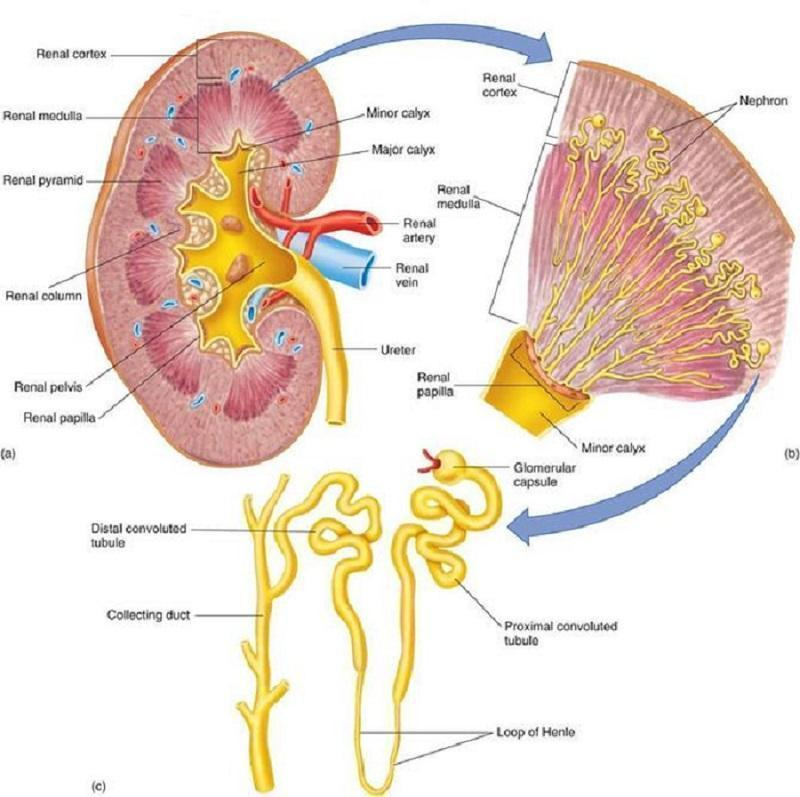
Structure of the kidney. From: Wikispaces. Human Physiology. 12. Urology.https://humanphysiology2011.wikispaces.com/12.+Urology
Kidney disease is a frequent and potentially severe complication in people with SCD. Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function, present for more than three months, with implications for health (KDIGO 2012) (See Table I below). It is more common in people with HbSS and HbSβº than in people with HbSC or HbSβ⁺; however, there is conflicting evidence relating to the relative prevalence of CKD in different SCD genotypes (Nath 2015, Yee 2011). The prevalence of CKD increases with age, affecting over 50% of people with SCD who are over 40 years of age (Gosmanova 2014).
Sickle cell nephropathy refers to the spectrum of kidney complications in SCD (Figure 1). One of the hallmarks of sickle cell nephropathy is hyperfiltration (a glomerular filtration rate (GFR) greater than 120 mL/min/1.73 m²) which has been described in 51% of people with SCD, particularly younger individuals, and those with higher levels of haemolysis (Haymann 2010). The inner kidney (medulla) is particularly prone to red blood cell sickling due to its acidotic and hypoxic environment (Figure 2). Damage is predominantly due to recurrent episodes of ischaemia and reperfusion injury or infarction leading to scarring (Hebbel 2014).
Glomerular damage (caused by hyperfiltration) leads to urinary protein leak. Renal tubule injury results in childhood enuresis and impaired ability to concentrate urine, with increased susceptibility to dehydration, which may precipitate a sickling crisis. Renal papillary necrosis due to infarction leads to haematuria, scarring and further impairment of function. The combined effect of glomerular and tubulointerstitial scarring leads to progressive decline in kidney function (Nasr 2006; Sharpe 2014).
The risk of end‐stage renal disease (ESRD), defined as requiring long‐term dialysis or transplantation, is around 12% (Powars 2005). Risk factors for ESRD include proteinuria, anaemia, hypertension, and HbSS genotype (Ataga 2014).
Assessment of kidney function
The gold standard for assessing how well the kidneys are working is direct measurement of the GFR. Generally, as kidney disease gets worse, the GFR drops (see Table I below). In people with SCD, a GFR greater than 120 mL/min/1.73 m² is an additional indicator of abnormal kidney function. However, direct measurement of GFR is invasive and time consuming and so estimations of GFR based on the serum creatinine are more commonly used. A number of equations exist, including the 'Modification of Diet in Renal Disease' (MDRD), CKI‐EPI, and Cockcroft–Gault equations (Botev 2009; Levey 1999; Levey 2009). Different estimated GFR calculations have been compared to the measured GFR in people with HbSS from the Caribbean and sub‐Saharan Africa, the CKI‐EPI was found to be the most accurate estimate in two small studies (Arlet 2012; Asnani 2013). In individuals with SCD, increased proximal tubule secretion of creatinine results in the serum creatinine level being a poor estimate of GFR (Asnani 2015).
Table I: GFR categories in CKD (KDIGO 2012)
|
GFR category (CKD stages) |
GFR (mL/min/1.73 m²) | Terms |
| G1 (Stage 1) | ≥ 90 | Normal or high |
| G2 (Stage 2) | 60 to 89 | Mildly decreased* |
| G3a (Stage 3a) | 45 to 59 | Mildly to moderately decreased |
| G3b (Stage 3b) | 30 to 44 | Moderately to severely decreased |
| G4 (Stage 4) | 15 to 29 | Severely decreased |
| G5 (Stage 5) | < 15 | Kidney failure |
| * Relative to young adult level | ||
Proteinuria, albumin‐to‐creatinine ratio (ACR) greater than 2.5 mg/mmol in men or 3.5 mg/mmol in women, or a protein‐to‐creatinine ratio (PCR) greater than 15 mg/mmol is sufficient for a diagnosis of CKD (see Table II below), and is an independent risk factor for kidney and cardiovascular mortality in the general population (Astor 2011; de Zeeuw 2004). Proteinuria can be classified as either a microalbuminuria (3 to 30 mg/mmol creatinine) or macroalbuminuria (greater than 30 mg/mmol creatinine). ACR and PCR correlate well with 24‐hour urinary protein excretion (Gaspari 2006).
Table II: Relationship among categories for albuminuria and proteinuria in CKD (KDIGO 2012)
| Categories | |||
| Measure | Normal to mildly increased (A1) | Moderately increased (A2) | Severely increased (A3) |
| AER (mg/24 hours) | < 30 | 30 to 300 | > 300 |
| PER (mg/24 hours) | < 150 | 150 to 500 | > 500 |
| ACR (mg/mmol) (mg/g) |
< 3 < 30 |
3 to 30 30 to 300 |
> 30 > 300 |
| PCR (mg/mmol) (mg/g) |
< 15 < 150 |
15 to 50 150 to 500 |
> 50 > 500 |
|
Abbreviations: A1 ‐ A3: albuminuria categories; ACR: albumin‐to‐creatinine ratio; AER: albumin excretion rate; PCR: protein‐to‐creatinine ratio; PER: protein excretion rate. Relationships among measurement methods within a category are not exact. The relationships between AER and ACR and between PER and PCR are based on the assumption that average creatinine excretion rate is approximately 1.0 g per day or 10 mmol per day. Creatinine excretion varies with age, sex, race and diet; therefore the relationship among these categories is approximate only. The conversions are rounded for pragmatic reasons. | |||
Prevalence
CKD stage 1 or 2 is present in 26.5% of children with SCD, and is defined as urinary structural or genetic abnormalities pointing to kidney disease or a GFR less than 90 mLmin/1.73 m² (Yee 2011). In a four‐decade observational study of 1052 people with HbSS, 11.6% of participants developed ESRD, and 29.4% of deaths in participants with SCD had prior ESRD (Powars 2005). Furthermore, 16% to 18% of overall mortality in SCD is attributable to kidney disease (Hamideh 2013; Nath 2015).
An albumin‐to‐creatinine ratio that is repeatedly higher than 3 mg/mmol is present in up to 20% of children with SCD and screening for microalbuminuria may be useful for identifying children with early sickle cell nephropathy (McKie 2007; Sharpe 2014). The prevalence of proteinuria increases with age and increased systolic blood pressure; it varies between 4.5% to 26% of people up to 21 years and from 26% to 68% in older people (Ataga 2014).
Description of the intervention
Recommended interventions for preventing kidney complications include avoiding dehydration and the chronic use of drugs toxic to the kidneys, such as non‐steroidal anti‐inflammatories.
However, as kidney damage is initiated directly by the sickling of red blood cells in the kidneys, reducing sickling will be expected to cut short the primary insult and, in the long term, slow the rate of progression of sickle cell nephropathy. This can be achieved by a direct reduction in the percentage of HbS in the blood through the use of red blood cell transfusions, or using strategies to increase HbF production such as hydroxyurea (hydroxycarbamide).
Inhibition of the renin‐angiotensin‐aldosterone system (RAAS) by angiotensin‐converting‐enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) can reduce kidney damage by lowering intraglomerular pressure, and reducing proteinuria. While there is a Cochrane Review on the use of ACEI in SCD (Sasongko 2015), the mechanisms of action of blood transfusions, hydroxyurea and ACEI may be synergistic, thus we are also including ACEI in the current review as they may be compared to, or used in combination with, red blood cell transfusion and hydroxyurea.
Red blood cell transfusions
Red blood cell transfusion is not a specific treatment for acute kidney injury in people with SCD unless it is required to treat other SCD complications (e.g. acute chest syndrome) (Yawn 2014). Chronic red blood cell transfusions, either given as simple (top‐up) or exchange transfusions, form part of the management of a number of SCD complications such as the primary prevention of strokes in children with abnormal transcranial dopplers (Adam 2008) or the prevention of further chest crises in people with recurrent episodes (Howard 2015).
Studies have suggested that red blood cell transfusions may be beneficial in preventing the progression of kidney disease in children (McKie 2007;Marsenic 2008). One study of 120 children with sickle haemoglobinopathies found that chronic red blood cell transfusions before the age of nine years was protective against the onset of microalbuminuria (Alvarez 2006).
Red blood cell transfusions have reduced complications and improved the quality of life in people with SCD, but are not without potentially serious complications. The benefits of transfusion therapy must be balanced against risks including infections, iron overload, acute or delayed haemolytic transfusion reactions, and increased complexity of compatibility testing (Chou 2013a; Chou 2013b; Porter 2013; Scheunemann 2010; Ubesie 2012). Frequent blood transfusions in SCD can also lead to alloimmunisation (Yazdanbakhsh 2012).
ACEI
ACEI prevent the formation of angiotensin II, a protein (peptide) which causes the narrowing of blood vessels (arteriolar vasoconstriction) and the release of a hormone (aldosterone). This hormone causes the kidney tubules (a part of the kidneys) to retain more water and thus expand circulating blood volume, resulting in an increase in blood pressure.
Angiotensin II raises the pressure within the glomeruli (the parts of the kidney that first filter the blood). This increased pressure damages the filtration barrier and allows larger proteins to be lost in the urine (Macconi 2006). Angiotensin II is also a component in the progression of kidney fibrosis which can lead to ESRD. ACEI reduce the pressure within the glomeruli and proteinuria independently of their anti‐hypertensive effect (Gansevoort 1995; Maki 1995), and were first shown to slow the decline in GFR in diabetic kidney disease (Lewis 1993).
Hypotension is a risk with ACEI. However, ACEI are less effective in lowering blood pressure in people of African or Carribean origin (Ventura 1985), the populations primarily affected by SCD. Therefore, people with SCD who do not normally have raised blood pressure and who are given ACEI may be at a lower risk of this side‐effect.
Hydroxyurea
Hydroxyurea has been used since the 1980s and shown in clinical trials to be beneficial in reducing vaso‐occlusive crises, chest crises and in improving survival in people with SCD (Field 2014). Children treated with hydroxyurea have been shown to have better kidney function than those treated with placebo, as assessed by the ability to concentrate urine (Alvarez 2012). In a non‐randomised study of children with SCD requiring hydroxyurea for standard indications, treatment for three years led to a mean (standard deviation (SD)) decrease in GFR from 167 (SD 46) mL/min/1.73 m² to 145 (SD 27) mL/min/1.73 m² (Aygun 2013), indicating an improvement in the hyperfiltration.
How the intervention might work
Red blood cell transfusions
In its simplest form, blood transfusions proportionally reduce HbS, prevent direct sickling in the kidney and local vaso‐occlusion, thereby reducing glomerular and tubular ischaemic damage to the kidney. However, a further mechanism by which transfusions could prevent kidney damage is by reducing sickling, so that the amount of haemolysis decreases and with it, the sequestration of nitric oxide. Nitric oxide is known to have an important local vasodilatory effect and nitric oxide sequestration by free Hb released during haemolysis is thought to contribute to the vasculopathy of sickle cell nephropathy (Potoka 2015). Finally haemolysis directly leads to endothelial damage, inflammation and dysfunction, a further mechanism of kidney disease which may play an important role in sickle cell nephropathy (Zafrani 2015).
ACEI
As described above, ACEI reduce proteinuria and slow kidney progression in other forms of CKD. It is plausible that this benefit would also apply in SCD, since ACE inhibition decreases the intraglomerular pressure which is raised in all stages of SCD‐related CKD. ACEI also appear to increase the expression of the protein nephrin, contributing to the restoration of the kidney filtration barrier (kidney filter size) (Ziyadeh 2008). Furthermore, ACE inhibition can reduce fibrogenesis and free radical‐induced oxidative stress, which may be protective against endothelial damage induced by ischaemia‐reperfusion injury (van der Meer 2010). However, in the context of medullary hypoperfusion or kidney ischaemia, this may lead to a fall in GFR, as is seen when ACEI are continued during acute kidney injury (AKI). If sickle cell nephropathy is considered as a form of recurrent ischaemic AKI, then ACEI could theoretically be damaging, at least in the context of acute crises.
Hydroxyurea
Hydroxyurea is likely to reduce kidney damage through a variety of mechanisms, which are not entirely understood and probably reflect pleiotropic effects (a drug's actions, usually unexpected, that are not the main mechanism of action and may be beneficial or harmful). It is known to modestly increase the level of HbF via a range of mechanisms, including epigenetic modifications (Pule 2015). The increase in HbF could diminish the primary kidney damage at both the glomerular and the tubular levels by reducing sickling and local ischaemia. In randomised controlled trials (RCTs) of hydroxyurea in SCD, the drug was found to increase total Hb and HbF levels and reduce vaso‐occlusive crises; however, its benefit could not be solely attributed to the rise in HbF, with other likely mechanisms including the effects on platelet count, white count, and red blood cell adhesion to endothelia (Charache 1995; Wang 2011). Hydroxyurea also decreases intravascular haemolysis which may ameliorate nitric oxide sequestration. Finally, a reduction in HbS erythrocyte adhesion by hydroxyurea (shown in vivo and in vitro) could lead to a reduction in kidney inflammation (Brun 2003; Hillery 2000; Styles 1997).
Why it is important to do this review
Glomerular damage is a cause of microalbuminuria and can develop at an early age in children with SCD; it increases in prevalence in adulthood. In people with SCD, outcomes are poor as a result of the progression to proteinuria and chronic kidney insufficiency (Lebensburger 2011). Up to 12% of people who develop sickle cell nephropathy (i.e. microalbuminuria) will develop kidney failure (Powars 2005). For these people, the development of ESRD and its treatment has a profound negative effect on their quality of life, and furthermore there are major resource implications.
While it has always been recognised that long‐term complications of SCD can occur, including kidney failure and pulmonary hypertension, the poor life expectancy of people with SCD in the past has resulted in a relatively small proportion of people suffering from these conditions. However, life expectancy for people with SCD has improved dramatically. In the 1970s people born with SCD had a median survival of 14.3 years, in the 1990s this increased to between 42 and 48 years; it is now predicted that 50% of people with SCD born after 2000 will reach their fifth decade of life (Sandhu 2015; Boyle 2016). Now more people are surviving long enough to develop long‐term complications with an expected rise in cases of kidney failure requiring dialysis or kidney transplant (renal replacement therapy).
Once CKD develops, it is associated with a poor outcome, and the identification of therapies which can prevent or slow down the decline in kidney function in people with SCD will be critical in reducing the number requiring renal replacement therapy.
Objectives
To assess the effectiveness of any intervention in preventing or reducing kidney complications or CKD in people with SCD including red blood cell transfusions, hydroxyurea and ACEI (either alone or in combination with each other).
Methods
Criteria for considering studies for this review
Types of studies
RCTs; we excluded cross‐over trials as these are not appropriate for long‐term outcomes.
Types of participants
People with all types of SCD of all ages and either gender.
Types of interventions
We included RCTs comparing all interventions, including red blood cell transfusions, hydroxyurea and ACEI (alone or in combination with each other) compared to each other, placebo or standard care.
Types of outcome measures
Primary outcomes
-
Reduction or prevention of kidney disease progression
incidence of end stage renal disease (ESRD measured as start of renal replacement therapy or death from kidney failure)
slower progression or improvement in GFR (including a reduction in hyperfiltration as evidenced by reduction of GFR into the normal range, measured by gold standard clearance methods, creatinine or creatinine based estimated GFR using MDRD equation, Cockcroft‐Gault, CKD‐EPI, or modified versions of these calculations)
slower progression or reduction in proteinuria (measured by random spot albumin‐to‐creatinine ratio, protein‐to‐creatinine ratio or 24‐hour urinary collection)
new evidence of kidney disease (based on histological examination of kidney tissue)
improvement in ability to concentrate urine (urine osmolality > 500 mOsm/kg H₂O after water deprivation)
-
Serious adverse events (SAEs)
transfusion complications (severe haemolytic reactions, transfusion‐related acute lung injury (TRALI), transfusion‐associated circulatory overload (TACO))
drug treatments (e.g. neutropenic sepsis, hospital admission secondary to drug complications)
SCD complications (e.g. acute chest syndrome, stroke, painful crisis) up to 30 days post‐transfusion or post‐drug treatment
Mortality due to any cause
If the data were available, we would have categorised kidney disease progression and mortality due to any cause according to short‐, medium‐, and long‐term outcomes. We would have reported the exact definition of these time frames over time periods that are common to as many trials as possible (for example, 0 to 5 years, 6 to 10 years, over 10 years).
Secondary outcomes
-
Other complications
transfusion‐related (alloimmunisation, infection from blood products, minor transfusion reactions, procedure‐related)
drug‐related adverse events (AEs) (neutropenia, thrombocytopenia, hypotension, hyperkalaemia, infection, allergic reaction, skin ulcers up to 60 days following ingestion)
Quality of life (measured on a validated scale)
Number of units or volume (mL) of red blood cells infused (regardless of intervention)
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status
Electronic searches
We identified trials from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND nephrology.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 13 April 2017.
In addition to this we searched the following databases for RCTs on 05 April 2016 (see Appendix 2).
the Cochrane Library: CENTRAL 2016, Issue 4; DARE & NHSEED 2015, Issue 2; HTA 2016, Issue 1 (www.cochranelibrary.com/)
MEDLINE (OvidSP, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, 1946 to 05 April 2016)
Embase (OvidSP, 1974 to 05 April 2016)
CINAHL (EBSCOHost, 193 to 05 April 2016)
PubMed (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, for recent records not yet added to MEDLINE, 1966 to 05 April 2016) (www.ncbi.nlm.nih.gov/sites/entrez)
Transfusion Evidence Library (1950 to 05 April 2016) (www.transfusionevidencelibrary.com)
LILACS (1982 to 05 April 2016) (lilacs.bvsalud.org/en/)
IndMed (1986 to 05 April 2016) (indmed.nic.in/indmed.html)
KoreaMed (1997 to 05 April 2016) (koreamed.org/)
PakMediNet (2001 to 05 April 2016) (www.pakmedinet.com/)
Web of Science (Conference Proceedings Citation Index‐ Science (CPCI‐S), 1990 to 05 April 2016t)
We searched the following trial databases for ongoing trials to 05 April 2016.
ClinicalTrials.gov (clinicaltrials.gov/)
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
We combined searches in MEDLINE and Embase with the recommended Cochrane RCT search filters (Lefebvre 2011) and in CINAHL with an RCT filter based on the Scottish Intercollegiate Guidelines Network (SIGN) RCT filter (www.sign.ac.uk/methodology/filters.html). Search strategies are presented in an appendix (Appendix 2).
Searching other resources
We handsearched the reference lists of the included trials in order to identify further relevant trials. We contacted lead authors of the included trials to identify any unpublished material, missing data or information regarding ongoing trials.
Data collection and analysis
Selection of studies
We selected trials according to chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Two review authors (NR, PF) independently screened all electronically‐derived citations and abstracts of papers identified by the search strategy for relevance. We excluded trials that were clearly irrelevant at this stage based on the abstract. Two review authors (NR, PF) independently and formally assessed the full texts of all potentially relevant trials for eligibility against the criteria outlined above. The two review authors discussed the results of trial selection and resolved any discrepancies between themselves. In the event that this was not possible, we referred the decision of eligibility to a third review author (LE). We reported the results of trial selection using a PRISMA flow diagram (Moher 2009). We would have sought further information from trial authors if the trial or abstract contained insufficient data to make a decision about eligibility. We used Covidence to help in the assessment of relevance, which included ascertaining whether the participants have SCD, and whether there are red blood cell transfusion, ACEI, or hydroxyurea treatment arms in the trial (Covidence 2015). We recorded the reasons why potentially‐relevant trials failed to meet the eligibility criteria.
Data extraction and management
Two review authors (NR, PF) conducted the data extraction according to Cochrane guidelines (Higgins 2011a). The review authors came to a consensus; if an agreement could not be reached, they would have consulted a third review author (LE). Data extraction forms were piloted in Covidence and two authors (NR, PF) extracted data independently for all the trials (Covidence 2015).
Two authors (NR and PF) independently extracted outcome data using templates modified to reflect the outcomes in this review. The review authors were not blinded to the names of authors, institutions, journals or the trial outcomes. In addition they used Covidence and the available tables in the Review Manager software to extract data on trial characteristics (Covidence 2015; RevMan 2014). We extracted the following information for each trial.
General information
Review author's name, date of data extraction, trial ID, first author of trial, author's contact address (if available), citation of paper, objectives of the trial.
Trial details
Trial design, location, setting, sample size, power calculation, treatment allocation, inclusion and exclusion criteria, reasons for exclusion, comparability of groups, length of follow‐up, stratification, stopping rules described, statistical analysis, results, conclusion, and funding.
Characteristics of participants
Age, gender, total number recruited, total number randomised, total number analysed, types of underlying disease, lost‐to‐follow‐up numbers, dropouts (percentage in each arm) with reasons, protocol violations, previous treatments, current treatment, prognostic factors, HbS levels, kidney complications.
Interventions
Experimental and control interventions, method of red blood cell transfusion (simple (top‐up), partial or full exchange transfusion), type of red blood cell transfusion (intermittent or chronic), or dose and duration of hydroxyurea or ACEI treatment.
Outcomes measured
Reduction or prevention of kidney disease progression (including incidence of ESRD, slower progression or improvement in GFR, slower progression or reduction in proteinuria, evidence of kidney disease), mortality due to any cause, SAEs (related to transfusion complications, drug treatments), SCD complications, other transfusion‐related complications, other AEs, quality of life, number of units or volume (mL) of red blood cells infused.
We used both full‐text versions and abstracts to extract data. For publications reporting on multiple trials, we originally planned to use one data extraction form for each trial. For each trial with multiple publications, we extracted data using one form. We contacted authors and trial groups for additional details if the available publications did not provide sufficient information.
One review author entered information into the Review Manager software and a second review author checked this for accuracy (RevMan 2014).
Assessment of risk of bias in included studies
We performed an assessment of all RCTs using the Cochrane 'Risk of bias’ tool according to chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Two review authors (NR, PF) worked independently to assess each element of potential bias listed below as high, low, or unclear risk of bias. We reported a brief description of the judgement statements upon which the authors have assessed potential bias in the 'Characteristics of included studies' table. We ensured that a consensus on the degree of risk of bias was met through a comparison of the review authors' statements and where necessary, through consultation with a third review author (LE). We used Cochrane’s tool for assessing risk of bias, that included the following domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias.
Measures of treatment effect
If data allowed, we undertook quantitative assessments using Review Manager (RevMan 2014).
For continuous outcomes we recorded the mean, SD and total number of participants in both the treatment and control groups. For continuous outcomes using the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). If continuous outcomes were reported using different scales we used standardised mean difference (SMD).
For dichotomous outcomes we recorded the number of events and the total number of participants in both the treatment and control groups. For dichotomous outcomes we reported the pooled risk ratio (RR) with a 95% CI. Where the number of observed events is small (less than 5% of sample per group), and where trials have balanced treatment groups, we reported the Peto odds ratio (OR) with 95% CI (Deeks 2011).
For mortality data, when available, we extracted and reported hazard ratios (HR). If HR were not available, we would have made every effort to estimate as accurately as possible the HR using the available data and a purpose built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007). We reported HR for other outcomes it they were reported in the studies.
We reported secondary outcomes as groups of transfusion‐related and drug‐related AEs. If this was not possible due to duplicate counting of the same participant who may have experienced more than one AE of the same category (e.g. more than one transfusion‐related AE). In this case, we reported subgroup categories of AEs separately and reported the 99% CI of the pooled RR to allow for multiple statistical testing.
Where appropriate, we reported the number‐needed‐to‐treat‐to‐benefit (NNTB) and the number‐needed‐to‐treat‐to‐harm (NNTH) with CIs. If we could not report the available data in any of the formats described above, we performed a narrative report, and if appropriate we presented the data in tables.
Unit of analysis issues
We did not expect to encounter unit of analysis issues as cluster randomised trials, and multiple observations for the same outcome are unlikely to be included in this review. We did not encounter any unit of analysis issues and should any trials of these designs have arisen, we would have treated these in accordance with the advice given in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If participants were randomised more than once we would have contacted the authors of the trial to provide us with data on outcomes associated with the initial randomisation.
Dealing with missing data
We dealt with missing data according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We contacted the lead author of the Foucan trial for additional data on creatinine clearance and also to confirm the actual number of participants that are included in the proteinuria analysis, at the time of review publication we had not received a response (Foucan 1998). We recorded the number of participants lost to follow‐up for each trial if possible. Where possible, we analysed data on an intention‐to‐treat (ITT) basis, but if insufficient data were available, we presented per protocol analyses (Higgins 2011b).
Assessment of heterogeneity
If the clinical and methodological characteristics of individual trials were sufficiently homogeneous, we would have combined the data to perform a meta‐analysis. We would have assessed statistical heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. We would have used the I² statistic to quantify the degree of potential heterogeneity and classify it as moderate if I² > 50%, or considerable if I² > 80%. We perceived that we would identify at least moderate clinical and methodological heterogeneity within the trials selected for inclusion, and hence we planned to use the random‐effects model throughout. If statistical heterogeneity was considerable, we would not have reported the overall summary statistic. We would have assessed potential causes of heterogeneity by sensitivity and subgroup analyses (Deeks 2011).
Assessment of reporting biases
We did not identify at least 10 trials for inclusion in a meta‐analysis, so we did not explore potential publication bias (small trial bias) by generating a funnel plot and using a linear regression test. We would have considered P < 0.1 as significant for this test (Sterne 2011).
Data synthesis
We presented the different comparisons separately. We performed analyses according to the recommendations of chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions using aggregated data for analysis (Deeks 2011). For statistical analysis, we entered data into the Review Manager software (RevMan 2014). One review author (PF) entered the data and a second (NR) checked for accuracy.
If meta‐analyses were feasible, we would have used the random‐effects model for pooling the data. For dichotomous outcomes we would have used the Mantel‐Haenszel method or the Peto method as necessary, and for continuous outcomes the inverse variance method (and SMDs as necessary). If I² had been > 80%, we would have presented results as a narrative, and commented on any trends in the data within the results section of the review.
Subgroup analysis and investigation of heterogeneity
There was insufficient data to perform subgroup analyses, however, If adequate data were available, we would have performed subgroup analyses according to Cochrane recommendations for each of the following outcomes in order to assess the effect on heterogeneity (Deeks 2011).
Age of participant (neonate, child (1 to 15 years), adult (16 years and older)
Genotype (homozygous SCD (SS), sickle beta thalassaemia (Sβº and Sβ⁺) and sickle haemoglobin C disease (SC))
Severe SCD complications (strokes, acute chest syndrome, painful crisis, priapism)
People with proteinuria (ACR > 3 mg/mmol or PCR > 5 mg/mmol versus others)
Presence of CKD according to recognised classifications of kidney disease
People with hyperfiltration (defined as GFR > 120 mL/min/1.73 m²)
Sensitivity analysis
There were insufficient data to perform a sensitivity analysis; however, If enough data were available we would have assessed the robustness of our findings by performing the following sensitivity analyses according to Cochrane recommendations where appropriate (Deeks 2011):
• including only those trials with a 'low risk of bias' (e.g. RCTs with methods assessed as low risk for random sequence generation and concealment of treatment allocation); • including only those trials with less than a 20% dropout rate.
Summary of findings table
We used the GRADE approach to create a 'Summary of findings' table, as suggested in chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We used the GRADE approach to rate the quality of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations.
Risk of bias: serious or very serious
Inconsistency: serious or very serious
Indirectness: serious or very serious
Imprecision: serious or very serious
Publication bias: likely or very likely
We reported separate 'Summary of findings' tables for each comparison for the following outcomes.
Reduction or prevention of kidney disease progression
SAEs related to transfusion, drug treatments and SCD complications
Mortality (all‐cause)
Quality of life
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See PRISMA flow diagram (Figure 3).
3.
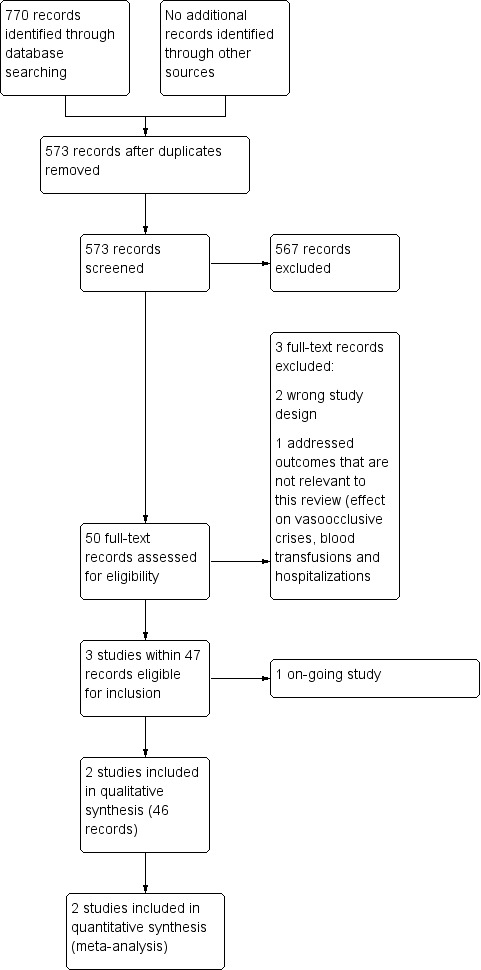
Study flow diagram.
In the searches for this review, we identified a total of 770 citations, which were reduced to 572 citations once duplicates were removed. Two review authors (NR, PF) excluded 567 citations on the basis of the abstract, and two authors (NR, PF) reviewed six full text articles for relevance. We included two trials (47 publications) and excluded three trials that were not relevant. We identified one ongoing trial, no trials are awaiting classification.
Included studies
We identified two trials with a total of 215 participants that met the predefined inclusion criteria (BABY HUG 2011; Foucan 1998).
Trial design and setting
Both trials were RCTs. One trial was conducted in 13 centres in the USA for a two‐year period between October 2003 and September 2009 (BABY HUG 2011). The second trial was conducted in 1996 for six months in an outpatient department in a university hospital centre on the Carribean island of Guadaloupe (Foucan 1998).
Trial size
One trial enrolled 196 participants who were followed for two years (BABY HUG 2011); and the other enrolled 22 participants who were followed for six months (Foucan 1998).
Participants
The Baby Hug trial enrolled children aged 9 to18 months with HbSS or HbSβº irrespective of disease severity (BABY HUG 2011). Children were excluded if they had a transfusion within two months or were on chronic transfusion therapy; had an abnormal transcranial doppler ultrasound (TCD) velocity; severe developmental delay (e.g. cerebral palsy or other mental retardation, stroke with neurological deficit); surgical splenectomy; previous or current treatment with hydroxyurea or another anti‐sickling drug (see Characteristics of included studies for a complete list of exclusion criteria).
In the Foucan trial, participants were 18 years of age or older, had HbSS disease, normal blood pressure and persistent microalbuminuria (Foucan 1998). Participants were excluded if they had hypertension, heart, kidney, liver, or systemic disease; pregnant; or taking anti‐inflammatory or antihypertensive medications.
Interventions
The Baby Hug trial compared hydroxyurea at 20 mg/kg per day to matching placebo (BABY HUG 2011). The Foucan trial compared the ACEI captopril at an initial dose of 6.25 mg per day during the first month, 12.5 mg per day during the second and the third months, and 25 mg per day after the third month to matching placebo (Foucan 1998).
Outcomes
In the Baby Hug trial the co‐primary outcome was the effect of hydroxyurea on splenic and liver function (as measured by GFR); and secondary outcomes included: growth and development; neuro‐development assessment; complications of sickle‐cell anaemia, and any other SAEs (BABY HUG 2011).
The Foucan trial measured the effects of captopril on the progression of albuminuria and blood pressure (Foucan 1998).
Source
Both trials received government funding.
Excluded studies
We excluded three trials: two were not randomised (NCT02373241; Steinberg 2003); and one recruited participants who did not have CKD and addressed outcomes that are not relevant to this review (effect on vaso‐occlusive crises, blood transfusions and hospitalizations) (Jain 2012).
Ongoing studies
We identified one ongoing trial on atorvastatin, due to be completed December 2017 (NCT01732718).
Risk of bias in included studies
Please refer to the figures for visual representations of the assessments of risk of bias across all trials and for each item in the included trials (Figure 4; Figure 5). See the risk of bias section in the 'Characteristics of included studies' section for further information about the bias identified within the individual trials.
4.
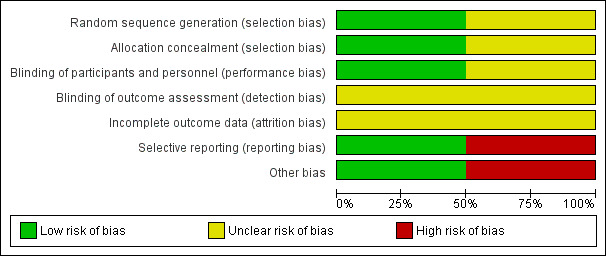
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias)
We considered one trial to be at low risk for selection bias as the telephone randomisation schedule was developed by the medical co‐ordinating centre (BABY HUG 2011).
We considered one trial to be at unclear risk for selection bias as there was no description of the method of randomisation (Foucan 1998).
Allocation concealment (selection bias)
We considered the Baby Hug trial to be at a low risk of bias for allocation concealment as allocation was done centrally by the drug distribution centre and hydroxyurea and placebo had the same packaging, appearance and taste (BABY HUG 2011).
The Foucan trial was considered to be at an unclear risk of bias as no description of allocation concealment was provided (Foucan 1998).
Blinding
Blinding of participants and personnel (performance bias)
We considered one trial to have a low risk of performance bias as participants, caregivers and medical co‐ordinating staff were masked to treatment (BABY HUG 2011).
We considered one trial to have an unclear risk of performance bias as there is no description of blinding of personnel (Foucan 1998). Also it is not clear if dosing schedules were similar in both groups.
Blinding of outcome assessment (detection bias)
We considered both trials to be at an unclear risk of detection bias. In one trial it is not clear if all assessors were blinded (BABY HUG 2011) and in the second trial no description of blinding of outcome assessment was provided (Foucan 1998).
Incomplete outcome data
We judged both trials to be at unclear risk for attrition bias. In the Baby Hug trial the co‐primary endpoints were reported only in participants with entry and exit values resulting in a GFR analysis with missing values for approximately 25% of participants (BABY HUG 2011).
In the Foucan trial, two participants, one in each group, withdrew within the first month; also one participant in the placebo group did not comply with treatment and another participant in the placebo group developed proteinuria during the third month (Foucan 1998). It is not clear if these participants were included in the six‐month analysis.
Selective reporting
We judged one trial to be at low risk of reporting bias as all outcomes were reported (BABY HUG 2011).
We found the Foucan trial to be at high risk of bias for selective reporting as creatinine clearance is an important marker of kidney disease progression, but no data on values at the end of six months were provided (Foucan 1998).
Other potential sources of bias
We considered the Foucan trial to be at high risk of other sources of bias as the sample size is small and not powered to detect a difference between groups, furthermore the follow‐up period was too short to assess long‐term benefits or harms (Foucan 1998).
No other sources of bias were detected for the Baby Hug trial (BABY HUG 2011).
Effects of interventions
Hydroxyurea versus placebo
This comparison includes one trial with 193 young children aged 8 to 19 months (BABY HUG 2011).
See also Table 3 for unadjusted HRs for SAEs and AEs reported in the Baby Hug trial (BABY HUG 2011).
1. Unadjusted HRs for SAEs and AEs reported in BABY HUG 2011.
| Unadjusted HRs reported in BABY HUG 2011 | ||
| Outcome | HR | 95% CI |
| Acute chest syndrome | 0.36 | 0.15 to 0.87 |
| Painful crisis | 0.54 | 0.36 to 0.83 |
| Hospitalisations | 0.73 | 0.53 to 1.00 |
| Neutropenia | 3.0 | 1.7 to 5.1 |
| Thrombocytopenia | 1.6 | 0.6 to 4.1 |
| Transfusions | 0.55 | 0.32 to 0.96 |
CI: confidence interval HR: hazard ratio
Primary outcomes
1. Reduction or prevention of kidney disease progression
We are very uncertain whether hydroxyurea reduces or prevents kidney disease progression (assessed by change in GFR ), or reduces hyperfiltration, in children aged 9 to 18 months, MD 0.58 (95% CI ‐14.60 to 15.76 (mL/ min per 1.73 m²)) (one study; 142 participants; very low‐quality evidence) (Analysis 1.1; Table 1). We downgraded the quality of evidence due to unclear risk of attrition bias (25% of participants were excluded from the analysis); imprecision (the estimate has wide confidence intervals including possible clinically significant harm or benefit); and indirectness (results apply only to small children aged 8 to 19 months).
1.1. Analysis.
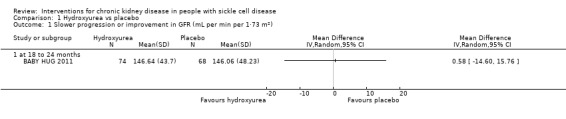
Comparison 1 Hydroxyurea vs placebo, Outcome 1 Slower progression or improvement in GFR (mL per min per 1·73 m²).
Hydroxyurea may improve the ability to concentrate urine in children aged 9 to 18 months, MD 42.23 (95% CI 12.14 to 72.32 mOsm/kg) (one study; 178 participants; low‐quality evidence) (Analysis 1.2; Table 1). We downgraded the quality of evidence due to imprecision (one study with 178 participants); and indirectness (results only apply to small children aged 8 to 19 months).
1.2. Analysis.
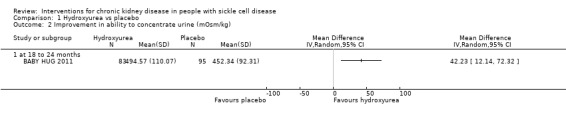
Comparison 1 Hydroxyurea vs placebo, Outcome 2 Improvement in ability to concentrate urine (mOsm/kg).
2. SAEs
We could not report the overall effect of hydroxyurea on SCD‐related SAEs. We therefore reported subgroup categories of SAEs separately and reported the 99% CI of the pooled RR to allow for multiple statistical testing.
Acute chest syndrome
Hydroxyurea may have little or no effect on the incidence of acute chest syndrome in children aged 9 to 18 months, RR 0.39 (99% CI 0.13 to 1.16) (one study, 193 participants, low‐quality evidence) (Analysis 1.3; Table 1). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit); and indirectness (results only apply to small children aged 8 to 19 months).
1.3. Analysis.
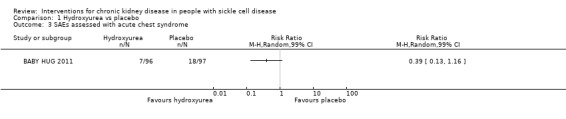
Comparison 1 Hydroxyurea vs placebo, Outcome 3 SAEs assessed with acute chest syndrome.
Painful crisis
Hydroxyurea may have little or no effect on the incidence of painful crises in children aged 9 to 18 months, RR 0.68 (99% CI 0.45 to 1.02) (one study, 193 participants, low‐quality evidence) (Analysis 1.4; Table 1). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit); and indirectness (results only apply to small children aged 8 to 19 months).
1.4. Analysis.
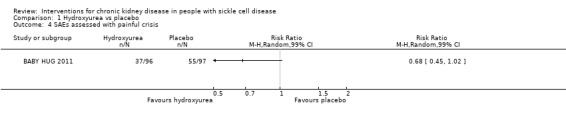
Comparison 1 Hydroxyurea vs placebo, Outcome 4 SAEs assessed with painful crisis.
Hospitalisations
Hydroxyurea may have little or no effect on hospitalisations in children aged 9 to18 months, RR 0.83 (99% CI 0.68 to 1.01) (one study, 193 participants, low‐quality evidence) (Analysis 1.5;Table 1). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit); and indirectness (results only apply to small children aged 8 to 19 months).
1.5. Analysis.
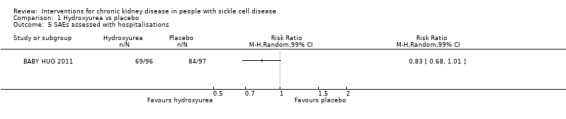
Comparison 1 Hydroxyurea vs placebo, Outcome 5 SAEs assessed with hospitalisations.
Stroke
Hydroxyurea may have little or no difference on the incidence of stroke in children aged 9 to 18 months, Peto OR 0.14 (99% CI 0.00 to 23.62) (one study, 193 participants, very low‐quality evidence) (Analysis 1.6). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit, and it is a rare event); and indirectness (results only apply to small children aged 8 to 19 months).
1.6. Analysis.
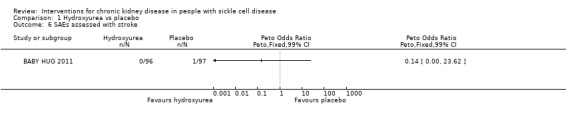
Comparison 1 Hydroxyurea vs placebo, Outcome 6 SAEs assessed with stroke.
3. Mortality due to any cause
No deaths were reported in either group (Table 1).
Secondary outcomes
1. Other complications
Neutropenia
Hydroxyurea may increase the risk of neutropenia in children aged 9 to 18 months, RR 2.53 (99% CI 1.43 to 4.47) (one study, 193 participants, low‐quality evidence) (Analysis 1.7). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant harm); and indirectness (results only apply to small children aged 8 to 19 months).
1.7. Analysis.
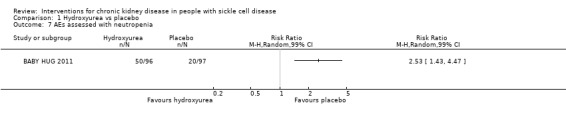
Comparison 1 Hydroxyurea vs placebo, Outcome 7 AEs assessed with neutropenia.
Thrombocytopenia
Hydroxyurea may make little or no difference in the risk of thrombocytopenia in children aged 9 to 18 months, RR 1.59 (99% CI 0.48 to 5.21) (one study, 193 participants, low‐quality evidence) (Analysis 1.8). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit or harm); and indirectness (results only apply to small children aged 8 to 19 months).
1.8. Analysis.
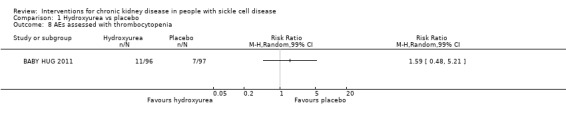
Comparison 1 Hydroxyurea vs placebo, Outcome 8 AEs assessed with thrombocytopenia.
2. Quality of life (measured on a validated scale)
This outcome was not reported.
3. Number of units or volume (mL) of red blood cells infused (regardless of intervention)
Number of participants transfused
Hydroxyurea may reduce the number of children aged 9 to 18 months requiring a transfusion, RR 0.61 (95% CI 0.38 to 0.99) (one study, 193 participants, low‐quality evidence) (Analysis 1.9). We downgraded the quality of evidence due to imprecision (the estimate has wide CIs including a possible clinically significant benefit); and indirectness (results only apply to small children aged 8 to 19 months).
1.9. Analysis.
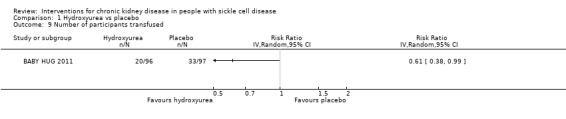
Comparison 1 Hydroxyurea vs placebo, Outcome 9 Number of participants transfused.
ACEI versus placebo
This comparison includes one trial with 22 adults (18 years and older) with normal blood pressure and microalbuminuria (Foucan 1998).
Primary outcomes
Reduction or prevention of kidney disease progression
We are very uncertain if ACEI reduce proteinuria in adults with normal blood pressure and microalbuminuria, MD ‐49.00 (95% CI ‐124.10 to 26.10 (mg per day)) (one study, 22 participants, very low‐quality evidence) (Analysis 2.1; Table 2). We downgraded the quality of evidence as there was high or unclear risk of bias in all domains; imprecision (the estimate has very wide CIs including clinically significant harm or benefit); and indirectness (small population of adults with normal blood pressure and albuminuria).
2.1. Analysis.
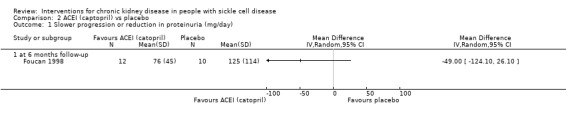
Comparison 2 ACEI (captopril) vs placebo, Outcome 1 Slower progression or reduction in proteinuria (mg/day).
We are very uncertain if ACEI reduce or prevent kidney disease as measured by creatinine clearance. The authors state that creatinine clearance remained constant over six months in both groups, but no comparative data were provided (very low‐quality evidence). We downgraded the quality of evidence for high or unclear risk of bias in all domains and indirectness (small population of adults with normal blood pressure and albuminuria). We contacted the authors for data on creatinine clearance but at review publication we had not yet received a response.
SAEs
SAEs outcomes were not reported in this trial.
Mortality due to any cause
Mortality was not reported.
Secondary outcomes
Other complications
One incidence of dry cough was reported in the ACEI group, RR 2.54 (99% CI 0.04 to 148.91) (one study, 22 participants, very low‐quality evidence) (Analysis 2.2). We downgraded the quality of evidence due to high or unclear risk of bias in all domains; imprecision (the estimate has very wide CIs and includes possible clinically significant harm); and indirectness (small population of adults with normal blood pressure and albuminuria).
2.2. Analysis.
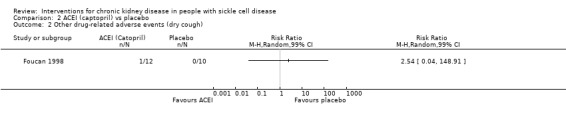
Comparison 2 ACEI (captopril) vs placebo, Outcome 2 Other drug‐related adverse events (dry cough).
Quality of life (measured on a validated scale)
Quality of life was not reported in the trial.
Number of units or volume (mL) of red blood cells infused (regardless of intervention)
Red blood cell transfusions were not reported in the trial.
Discussion
Summary of main results
Hydroxyurea versus placebo
One trial with 193 children aged 9 months to 18 months was included in this comparison (Table 1):
we are very uncertain whether hydroxyurea improves glomerular filtration rate (GFR) or reduces hyperfiltration in young children aged 9 to 18 months;
hydroxyurea may improve the ability to concentrate urine mOsm/kg in young children aged 9 to 18 months;
hydroxyurea may have little or no difference on the incidence of acute chest syndrome, painful crises and hospitalisations in young children aged 9 to 18 months.
Angiotensin converting enzyme inhibitor (ACEI) versus placebo
One trial with 22 adults with normal blood pressure and microalbuminuria was included in this comparison (Table 2):
we are very uncertain if ACEI reduce proteinuria or reduce or prevent kidney disease as measured by creatinine clearance in adults with normal blood pressure and albuminuria.
Overall completeness and applicability of evidence
This review provides the most up‐to‐date assessment of the effectiveness and safety of interventions in preventing or reducing kidney complications in people with sickle cell disease (SCD).
The results of this review can only be interpreted in consideration of the following factors:
the findings in this review can only be generalised to hydroxyurea treatment in young children aged 9 to 18 months;
the findings of this review cannot be generalised to adults taking ACEI as the trial had too few participants and too short a follow‐up time;
due to lack of evidence, this review cannot comment on management for children aged over 18 months and any known genotype of SCD, other than HbSS; and adults with any known genotype of SCD.
Quality of the evidence
Overall the quality of the evidence was rated low to very low across different outcomes according to GRADE methodology (Table 1; Table 2).
Potential biases in the review process
To our knowledge, our review process is free from bias. We conducted a comprehensive search and searched data sources (including multiple databases, and clinical trial registries) to ensure that all relevant trials would be captured. There were no restrictions for the language in which the paper was originally published. The relevance of each paper was carefully assessed and all screening and data extractions were performed independently and in duplicate. We pre‐specified all outcomes and subgroups prior to analysis. There were insufficient numbers of included trials to conduct meta‐analyses or assess publication bias.
Agreements and disagreements with other studies or reviews
The Cochrane Review on ACE inhibitors for proteinuria and microalbuminuria in people with SCD (Sasongko 2015) identified a single study meeting their inclusion criteria (Foucan 1998). This study is one of the two which we included in this review (the rationale for the overlap between the objectives of the 2015 review and this review was to identify any studies in which ACEI and blood transfusions were compared as treatment modalities in preventing progression of kidney disease). We have rated the quality of evidence as very low according to GRADE methodology. Both reviews have similar conclusions where Sasongko concludes that "there is not enough evidence to show that the administration of ACE inhibitors is associated with a reduction of microalbuminuria and proteinuria in people with sickle cell disease, although a potential for this was seen" (Sasongko 2015). We conclude that we are very uncertain whether giving ACEI to adults with SCD and with normal blood pressure and microalbuminuria has any effect on preventing or reducing kidney complications.
There were no studies identified in which red blood cell transfusions were used to prevent the development of chronic kidney disease.
A small (N = 23), uncontrolled study in older children (mean (standard deviation) age of 7.4 (3.5) years (Aygun 2013) showed a reduction in hyperfiltration after three years of treatment with hydroxyurea, although GFR still remained elevated. The authors suggested that escalating children to the maximum tolerated dose could have been a factor (amongst other variables) in GFR improvement, whereas in the Baby Hug trial, a 20 mg dose of hydroxyurea was administered with no dose escalation (BABY HUG 2011). However, the results reported by Aygun can only be confirmed by an adequately powered randomised dose‐escalation trial (Aygun 2013).
Authors' conclusions
Implications for practice.
Based on current evidence, in young children aged 9 to 18 months, we are very uncertain if hydroxyurea improves GFR or reduces hyperfiltration, it may improve young children's ability to concentrate urine but may make little or no difference on the incidence of acute chest syndrome, painful crises and hospitalisations.
Based on current evidence, we are very uncertain if giving ACEI to adults with normal blood pressure and microalbuminuria has any effect on preventing or reducing kidney complications.
This review identified no trials that looked at red blood cell transfusions or any other interventions to prevent or reduce kidney complications.
Due to lack of evidence this review cannot comment on management of children aged over 18 months or adults with SCD disease.
Implications for research.
We estimated that an adjusted sample size of 2986 participants would be needed to show a 90% chance of detecting, as significant at the 5% level, a decrease in the incidence of proteinuria from 20% in the control group (McKie 2007; Sharpe 2014) to 15% in the experimental group with 5% non‐compliance or cross‐over rate. As well, an adjusted sample size of 2356 participants is required to have a 90% chance of detecting, as significant at the 5% level, a decrease in the incidence of end‐stage renal disease from 12% in the control group (Powars 2005) to 8% in the experimental group with a 10% non‐compliance or cross‐over rate (estimates calculated using Sealed Envelope). This would mean if this treatment is effective we could detect its ability to prevent four people developing end‐stage renal disease for every 100 people treated (a reduction of 33%).
People with SCD are living longer and now have an increased risk of developing chronic kidney disease during their lifetime. Both this Cochrane Review and the Cochrane Review on ACE inhibitors for proteinuria and microalbuminuria in people with SCD (Sasongko 2015) have identified a striking lack of adequately designed and powered studies, and no ongoing trials which address this critical question. Trials of hydroxyurea, ACEI or red blood cell transfusion in older children and adults are urgently needed to determine any effect on prevention or reduction kidney complications in people with SCD. Also longer‐term trials with adequate power comparing hydroxyurea, ACEI or red cell transfusions to placebo or other interventions are urgently required.
Acknowledgements
We thank the editorial base of the Cochrane Cystic Fibrosis and Genetic Disorders Group for their help and support.
We thank the National Institute for Health Research (NIHR). This review is part of a series of reviews that have been partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components. This research was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. Glossary
Alloimmunisation An immune response to foreign antigens as a result of exposure to donor blood transfusions
Enuresis Inability to control urination and includes bedwetting by children
Epigenetic Traits that are not determined by the DNA code itself but rather by modifications of the DNA bases or of proteins associated with DNA
Extravascular Outside the blood vessel or vascular system
Glomerular Network of filters in the kidney that filter waste from the blood
Glomerulosclerosis Scarring or hardening of the glomeruli, the tiny blood vessels in the kidney
Haematopoiesis The production of red blood cells, white blood cells, and platelets from stem cells within the bone marrow
Hypoxia Lack of oxygen reaching the cells of the kidney
Intravascular Within the blood vessel or vascular sytem
Ischaemia Restriction of blood supply to tissues
Nephropathy Damage to or disease of the kidney
Renal papillary necrosis This is a disorder of the kidneys in which all or part of the kidney papillae die. The kidney papillae are the areas where the openings of the collecting ducts enter the kidney, and where the urine flows into the ureters
Renal tubules Small tube‐shaped structures that remove salt, excess fluids, and waste products from the blood
Splenic sequestration This happens when large pools of sickled red blood cells are trapped in the spleen resulting in damage to the spleen
Appendix 2. Search strategies
CENTRAL (the Cochrane Library) #1 MeSH descriptor: [Anemia, Sickle Cell] explode all trees #2 MeSH descriptor: [Hemoglobin, Sickle] this term only #3 ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbAS or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases") #4 (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*) #5 (sickle and SCD) #6 ((Hb S or HbS or sickle) near/3 (disease* or thalass?emi*)) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Kidney Diseases] explode all trees #9 MeSH descriptor: [Urologic Diseases] this term only #10 ((kidney or renal) near/5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)) #11 ("end‐stage renal" or "end‐stage kidney" or "endstage renal" or "endstage kidney" or "chronic kidney" or "chronic renal") #12 (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD) #13 MeSH descriptor: [Hematuria] this term only #14 MeSH descriptor: [Proteinuria] explode all trees #15 (proteinuria* or hematuria* or haematuria* or hemoglobinuria* or haemoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin near/2 creatine)) #16 MeSH descriptor: [Renal Replacement Therapy] explode all trees #17 ((renal or kidney) near/3 (transplant* or replacement*)) #18 (predialysis or pre‐dialysis or dialysis or hemodialysis or haemodialysis or hemofiltration or haemofiltration or hemodiafiltration or haemodiafiltration) #19 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #7 and #19 #21 sickle cell nephropathy #22 #20 or #21
MEDLINE (OvidSP) 1. exp Anemia, Sickle Cell/ 2. Hemoglobin, Sickle/ 3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw,kf. 4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw,kf. 5. (sickle and SCD).tw,kf. 6. ((Hb S or HbS or sickle) adj3 (disease* or thalass?emi*)).tw,kf. 7. or/1‐6 8. exp Kidney Diseases/ 9. Urologic Diseases/ 10. ((kidney or renal) adj5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)).tw,kf. 11. (end‐stage renal or end‐stage kidney or endstage renal or endstage kidney or chronic kidney or chronic renal).tw,kf. 12. (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD).tw,kf. 13. Hematuria/ 14. exp Proteinuria/ 15. (proteinuria* or h?ematuria* or h?emoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin adj2 creatine)).tw,kf. 16 exp Renal Replacement Therapy/ 17. ((renal or kidney) adj3 (transplant* or replacement*)).tw,kf. 18. (predialysis or pre‐dialysis or dialysis or h?emodialysis or h?emofiltration or h?emodiafiltration).tw,kf. 19. or/8‐18 20. 7 and 19 21. sickle cell nephropathy.tw,kf. 22. 20 or 21 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. randomi*.tw. 26. placebo.ab. 27. clinical trials as topic.sh. 28. randomly.ab. 29. groups.ab. 30. trial.tw. 31. or/23‐30 32. exp animals/ not humans/ 33. 31 not 32 34. 22 and 33
Embase (OvidSP) 1. exp Sickle Cell Anemia/ 2. Hemoglobin S/ 3. (h?emoglobin S or h?emoglobin SC or h?emoglobin SE or h?emoglobin SS or h?emoglobin C disease or h?emoglobin D disease or h?emoglobin E disease or Hb SC or HbSC or HbAS or HbSS or HbAC or Hb SE or Hb SS or Hb C disease or Hb D disease or Hb E disease or SC disease*).tw,kw. 4. (sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*).tw,kw. 5. (sickle and SCD).tw,kw. 6. ((Hb S or HbS or sickle) adj3 (disease* or thalass?emi*)).tw,kw. 7. or/1‐6 8. exp Kidney Disease/ 9. Urinary Tract Disease/ 10. ((kidney or renal) adj5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)).tw,kw. 11. (end‐stage renal or end‐stage kidney or endstage renal or endstage kidney or chronic kidney or chronic renal).tw,kw. 12. (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD).tw,kw. 13. Hematuria/ 14. exp Proteinuria/ 15. (proteinuria* or h?ematuria* or h?emoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin adj2 creatine)).tw,kw. 16. exp Renal Replacement Therapy/ 17. ((renal or kidney) adj3 (transplant* or replacement*)).tw,kw. 18. (predialysis or pre‐dialysis or dialysis or h?emodialysis or h?emofiltration or h?emodiafiltration).tw,kw. 19. or/8‐18 20. 7 and 19 21. sickle cell nephropathy.tw,kw. 22. 20 or 21 23. crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ 24. (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or doubl* blind* or singl* blind* or assign* or allocat* or volunteer*).mp. 25. 23 or 24 26. 22 and 25 27. limit 26 to embase
CINAHL (EBSCOHost) S1 (MH "Anemia, Sickle Cell+") S2 TX ("hemoglobin S" or "haemoglobin S" or "hemoglobin SC" or "haemoglobin SC" or "hemoglobin SE" or "haemoglobin SE" or "hemoglobin SS" or "haemoglobin SS" or "hemoglobin C disease" or "hemoglobin D disease" or "hemoglobin E disease" or "haemoglobin C disease" or "haemoglobin D disease" or "haemoglobin E disease" or "Hb SC" or HbSC or HbAS or HbSS or HbAC or "Hb SE" or "Hb SS" or "Hb C disease" or "Hb D disease" or "Hb E disease" or "SC disease" or "SC diseases" OR sickle cell* or sicklemia or sickled or sickling or meniscocyt* or drepanocyt*) S3 TX ((Hb S or HbS or sickle) N3 (disease* or thalass?emi*)) S4 S1 OR S2 OR S3 S5 (MH "Kidney Diseases+") S6 (MH "Urologic Diseases") S7 ((kidney or renal) N5 (disease* or injur* or insufficienc* or function* or dysfunction* or abnormal* or damage* or failure* or complication* or manifestation*)) S8 ("end‐stage renal" or "end‐stage kidney" or "endstage renal" or "endstage kidney" or "chronic kidney" or "chronic renal") S9 (ESRF or ESKF or ESRD or ESKD or CKF or CKD or CRF or CRD or CAPD or CCPD or APD) S10 (MH "Hematuria") S11 (MH "Proteinuria+") S12 (proteinuria* or hematuria* or haematuria* or hemoglobinuria* or haemoglobinuria* or erythrocyturia* or albuminuria* or microalbuminuria* or macroalbuminuria* or (albumin N2 creatine)) S13 (MH "Renal Replacement Therapy+") S14 ((renal or kidney) N3 (transplant* or replacement*)) S15 (predialysis or pre‐dialysis or dialysis or hemodialysis or haemodialysis or hemofiltration or haemofiltration or hemodiafiltration or haemodiafiltration) S16 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 S17 S4 AND S16 S18 sickle cell nephropathy S19 S17 OR S18 S20 (MH Clinical Trials+) S21 PT Clinical Trial S22 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*)) S23 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) S24 TI randomi* OR AB randomi* S25 MH RANDOM ASSIGNMENT S26 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three)) S27 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) ) S28 MH PLACEBOS S29 TI placebo* OR AB placebo* S30 MH QUANTITATIVE STUDIES S31 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 S32 S19 AND S31
PubMed #1 ("hemoglobin S" OR "haemoglobin S" OR "hemoglobin SC" OR "haemoglobin SC" OR "hemoglobin SE" OR "haemoglobin SE" OR "hemoglobin SS" OR "haemoglobin SS" OR "hemoglobin C disease" OR "hemoglobin D disease" OR "hemoglobin E disease" OR "haemoglobin C disease" OR "haemoglobin D disease" OR "haemoglobin E disease" OR "Hb SC" OR HbSC OR HbAS OR HbSS OR HbAC OR "Hb SE" OR "Hb SS" OR "Hb C disease" OR "Hb D disease" OR "Hb E disease" OR "SC disease" OR "SC diseases" OR "sickle cell" OR "sickle cells" OR sicklemia OR sickled OR sickling OR meniscocyt* OR drepanocyt* OR (sickle and SCD)) #2 ((Hb S OR HbS OR sickle) AND (disease* OR thalass?emi*)) #3 #1 OR #2 #4 ((kidney OR renal) AND (disease* OR injur* OR insufficienc* OR function* OR dysfunction* OR abnormal* OR damage* OR failure* OR complication* OR manifestation*)) #5 ("end‐stage renal" OR "end‐stage kidney" OR "endstage renal" OR "endstage kidney" OR "chronic kidney" OR "chronic renal" OR ESRF OR ESKF OR ESRD OR ESKD OR CKF OR CKD OR CRF OR CRD OR CAPD OR CCPD OR APD) #6 (proteinuria* OR hematuria* OR haematuria* OR hemoglobinuria* OR haemoglobinuria* OR erythrocyturia* OR albuminuria* OR microalbuminuria* OR macroalbuminuria* OR (albumin AND creatine)) #7 ((renal OR kidney) AND (transplant OR transplants OR transplantation OR transplantations OR transplanted OR replacement*)) #8 (predialysis OR pre‐dialysis OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration) #9 #4 OR #5 OR #6 OR #7 OR #8 #10 #3 AND #9 #11 sickle cell nephropathy #12 #10 OR #11 #13 (random* OR blind* OR "control group" OR placebo* OR "controlled study" OR groups OR trial* OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR pubmed OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #14 #12 AND #13
Transfusion Evidence Library sickle AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy)
LILACS tw:(sickle AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy)) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
IndMed (sickle OR sicklemia OR sickled OR sickling OR SC disease) AND (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy) AND (randomized OR randomised OR randomly OR random OR blind OR blinded OR trial OR placebo OR control group OR groups)
KoreaMed & PakMediNet "randomized controlled trial" [PT] AND sickle [ALL]
Web of Science CPCI‐S TOPIC: (sickle OR sicklemia OR sickled OR sickling) AND TOPIC: (kidney OR renal OR dialysis OR hemodialysis OR haemodialysis OR hemofiltration OR haemofiltration OR hemodiafiltration OR haemodiafiltration OR proteinuria OR hematuria OR haematuria OR hemoglobinuria OR haemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy) AND TOPIC: (random* OR blind* OR control group OR placebo OR controlled study OR groups OR trial OR trials OR systematic review OR meta‐analysis OR metaanalysis OR medline OR pubmed OR cochrane OR embase)
ClinicalTrials.gov Search Terms: kidney OR renal OR dialysis OR hemodialysis OR hemofiltration OR hemodiafiltration OR proteinuria OR hematuria OR hemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy Condition: sickle cell anemia Study Type: Interventional Studies
WHO ICTRP Title: kidney OR renal OR dialysis OR hemodialysis OR hemofiltration OR hemodiafiltration OR proteinuria OR hematuria OR hemoglobinuria OR erythrocyturia OR albuminuria OR microalbuminuria OR macroalbuminuria OR nephropathy Condition: sickle cell anemia Recruitment Status: ALL
Data and analyses
Comparison 1. Hydroxyurea vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Slower progression or improvement in GFR (mL per min per 1·73 m²) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement in ability to concentrate urine (mOsm/kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 at 18 to 24 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SAEs assessed with acute chest syndrome | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 4 SAEs assessed with painful crisis | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 5 SAEs assessed with hospitalisations | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 6 SAEs assessed with stroke | 1 | Peto Odds Ratio (Peto, Fixed, 99% CI) | Totals not selected | |
| 7 AEs assessed with neutropenia | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 8 AEs assessed with thrombocytopenia | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected | |
| 9 Number of participants transfused | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 2. ACEI (captopril) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Slower progression or reduction in proteinuria (mg/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 at 6 months follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Other drug‐related adverse events (dry cough) | 1 | Risk Ratio (M‐H, Random, 99% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BABY HUG 2011.
| Methods |
Study design: RCT Study grouping: parallel group |
|
| Participants |
Baseline characteristics Hydroxyurea
Placebo
Inclusion criteria: participants aged 9 ‐ 18 months were recruited between October 2003, and September 2007, at 13 trial centres in the USA; eligible participants had HbSS or Sβ⁰thalassaemia, and were enrolled irrespective of clinical severity. Exclusion criteria: transfusion within two months; height, weight, or head circumference less than the 5th percentile; MDI) less than 70; abnormal TCD velocity; chronic transfusion therapy; cancer; severe developmental delay (e.g. cerebral palsy or other mental retardation); grade III/IV intraventricular haemorrhage; stroke with neurological deficit; surgical splenectomy; participating in other clinical intervention trials; probable or known diagnosis of haemoglobin S‐hereditary persistence of fetal haemoglobin; known HbSβ⁺ thalassaemia (haemoglobin A present); any condition or chronic illness, which in the opinion of the principal investigator, makes participation unadvised or unsafe; inability or unwillingness to complete baseline (pre‐enrolment) studies, including blood or urine specimen collection, liver‐spleen scan, abdominal sonogram, neurological examination, neuropsychological testing, or transcranial doppler ultrasound (interpretable study not required, but confirmed velocity greater than 200 cm/second results in ineligibility); previous or current treatment with hydroxyurea or another anti‐sickling drug (additional exclusion criteria from trial registration NCT00006400). |
|
| Interventions |
Hydroxyurea: 20 mg/kg/day; local pharmacists reconstituted powder with syrup and water to a concentration of 100 mg/mL, and dispensed a 35‐day supply. There was no dose escalation. Placebo: hydroxyurea and placebo powders had the same appearance and packaging and the liquid formulations had the same appearance and taste. Hydroxyurea and placebo were distributed to clinical centres in encoded kits. |
|
| Outcomes |
Co‐primary outcomes: splenic and liver function (as measured by GFR) Secondary outcomes: investigations of the brain, lungs, hepatobiliary system, and growth and development; monitoring of height, weight, and head circumference; neuro‐development assessment (Bayley Developmental and Vineland Adaptive Behavior Scales); adverse clinical events included known complications of sickle‐cell anaemia, such as pain, dactylitis, acute chest syndrome, stroke, priapism, sepsis or bacteraemia, splenic sequestration, hospitalisation, and transfusion; SAEs. |
|
| Identification |
Sponsorship source: the US National Heart, Lung, and Blood Institute; and the National Institute of Child Health and Human Development Country: USA Setting: 13 medical centres Authors name: Prof W C Wang MD Institution: St Jude Children's Research Hospital, Memphis, TN, USA Email: winfred.wang@stjude.org Address: Hematology MS 800, Room R5036 St. Jude Children's Research Hospital 262 Danny Thomas Place Memphis, TN 38105‐3678 |
|
| Notes | 179 (93%) participants who completed at least 18 months of the trial and at least one exit assessment were analysed; 167 (86%) completed the full study. Hydroxyurea: 4 withdrawals: 3 lost to follow‐up; 1 incorrect diagnosis; 91 analysed. Placebo: 9 withdrawals: 4 declined further participation; 2 moved; 2 lost to follow‐up; 1 placed on chronic transfusion; 88 analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation sequence was pre‐decided by a randomisation schedule developed for each clinical site by the medical coordinating centre. Double‐blind randomisation was done with an automated telephone response system and the use of a random three digit kit number for each enrolled participant." |
| Allocation concealment (selection bias) | Low risk | "The kit number, which was linked to the assignment sequence, was used by the drug distribution centre to ship the appropriate study drug to the clinical site pharmacy. Hydroxycarbamide and placebo powders had the same appearance and packaging and the liquid formulations had the same appearance and taste. Hydroxyurea and placebo were distributed to clinical centres in encoded kits." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Hydroxyurea and placebo were distributed to clinical centres in encoded kits. Local pharmacists reconstituted powder with syrup and water to a concentration of 100 mg/mL, and dispensed a 35‐day supply. As in the HUSOFT trial, there was no dose escalation. Participants, caregivers, and medical coordinating centre staff were masked to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "An unmasked so‐called primary endpoint person monitored laboratory values and assisted in clinical management. Masked readings of splenic uptake on ⁹⁹ m Tc‐sulphur colloid liver‐spleen scans were categorised qualitatively as normal, decreased (but present), or absent." While the Methods state "double blind randomisation", it is not clear whether this is at the level of the outcome assessors as well as at the level of the drug administration.The statement about an unmasked primary endpoint assessor monitoring laboratory values is suggestive of a risk of bias; however; they may have only monitored for safety, whereas the splenic readings were done by someone who was blinded to the study drug allocation. It is not clear whether the assessor of the GFR was blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States that all participants randomly assigned to a treatment group were analysed for the co‐primary endpoints ‐ but also 'Total number of participants assessed for each endpoint. N differs from the number reported in table one because only entry values that are paired with exit values from the same participants are included. Both co‐primary endpoints are per protocol analysis and only include participants with paired entry and exit values. There were approximately 25% of participants with no entry/exit GFR values. 'All other outcomes reported as intention to treat'. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes stated in the methods were reported in the results. |
| Other bias | Low risk | No other sources of bias were detected from the Baby Hug trial |
Foucan 1998.
| Methods |
Study design: RCT Study grouping: parallel group |
|
| Participants |
Baseline characteristics ACEI (captopril)
Placebo
Inclusion criteria: homozygous for haemoglobin SS; 18 years of age or older; diagnosis of sickle cell anaemia based on clinical and biological data including haemoglobin electrophoresis;urinary albumin excretion between 30 and 300 mg per 24 hours on three separate occasions during the 6‐month period preceding the study. Exclusion criteria: non‐HbSS genotype; age < 18 years; hypertension (blood pressure > 140/90 mm Hg); evidence of heart, kidney, liver, or systemic disease; pregnant; taking anti‐inflammatory or antihypertensive medications. Pre‐treatment: no statistical differences. |
|
| Interventions |
Intervention characteristics ACEI (captopril):
Placebo:
|
|
| Outcomes | Outcomes: efficacy of ACE inhibitors in the progression of albuminuria and their effects on blood pressure in people with sickle cell anemia. | |
| Identification |
Sponsorship source: supported by grants from the Programme Hospitalier de Recherche Clinique (PHRC), France. Country: France (Guadeloupe) Setting: outpatients in one hospital Comments: Centre Hospitalo Universitaire (CHU) of Pointe‐a`‐Pitre in Guadeloupe in 1996 Authors name: Lydia Foucan Institution: University Hospital, Pointe‐a‐Pitre, Guadeloupe; the Sickle Cell Center of Guadeloupe Email: lydia.foucan@chu‐guadeloupe.fr Address: Departement d’Information Medicale et Sante Publique, Centre Hospitalier Universitaire de Pointe‐a‐Pitre 97159, Guadeloupe, French West Indies. |
|
| Notes | Blood pressure was measured by the automated oscillometric method (Dynamap) after 5 minutes of rest in a half‐sitting position. Systolic pressure, diastolic pressure, and mean arterial pressure (mBP) were measured as the average of three measurements taken at 5‐minute intervals. We contacted the lead author of Foucan 1998 for additional data on creatinine clearance and also to confirm the actual number of participants that are included in the proteinuria analysis, at the time of review publication we had not received a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no description of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were randomly assigned to two groups, and received captopril or an indistinguishable placebo". Judgement comment: participants may have been blinded to treatment ‐ but not clear if dosing was done similarly in both arms. No description or statement regarding blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: no description if outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All patients were included in an intention‐to‐treat analysis." Judgement comment: 1 in the captopril group had an unusual pain in the shoulder and discontinued treatment on the sixth day, and 1 in the placebo group was unavailable for follow‐up after the first month. These 2 participants were included in the results for as long as they participated.Does not appear that they were included in 6‐month analysis even though state intention‐to‐treat. Very small sample size so all results should be included. |
| Selective reporting (reporting bias) | High risk | "Creatinine clearance was calculated by the modified Cockroft and Gault formula (10,11). All measurements were repeated at baseline and at 1, 3, and 6 months." "creatinine concentrations and creatinine clearance remained constant throughout the study in both groups (data not shown)." Judgement comment: creatinine clearance important marker of kidney progression but data not shown. |
| Other bias | High risk | Judgement comment: this is a small sample size and likely not powered to detect any differences. Also follow‐up is too short to assess longer‐term AEs or actual effects on kidney disease progression. |
ACE: angiotensin converting enzyme AEs: adverse events GFR: glomerular filtration rate MDI: mental developmental index RCT: randomised controlled trial SAEs: serious adverse events SD: standard deviation TCD: transcranial doppler ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jain 2012 | Participants did not have chronic kidney disease. Trial addressed outcomes that are not relevant to this review (effect on vaso‐occlusive crises, blood transfusions and hospitalizations). |
| NCT02373241 | Wrong study design; not randomised. |
| Steinberg 2003 | Wrong study design; not randomised. |
Characteristics of ongoing studies [ordered by study ID]
NCT01732718.
| Trial name or title | The Effect of Atorvastatin on Endothelial Dysfunction and Albuminuria in Sickle Cell Disease (in the Grant Entitled: Endothelial Dysfunction in the Pathogenesis of Sickle Cell Nephropathy) |
| Methods | Phase 2 randomised cross‐over assignment; double‐blind (participant, care provider, investigator, outcomes assessor) trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
Patients will also be encouraged to avoid grape fruit juice and red yeast rice for the duration of the study. Atorvastatin is contraindicated during pregnancy and breast‐feeding. |
| Interventions | Atorvastatin 40 mg tablet once daily for 6 weeks Placebo (for atorvastatin) 1 tablet once daily for 6 weeks |
| Outcomes | Primary: change from baseline in endothelial function at 6 weeks Secondary: change from baseline in plasma markers of endothelial activation; change from baseline in heme oxygenase activity; change from baseline in plasma levels of sFLT‐1; change from baseline in monocyte activation;change from baseline in renal function; occurrence of AEs; change from baseline in rho/rho kinase activity; change from baseline in plasma levels of VEGF; change from baseline in absolute cell counts; change from baseline in TF expression; change from baseline in TF‐mediated sFLT release from monocytes; change from baseline to week 6 in TR jet |
| Starting date | September 2013 |
| Contact information | Kenneth Ataga, MD, University of North Carolina, Chapel Hill |
| Notes | Completion date December 2017; R01HL111659 ( US NIH Grant/Contract Award Number ) |
ACE: angiotensin converting enzyme AEs: adverse events ALT: alanine aminotransferase ARB: angiotensin blockers GGT: gamma glutamyl transferase INR: International Normalized Ratio LDL: low‐density lipoprotein PT: prothrombin time PTT: partial thromboplastin time sFLT‐1: sSoluble fms‐like tyrosine kinase‐1 TF: tissue factor TR: tricuspid regurgitant VEGF: vascular endothelial growth factor
Differences between protocol and review
We adjusted the following statement for reporting 99% confidence intervals for SAEs and other adverse events: "We reported secondary outcomes as groups of transfusion‐related and drug‐related adverse event. If this was not possible due to duplicate counting of the same participant who may have experienced more than one adverse event of the same category (e.g. more than one transfusion‐related adverse event). In this case, we reported subgroup categories of adverse events separately and reported the 99% CI of the pooled RR to allow for multiple statistical testing".
Contributions of authors
Noemi Roy: searching; selection of trials; eligibility assessment; content expert, and review content development
Patricia Fortin: searching; selection of trials; eligibility assessment; and review content development
Katherine Bull: protocol development and content expert
Carolyn Doree: protocol development, search methods and strategies
Sally Hopewell: methodological expert and review development
Marialena Trivella: statistical and methodological expert and review development
Lise Estcourt: review conception, review development and content expert
Sources of support
Internal sources
-
NHS Blood and Transplant, Research and Developmen, UK.
To fund the work of the Systematic Review Initiative (SRI)
External sources
-
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
To provide funding for systematic reviewers and methodological support from the Centre for Statistics in Medicine, Oxford
Declarations of interest
Noemi Roy: none known.
Patricia Fortin: funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Katherine Bull: none known.
Carolyn Doree: none known.
Sally Hopewell: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Marialena Trivella: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Lise Estcourt: partly funded by the NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
New
References
References to studies included in this review
BABY HUG 2011 {published data only}
- Adams RJ, Barredo J, Bonds DR, Brown C, Casella J, Daner L, et al. TCD in infants: A report from the BABY HUG trial. Blood 2005; Vol. 106, issue 11. [Abstract no: 952; CENTRAL: 593091; CRS: 5500100000003045]
- Adams RJ, Luden J, Miller S, Wang W, Rees R, Li D, et al. TCD in infants: a report from the Baby Hug study. 28th Annual Meeting of the National Sickle Cell Disease Program; 2005 April 9‐13; Cincinnati, Ohio.. 2005:105. [CENTRAL: 592981; CRS: 5500100000002944]
- Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi‐center placebo‐controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatric Blood & Cancer 2012;59(4):668‐74. [CENTRAL: 848700; CRS: 5500125000000525; PUBMED: 22294512] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong FD, Elkin TD, Brown RC, Glass P, Rana S, Casella JF, et al. Developmental function in toddlers with sickle cell anemia. Pediatrics 2013;131(2):e406‐14. [CENTRAL: 853612; CRS: 5500125000000530; PUBMED: 23296434] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong FD, Elkin TD, Brown RC, Glass P, Rees RC, Wang WC, et al. Neurodevelopment in infants with sickle cell anemia: baseline data from the Baby HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 713; CENTRAL: 723732; CRS: 5500100000003393]
- Armstrong FD, Rees RC, Li D, Bonner M, Elkin D, Strouse JJ, et al. Baseline developmental function by age for children in the pediatric hydroxyurea phase 3 clinical trial (Baby Hug). 28th Annual Meeting of the National Sickle Cell Disease Program; 2005 April 9‐13; Cincinnati, Ohio. 2005:137. [CENTRAL: 592985; CRS: 5500100000002948]
- Kalpatthi R, Thompson B, Lu M, Wang WC, Patel N, Kutlar A, et al. Comparison of hematologic measurements between local and central laboratories: data from the BABY HUG trial. Clinical Biochemistry 2013;46(3):278‐81. [CENTRAL: 977455; CRS: 5500125000000527; PUBMED: 23123915] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebensburger JD, Miller ST, Howard TH, Casella JF, Brown RC, Lu M, et al. Influence of hemoglobin level on clinical findings in infants with sickle cell anemia; data from BABY HUG. 52nd ASH Meeting and Exposition; 2010 Dec 4‐7; Orlando, Florida. 2010. [Abstract no: 1631; CENTRAL: 783468; CRS: 5500100000005749]
- Lebensburger JD, Miller ST, Howard TH, Casella JF, Brown RC, Lu M, et al. Influence of severity of anemia on clinical findings in infants with sickle cell anemia: analyses from the BABY HUG study. Pediatric Blood & Cancer 2012;59(4):675‐8. [CENTRAL: 854381; CRS: 5500125000000528; PUBMED: 22190441] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman HM, Connolly MA, Kalpatthi R, Ware RE, Wang WC, Luchtman‐Jones L, et al. Immunologic effects of hydroxyurea in sickle cell anemia. Pediatrics 2014;134(4):686‐95. [CENTRAL: 1053679; CRS: 5500135000000943; PUBMED: 25180279] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman HM, Connolly MA, Ware RE, Luchtman‐Jones L, Goldsmith JC. Effects of hydroxyurea (HU) on lymphocyte subsets and the immune response to pneumococcal, measles, mumps and rubella vaccination in the pediatric hydroxyurea phase III clinical trial ‐ BABY HUG ‐ (ClinicalTrials.gov Identifier: NCT00006400). Blood 2012; Vol. 120, issue 21. [Abstract no: 243; CENTRAL: 977456; CRS: 5500125000000532]
- Manwani D. Hydroxycarbamide for very young children with sickle cell anaemia: No effect on the primary outcomes of spleen or kidney function, but evidence for decreased pain and dactylitis, with minimal toxicity. Evidence‐based Medicine 2012;17(2):37‐8. [CENTRAL: 896682; CRS: 5500050000000484; EMBASE: 2012199164] [DOI] [PubMed] [Google Scholar]
- McCarville MB, Luo Z, Huang X, Rees RC, Rogers ZR, Miller ST, et al. Abdominal ultrasound with scintigraphic and clinical correlates in infants with sickle cell anemia: baseline data from the BABY HUG trial. AJR. American Journal of Roentgenology 2011;196(6):1399‐404. [CENTRAL: 799797; CRS: 5500100000005750] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville MB, Rees RC, Rogers ZR, Kalpatthi R, Miller ST, Wang WC, et al. Adbominal ultrasound findings in infants with sickle cell anemia; baseline data from the BABY HUG Trial. 3rd Annual Sickle Cell Disease Research and Educational Symposium and Annual Sickle Cell Disease Scientific Meeting; 2009 Feb 18‐20. 2009. [Abstract no: 212; CENTRAL: 744101; CRS: 5500100000003432]
- McGann PT, Flanagan JM, Howard TA, Dertinger SD, He J, Kulharya AS, et al. Genotoxicity associated with hydroxyurea exposure in infants with sickle cell anemia: results from BABY‐HUG phase III clinical trial. 53rd ASH Annual Meeting and Exposition; 2011 Dec 10‐13; San Diego, California. 2011. [Abstract no: 8; CENTRAL: 848826; CRS: 5500100000005753]
- McGann PT, Flanagan JM, Howard TA, Dertinger SD, He J, Kulharya AS, et al. Genotoxicity associated with hydroxyurea exposure in infants with sickle cell anemia: results from the BABY‐HUG Phase III Clinical Trial. Pediatric Blood & Cancer 2012;59(2):254‐7. [CENTRAL: 854422; CRS: 5500125000000524; PUBMED: 22012708] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ST, Barredo J, Brown C, Bonds DR, Casella JF, Li D, et al. Renal concentrating ability in infants with sickle cell anemia; baseline data from Baby Hug, a multicenter trial. 29th Annual Meeting of the National Sickle Cell Disease Program; 2006 April 8‐12; Memphis, USA. 2006. [Abstract no: 141; CENTRAL: 593068; CRS: 5500100000003026]
- Miller ST, Rey K, He J, Flanagan J, Fish BJ, Rogers ZR, et al. Massive accidental overdose of hydroxyurea in a young child with sickle cell anemia. Pediatric Blood & Cancer 2012;59(1):170‐2. [CENTRAL: 848891; CRS: 5500100000010583] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ST, Wang WC, Iyer R, Rana S, Lane P, Ware RE, et al. Urine concentrating ability in infants with sickle cell disease: baseline data from the phase III trial of hydroxyurea (BABY HUG). Pediatric Blood & Cancer 2010;54(2):265‐8. [CENTRAL: 744105; CRS: 5500100000003435] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ST, Wang WC, Iyer RV, Rana SR, Lane PA, Ware RE, et al. Urine concentrating ability in infants with sickle cell anemia: baseline data from the Baby HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 1413; CENTRAL: 723730; CRS: 5500100000003391]
- Miller ST, Ware RE, Kutlar A, Alvarez OA, Iyer RV, Sarnaik SA, et al. Serum cystatin‐C levels in infants with sickle cell anemia: baseline data from the BABY HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 4791; CENTRAL: 723725; CRS: 5500100000003386]
- Pavlakis SG, Rees RC, Huang X, Brown RC, Casella JF, Iyer RV, et al. Transcranial doppler ultrasonography (TCD) in infants with sickle cell anemia: baseline data from the BABY HUG trial. Pediatric Blood & Cancer 2010;54(2):256‐9. [CENTRAL: 744103; CRS: 5500100000003434] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Houston PE, Wang WC, Iyer RV, Goldsmith J, Casella JF, et al. Hydroxyurea and growth in young children with sickle cell disease. Pediatrics 2014;134(3):465‐472, Supplemental information. http://pediatrics.aappublications.org/content/134/3/465.supplemental. [CRS: 5500135000001451] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Houston PE, Wang WC, Iyer RV, Goldsmith J, Casella JF, et al. Hydroxyurea and growth in young children with sickle cell disease. Pediatrics 2014;134(3):465‐72. [CRS: 5500135000000945; PUBMED: 25157002] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ZR, Capparelli EV, Thompson B, Ware RE, Wang WC, Iyer RV, et al. Pharmacokinetics of hydroxyurea in young children with sickle cell anemia: a report from the Baby Hug trial. 29th Annual Meeting of the National Sickle Cell Disease Program; 2006 April 8‐12; Memphis, USA. 2006:157. [CENTRAL: 593070; CRS: 5500100000003028]
- Rogers ZR, Rees RC, Files B, Iyer RV, Shulkin BL, Shalaby‐Rana E, et al. Spleen function in infants with sickle cell anemia : baseline data from the BABY HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 1416; CENTRAL: 723728; CRS: 5500100000003389]
- Rogers ZR, Rees RC, Files B, Iyer RV, Shulkin BL, Shalaby‐Rana E, et al. Spleen function in infants with sickle cell anemia: baseline data from the Baby Hug trial. 3rd Annual Sickle Cell Disease Research and Educational Symposium and Annual Sickle Cell Disease Scientific Meeting; 2009 Feb 18‐20. 2009. [Abstract no: 199; CENTRAL: 744100; CRS: 5500100000003431]
- Rogers ZR, Rees RR, Wang WC, Li D, Iyer RV, Rana S, et al. Evaluation of splenic function in infants with sickle cell anemia in the Baby Hug trial. 28th Annual Meeting of the National Sickle Cell Disease Program; 2005 April 9‐13; Cincinnati, Ohio. 2005:106. [CENTRAL: 592983; CRS: 5500100000002946]
- Rogers ZR, Thompson B, Ware RE, Wang WC, Iyer RV, Miller ST, et al. Pharmacokinetics of hydroxyurea in young children with sickle cell anemia: a report from the BABY HUG trial. Blood 2005; Vol. 106, issue 11. [Abstract no: 3184; CENTRAL: 580961; CRS: 5500100000002906]
- Sheehan VA, Luo Z, Flanagan JM, Howard TA, Thompson BW, Wang WC, et al. Genetic modifiers of sickle cell anemia in the BABY HUG cohort: influence on laboratory and clinical phenotypes. American Journal of Hematology 2013;88(7):571‐6. [CENTRAL: 983421; CRS: 5500125000000710; PUBMED: 23606168] [DOI] [PubMed] [Google Scholar]
- Thompson BW, Miller ST, Rogers ZR, Rees RC, Ware RE, Waclawiw MA, et al. The pediatric hydroxyurea phase III clinical trial (BABY HUG): challenges of study design. Pediatric Blood & Cancer 2010;54(2):250‐5. [CENTRAL: 744102; CRS: 5500100000003433] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BW, Wang WC, Miller ST, Rogers ZR, Ware RE, Thornburg CD, et al. The physiological and clinical effects of interrupting a treatment regimen of hydroxyurea in young children with sickle cell anemia (SCA). 53rd ASH Annual Meeting and Exposition; 2011 Dec 10‐13; San Diego, California. 2011. [Abstract no: 2134; CENTRAL: 848934; CRS: 5500100000010730]
- Thornburg CD, Files BA, Luo Z, Miller ST, Kalpatthi R, Iyer R, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012;120(22):4304‐10; quiz 4448. [CENTRAL: 853818; CRS: 5500125000000526; PUBMED: 22915643] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg CD, Rogers ZR, Jeng MR, Rana SR, Iyer RV, Faughnan L, et al. Adherence to study medication and visits: data from the BABY HUG trial. Pediatric Blood & Cancer 2010;54(2):260‐4. [CENTRAL: 789886; CRS: 5500100000003523; EMBASE: 2010057733] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg CD, Rogers ZR, Wang W, Jeng M, Rana SR, Iyer RV, et al. Study drug and visit adherence: data from the Baby HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 1275; CENTRAL: 723731; CRS: 5500100000003392]
- Wang W, Luo Z, Alvarez O, Fixler J, T Miller S, Ware RE, et al. Effects of hydroxyurea in asymptomatic infants with sickle cell anemia: Analysis F from the BABY HUG trial. American Journal of Hematology 2012;87(7):E20‐1. [CENTRAL: 1027771; CRS: 5500050000000485; EMBASE: 71030870] [Google Scholar]
- Wang W, Rees RC, Miller ST, Brown RC, Casella JF, Iyer RV, et al. Transcranial doppler (TCD) ultrasonography in infants with sickle cell anemia: baseline data from the BABY HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 1436; CENTRAL: 723726; CRS: 5500100000003387]
- Wang WC, Oyeku SO, Luo Z, Boulet SL, Miller ST, Casella JF, et al. Hydroxyurea is associated with lower costs of care of young children with sickle cell anemia. Pediatrics 2013;132(4):677‐83. [CENTRAL: 962768; CRS: 5500125000000529; PUBMED: 23999955] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle‐cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377(9778):1663‐72. [CENTRAL: 778254; CRS: 5500100000005761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yeku SO, Luo Z, Boulet SL, Miller ST, Fish B, et al. Costs associated with the care of very young children with sickle cell anemia (SCA): analysis from the BABY HUG study. 53rd ASH Annual Meeting and Exposition; 2011 Dec 10‐13; San Diego, California. 2011. [Abstract no: 171; CENTRAL: 848827; CRS: 5500100000005763]
- Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, Casella JF, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. Blood 2008; Vol. 112, issue 11. [Abstract no: 1414; CENTRAL: 723729; CRS: 5500100000003390]
- Ware RE, Rees RC, Sarnaik SA, Iyer RV, Alvarez OA, Casella JF, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. Journal of Pediatrics 2010;156(1):66‐70. [CENTRAL: 730927; CRS: 5500100000003405; PUBMED: 19880138] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn L, Debenham E, Faughnan L, Martin B, Kelly T, Reed C, et al. Recruitment in the baby hug pediatric hydroxyurea phase 3 clinical trial. 35th Anniversary Convention of the National Sickle Cell Disease Program; 2007 Sep 17‐22; Washington Dc, USA. 2007:245. [CENTRAL: 623757; CRS: 5500100000003180]
- Wynn L, Miller S, Faughnan L, Luo Z, Debenham E, Adix L, et al. Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study. Contemporary Clinical Trials 2010;31(6):558‐63. [CENTRAL: 769171; CRS: 5500100000005768] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn LW, Faughnan L, Li D, Wang W, Martin B, Kelly T, et al. Recruitment of infants with sickle cell anemia to a phase III trials: data from the BABY HUG study. Blood 2008; Vol. 112, issue 11. [Abstract no: 1429; CENTRAL: 723727; CRS: 5500100000003388]
Foucan 1998 {published data only}
- Foucan L, Bourhis V, Bangou J, Merault L, Etienne‐Julan M, Salmi RL. A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. American Journal of Medicine 1998;104(4):339‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Jain 2012 {published data only}
- Jain DL, Sarathi V, Desai S, Bhatnagar M, Lodha A. Low fixed‐dose hydroxyurea in severely affected Indian children with sickle cell disease. Hemoglobin 2012;36(4):323‐32. [DOI] [PubMed] [Google Scholar]
NCT02373241 {published data only}
- NCT02373241. Preventing sickle cell kidney disease [Chronobiology and Chronopharmacology to Prevent Sickle Cell Kidney Disease]. clinicaltrials.gov/ct2/show/NCT02373241 Date first received: 5 February 2015.
Steinberg 2003 {published data only}
- Steinberg MH, Barton F, Castro, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and Morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003;289(13):1645‐51. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01732718 {published data only}
- NCT01732718. Effect of atorvastatin on endothelial dysfunction and albuminuria in sickle cell disease (ENDO) [The Effect of Atorvastatin on Endothelial Dysfunction and Albuminuria in Sickle Cell Disease (in the Grant Entitled: Endothelial Dysfunction in the Pathogenesis of Sickle Cell Nephropathy)]. clinicaltrials.gov/ct2/show/NCT01732718 Date first received: 9 November 2012.
Additional references
Adam 2008
- Adam S, Jonassaint J, Kruger H, Kail M, Orringer EP, Eckman JR, et al. Surgical and obstetric outcomes in adults with sickle cell disease. American Journal of Medicine 2008;121(10):916‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akinsheye 2011
- Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin C T, Sebastiani P, et al. Fetal hemoglobin in sickle cell anemia. Blood 2011;118(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez 2006
- Alvarez O, Montane B, Lopez G, Wilkinson J, Miller T. Early blood transfusions protect against microalbuminuria in children with sickle cell disease. Pediatric Blood & Cancer 2006;47:71‐6. [DOI] [PubMed] [Google Scholar]
Alvarez 2012
- Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi‐center placebo‐controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatric Blood & Cancer 2012;59(4):668‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arlet 2012
- Arlet JB, Ribeil JA, Chatellier G, Eladari D, Seigneux S, Souberbielle JC, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrology 2012;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asnani 2013
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in Homozygous sickle cell disease: Utility of serum creatinine based estimating equations. PLoS ONE 2013;8(7):e69922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asnani 2015
- Asnani MR, Reid ME. Renal function in adult Jamaicans with homozygous sickle cell disease. Hematology 2015;20(7):422‐8. [DOI] [PubMed] [Google Scholar]
Astor 2011
- Astor BC, Matsushita K, Gansevoort RT, Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end‐stage renal disease. A collaborative meta‐analysis of kidney disease population cohorts. Kidney International 2011; Vol. 79, issue 12:1331‐40. [DOI] [PMC free article] [PubMed]
Ataga 2014
- Ataga KI, Derebail VK, Archer DR. The glomerulopathy of sickle cell disease. American Journal of Hematology 2014;89:907‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aygun 2013
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. American Journal of Hematology 2013;88(2):116‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Botev 2009
- Botev R, Mallie´ JP, Couchoud C, Schuck O, Fauvel JP, Wetzels JFM, et al. Estimating glomerular filtration rate: Cockcroft–Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clinical Journal of the American Society of Nephrology 2009;4(5):899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2016
- Boyle SM, Jacobs B, Sayani FA, Hoffman B. Management of the dialysis patient with sickle cell disease. Seminars in Dialysis 2016;29(1):62‐70. [DOI] [PubMed] [Google Scholar]
Brun 2003
- Brun M, Bourdoulous S, Couraud PO, Elion J, Krishnamoorthy R, Lapoumeroulie C. Hydroxyurea down regulates endothelin‐1 gene expression and up regulates ICAM‐1 gene expression in cultured human endothelial cells. Pharmacogenomics Journal 2003;3(4):215‐26. [DOI] [PubMed] [Google Scholar]
Charache 1995
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. New England Journal of Medicine 1995;332(20):1317‐22. [DOI] [PubMed] [Google Scholar]
Chou 2013a
- Chou ST. Transfusion therapy for sickle cell disease: a balancing act. Hematology / the Education Program of the American Society of Hematology 2013;2013(1):439‐46. [DOI] [PubMed] [Google Scholar]
Chou 2013b
- Chou ST, Jackson T, Vege S, Smith‐Whitley K, Friedman DF, Whesthoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh‐matched minority donors. Blood 2013;122(6):1062‐71. [DOI] [PubMed] [Google Scholar]
Covidence 2015 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Version accessed prior to 12 April 2017. Melbourne, Australia: Veritas Health Innovation, 2015.
de Zeeuw 2004
- Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment?. Kidney International. Supplement 2004;92:S2‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Field 2014
- Field JJ, Nathan DG. Advances in sickle cell therapies in the hydroxyurea era. Molecular Medicine 2014;20 Suppl 1:S37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frenette 2007
- Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. Journal of Clinical Investigation 2007;117(4):850‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gansevoort 1995
- Gansevoort RT, Sluiter WJ, Hemmelder MH, Zeeuw D, Jong PE. Antiproteinuric effect of blood‐pressure‐lowering agents: a meta‐analysis of comparative trials. Nephrology, Dialysis, Transplantation 1995;10(11):1963‐74. [PubMed] [Google Scholar]
Gaspari 2006
- Gaspari F, Perico N, Remuzzi G. Timed urine collections are not needed to measure urine protein excretion in clinical practice. American Journal of Kidney Diseases 2006;47(1):1‐7. [DOI] [PubMed] [Google Scholar]
Gosmanova 2014
- Gosmanova EO, Zaidi S, Wan JY, Adams‐Graves PE. Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. Journal of Investigative Medicine 2014;62(5):804‐7. [DOI] [PubMed] [Google Scholar]
Gravitz 2014
- Gravitz L, Pincock S. Sickle‐cell disease. Nature 2014;515(7526):S1. [DOI] [PubMed] [Google Scholar]
Grosse 2011
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa. A neglected cause of early childhood mortality. American Journal of Preventive Medicine 2011;41(6 Suppl 4):S398‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamideh 2013
- Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009). Blood Cancer 2013;60(9):1482‐86. [DOI] [PubMed] [Google Scholar]
Haymann 2010
- Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clinical Journal of the American Society of Nephrology 2010;5(5):756‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebbel 2014
- Hebbel RP. Ischemia‐reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematology/oncology clinics of North America 2014;28(2):181‐98. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillery 2000
- Hillery CA, Du MC, Wang WC, Scott JP. Hydroxyurea therapy decreases the in vitro adhesion of sickle erythrocytes to thrombospondin and laminin. British Journal of Haematology 2000;109(2):322‐7. [DOI] [PubMed] [Google Scholar]
Howard 2015
- Howard J, Hart N, Roberts‐Harewood M, Cummins M, Awogbade M, Davis B. Guideline on the management of acute chest syndrome in sickle cell disease. British Journal of Haematology 2015;169(4):492‐505. [DOI] [PubMed] [Google Scholar]
Kato 2006a
- Kato GJ, Hsieh M, Machado R, Taylor J 6th, Little J, Butman JA, et al. Cerebrovascular disease associated with sickle cell pulmonary hypertension. American Journal of Hematology 2006;81(7):503‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2006b
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, Morris CR, et al. Lactate dehydrogenase as a biomarker of hemolysis‐associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 2006;107(6):2279‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO 2012
Lebensburger 2011
- Lebensburger J, Johnson SM, Askenazi DJ, Rozario NL, Howard TH, Hilliard LM. Protective role of hemoglobin and fetal hemoglobin in early kidney disease for children with sickle cell anemia. American Journal of Hematology 2011;86(5):430‐2. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levey 1999
- Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. Journal of the American Society of Nephrology 1999;10(11):2426‐39. [DOI] [PubMed] [Google Scholar]
Levey 2009
- Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro III AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine 2009;150(9):604‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1993
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New England Journal of Medicine 1993;329(20):1456‐62. [DOI] [PubMed] [Google Scholar]
Macconi 2006
- Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, et al. Permselective dysfunction of podocyte‐podocyte contact upon angiotensin II unravels the molecular tTarget for renoprotective iIntervention. American Journal of Pathology 2006;168(4):1073‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maki 1995
- Maki DD, Ma JZ, Louis TA, Kasiske BL. Long‐term effects of antihypertensive agents on proteinuria and renal function. Archives of Internal Medicine 1995;155(10):1073‐80. [PubMed] [Google Scholar]
Marsenic 2008
- Marsenic O, Couloures KG, Wiley JM. Proteinuria in children with sickle cell disease. Nephrology, Dialysis, Transplantation 2008;23(2):715‐20. [DOI] [PubMed] [Google Scholar]
McKie 2007
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. Journal of Pediatric Hematology/Oncology 2007;29(3):140‐4. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐Analyses:The PRISMA Statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Nasr 2006
- Nasr SH, Markowitz GS, Sentman RL, D'Agati VD. Sickle cell disease, nephrotic syndrome, and renal failure. Kidney International 2006;69(7):1276‐80. [DOI] [PubMed] [Google Scholar]
Nath 2015
- Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nature Reviews. Nephrology 2015;11(3):161‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Clinical knowledge summaries: Sickle cell disease. cks.nice.org.uk/sickle‐cell‐disease#!backgroundsub:3. London: NICE, (accessed 15 August 2016).
Okafor 2013
- Okafor UH, Aneke E. Outcome and challenges of kidney transplant in patients with sickle cell disease. Journal of Transplantation 2013;2013:614610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar M, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Piel 2012
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model‐based map and population estimates. Lancet 2012;381(9861):142‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pleasants 2014
- Pleasants S. Epidemiology: a moving target. Nature 2014;515(7526):S2‐3. [DOI] [PubMed] [Google Scholar]
Porter 2013
- Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. ASH Education Program Book 2013;1:447‐56. [DOI: 10.1182/asheducation-2013.1.447] [DOI] [PubMed] [Google Scholar]
Potoka 2015
- Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 2015;308(4):L314‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powars 2005
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4‐decade observational study of 1056 patients. Medicine 2005;84(6):363‐76. [DOI] [PubMed] [Google Scholar]
Pule 2015
- Pule GD, Mowla S, Novitzky N, Wiysonge CS, Wonkam A. A systematic review of known mechanisms of hydroxyurea‐induced fetal hemoglobin for treatment of sickle cell disease. Expert Review of Hematology 2015;8(5):669‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2010
- Rees DC, Williams TN, Gladwin MT. Sickle‐cell disease. Lancet 2010;376(9757):2018‐31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rumaney 2014
- Rumaney MB, Ngo Bitoungui VJ, Vorster AA, Ramesar R, Kengne AP, Ngogang J, et al. The co‐inheritance of alpha‐thalassemia and sickle cell anemia Is associated with better hematological indices and lower consultations rate in Cameroonian patients and could improve their survival. PLoS ONE 2014;9:e100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandhu 2015
- Sandhu MK, Cohen A. Aging in sickle cell disease: co‐morbidities and new issues in management. Hemoglobin 2015;39(4):221‐4. [DOI] [PubMed] [Google Scholar]
Sasongko 2015
- Sasongko TH, Nagalla S, Ballas SK. Angiotensin‐converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD009191.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheunemann 2010
- Scheunemann LP, Ataga KI. Delayed hemolytic transfusion reaction in sickle cell disease. American Journal of the Medical Sciences 2010;339(3):266‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane handbook for systematic reviews of interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sealed Envelope [Computer program]
- Sealed Envelope Ltd. Power calculator for binary outcome superiority trial. Available from: https://www.sealedenvelope.com/power/binary‐superiority. Version accessed 06 February 2017. Sealed Envelope Ltd, 2012.
Sharpe 2014
- Sharpe CC, Thein SL. How I treat renal complications in sickle cell disease. Blood 2014;123(24):3720‐6. [DOI] [PubMed] [Google Scholar]
Sparkenbaugh 2013
- Sparkenbaugh E, Pawlinski R. Interplay between coagulation and vascular inflammation in sickle cell disease. British Journal of Haematology 2013;162(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinberg 2012
- Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. American Journal of Hematology 2012;87(8):795‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s) on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Styles 1997
- Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, et al. Decrease of very late activation antigen‐4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood 1997;89(7):2554‐9. [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ubesie 2012
- Ubesie A, Emodi I, Ikefuna A, Ilechukwu G, Ilechukwu G. Prevalence of human immunodeficiency virus transmission among transfused children with sickle cell anemia in Enugu Nigeria. Annals of Medical and Health Sciences Research 2012;2(2):109‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Meer 2010
- Meer IM, Cravedi P, Remuzzi G. The role of renin angiotensin system inhibition in kidney repair. Fibrogenesis & Tissue Repair 2010;3:7. [DOI: 10.1186/1755-1536-3-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ventura 1985
- Ventura HO, Frohlich ED, Messerli FH, Kobrin I, Kardon MB. Cardiovascular effects and regional blood flow distribution associated with angiotensin converting enzyme inhibition (captopril) in essential hypertension. American Journal of Cardiology 1985;55(8):1023‐6. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, et al. Hydroxycarbamide in very young children with sickle‐cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377(9778):1663‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yawn 2014
- Yawn BP, Buchanan GR, Afenyi‐Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: Summary of the 2014 evidence‐based report by expert panel members. JAMA 2014;312(10):1033‐48. [DOI] [PubMed] [Google Scholar]
Yazdanbakhsh 2012
- Yazdanbakhsh K, Ware RE, Noizat‐Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood 2012;120(3):528‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yee 2011
- McPherson Yee M, Jabbar SF, Osunkwo I, Clement L, Lane PA, Eckman JR, et al. Chronic kidney disease and albuminuria in children with sickle cell disease. Clinical Journal of The American Society of Nephrology 2011;6(11):2628‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zafrani 2015
- Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. American Journal of Kidney Diseases 2015;66(6):1083‐94. [DOI] [PubMed] [Google Scholar]
Ziyadeh 2008
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Current Diabetes Reviews 2008;4(1):39‐45. [DOI] [PubMed] [Google Scholar]


