Abstract
Seymour Island, Antarctic Peninsula, was once called the ‘Rosetta Stone’ of Southern Hemisphere palaeobiology, because this small island provides the most complete and richly fossiliferous Palaeogene sequence in Antarctica. Among fossil marine vertebrate remains, chondrichthyans seemingly were dominant elements in the Eocene Antarctic fish fauna. The fossiliferous sediments on Seymour Island are from the La Meseta Formation, which was originally divided into seven stratigraphical levels, TELMs 1–7 (acronym for Tertiary Eocene La Meseta) ranging from the upper Ypresian (early Eocene) to the late Priabonian (late Eocene). Bulk sampling of unconsolidated sediments from TELMs 5 and 6, which are Ypresian (early Eocene) and Lutetian (middle Eocene) in age, respectively, yielded very rich and diverse chondrichthyan assemblages including over 40 teeth of carpet sharks representing two new taxa, Notoramphoscyllium woodwardi gen. et sp. nov. and Ceolometlaouia pannucae gen. et sp. nov. Two additional teeth from TELM 5 represent two different taxa that cannot be assigned to any specific taxon and thus are left in open nomenclature. The new material not only increases the diversity of Eocene Antarctic selachian faunas but also allows two previous orectolobiform records to be re-evaluated. Accordingly, Stegostoma cf. faciatum is synonymized with Notoramphoscyllium woodwardi gen. et sp. nov., whereas Pseudoginglymostoma cf. brevicaudatum represents a nomen dubium. The two new taxa, and probably the additional two unidentified taxa, are interpreted as permanent residents, which most likely were endemic to Antarctic waters during the Eocene and adapted to shallow and estuarine environments.
Keywords: Chondrichthyes, Hemiscyllidae, Orectolobidae, La Meseta Formation, Southern Ocean, Palaeogene
Introduction
Living carpet sharks, the Orectolobiformes, comprise about 43 species in seven families (Ebert et al. 2013) that are distributed circum-globally in warm-temperate and tropical seas, ranging from intertidal to deep waters, but do not reach cold boreal or subantarctic waters. The modern tropical Indo-West Pacific harbours the greatest diversity and endemism of modern Orectolobiformes. Almost all species are benthic and have a strong association to the substrate mostly on coral reefs, with the exception of the whale shark, Rhincodon typus (Smith, 1828), which is highly migratory and pelagic. Only two species (Ginglymostoma cirratum Bonnaterre, 1788, and the whale shark) occur in the Atlantic Ocean. Despite being mostly benthic and sharing a suite of synapomorphies, they display a large variety in size (40 cm to 10 m) and shape (dorsoventrally flattened to more streamlined shaped) (Goto 2001). The dentition is also very varied and ranges from clutching-type (adapted for grasping small prey) to crunching-type (adapted for breaking shelled prey), and cutting-type in larger forms (Goto 2001; Ramsay & Wilga 2007). Additionally, the arrangement and thus replacement pattern of teeth differs among orectolobiform taxa (e.g. imbricate or in defined and well-separated rows; Goto 2001). The tooth design generally consists of a hemiaulacorhize root (nutritive root grove opens only anteriorly) and a labial apron in most species; it is very generalized and can be considered plesiomorphic for elasmobranchs.
Orectolobiforms belong to one of the two major clades within elasmobranchs, the Galeomorphii, which includes the Heterodontiformes (bull-head sharks), Lamniformes (mackerel sharks) and Carcharhiniformes (requiem sharks). The systematic affinities of orectolobiforms within this clade still are controversial. They are either considered the sister of lamniform sharks (e.g. Winchell et al. 2004) or sister to a clade [Carcharhiniformes + Lamniformes] (Maisey et al. 2004; Naylor et al. 2012).
The fossil record of orectolobiforms is very patchy and consists predominantly of isolated teeth, with the oldest records coming from the Early Jurassic (Toarcian), some 190 million years ago (Thies 1983; Kriwet 2003). Most modern-level shark clades like the Orectolobiformes originated in the Jurassic (Kriwet et al. 2009). A first diversification event seemingly occurred in the Jurassic (Underwood & Ward 2004) with subsequent rapid radiations in the Cretaceous (Underwood 2006; Maisey 2012) although the familial assignment of many extinct taxa, especially those from the Jurassic and Cretaceous, mostly remains ambiguous. Orectolobiforms were heavily affected by the K/Pg boundary event and many characteristic Cretaceous genera (e.g. Cantioscyllium, Ganntouria, Paraginglymostoma and Plicatoscyllium) went extinct (Kriwet & Benton 2004). In the Palaeogene, the diversity of orectolobiforms continuously increased and in the Eocene a first post-Cretaceous diversity peak seemingly occurred. This, however, needs to be evaluated with further research. Modern forms did not diversify until 10–15 million years ago according to recent molecular phylogenetic analyses (Corrigan & Beheregaray 2009), although orectolobids and ginglymostomatids, for instance, already appeared in the Early and Late Cretaceous, respectively (Maisey 2012).
The intention of this paper is to present new orectolobiform remains and a revision of previously described carpet sharks from the Eocene of Seymour Island, which is located at the north-eastern tip of the Antarctic Peninsula. Most of the material presented here was obtained by screen-washing of bulk samples, but some teeth were obtained by surface collecting. Seymour Island yields the most diverse Palaeogene fish fauna from the Southern Hemisphere to date that provides a unique opportunity to study composition and faunal turnover in Eocene ecosystems (Kriwet 2005; Kriwet et al. 2016). Here, middle and upper Eocene marine sediments of the La Meseta Formation have yielded a very diverse chondrichthyan fauna (e.g. Welton & Zinsmeister 1980; Jerzmanska 1988; Eastman & Grande 1989, 1991; Long 1992a, b; Balushkin 1994; Cione & Reguero 1995, 1998; Doktor et al. 1996; Kriwet 2005; Kriwet et al. 2016). Long & Stilwell (2000) presented additional Eocene elasmobranchs from East Antarctica. So far, 24 Eocene species in 15 families have been described (for complete species lists see Kriwet (2005) and Reguero et al. (2013)). Long (1992a) also reported a few teeth of orectolobiform sharks that he assigned to two different taxa, Stegostoma cf. faciatum (Hermann, 1783) and Pseudogin-glymostoma cf. brevicaudatum (Günther, 1867), respectively. The new material described here allows these to be re-evaluated and revised, and enables us to introduce new taxa, which characterize extinct and endemic Eocene groups for the Southern Hemisphere.
Localities and geological setting
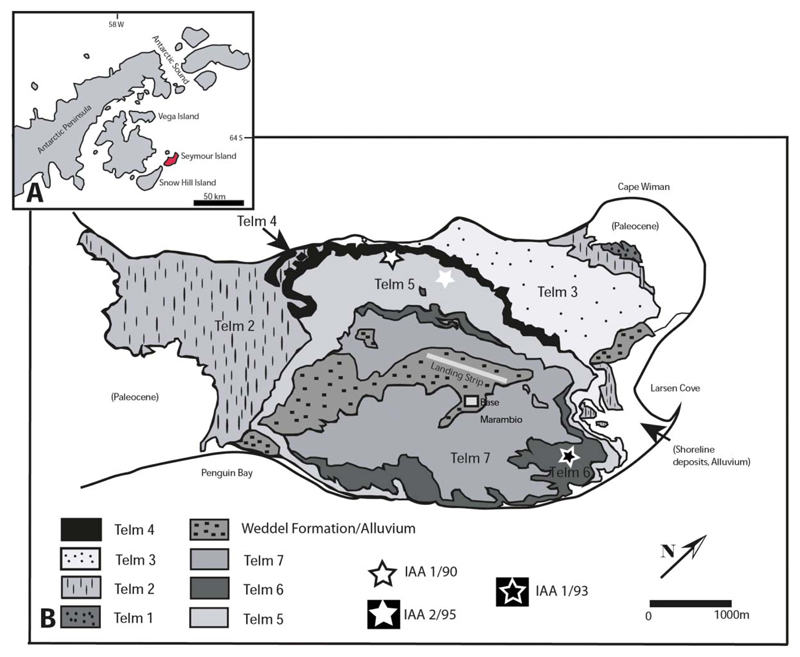
Seymour Island (Fig. 1) is situated at the north-eastern tip of the Antarctic Peninsula (64°15′S, 56°45′W). It is a small and ice-free island, with five geological formations, which span from the Late Cretaceous (Lopez de Bertodano Fm) to the Pliocene–Quaternary (Weddell Fm). The La Meseta Fm is a 6.5 km wide fossiliferous sequence in the northern part of Seymour Island. It consists of about 720 m loosely consolidated, silty sandstones. Elliot & Trautman (1982) grouped the La Meseta Fm into three transgressive–regressive cycles (Units I to III). Later Sadler (1988) subdivided the La Meseta Fm into seven units named TELM 1 to TELM 7 (acronym for Tertiary Eocene La Meseta). The La Meseta Fm forms an incised valley system with marine, deltaic and estuarine deposits (Marenssi et al. 1998). Deposition occurred from the upper Ypresian (Early Eocene) to the late Priabonian (Late Eocene), according to 87Sr/86Sr isotope data (Dingle & Lavelle 1998; Dutton et al. 2002; Ivany et al. 2008). Results of geochemical analyses of fossil shell material suggest a considerable cooling event (Gazdzicki et al. 1992) at the time of deposition of the upper part of the formation (TELMs 6 and 7).
Figure 1.
Geological map of Seymour Island, Antarctica A, index map of Seymour Island; B, geological map of Seymour Island, showing the outcrop of TELM 5–6 with the localities IAA 1/90, IAA 2/95 and IAA 1/93 of the Eocene La Meseta Formation.
Recently, a new unit, the Submeseta Formation, was defined by Montes et al. (2013) by inclusion of the uppermost part of the early/middle Eocene to ?earliest Oligocene La Meseta Formation (Elliot & Trautman 1982; Ivany et al. 2008). The Submeseta Formation is divided into three members named from base to top: Submeseta I (TELM 6), Submeseta II (TELM 7 in partem), and Submeseta III (TELM 7 in partem), with the base of this unit being at 43.4 Ma (upper Lutetian), and the top at 33.9 Ma (Priabonian/Rupelian) (Montes et al. 2013).
The material described herein originated from three different localities in two TELMs. Most of the orectolobiform teeth come from TELM 5, which is Ypresian (early Eocene) in age, at the locality IAA 1/90 (64°14′04.67ʺS; 56°39′56.38ʺW, also known as the ‘Ungulate Site’) (Bond et al. 1990), and the second most abundant material was collected at locality IAA 2/95 (64°13′58ʺS; 56°39′06ʺW or ‘Marsupial Site’, due to discovery of marsupial remains). TELM 5 consists of laminated fine-grained sandstones and silty clays with interbedded conglomeratic sandstones (Sadler 1988). Localities IAA 1/90 and IAA 2/95 are situated on the north-western side of Seymour Island. Bond et al. (1990) interpreted locality IAA 1/90 as middle Eocene in age. The thin shell beds in which localities IAA 1/90 and IAA 2/95 are located are dominated by naticid gastropods (Bomfleur et al. 2015; McLoughlin et al. 2016). The conglomeratic lenses are less than 1 m thick. Locality IAA 1/90 is interpreted as a nearshore, shallow-marine environment (Stilwell & Zinsmeister 1992).
Only two specimens were collected at locality IAA 1/93 (64°13′51.8ʺS; 56°35′53.14ʺW) in TELM 6, which is of Lutetian (middle Eocene) age. The stratigraphical unit consists of medium to fine grained sandstones with intervals of laminated fine-grained sand and silty clays (Sadler 1988) and was deposited in a low energy and shallow marine environment. According to Ivany et al. (2008) sea surface temperatures for TELMs 5 and 6 ranged from 10°C to 15°C. Subsequent cooling took place in the upper part of TELM 6, with estimated sea surface temperatures of approximately 7°C.
Material and methods
Argentinian–Swedish field parties, as a joint project of the Instituto Antártico Argentino (DNA-IAA) and the Swedish Polar Research Secretary (SPFS), collected the material for this study during three summer campaigns from 2011 to 2013 on Seymour Island. The described material consists exclusively of isolated shark teeth.
Approximately 1100 kg of dry-screened sediments (mesh sizes 10 mm, 5 mm, 2.5 mm) were collected in the field for further processing in the laboratory. Of these 1100 kg, about 350 kg of concentrated sediment from localities IAA 1/90 (Ungulate Site), IAA 2/95 (Marsupial Site) and IAA 1/93 were further processed with mesh sizes of 2 mm, 0.5 mm and 0.1 mm and picked for orectolobiform teeth. All teeth were cleaned with Rewoquat®, mounted on stubs, and subsequently sputter-coated with gold (Sputter coater SC 500) for study in a scanning electron microscope (JEOL 6400). Photographs of each tooth were taken in labial, lingual, profile and occlusal views. Additional photographs of the basal root face were taken with a digital microscope (Keyence VHX-1000D) of selected teeth.
There is disagreement as to the systematic content of Orectolobiformes (Applegate 1972; Compagno 1973; Dingerkus 1986; Herman et al. 1992). Here, we follow the taxonomy, systematic arrangement and dental terminology of extinct and extant taxa provided by Cappetta (2012). Orectolobiform material described here is deposited in the Palaeozoological collection of the Swedish Museum of Natural History (Department of Palaeobiology), with registration numbers prefixed by ‘NRM-PZ’.
Systematic palaeontology
Class Chondrichthyes Huxley, 1880
Subclass Elasmobranchii Bonaparte, 1838
Cohort Euselachii Hay, 1902
Subcohort Neoselachii Compagno, 1977
Superorder Galeomorphii Compagno, 1973
Order Orectolobiformes Applegate, 1972
Family Hemiscylliidae Gill, 1862
Genus Notoramphoscyllium gen. nov.
Type species. Notoramphoscyllium woodwardi sp. nov.
Etymology. Combines the Greek substantive ‘noto’, ‘n τος’ meaning ‘south’, the Greek substantive ‘rhampho’, ‘pάμφος’ meaning ‘beak’, and the Greek sub-stantive ‘skylion’, ‘σκύλος’ meaning ‘dogfish’, in allusion to the unique morphological characters and occurrence in the Southern Ocean during the Eocene.
Diagnosis. Extinct hemiscyllid shark only known by isolated teeth that are characterized by the following combination of dental characters: gradient monognathic heterodonty; main cusp triangular, with a broad base in labial view, slightly lingually inclined; labial and lingual crown faces devoid of ornamentation; lateral cusplets low, triangular, and well separated from the main cusp; cutting edges delicate to almost absent; crown with more or less concave depression at transition from cusp to apron; apron jutting over the root, very prominent, well detached from labial crown face in labial view and directed basally but not reaching the basal plane of the root in profile view; anterior edge of apron very bulky in profile view; basal labial margin of apron varies from convex in anterior teeth to being slightly concave in lateral to posterior teeth; anterior margin of apron curved basally; root high with sub-rectangular outline in profile view; diverging root lobes form an anteriorly open, V-shaped basal plane with well-developed labial depression; central nutrient foramen large; supposed lower jaw teeth higher and more slender than supposed upper jaw teeth, which are shorter and more compact; lateral cusplets of presumed upper jaw teeth not as distinctive as in those assigned to the lower jaw.
Differential diagnosis. Teeth of Notoramposcyllium woodwardi gen. et sp. nov. differ from teeth of Ginglymostoma, Hologinglymostoma and Nebrius in the absence of numerous lateral cusplets. The ornamented labial crown face distinguishes teeth of Protoginglymostoma and Pseudoginglymostoma. Teeth of Orectoloboides, Pararhincodon and Parascyllium differ from the new taxon in lacking a well-defined apron. Orectolobus and Squatiscyllium resemble teeth of Squatina, lacking lateral cusplets and a broad apron. Teeth of Hemiscyllium differ in lacking lateral cusplets, having a basal part of the crown which is practically as high as the cusp, and an apron that reaches the basal plane in profile view. Teeth of Chiloscyllium and Eostegostoma can be distinguished from the new taxon in having well-defined cutting edges. The combination of a very flat labial crown face without a depression above the apron, continuous lateral cutting edges, basally concave margin of the apron, and a generally labially displaced central foramen (in relation to the connection of root lobes posteriorly in basal view) also distinguishes teeth of Chiloscyllium. Teeth of Eometlaouia are laterally elongated with lateral cusplets, which begin high on the crown and have a short apron, which is distinct from the crown. Teeth of Brachaelurus differ in the presence of low and comparably slender lateral cusplets. Teeth of Palaeorhincodon and Rhincodon differ in having a well-marked cutting edge, a widely and weakly prominent apron and a high root.
Notoramphoscyllium woodwardi sp. nov. (Figs 2–8)
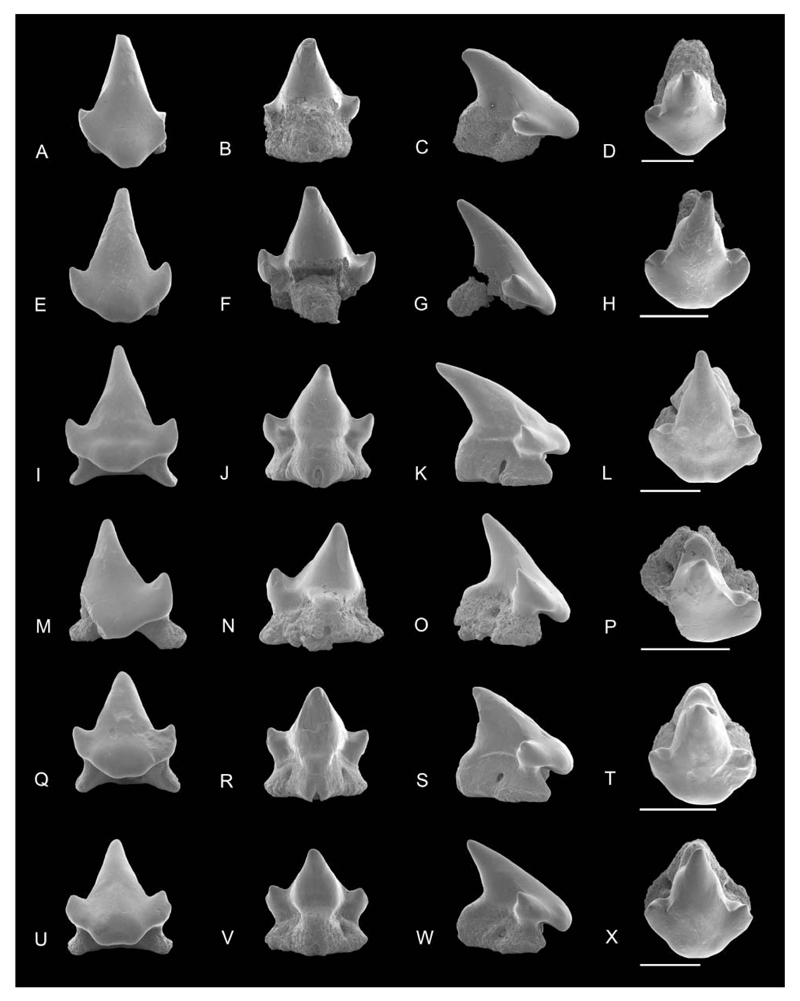
Figure 2.
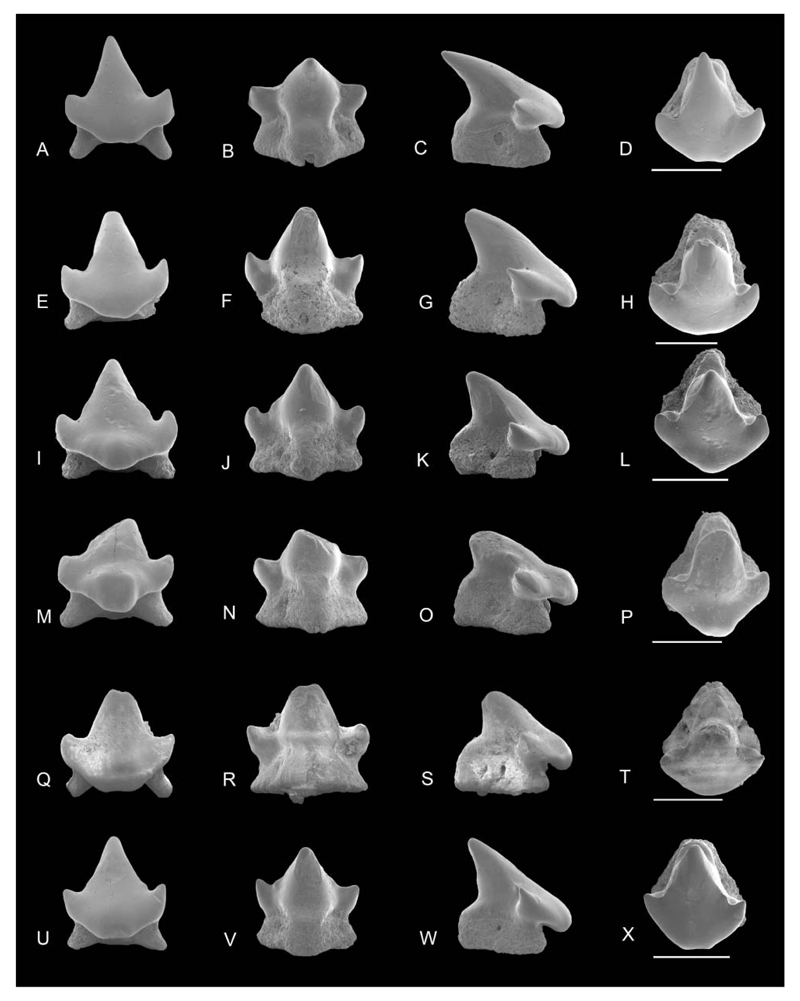
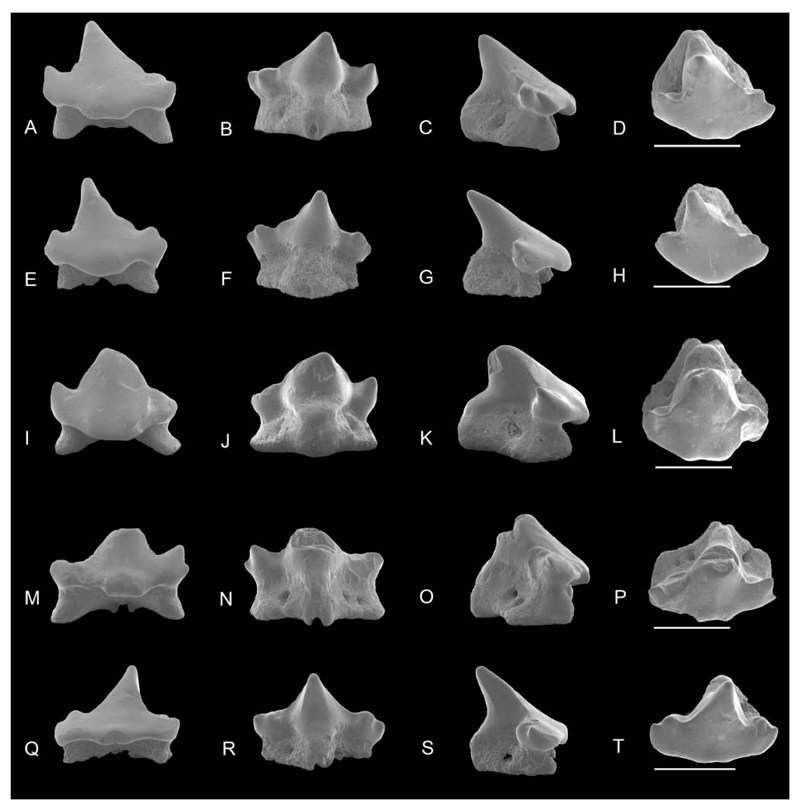
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov. A–H, upper anterior teeth: NRM-PZ P.16011 in A, labial, B, lingual, C, profile, D, occlusal views; NRM-PZ P.16012 in E, labial, F, lingual, G, profile, H, occlusal views. I–X, upper anterolateral teeth: NRM-PZ P.16013 in I, labial; J, lingual; K, profile; L, occlusal views; NRM-PZ P.16014 in M, labial, N, lingual, O, profile, P, occlusal views; holotype NRM-PZ P.16015 in Q, labial, R, lingual, S, profile, T, occlusal views; NRM-PZ P.16016 in U, labial, V, lingual, W, profile, X, occlusal views. Scale bars = 1 mm.
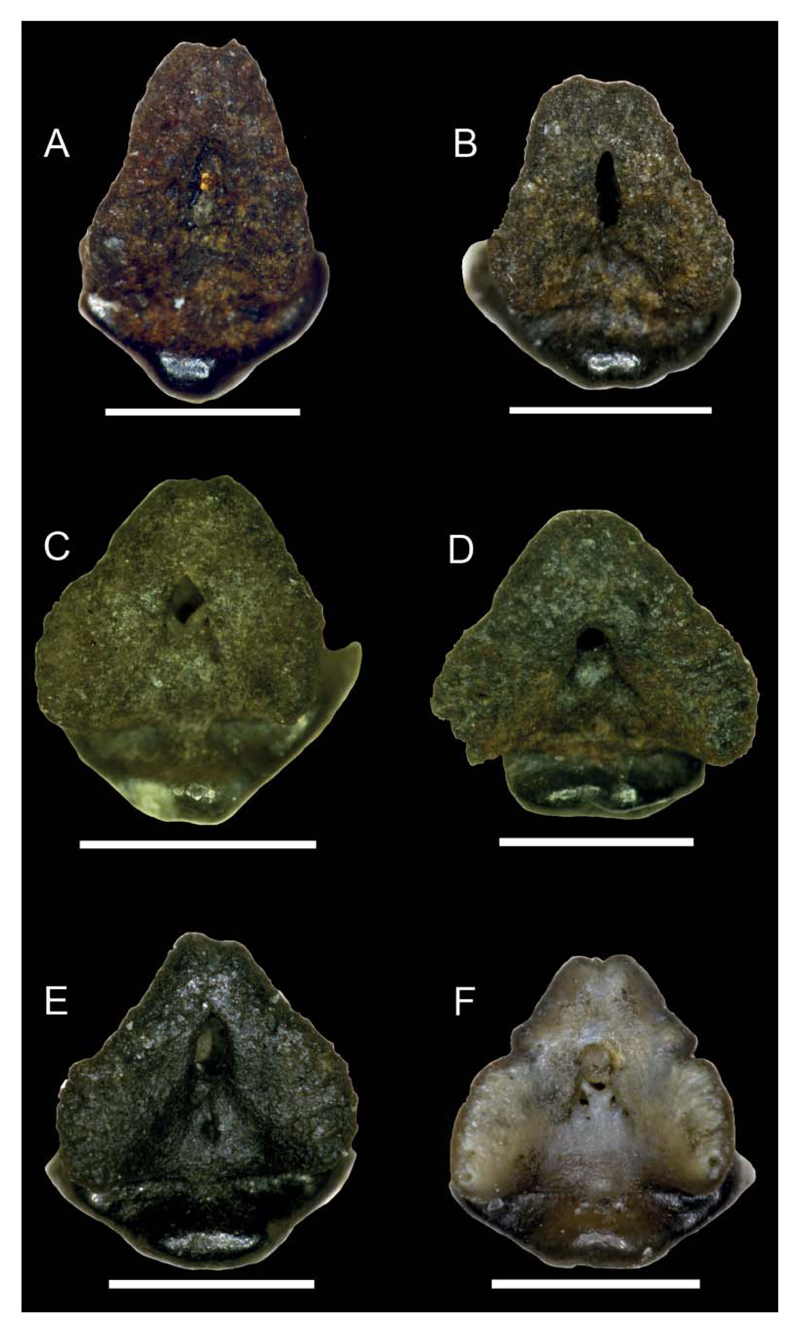
Figure 8.
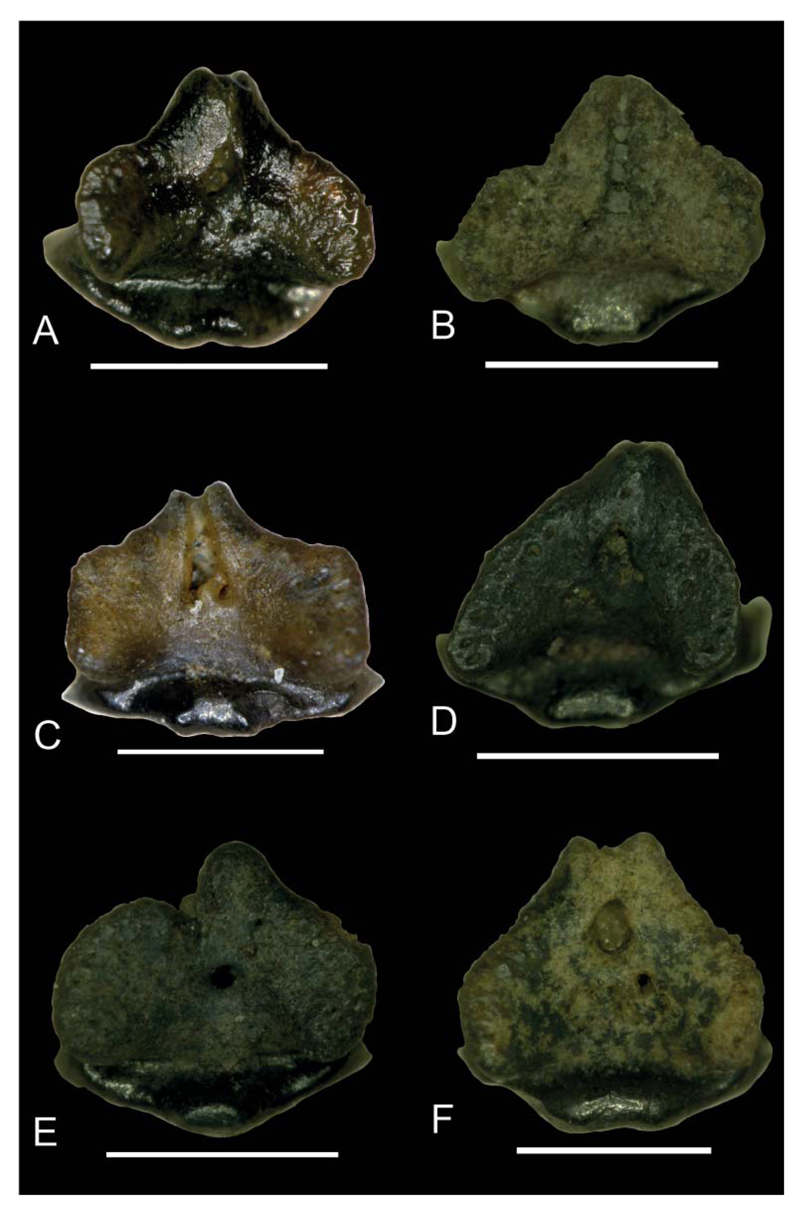
Images taken with a Keyence Digital Microscope DHX-1000D of Notoramphoscyllium woodwardi gen. et sp. nov.; all specimens in basal view; A, NRM-PZ P.16011; B, NRM-PZ P.16031; C, NRM-PZ P.16026; D, NRM-PZ P.15916; E, NRM-PZ P.16016; F, NRM-PZ P.16013. Scale bars = 1 mm.
1992a Stegostoma cf. fasciatum Hermann, 1783; Long 22, fig. 8A–C.
Holotype. NRM-PZ P.16015 (Fig. 2Q–T), an anterolateral tooth, presumably from the upper jaw.
Paratypes. Two anterior upper teeth, NRM-PZ P.16011–16012; five anterolateral upper teeth, NRM-PZ P.16013–16017, four lateral upper teeth, NRM-PZ P.16018–16022; three more lateral to posterior directed upper teeth, NRM-PZ P.16023–16025; two anterior lower teeth, NRM-PZ P.16026–16027; three anterolateral teeth, NRM-PZ P.16028–16030; eight lateral lower teeth, NRM-PZ P.15916, NRM-PZ P.16031–16037; four more lateral to posterior teeth, NRM-PZ P.16038–16041.
Type horizon. TELM 5, Natica-Horizon, Cucullaea I Allomember, La Meseta Formation.
Type locality. IAA 1/90, Ungulate Site.
Geographical distribution. IAA 1/90, Ungulate Site (64°14′04.67ʺS; 56°39′56.38ʺW) and IAA 2/95, Marsupial Site, (64°13′58ʺS; 56°39′06ʺW) and IAA 1/93 (64°13′51.8ʺS; 56°35′53.14ʺW) Antarctica.
Stratigraphical range. TELM 5, Ypresian, early Eocene (two teeth described by Long 1992a and 31 teeth, respectively), and TELM 6, Lutetian, middle Eocene (only one tooth).
Etymology. In honour of the famous English palaeoichthyologist Sir Arthur Smith Woodward, who was the first to describe fossil fish remains from Seymour Island collected during the 1901–1903 Swedish South Polar Expedition (Woodward 1908).
Diagnosis. As for the genus.
Description. Anterior teeth. Upper anterior teeth are depicted in Figure 2A–H, lower teeth in Figure 5A–H.
Figure 5.
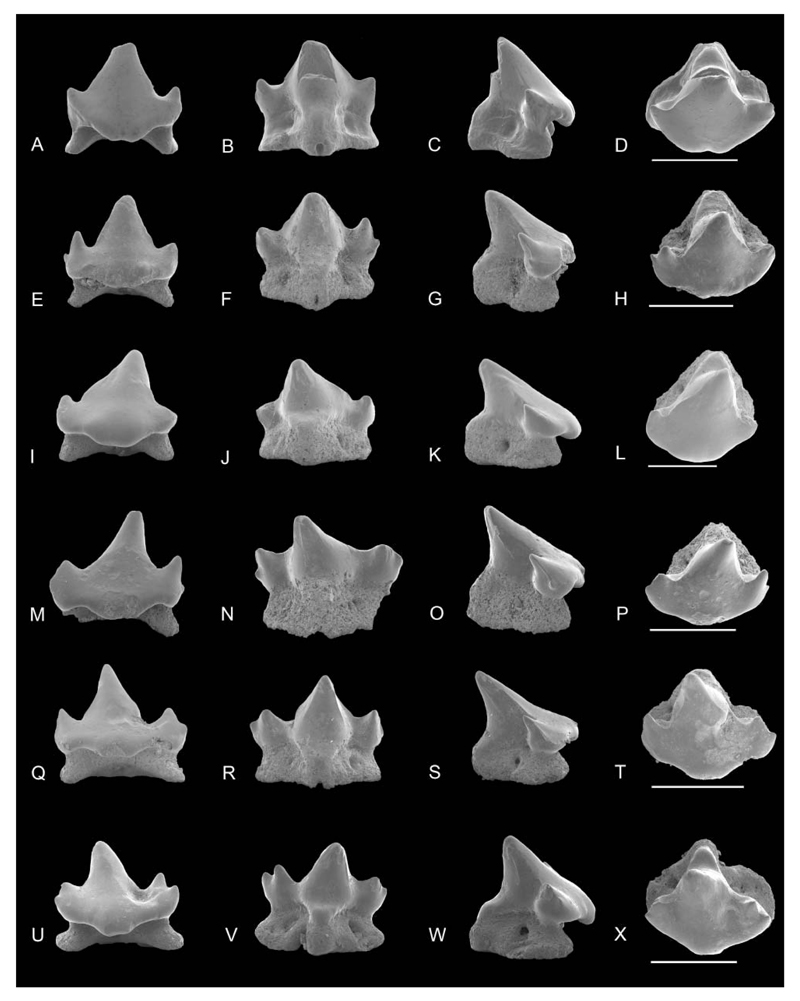
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov. A–H, lower anterior teeth: NRM-PZ P.16026 in A, labial, B, lingual, C, profile, D, occlusal views; NRM-PZ P.16027 in E, labial, F, lingual, G, profile, H, occlusal views. I–X, lower anterolateral to lateral teeth: NRM-PZ P.16028 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16029 in M, labial, N, lingual, O, profile, P, occlusal, views; NRM-PZ P.16030 in Q, labial, R, lingual, S, profile, T, occlusal views. U–X, lower lateral teeth; NRM-PZ P.16031 in U, labial, V, lingual, W, profile, X, occlusal views. Scale bars = 1 mm.
Anterior. Anterior teeth are higher than wide in labial view with a comparably tall and triangular blunt main cusp lacking ornamentation. In the anteriormost tooth, the cusp is straight, becoming slightly inclined distally in subsequent anterior teeth along the jaw. In profile view, the cusp is slightly undulating to sigmoidal. Cutting edges are weak near the base of the main cusp or are entirely lacking. The well-separated lateral cusplets have almost perpendicular to oblique lateral edges in labial view. They are low and acuminate with the distal being more delicate. The transition between cusp and apron, which is short but prominent and well detached, is dented in profile view. The apron is flexed basally but does not reach the base of the root. In labial view the apron is narrow with a rounded basal edge and concave lateral margins. In assumed lower teeth, the basal apron edge may be concave. The median lingual protuberance is short and broad with a rounded lingual margin covering the lingual portion of the root. The root is deeper than wide, high and almost rectangular in profile view, and has laterally compressed root lobes at the level of the lingual protuberance. The central foramen is placed lingually and is slightly elongated.
Anterolateral and lateral teeth. Upper anterolateral and lateral teeth are depicted in Figures 2I–X and 3A and Figure 3E–X, respectively; lower teeth in Figures 5I–X to 7D. These teeth are higher than broad but not as high as anterior teeth and also lack ornamentation. The main cusp is triangular, high, and basally broad. In profile view, it is straight to slightly sigmoid and strongly inclined lingually (e.g. Fig. 2U). The cusp is slightly distally inclined. The cutting edges are weak to blunt and, if present, do not reach the apex of the crown. Lateral cusplets are well separated from the main cusp, low and blunt. The distal cusplet, which is separated by a narrow notch from the cusp, is higher than the mesial one in assumed lower teeth. The transition between cusp and apron is also dented in most teeth (e.g. Fig. 2I). The broad, knobby and short apron, which does not reach the basal plane of the root, is well detached from the labial crown face with a generally semi-circular basal edge (e.g. Figs 2A, I, 5A), which is slightly concave in more lateral teeth. The median lingual protuberance is short but prominent. The root is high and almost heart-shaped in basal view with well-separated, short and rounded root lobes diverging anteriorly. In profile view the posterior margin of the root is almost vertical whereas the anterior margin is more or less oblique. There is a single rounded to drop-shaped marginolingual foramen in deep indents. The mediolingual foramen is small, circular, and located close to the basal margin of the lingual protuberance (e.g. Fig. 3J). In some teeth, the mediolingual foramen opens basally (e.g. Fig. 2R) so that the root appears almost holaulocorhize.
Figure 3.
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov. A–D, upper anterolateral teeth: NRM-PZ P.16017 in A, labial, B, lingual, C, profile, D, occlusal views. E–X, upper lateral teeth: NRM-PZ P.16018 in E, labial, F, lingual, G, profile, H, occlusal views; NRM-PZ P.16019 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16020 in M, labial, N, lingual, O, profile, P, occlusal views; NRM-PZ P.16021 in Q, labial, R, lingual, S, profile, T, occlusal views; NRM-PZ P.16022 in U, labial, V, lingual, W, profile, X, occlusal views. Scale bars = 1 mm.
Figure 7.
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov. A–D, lower teeth, lateral to posterior, NRM-PZ P.16037 in A, labial, B, lingual, C, profile, D, occlusal views. E–T, more lateral to posterior teeth; NRM-PZ P.16038 in E, labial, F, lingual, G, profile, H, occlusal views; NRM-PZ P.16039 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16040 in M, labial, N, lingual, O, profile, P, occlusal views; NRM-PZ P.16041 in Q, labial, R, lingual, S, profile, T, occlusal views. Scale bars = 1 mm.
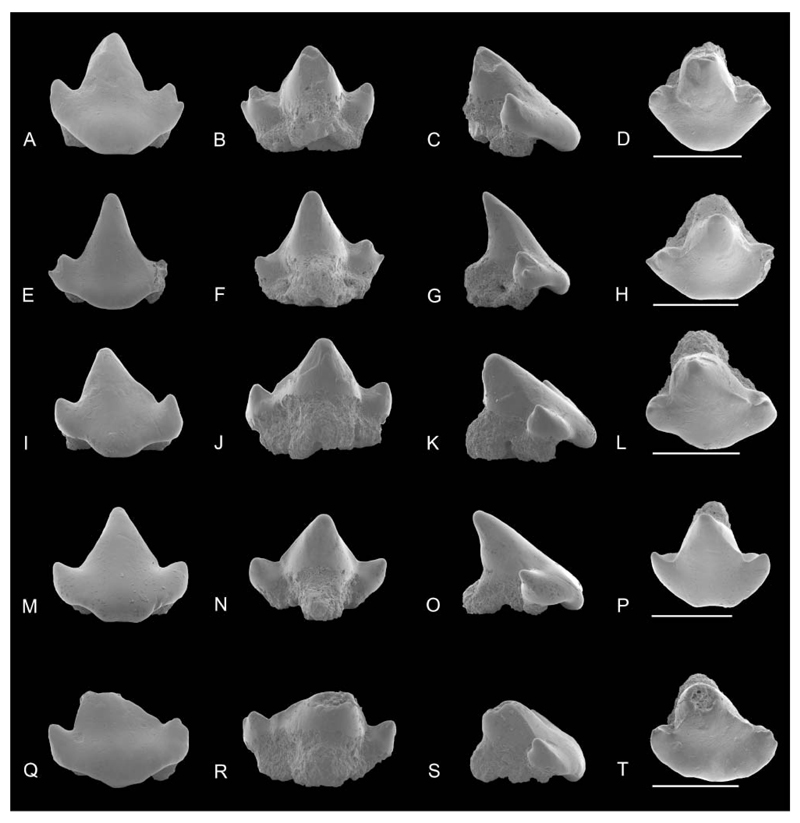
Posterior teeth. Upper posteriors are depicted in Figure 4, lower teeth in Figure 7E–T. These teeth have a sigmoid, triangular, and broad but comparably lower main cusp, which lacks ornamentation. The cutting edge is weakly developed in the lower third of the main cups and does not reach far upwards. The lateral cusplets are small and well separated from the main cusp in upper, but broadly united in lower posteriors. The apron is broad, prominent, and semicircular to slightly concave medially. It is less well detached from the labial crown face as in anterior and lateral teeth but does not reach the basal plane of the root. The marginolingual foramen is medium-sized and rounded. The mediolingual foramen is large and rounded. The basal root face is V-shaped. The central nutrient foramen is large and round to elliptical in outline (Fig. 8).
Figure 4.
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov., upper posterior lateral teeth, NRM-PZ P.16023 in A, labial, B, lingual, C, profile, D, occlusal views; NRM-PZ P.16024 in E, labial, F, lingual, G, profile, H, occlusal views; NRM-PZ P.16025 in I, labial, J, lingual, K, profile, L, occlusal views. Scale bars = 1 mm.
Dental variation. A total of 32 teeth are assigned to the new taxon. Fifteen teeth are tentatively identified as coming from the upper jaws, whereas 17 represent lower jaw teeth. The morphological variation specifies a gradient monognathic dentition as is typical for orectolobiforms but also indicates differences between upper and lower teeth. The height/width ratio is c. 1.17 for anterior teeth, decreasing laterally (c. 0.99). All teeth except for the most anterior display a more or less distally inclined main cusp with an almost flat labial face, which is slightly convex from side to side. Lower lateral teeth (Figs 5U–7D) differ from lower anteriors in a more massive but lower and mesiodistally broader crown with smaller mesial cusplets. The transition between cusp and apron is less well marked in lower posteriors so that the labial crown face and apron form an almost continuous plane. Additionally, a second, incipient lateral cusplet may be present at the base of the mesial cusplet in lower posteriors. The root lobes slightly broaden in lateral tooth positions. They are rather long and form an acute angle in basal view in anterior teeth, whereas the angle between the root lobes is obtuse in more lateral files, giving the root a heart-shaped outline. In profile view, the anterior margin of the root is perpendicular to oblique. The central foramen is positioned lingually and an elongated oval to rounded (Fig. 8A–F).
Remarks. So far, 19 orectolobiform genera have been identified from Cenozoic strata worldwide representing seven families (Hemiscylliidae, Orectolobidae, Parascylliidae, Brachaeluridae, Stegostomatidae, Ginglymostomatidae, Rhincodontidae), all with living members (Cappetta 2012). The teeth of the new taxon described here, Notoramphoscyllium woodwardi gen. et sp. nov., resemble those of Cenozoic and living members of Chiloscyllium Müller & Henle, 1837 and Hemiscyllium (Smith 1837), but differ significantly from these in the combination of characters discussed above (see also differential diagnosis above). The completely smooth crown faces without any ornamentation patterns distinguish the teeth of this new taxon from teeth of Cretaceous orectolobiforms with similar tooth morphologies but which generally have a strongly folded enameloid crown (Cappetta 2012).
The teeth of the new taxon also deviate from teeth of other Eocene orectolobiforms in having an asymmetrical, smooth main cusp with very weak to almost absent cutting edges not reaching the apex, a low crown shoulder, a protruding and very prominent apron that juts over the root almost horizontally because of the depression between cusp and apron, a high almost rectangular root in profile view, a very convex root face in labial view, and a lingually positioned and small central foramen.
The new taxon is characterized by a marked gradient monognathid heterodonty, similar to that of other orectolobiforms. Additionally, it also displays morphological dental variations that may indicate differences between lower and upper jaw teeth. Nevertheless, it cannot be precluded that this dental variability indicates sexual dimorphism. Ontogenetic differences can be excluded because all teeth are more or less of the same size and no small teeth referable to this taxon were recovered. The general morphology of all teeth clearly supports our interpretation that they belong to a single taxon rather than distinct orectolobiforms.
Long (1992a) assigned two teeth from TELM 4 to Stegostoma cf. fasciatum, one of which was figured in his paper. An asymmetrical, smooth main cusp with low and also asymmetrical lateral cusplets, a very concave root base in labial view, and a lingually placed central foramen characterize this specimen. These traits distinguish this tooth, however, from teeth of Stegostoma but concur well with teeth of Notoramphoscyllium woodwardi gen. et sp. nov. The basally open nutritive groove between the root lobes of the specimen (Long 1992a) is expected to be within the variety of dental morphologies present in the new taxon as some teeth described here also display basally open nutritive grooves. Nevertheless, teeth of the new taxon resemble teeth of Stegostoma in characters such as the labial apron not reaching the base of the root, and the high compressed root at the level of the lingual protuberance.
We assign the teeth of Notoramphoscyllium woodwardi gen. et sp. nov. to the family Hemiscyillidae despite the varied combination of characters that are also found in other orectolobiforms, because of their general resemblance to teeth of Chiloscyllium and Hemiscyllium. This family is known to date since the Aptian (Early Cretaceous) as fossils only from Europe, North America, the Near East and the West Indies (Cappetta 2012). Thus, the new taxon represents the first fossil record of a hemiscylliid carpet shark from the Southern Hemisphere.
Family Orectolobidae Jordan & Fowler 1903 Genus Coelometlaouia gen. nov.
Type species. Coelometlaouia pannucea sp. nov.
Diagnosis. Orectolobid shark characterized by the following combination of dental characters: gradient monognathic heterodonty; main cusp of anterior teeth triangular and low, almost as high as crown shoulder; main cusp and lateral cusplets evenly inclined lingually; crown shoulder significantly mesiodistally expanded; labial and lingual crown faces of all teeth devoid of ornamentation; labial face in profile view straight to slightly sigmoidal; lingual crown face significantly convex from side to side; lateral cusplets well separated from base of the main cusp by a concave notch in all teeth; lateral cusplets very asymmetrical with mesial cusplet low and very triangular whereas the distal cusplet is very slender, higher, and more acute; generally an incipient, low additional cusplet developed mesially in teeth of anterior and lateral files; additional, very low cusplet present in more posterior teeth; apron very short, broad, almost rectangular in labial view with characteristic concave lateral margins, and only slightly jutting over the root in profile view; anterior margin of apron characteristically curved downwards but not reaching the basal root plane in profile view; cutting edges continuous between cusplets and cusp but not reaching the apex of main cusp; lingual protuberance prominent, oblique in profile view, and completely enameloid-covered; root in basal view heart-shaped in all tooth positions with diverging, broad root lobes enclosing an anterior labial depression; pair of marginolingual foramina small and rounded; mediolingual foramen small and located near the base of the lingual protuberance in the edge where the root lobes meet; root lobes separated at level of lingual protuberance forming a more or less well-developed open nutritive groove.
Differential diagnosis. Differs from Ginglymostoma, Hologinglymostoma and Nebrius in having only one pair of lateral cusplets instead of numerous and in being smaller in the overall size. Palaeorhincodon and Rhincodon differ in having a well-defined cutting edge and weakly prominent and wide apron. Protoginglymostoma and Pseudoginglymostoma differ in possessing ornamentation on the labial crown face of the teeth. Stegostoma differs in having very high lateral cusplets. The hooked apex of lateral cusplets in Deplitoscyllium separates this taxon from the new species. Absence of an apron separates Pararhincodon and Parascyllium from Coelometlaouia gen. nov. Chiloscyllium and Eostegostoma differ in possessing a well-marked cutting edge and the higher principal cusp. Hemiscyllium differs in lacking lateral cusplets and having a basal crown part which is as high as the cusp. Teeth of Orectolobus and Squatiscyllium resemble that of Squatina, lacking lateral cusplets and a broad apron. Orectoloboides lacks a labial bulge overhanging the root. Coleometlaouia gen nov. shares some similarities with Eometlaouia but differs in having a higher root and higher lateral cusplets. Teeth of Coelometlaouia gen. nov. readily can be differentiated from teeth of Notoramphoscyllium gen. nov. in the well-marked, but asymmetrical long lateral cutting edges, comparably shorter apron with downwardly curved anterior edge, high root with distinct root lobes in profile view, an open nutritive grove separating the strongly diverging root lobes in almost all teeth with a labially placed central foramen, and high lateral cusplets, which are detached from the base of the cusp by a concave notch.
Etymology. Composed of the Greek word ‘coelo’, ‘kοίλος’ meaning ‘concave’ in reference to the tooth shape and ‘metlaouia’, because of dental similarities to the extinct carpet shark Eometlaouia.
Coelometlaouia pannucea sp. nov. (Figs 9–11)
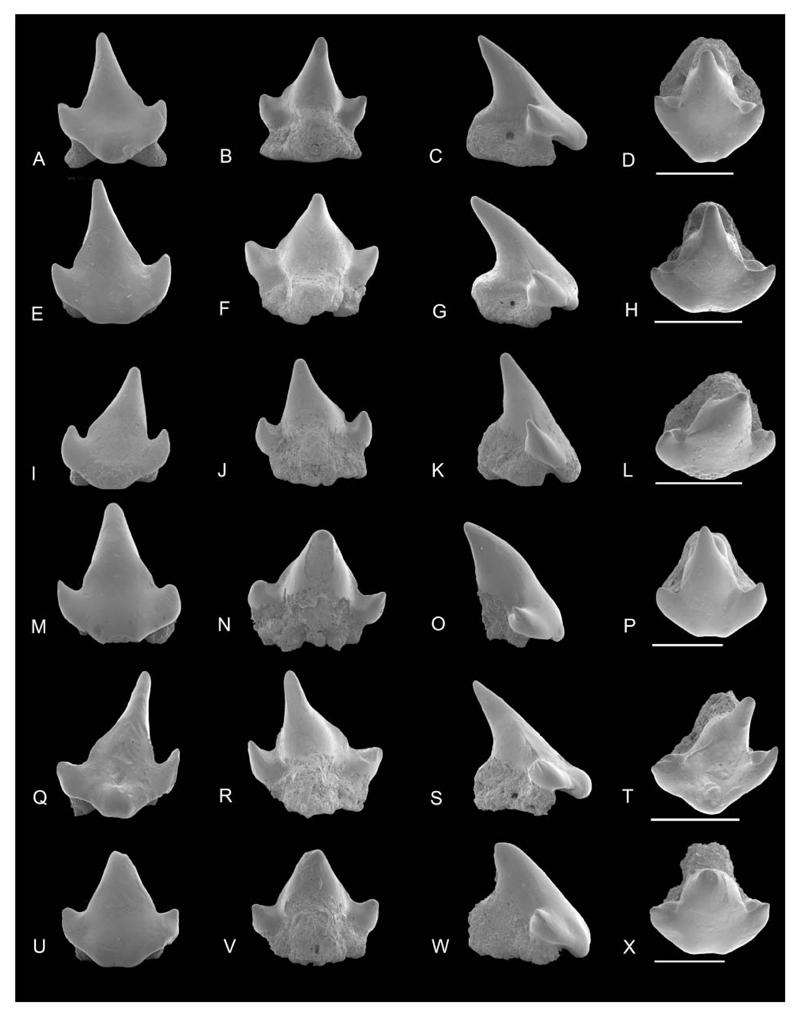
Figure 9.
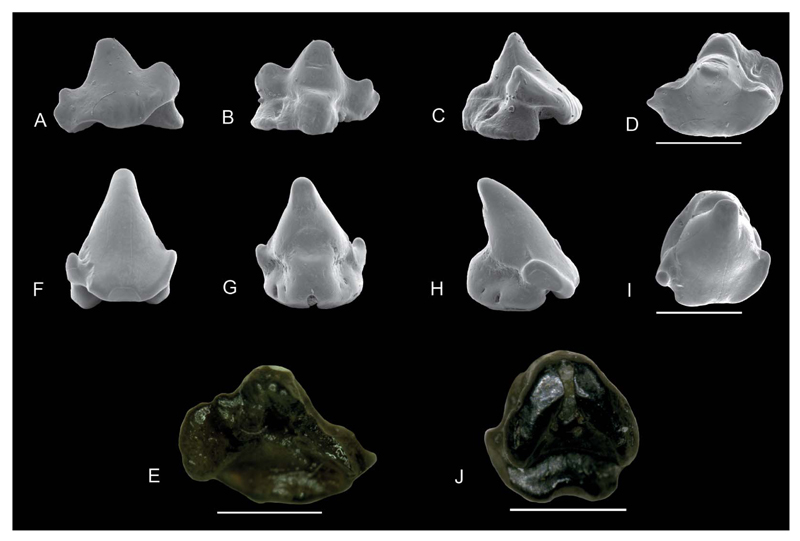
SEM images of Coelometlaouia pannucea gen. et. sp. nov. A–H, anterior teeth: NRM-PZ P.16042 in A, labial, B, lingual, C, profile, D, occlusal views; NRM-PZ P.16043 in E, labial, F, lingual, G, profile, H, occlusal views. I–P, anterolateral teeth: NRM-PZ P.16044 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16045 in M, labial, N, lingual, O, profile, P, occlusal views. Q–X, lateral teeth: holotype NRM-PZ P.16046 in Q, labial, R, lingual, S, profile, T, occlusal views; NRM-PZ P.16047 in U, labial, V, lingual, W, profile, X, occlusal views. Scale bars = 1 mm.
Figure 11.
Images taken with a Keyence Digital Microscope VHX-1000D of Coelometlaouia pannucea gen. et. sp. nov.; all specimens in basal view. A, NRM-PZ P.16042; B, NRM-PZ P.16046; C, NRM-PZ P.16051; D, NRM-PZ P.16048; E, NRM-PZ P.16047; F, NRM-PZ P.16050. Scale bars = 1 mm
Holotype. NRM-PZ P.16046 (Fig. 9Q–T), an anterolateral tooth.
Paratypes. Two anterior teeth, NRM-PZ P.16042– 16043; two anterolateral teeth, NRM-PZ P.16044–16045; three lateral teeth, NRM-PZ P.16046–16048; four more lateral to posterior teeth, NRM-PZ P.16049–16052.
Type horizon. TELM 5, Natica-Horizon, Cucullaea I Allomember, La Meseta Formation.
Type locality. IAA 2/95, Marsupial Site.
Geographical distribution. IAA 1/90, Ungulate Site (64°140′4.67ʺS; 56°39′56.38ʺW) and IAA 2/95, Marsupial Site, (64°13′58ʺS; 56°39′06ʺW) and IAA 1/93 (64°13′51.8ʺS; 56°35′53.14ʺW); Antarctica.
Stratigraphical range. TELM 5, Ypresian, early Eocene (10 teeth) and TELM 6, Lutetian, middle Eocene (one tooth only).
Etymology. The species name is derived from the Latin adjective ‘pannucea’ meaning ‘ragged’, in allusion to the morphology of the tooth crown in labial view.
Diagnosis. As for the genus.
Description. Anterior teeth.
Anterior. Anterior teeth are wider than high (c. 1.5 mm). The main cusp is triangular with a broad base labially, being slightly higher than its base. The mesial cutting edge is longer than the distal. The labial and lingual crown faces lack ornamentation. The labial crown face is slightly undulating to sigmoidal in profile view. The transition between cusp and apron is dented in profile view. The apron is short with concave lateral margins and an indented basal margin labially. The short apron juts only slightly over the root (Fig. 9C). The anterior edge is curved basally rather than being directed basally. The lingual crown face is convex and perpendicular in profile view. The cutting edges start halfway along the main cusp and are continuous with the cutting edges of the lateral cusplets. A single pair of low, pointed, delicate lateral cusplets is present in the anteriormost teeth (e.g. Fig. 9A). The mesial cusplet is divergent and low, the distal more erect. In another anterior tooth, an incipient additional cusplet is developed mesially (Fig. 9E). In this particular tooth, the mesial cusplet is erect, slender, and acuminate whereas the distal one is low and diverges laterally. It is unclear whether these two anterior teeth indicate some sort of dignathic or sexual heterodonty.
The lateral cusplets are well separated from the cusp base by a broad and concave notch. The lateral edges are convex in labial view due to the incipient second pair of cusplets. In profile view the tips of the lateral cusplets are acuminate with a circular basis and offset apex (Fig. 9G). The lingual protuberance is short, oblique in profile view and enameloid covered. The root is massive and high, with two clearly separated long and slender lobes, flaring labially. The basal margin of the root is concave in labial view. The marginolingual foramina are large and rounded, and situated in deep indentations. In lingual view, root lobes are oblique with a basal small and rounded lingual foramen. The central nutrient foramen connects to the mediolingual foramen by a narrow, open nutritive groove. Smaller foramina are present on the edge of the root lobe (Fig. 11).
Anterolateral and lateral teeth. These teeth are distinctly wider than high. The main cusp is triangular labially and inclined distally. The mesial cutting edge is longer than the distal, more oblique and may bear a low basal bulge (e.g. Fig. 9M). The main cusp is slightly displaced distally in more lateral positions, with an almost perpendicular distal margin (e.g. in the holotype, Fig. 9M). The labial crown face is smooth and straight to slightly sigmoidal in profile view. The holotype displays a depression between main cusp and apron in profile view, similar to Notoramphoscyllium woodwardi gen. et sp. nov. (Fig. 9S). Nevertheless, the holotype differs from Coelometlaouia pannucea gen. et sp. nov. in significant characters such as crown form, morphology of the lateral cusplets, well-developed cutting edges not reaching the apex of the main cusp, and the apron being shorter and not bulky.
The lateral cusplets are well separated from the main cusp by a concave notch. The distal cusplet is low and blunt, the mesial one acuminate and erect. A second incipient cusplet may be developed mesially and distally (e.g. Fig. 9Q). The apices of the lateral cusplets taper apically and are acuminate and rounded in cross section. In profile view, the lateral cusplets are inclined lingually, the apices parallel to the cutting edges. The lingual protuberance is short, broadly rounded, oblique in profile view and completely covered with enameloid. The root is high, with slender, but short and widely separated, laterally flaring root lobes. Consequently, the root is broadly concave in labial view with a well-developed depression below the apron. The anterior margin is concave in profile view. Marginolingual foramina are large, rounded, and located in deep indentations of the root margins. The mediolingual foramen is small and located close to the basal edge of the lingual protuberance. In basal view, the root is heart-shaped with distinctly incised lateral margins. The central foramen is located labially and connected to the lingual root foramen by an almost completely open nutritive groove (holaulacorhize).
Posterior teeth. More posterior teeth are mesiodistally elongated with slender, acuminate main cusps and low lateral cusplets (Fig. 10E–G, M–T. In labial view, the main cusp is displaced distally. As in all other teeth, they lack ornamentation and display longer mesial than distal cutting edges. Consequently, the main cusp is also distally inclined. The lingual crown face is slightly concave in profile view because the cusp is more inclined lingually than in anterior teeth. Cutting edges are blunt and do not reach the apex. Lateral cusplets are low but well separated from the main cusp. Additional, almost completely reduced cusplets are present (Fig. 10E, M). The apron is short, jutting only slightly over the root. The basal area of the crown shoulder is bulge-like so that the crown has a low rim (Fig. 10Q–T). The lingual protuberance is short, broad, and very steep in profile view. Root lobes are thin, short and well separated. Lateral margins are almost perpendicular to faintly concave medially in labial view. In lingual view the root is almost rectangular. Marginolingual foramina are small and rounded (Fig. 10O). The mediolingual foramen is small, elliptically shaped, and basally open. The root is heart-shaped to almost circular in basal view with a shallow open nutritive grove separating the root lobes.
Figure 10.
SEM images of Coelometlaouia pannucea gen. et. sp. nov. A–D, lateral teeth: NRM-PZ P.16048 in A, labial, B, lingual, C, profile, D, occlusal views. E–T, more lateral to posterior teeth: NRM-PZ P.16049 in E, labial, F, lingual, G, profile, H, occlusal views; NRM-PZ P.16050 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16051 in M, labial, N, lingual, O, profile, P, occlusal views; NRM-PZ P.16052 in Q, labial, R, lingual, S, profile, T, occlusal views. Scale bars = 1 mm.
Remarks. The teeth of Notoramphoscyllium woodwardi gen. et sp. nov. are superficially similar to teeth of Coelometlaouia pannucea gen. et sp. nov. but can readily distinguished by several characters (see differential diagnosis above). The overall morphology of the teeth of Coelometlaouia pannucea also resembles that of teeth of the genus Eometlaouia, and the extant orectolobid Ginglymostoma cirratum to some extent. However, teeth of Coelometlaouia pannucea gen. et sp. nov. differ from Eometlaouia numidica (Arambourg, 1952) by having a distinctive, short and straight apron, higher lateral cusplets, and a higher root. The labial crown face is smooth in Coelometlaouia pannucea gen. et sp. nov., lacking a median ridge, which is located on the upper part of the main cusp as in E. numidica. The closest species seems to be Eometalaouia delpiti (Noubhani & Cappetta 2002), but Coelometlaouia pannucea gen. et sp. nov. differs in having a high root, a less developed apron with a basally curved anterior margin rather than being directed completely basally, and a straight labial crown face in profile view, which lacks a well-marked concave depression between cusp and apron. Teeth of Ginglymostoma, Hologinglymostoma and Nebrius differ in the presence of more than a single pair of lateral cusplets in all tooth positions.
Orectolobiformes gen. et sp. indet. A (Fig. 12A–E)
Figure 12.
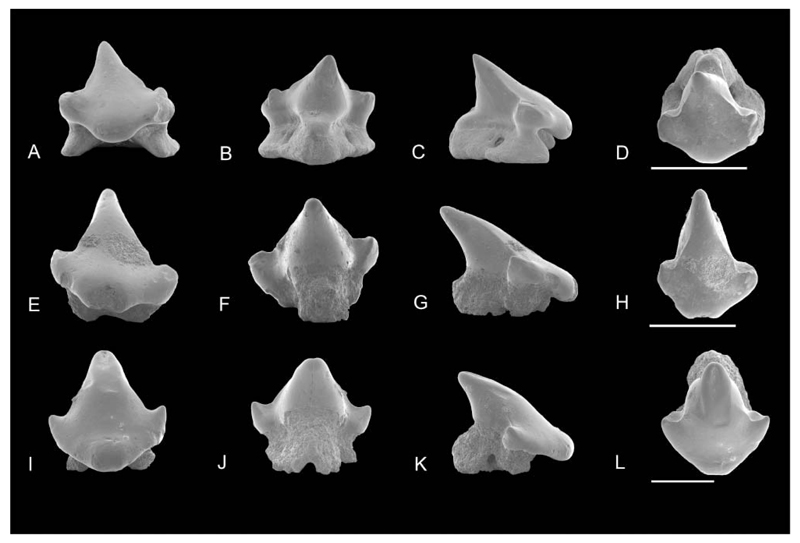
SEM and Keyence Digital Microscope images. A–D, Orectolobiformes gen. et sp. indet A: NRM-PZ P.16053 in A, labial, B, lingual, C, profile, D, occlusal views; F–I, Orectolobiformes gen. et sp. indet B: NRM-PZ P.16054 in F, labial; G, lingual; H, profile; I, occlusal views. Images with a microscope camera; E, Orectolobiformes gen. et sp. indet A, NRM-PZ P.16053 in basal view; J, Orectolobiformes gen. et sp. indet B, NRM-PZ P.16054 in basal view. Scale bars = 1 mm.
Material. NRM-PZ P.16053, a single lateral tooth.
Age. TELM 5, Ypresian, early Eocene.
Locality. Natica-Horizon, Cucullaea I Allomember, IAA 1/90, Ungulate Site, (64°14′04.67ʺS; 56°39′56.38ʺW)
Description. The tooth is distinctly elongated mesiodistally (less than 1.5 mm) and broader than high. The main cusp is low, broadly triangular, and is slightly inclined distally. The apex is lingually abraded. In profile view, the main cusp is not inclined lingually but also triangular with an almost vertical lingual face and a labial face, which is perpendicular apically and then continues obliquely into the apron (Fig. 12C). The labial face displays short and vertical enameloid folds along the basal margin of the apron (Fig. 12C), whereas the lingual crown face is unornamented. The cutting edges start at the apex, are blunt, and are continuous between cusp and lateral cusplets. The crown shoulder reaches up to one third in height of the main cusp.
Mesially, a small and pointed lateral cusplet is well separated from the main cusp by a broad convex notch. A second, almost completely reduced incipient cusplet is developed at its base, which is broadly united with the first mesial cusplet. The lateral edge of the crown appears concavely arched in labial view. The distal cusplet is very low and broad so that the distal crown shoulder appears heel-like. The distal portion of the crown shoulder is higher than the mesial one. In profile view, the crown shoulder and associated lateral cusplets are very broad with the same outline as the main cusp.
The apron is very broad at the base, short and convex. It juts significantly over the root in a horizontal basal plane (Fig. 12C). The apron bears fine and long enameloid folds, which span the entire length of the basal margin. The lingual protuberance is well developed, short, and broad. It is slightly oblique in profile view and covered with enameloid (Fig. 12B, C). The root is low and mesially damaged, lacking the corresponding root lobe. One short and externally curved root lobe is present in labial and profile views. The root is slightly displaced lingually. In labial view, the basal plane of the root is broadly concave, while the basal plane of the distal root lobe is flat. The mesial marginolingual foramen is elliptical and large, located in a deep notch of the root. The mediolingual foramen is small and placed in the middle portion of the protuberance. The basal root face is V-shaped, but one root lobe is damaged. The central basal foramen is nearly triangular and large. This foramen is slightly shifted towards the lingual portion of the root.
Remarks. The single tooth has a unique morphology, differing from all other teeth in this study but displaying the general morphology of other Orectolobiformes especially in the hemiaulacorhize vascularization pattern of the root. The horizontally directed basal plane of the apron and the presence of enameloid folds along the edge of the apron are, however, unique in comparison to the other La Meseta orectolobiform sharks. The prominent and thick apron resembles that of Delpitoscyllium africanum (Leriche, 1927), but the central cusp and the lateral cusplets are vertical, whereas they are significantly inclined lingually and more elongated in D. africanum. No vertical median ridge can be found in the described specimen as in teeth of Pseudoginglyomostoma Dingerkus, 1986, and they also differ in overall shape of the main cusp. In labial view the general form of the tooth is similar to Chiloscyllium but differs in having a short root and a distinctive apron, horizontally directed and bearing fine and long enameloid folds. Moreover, the cutting edges are blunt, whereas they are sharp in Chiloscyllium.
Teeth of the species belonging to the genus Palaeorhincodon differ completely from this specimen, because they have a globular root and slender and pointed lateral cusplets. A rather high and sharp main cusp, which is broad at the base and flanked by a pair of low lateral cusplets characterizes teeth of Eometlaouia. Teeth of Ginglyomostoma and Nebrius are multicuspid with several well-developed lateral cusplets and easy to distinguish from the La Meseta specimen. The tooth therefore probably represents an as yet undescribed genus or species, but with only one specimen recovered, the true affinities of the specimen cannot be properly resolved.
Orectolobiformes gen. et sp. indet. B (Fig 12F–J)
Material. NRM-PZ P.16054, a single oral tooth.
Age. TELM 5, Ypresian, early Eocene.
Locality. TELM 5, Natica-Horizon, Cucullaea I Allomember, IAA 2/95, Marsupial Site, (64°13′58ʺS; 56°39′06ʺW).
Description. The single specimen, which probably represents an anterior tooth, is significantly higher than wide (less than 1.5 mm). The main cusp is almost symmetrical, high, stout, and very triangular in labial view with a rounded and worn apex (Fig. 12F). In labial view the crown face is smooth, with faint vertical scratches in the median region of the main cusp. The main cusp is bulky, with a labial face almost flat basally and convex apically. In profile view, the labial face is slightly sigmoidal. On the labial crown face, at the height of the lateral cusplet, the enameloid shows some deep irregularities (Fig. 12F). The lingual face is slightly inclined lingually with a concave lingual face in profile view. The lingual crown face is smooth and shows some abrasions apically. One pair of very small and diverging lateral cusplets is present. The mesial lateral cusplet is small, rounded and clearly separated from the main cusp, whereas the distal cusplet inserts higher on the crown, is very low, and almost completely reduced. The cutting edge is very blunt and only present at the basally between the main cusp and lateral cusplet. The largest part of the main cusp lacks any cutting edge and the cross section therefore is sub-circular to circular. The convex and short apron overhangs the labial root face significantly and is broadly united with the labial crown face. A large trapezoidal impression is present on the apron, extending to the basal edge (Fig. 12F). The apron is inclined basally but does not reach the basal plane of the root (Fig. 12H). A high marginal rim at the distal margin is present, which runs from the lateral cusplets to the trapezoid impression and mesiodorsally until it reaches the base of the other cusplet (Fig. 12F). The median lingual protuberance is well developed, rounded on its upper part and short. The root is globular and short. In labial view the very small and rounded root lobes are apparent.
The crown–root junction is slightly incised laterally so that the crown overhangs the root on three sides (labially, mesially, distally). There are two small and oval marginolingual foramina of different sizes near the basal root but not set into the shallow lateral notches of the root. The mediolingual foramen is large and rounded. The lingual protuberance is very short and covered with enameloid dorsally. The basal root face is concave in labial view. The root lobes are short, slender, and labially divergent. In basal view, the root almost has a V-shaped outline. The nutritive groove is completely open but remains shallow and connects the mediolingual foramen and the central-basal foramen. Labially, there are several smaller rounded foramina in the convex labial depression of the root between the root lobes.
Remarks. The tooth possesses a unique combination of characters, which are different from all other Eocene La Meseta taxa. The unidentified species differs from Hemiscyllium and Chiloscyllium in having a more globular root and a thick, almost circular crown in cross section. The lateral cusplets are very reduced, as in Hemiscyllium, but the main cusp is broader and the basal shoulder of the crown is not as high as the cusp.
The described tooth is easily distinguished from those of Ginglymostoma and Nebrius, because it is not multicuspid and differs in the overall shape and the arrangement and morphology of lateral cusplets. It also can be distinguished from teeth of all other extant orectolobiform sharks by its combination of characters such as the broadly triangular main cusp with low and incipient lateral cusplets closely set to the main cusp, the very short cutting edges, the rim-like edge delimitating the basal edge of the labial tooth crown, and the holaulacorhize vascularization of the root.
A mainly triangular cusp flanked by a pair of lateral cusplets and a low apron concave basally, characterizes teeth of Pseudoginglymostoma. The presence of a single very reduced cusplet accompanying the main cusp laterally clearly separates this specimen from the aforementioned species.
This small tooth shows some similarities in morphology to teeth of Palaeorhincodon, but differs in having a concave instead of a strongly convex labial crown face and lacking cutting edges. Additionally, the crown of the Antarctic specimen is rather bulky, whereas it is less massive and less triangular with a broad base in teeth of Palaeorhincodon spp. It resembles teeth of some Jurassic orectolobiforms such as Dorsetoscyllium Underwood & Ward, 2004, and to some extent Heterophorcynus Underwood & Ward, 2004, but differs significantly in the combination of low and incipient lateral cusplets, the labial rim-like margin, and the holaulacorhize root vascularization pattern.
Discussion
The fossil record of orectolobiform sharks generally is poor. Most of the extant genera have a rather young fossil record, like Rhincodon, the whale shark, which is known back to the Miocene. In contrast, the oldest orectolobiform dates back to the Lower Jurassic of Germany (Thies 1983) and belongs to the family Brachaeluridae (Cappetta 2012). The family Hemiscyllidae Gill, 1862 is comparatively well represented in the fossil record by both articulated skeletons from the Upper Cretaceous of Lebanon (Signeux 1949; Cappetta 1980) and isolated teeth from a variety of fossil sites. The remaining families of fossil carpet sharks are mainly known from teeth, but they share a very poor fossil record (Cappetta 2012; Maisey 2012). Furthermore, they were more widespread in the past than today.
The La Meseta Formation, Seymour Island, has yielded the most abundant Palaeogene chondrichthyan fauna from the Southern Hemisphere to date (e.g. Kriwet 2005; Kriwet et al. 2016). With the exception of rare orectolobiform teeth that were previously reported by Long (1992a), this is the first detailed description of orectolobiform shark discoveries from the Eocene of Antarctica. These increase our knowledge of the selachian diversity in the Southern Ocean during the Eocene greenhouse climate but also allow us to re-evaluate previous identifications by Long (1992a), who assigned two teeth from the La Meseta Formation to Stegostoma cf. fasciatum and a single tooth to Pseudoginglymostoma cf. brevicaudatum. The figured tooth identified as representing the zebra shark, Stegostoma cf. fasciatum, displays all characteristic dental traits of the new taxon described here, Notoramphoscyllium woodwardi gen. et sp. nov., as outlined above and we thus synonymize both Antarctic records.
Teeth of Pseudoginglymostoma brevicaudatum have a high and triangular main cusp, one pair of lateral cusplets, and are characterized by a short, vertical ridge located apically on the main cusp of anterior to lateral teeth and numerous irregular labial folds on more posterior teeth. The cusplets of P. brevicaudatum are well developed, diverging, and insert low at the main cusp. The apron is not very prominent and distinctly bifid. Conversely, the lateral cusplets insert high at the cusp of the specimen described and figured by Long (1992a). Moreover, the Antarctic tooth figured by Long (1992a) resembles an open fan, the lateral cusplets are very low and broad, the same as the main cusp, and are poorly individualized. In the material examined, comprising several thousand isolated teeth of chondrichthyans from the La Meseta Fm of Seymour Island, no additional specimen had been found that could be assigned to Pseudoginglymostoma. Additionally, the tooth figured by Long (1992a) cannot be assigned to any of the two new taxa described here. Therefore, we consider the identification of the tooth as P. brevicaudatum a doubtful taxonomic assignment (nomen dubium).
The two new taxa described here are easily distinguishable from all other known orectolobiform taxa by the combination of characters and are assigned to Hemiscylliidae and Orectolobidae, respectively, based on characters described above. Nevertheless, the teeth of both taxa are rather similar and it cannot be excluded that both belong to the same family.
The two additional teeth presented here (Fig. 12) but left in open nomenclature display characteristic features that allow us to identify them as separate, probably new taxa. More material, however, is necessary for an unambiguous taxonomic designation. Nevertheless, the diversity of orectolobiform sharks was significantly higher during the Eocene in Antarctic waters than previously presumed. Obviously these orectolobiforms represent endemic taxa, which agrees well with current distribution patterns of carpet sharks showing rather restricted occurrences.
Palaeoecology and tropical warm water migrants
The Eocene is characterized by major plate-tectonic and climatic changes such as the Paleocene–Eocene Thermal Maximum and the Early Eocene Climatic Optimum (EECO). Palaeo-temperatures seemingly decreased from the early to the late Eocene in Antarctica (Ivany et al. 2008) that correlates with a decrease in chondrichthyan diversity (Kriwet et al. 2016).
The La Meseta Fm is dominated by a cool- to warm-temperate chondrichthyan fauna (Kriwet et al. 2016). This is supported by the presence of taxa such as Lamna cf. nasus (Bonnaterre, 1788), the probeagle shark, which is widely distributed in temperate oceans today (Compagno et al. 2005), or the extinct and extant sand tiger sharks, Striatolamia macrota (Agassiz, 1843) and Carcharias taurus (Rafinesque, 1810), respectively, that were (and are) widespread in subtropical, temperate and cool-temperate waters (Compagno et al. 2005; Cappetta 2012).
The orectolobiform remains that form the focus of this study were recovered from sediments of the Cucullaea I Allomember in TELM 5 and additional sediments of TELM 6, which are early to middle Eocene in age, respectively. The sediments of both stratigraphical units were deposited in a nearshore, ebb-tidal and delta-barrier island complex, which was strongly influenced by waves and tidal currents (Stilwell & Zinsmeister 1992). Sea surface temperatures of TELM 5 ranged from 10°C to 11°C, according to Ivany et al. (2008), and those of TELM 6 reached 15–16°C at the base from where the orectolobiform remains were recovered. The low temperatures in TELM 5 are striking, the one assumed for TELM 6 agreeing better with modern occurrence patterns of orectolobiforms. Deposits of TELM 5 nevertheless yielded diverse marine vertebrate faunas including teeth and placoid scales of sharks, rays and skates, and teeth, otoliths and bones of teleostean fishes, which are more similar to associations found in modern warm to tropical seas (for a complete species list see Reguero et al. (2013)). The occurrence of orectolobiforms in these associations agrees well with the general faunal composition of elasmobranchs for such an environment.
A significant cooling event occurred at the end of TELMs 6 and 7 (middle–late Eocene) with estimated sea surface temperatures of approximately 5°C (Ivany et al. 2008). Kriwet et al. (2016) demonstrated that cartilaginous fishes did not disappear until the onset of the Antarctic ice sheet, when temperatures slightly increased to c. 7–8°C (e.g. Marenssi et al. 2002; Ivany et al. 2008). A more recent investigation suggests slightly higher temperatures for southern high latitude seas, with 16.8°C during the Lutetian stage (45 Ma) and 12.6°C during TELM 6 (42 Ma) (Douglas et al. 2014). The lack of orectolobiform sharks in upper levels of the La Meseta Fm is in good accordance with the low seawater temperatures.
The occurrence of these small, mostly bottom-dwelling sharks can be used not only as an indicator for temperature ranges and water depth estimations, but also for habitat reconstructions in the Eocene of the Southern Ocean. Extant elasmobranchs often live in distinct depth ranges and prefer certain habitats. Long (1992b) concluded that the La Meseta elasmobranchs inhabited different bathymetric and ecological zones, with shallow-water and also some deep-water sharks, like squaliforms. Modern orectolobiform sharks are mostly bottom dwelling, preferring coral and rocky reefs, and living not deeper than 150 m, with some exceptions like the whale shark, which is pelagic and widely distributed. Extant orectolobiform sharks therefore are considered to be generally coastal residents, which are defined as species that solely inhabit coastal waters or spend the majority of their life in coastal habitats. For the extant Ginglymostoma cirratum and Orectolobus halei (Whitley 1940) only horizontal movements are known (Speed et al. 2010). Castro & Rosa (2005) recorded seasonal movements of G. cirratum, which are driven by sex-specific differences in habitat use, during the mating season. Females seem to aggregate in the shallow waters to avoid mating (Carrier et al. 1994). The affinity of orectolobiform sharks to the Indo-West Pacific is probably due to the diversity of coral reefs there and their low migratory tendency (Springer 1982). Thus, orectolobiform sharks are primarily non-migratory fishes, since they prefer warm temperatures and feed mostly on resident/stationary animals like small squids, molluscs and lobsters. These sharks will stay all their lives more or less in the same tropical region and are considered predominantly endemic. Long (1992b) considered the occurrence of orectolobiform sharks in Antarctic waters during the Eocene to represent occasional immigrants into the Southern Ocean, which is not supported by observations of living orectolobiformes as outlined above and our study here. The two new, and probably the additional two unidentified taxa are interpreted as permanent residents, which most likely were endemic to Antarctic waters during the Eocene.
Conclusions
Almost all teeth of the four described orectolobiforms come from the same stratigraphical level but two different sites in the upper part of the Cucullaea I Allomember in TELM 5, which is Ypresian (late early Eocene) in age (Montes et al. 2013; Schwarzhans et al. 2016), indicating a diverse orectolobiform assemblage in the Ypresian that differs taxonomically from contemporaneous sites elsewhere in the world. The teeth figured by Long (1992a) are not from TELM 4 but actually from TELM 5, which is also Ypresian in age. Both new taxa also occur in TELM 6 (this study), which is Lutetian in age. So far, these taxa have not been found in the lower and uppermost stratigraphical levels of the La Meseta Fm or outside Seymour Island. Moreover, none of the four taxa can be assigned to any known orectolobiform taxon and we therefore consider them to represent endemic sharks to Antarctic waters during the Ypresian and Lutetian with a short stratigraphical range according to our current knowledge. This, however, is more likely a collecting bias rather than a real pattern because bulk samples that were screen-washed and sorted for micro-teeth were only obtained from the sites investigated here.
It was not possible to resolve the systematic position of all four orectolobiform taxa reported here. Teeth of Coelometlaouia gen. nov. and Notoramphoscyllium gen. nov. display general morphological traits of Hemiscyllidae and Orectolobidae, respectively. However, we cannot dismiss the possibility that both belong to a different, yet unrecognized family of Southern Hemisphere orectolobiforms.
Figure 6.
SEM images of Notoramphoscyllium woodwardi gen. et sp. nov., lower lateral teeth, NRM-PZ P.15916 in A, labial, B, lingual, C, profile, D, occlusal views; NRM-PZ P.16032 in E, labial, F, lingual, G, profile, H, occlusal views; NRM-PZ P.16033 in I, labial, J, lingual, K, profile, L, occlusal views; NRM-PZ P.16034 in M, labial, N, lingual, O, profile, P, occlusal views; NRM-PZ P.16035 in Q, labial, R, lingual, S, profile, T, occlusal views; NRM-PZ P.16036 in U, labial, V, lingual, W, profile, X, occlusal views. Scale bars = 1 mm.
Acknowledgements
The Argentinian Antarctic Institute (IAA-DNA), Argentinian Air Force and Swedish Polar Research Secretariat (SPFS) are acknowledged for logistic support for field-work on Seymour Island. The authors are grateful to Martin de los Reyes, Museo de La Plata, for picking the small fractions in the laboratory. The authors would like to thank Patricia Holroyd, Museum of Paleontology, University of California, Berkeley, for access to the Woodburne collection of La Meseta specimens and for the possibility to study comparative material. Financial support for this project is provided by The Austrian Science Fund (FWF: P26465-B25 to JK), Swedish Research Council (VR grant 2009-4447 to TM), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET grant PIP 0462 to MR), and the Argentinian National Agency for Promotion of Science and Technology (ANPCyT grant PICTO 0093/2010 to MR). We also want to thank C. Underwood and G. Case for their constructive reviews, and the Associate Editor Z. Johanson for all her support and suggestions that greatly improved the manuscript.
References
- Agassiz JLR. Recherches sur les Poissons Fossiles. Vol. 5. Imprimerie de Petitpierre; Neuchâtel: 1833–1844. p. 1420. with supplements. [Google Scholar]
- Applegate SP. A revision of the higher taxa of Orectoloboids. Journal of the Marine Biological Association of India. 1972;14:743–751. [Google Scholar]
- Arambourg C. Les vertébrés fossiles des gisements de phosphates (Maroc-Algérie-Tunisie) Notes et Mémoires du Service Géologique du Maroc. 1952;92:1–372. [Google Scholar]
- Balushkin AV. Proeleginops grandeastmanorum gen. et sp. nov. (Perciformes, Notothenioidei, Eleginopsidae) from the Late Eocene of Seymour Island (Antarctica) is a fossil notothenioid, not a gadiform. Journal of Ichthyology. 1994;34:10–23. [Google Scholar]
- Bomfleur B, Mörs T, Ferraguti M, Reguero MA, McLoughlin S. Fossilized spermatozoa preserved in a 50-Myr-old annelid cocoon from Antarctica. Biology Letters. 2015;11:20150431. doi: 10.1098/rsbl.2015.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte CL. Selachorum tabula analytica. Nuovi Annali delle Scienze Naturali. 1838;2:195–214. [Google Scholar]
- Bond M, Pascual R, Reguero MA, Santillana SN, Marenssi SA. Los primeros “ungulados” extinguidos sudamericanos de la Antártida. Ameghiniana. 1990;26:240. [Google Scholar]
- Bonnaterre JP. Ichthyologie. Panckoucke; Paris: 1788. Tableau encyclopédique et methodique des trois règnes de la nature; p. 215. [Google Scholar]
- Cappetta H. Les sélaciens du Crétacé supérieur du Liban. I: Requins. Palaeontographica Abteilung A. 1980;168:69–148. [Google Scholar]
- Cappetta H. Chondrichthyes: Mesozoic and Cenozoic Elasmobranchii: Teeth. In: Schultze H-P, editor. Handbook of Paleoichthyology. 3E. Verlag Dr. Friedrich Pfeil; Munich: 2012. pp. 1–512. [Google Scholar]
- Carrier JC, Pratt HL, Jr, Martin LK. Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum. Copeia. 1994;3:646–656. [Google Scholar]
- Castro AL, Rosa RS. Use of natural marks on population estimates of the nurse shark, Ginglymostoma cirratum, at Atol das Rocas Biological Reserve, Brazil. Environmental Biology of Fishes. 2005;72:213–221. [Google Scholar]
- Cione AL, Reguero MA. Extension of the range of hexanchid and isurid sharks in the Eocene of Antarctica and comments on the occurrence of hexanchids in recent waters of Argentina. Ameghiniana. 1995;32:151–157. [Google Scholar]
- Cione AL, Reguero MA. A middle Eocene basking shark (Lamniformes, Cetorhinidae) from Antarctica. Antarctic Science. 1998;10:83–88. [Google Scholar]
- Compagno LJV. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society. 1973;53(Supplement 1):15–61. [Google Scholar]
- Compagno LJV. Phyletic relationships of living sharks and rays. American Zoologist. 1977;17:303–322. [Google Scholar]
- Compagno LJV, Dando M, Fowler S. A field guide to the sharks of the world. Harper Collins; London: 2005. p. 368. [Google Scholar]
- Corrigan S, Beheregaray LB. A recent shark radiation: molecular phylogeny, biogeography and speciation of wobbegong sharks (family: Orectolobidae) Molecular Phylogenetics and Evolution. 2009;52:205–216. doi: 10.1016/j.ympev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Dingerkus G. Interrelationships of orectolobiform sharks (Chondrichthyes: Selachii). In: Uyeno T, Arai R, Taniuchi T, Matsuura K, editors. Proceedings of the Second International Conference on Indo-Pacific Fishes; Tokyo. Ichthyological Society of Japan; 1986. pp. 227–245. [Google Scholar]
- Dingle RV, Lavelle M. Late Cretaceous–Cenozoic climatic variations of the northern Antarctic Peninsula: new geochemical evidence and review. Palaeogeography, Palaeoclimatology, Palaeoecology. 1998;141:215–232. [Google Scholar]
- Doktor M, Gazdzicki A, Jerzmanska A, Porebski SJ, Zastawniak E. A plant-and-fish assemblage from the Eocene La Meseta Formation of Seymour Island (Antarctic Peninsula) and its environmental implications. Palaeontologia Polonica. 1996;55:127–146. [Google Scholar]
- Douglas PM, Affek HP, Ivany LC, Houben AJ, Sijp WP, Sluijs A, Schouten S, Pagani M. Pronounced zonal heterogeneity in Eocene southern high-latitude sea surface temperatures. Proceedings of the National Academy of Sciences. 2014;111:6582–6587. doi: 10.1073/pnas.1321441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton AL, Lohmann KC, Zinsmeister WJ. Stable isotope and minor element proxies for Eocene climate of Seymour Island, Antarctica. Paleoceanography. 2002;17:6–1. [Google Scholar]
- Ebert DA, Fowler S, Compagno LJV. Sharks of the World – A fully illustrated guide. Wild Nature Press; Plymouth: 2013. p. 528. [Google Scholar]
- Eastman JT, Grande L. Evolution of the Antarctic fish fauna with emphasis on the recent notothenioids. Geological Society, London, Special Publications. 1989;47:241–252. [Google Scholar]
- Eastman JT, Grande L. Late Eocene gadiform (Teleostei) skull from Seymour Island, Antarctic Peninsula. Antarctic Science. 1991;3:87–95. [Google Scholar]
- Elliot DH, Trautman TA. Lower Tertiary strata on Seymour Island, Antarctic Peninsula. In: Craddock C, editor. Antarctic Geoscience. University of Wisconsin Press; Madison: 1982. pp. 287–297. [Google Scholar]
- Gazdzicki A, Gruszczynski M, Hoffman A, Malkowski K, Marenssi SA, Halas S, Tatur A. Stable carbon and oxygen isotope record in the Paleogene La Meseta formation, Seymour Island, Antarctica. Antarctic Science. 1992;4:461–468. [Google Scholar]
- Gill T. Analytical Synopsis of the Order of Squali; and Revision of the Nomenclature of the Genera. Annals of the Lyceum of Natural History of New York. 1862;7:367–408. [Google Scholar]
- Goto T. Comparative anatomy, phylogeny and cladistic classification of the order Orectolobiformes (Chondrichthyes, Elasmobranchii) Memoirs of the Graduate School of Fisheries Sciences Hokkaido University. 2001;48:1–100. [Google Scholar]
- Günther A. An account of the fishes of the states of Central America, based on collections made by Capt. J. M. Dow F Godman, Esq., and O. Salvin, Esq. Transactions of the Zoological Society of London. 1867;6:377–494. pls 63–87. [Google Scholar]
- Hay OP. Bibliography and catalogue of the fossil vertebrata of North America. United States Geological Survey, Bulletin. 1902;179:1–868. [Google Scholar]
- Herman J, Hovestadt-Euler M, Hovestadt DC. Part A: Selachii. No. 4: Order Orectolobiformes – Families: Brachaeluridae, Ginglymostomidae, Hemiscylliidae, Orectolobidae, Parascylliidae, Rhiniodontidae, Stegostomidae. Order Pristiphoriformes – Family: Pristiophoridae. Order Squatiniformes – Family: Squatinidae. Bulletin de l’Institut Royal des Sciences naturelles de Belgique. 1992;65:193–254. [Google Scholar]
- Hermann J. In: Tabula affinitatum animalium olim academico specimine edita, nunc uberiore commentario illustrata com annotationibus ad historiam naturalem animalium augendam facientibus. Treuttel JG, editor. Argentorati; Strasbourg: 1783. p. 370. [Google Scholar]
- Huxley TH. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Zoological Society of London. 1880;43:649–662. [Google Scholar]
- Ivany LC, Lohmann KC, Hasiuk F, Blake DB, Glass A, Aronson RB, Moody RM. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. Geological Society of America Bulletin. 2008;120:659–678. [Google Scholar]
- Jerzmanska A. Isolated vertebrae of teleostean fishes from the Paleogene of Antarctica. Polish Polar Research. 1988;9:421–435. [Google Scholar]
- Jordan DS, Fowler HW. A review of the elasmobranchiate fishes of Japan. Proceedings of the United States National Museum. 1903;26:593–674. [Google Scholar]
- Kriwet J. First record of an Early Cretaceous shark (Chondrichthyes, Neoselachii) from Antarctica. Antarctic Science. 2003;15:507–511. [Google Scholar]
- Kriwet J. Additions to the Eocene selachian fauna of Antarctica with comments on Antarctic selachian diversity. Journal of Vertebrate Paleontology. 2005;25:1–7. [Google Scholar]
- Kriwet J, Benton MJ. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous–Tertiary boundary. Palaeogeography, Palaeoclimatology, Palaeoecology. 2004;214:181–194. [Google Scholar]
- Kriwet J, Kiessling W, Klug S. Diversification trajectories and evolutionary life-history traits in early sharks and batoids. Proceedings of the Royal Society of London, Series B. 2009;276:945–951. doi: 10.1098/rspb.2008.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriwet J, Engelbrecht A, Mörs T, Reguero R, Pfaff C. Ultimate Eocene (Priabonian) chondrichthyans (Holocephali, Elasmobranchii) of Antarctica. Journal of Vertebrate Paleontology. 2016:e1160911. doi: 10.1080/02724634.2016.1160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche M. Les Poissons de la Molasse suisse. Mémoires de la Société Paléontologique Suisse. 1927;46:1–55. [Google Scholar]
- Long DJ. Sharks from the La Meseta Formation (Eocene), Seymour Island, Antarctic Peninsula. Journal of Vertebrate Paleontology. 1992a;12:11–32. [Google Scholar]
- Long DJ. Paleoecology of Eocene Antarctic sharks. Antarctic Research Series. 1992b;56:131–139. [Google Scholar]
- Long DJ, Stilwell JD. Fish remains from the Eocene of Mount Discovery, East Antarctica. Antarctic Research Series. 2000;76:349–353. [Google Scholar]
- Maisey JG. What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. Journal of Fish Biology. 2012;80:918–951. doi: 10.1111/j.1095-8649.2012.03245.x. [DOI] [PubMed] [Google Scholar]
- Maisey JG, Naylor GJ, Ward DJ. Mesozoic elasmobranchs, neoselachian phylogeny and the rise of modern elasmobranch diversity. Mesozoic Fishes. 2004;3:17–56. 16 figs., 2 tabs, 1 app. [Google Scholar]
- Marenssi SA, Santillana SN, Rinaldi CA. Stratigraphy of the La Meseta Formation (Eocene), Marambio (Seymour) Island, Antarctica. In: Casadio S, editor. Paleogeno de America del Sur y de la Peninsula Antartica: Asociacion Paleontologica Argentina. Vol. 5. Publicacion Especial; Buenos Aires, Argentina: 1998. pp. 137–146. [Google Scholar]
- Marenssi SA, Net LI, Santillana SN. Provenance, environmental and paleogeographic controls on sandstone composition in an incised-valley system: the Eocene La Meseta Formation, Seymour Island, Antarctica. Sedimentary Geology. 2002;150:301–321. [Google Scholar]
- McLoughlin S, Bomfleur B, Mörs T, Reguero M. Fossil clitellate annelid cocoons and their microbiological inclusions from the Eocene of Seymour Island, Antarctica. Palaeontologia Electronica. 2016;19(1.11A):1–27. [Google Scholar]
- Montes M, Nozal F, Santillana S, Marenssi, Olivero E. Mapa Geológico de la Isla Marambio (Seymour); escala 1:20.000. Serie Cartográfica Geocientífica Antártica. Con texto complementario. Instituto Geólogico y Minero de España, Instituto Antártico Argentino; Madrid, Buenos Aires: 2013. [Google Scholar]
- Müller J, Henle FGJ. Über die Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Berichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin. 1837;1837:111–118. [Google Scholar]
- Naylor GJP, Caira JN, Jensen K, Rosana KA, Straube N, Lakner C. Elasmobranch phylogeny: A mitochondrial estimate based on 595 species. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. 2nd edition. CRC Press; Boca Raton: 2012. pp. 31–56. [Google Scholar]
- Noubhani A, Cappetta H. Metlaouia Noubhani and Cappetta, 1997 [Chondrichthyes: Orectolobiformes] preoccupied by Metlaouia Dumont, 1928 [Insecta: Lepidoptera] Acta Palaeontologica Polonica. 2002;47:684. [Google Scholar]
- Rafinesque CS. Caratteri di alcuni nuovi generi e nuove specie di animali e pinate della Sicilia, con varie osservazioni sopra i medisimi. San Filippo, Palermo: 1810. [Google Scholar]
- Ramsay JB, Wilga CD. Morphology and mechanics of the teeth and jaws of white–spotted bamboo sharks (Chiloscyllium plagiosum) Journal of Morphology. 2007;268:664–682. doi: 10.1002/jmor.10530. [DOI] [PubMed] [Google Scholar]
- Reguero M, Goin F, Acosta Hospitaleche C, Marenssi S, Dutra T. South America/West Antarctica: Pacific Affinities of the Weddellian Marine/Coastal Vertebrates. In: Reguero M, Goin F, Acosta Hospitaleche C, Dutra T, Marenssi S, editors. Late Cretaceous/Paleogene West Antarctica terrestrial biota and its intercontinental affinities. Springer; Dordrecht: 2013. pp. 27–54. [Google Scholar]
- Sadler PM. Geometry and stratification of uppermost Cretaceous and Paleogene units on Seymour Island, northern Antarctic Peninsula. Geological Society of America Memoirs. 1988;169:303–320. [Google Scholar]
- Schwarzhans W, Mörs T, Engelbrecht A, Reguero M, Kriwet J. Before the freeze: otoliths from the Eocene of Seymour Island, Antarctica, reveal dominance of gadiform fishes (Teleostei) Journal of Systematic Palaeontology. 2016 doi: 10.1080/14772019.2016.1151958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signeux J. Notes paleoichthyologiques. I. Observations sur le genre Scapanorhynchus et ses relations. Bulletin du Muséum National d’Histoire Naturelle, série 2. 1949;21:633–638. figs 1–3. [Google Scholar]
- Smith A. Descriptions of new, or imperfectly known objects of the animal kingdom, found in the south of Africa. South African Commercial Advertiser. 1828;3:2. [Google Scholar]
- Smith A. On the necessity for a revision of the groups included in the Linnaean genus Squalus. Proceedings of the Zoological Society of London. 1837;5:85–86. [Google Scholar]
- Speed CW, Field IC, Meekan MG, Bradshaw CJ. Complexities of coastal shark movements and their implications for management. Marine Ecology Progress Series. 2010;408:275–293. [Google Scholar]
- Springer VG. Pacific plate biogeography with special reference to shorefishes. Smithsonian Contributions to Zoology. 1982;465:1–82. [Google Scholar]
- Stilwell JD, Zinsmeister WJ. Molluscan systematics and biostratigraphy. Lower Tertiary La Meseta Formation, Seymour Island, Antarctic Peninsula. Antarctic Research Series. 1992;55:1–192. [Google Scholar]
- Thies D. Jurazeitliche Neoselachier aus Deutschland und S-England. Courier Forschungsinstitut Senckenberg. 1983;58:1–11. [Google Scholar]
- Underwood C. Diversification of the Neoselachii (Chondrichthyes) during the Jurassic and Cretaceous. Paleobiology. 2006;32:215–235. [Google Scholar]
- Underwood CJ, Ward DJ. Neoselachian sharks and rays from the British Bathonian (Middle Jurassic) Palaeontology. 2004;47:447–501. [Google Scholar]
- Whitley GP. The fishes of Australia. Part 1. The sharks, rays, devil fishes and other primitive fishes of Australia and New Zealand. Royal Zoological Society of New South Wales; Sydney: 1940. p. 280. [Google Scholar]
- Winchell CJ, Martin AP, Mallatt J. Phylogeny of elasmobranchs based on LSU and SSU ribosomal RNA genes. Molecular Phylogenetics and Evolution. 2004;31:214–224. doi: 10.1016/j.ympev.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Welton BJ, Zinsmeister WJ. Eocene neoselachians from the La Meseta Formation, Seymour Island, Antarctic Peninsula. Contributions in Science Natural History Museum of Los Angeles County. 1980;329:1–10. [Google Scholar]
- Woodward AS. On fossil fish-remains from Snow Hill and Seymour Islands. Vol. 3. Wissenschaftliche Ergebnisse der schwedischen Südpolar Expedition, 1901–1903; 1908. pp. 1–4. [Google Scholar]