Abstract
Supported metal catalyst was designed, characterized and tested for aqueous phase heterogeneous hydrogenation of vinyl acetate with parahydrogen to produce 13C-hyperpolarized ethyl acetate for potential biomedical applications. The Rh/TiO2 catalyst with 23.2 wt% loading produced strong hyperpolarized 13C-enriched ethyl acetate-1-13C detected at 9.4 T. Approximately 14-fold 13C signal enhancement was detected using ~50% parahydrogen gas without taking into account relaxation losses before and after polarization transfer via magnetic field cycling from nascent parahydrogen-derived protons to 13C nuclei. This first observation of 13C PHIP-hyperpolarized products over supported metal catalyst in the aqueous medium opens up new possibilities for production of catalyst-free aqueous solutions of nontoxic hyperpolarized contrast agents for a wide range of biomolecules amenable to Parahydrogen Induced Polarization via Side Arm Hydrogenation (PHIP-SAH) approach.
Keywords: parahydrogen, PHIP, 13C NMR, hyperpolarization, polarization transfer
Graphical Abstract
Supported metal catalyst was designed, characterized and tested for aqueous phase heterogeneous hydrogenation of vinyl acetate with parahydrogen to produce 13C-hyperpolarized ethyl acetate for potential biomedical applications. Approximately 14-fold 13C signal enhancement was detected using ~50% parahydrogen gas at 9.4 T.
Nowadays, the methods based on magnetic resonance (NMR, MRI) are routinely used as sensitive analytical tools in chemistry and medicine. However, despite their impressive achievements, a potentially wider reach of these techniques is limited by their inherently limited sensitivity associated with the low nuclear spin polarization at thermal equilibrium. The advent of hyperpolarization NMR techniques significantly expands the range of potentially feasible MR applications.[1–9] One of hyperpolarization techniques is the dynamic nuclear polarization (DNP) that allows one to hyperpolarize a wide range of 13C-labeled compounds[10,11] and utilize them for metabolic NMR/MRI.[12–14] However, the main limitations of the DNP technique are (i) a long hyperpolarization time and (ii) high hyperpolarizer cost. Parahydrogen-induced polarization (PHIP) is an alternative hyperpolarization technique using singlet spin order of parahydrogen.[15–17] Pairwise addition of parahydrogen (p-H2), that is addition of the two H atoms from one p-H2 molecule to magnetically nonequivalent positions of substrate molecule, creates hyperpolarization on the nascent protons in the product molecule. Importantly, the polarization transfer from nascent parahydrogen atoms via spin-spin couplings to heteronuclei (e.g. to 13C) results in hyperpolarized (HP) contrast agents with the lifetimes sufficient for their biomedical applications.[18–20]
PHIP allows production of HP liquids that can be directly used for signal enhancement in various NMR/MRI applications.[18,20] Despite the relative synthesis complexity of 13C-labeled PHIP precursor compounds, many successful examples with high polarization levels on 13C nuclei (P13C>10%) in biomolecules have been reported: 13C-succinate,[21] 13C-tetrafluoropropionate,[22] and more recently 13C-phospholactate.[23] While the corresponding pioneering in vivo studies are encouraging,[22,24,25] they were performed using injections of liquid HP contrast agent containing homogeneous Rh-based catalyst,[26] which is potentially toxic, and thus it is a definite roadblock for ultimate in-human translation. Two alternative approaches can potentially address this obstacle: (i) catalyst removal by filtration, or (ii) utilization of heterogeneous catalyst for pairwise p-H2 addition (HET-PHIP).[9,27–29] The second approach is more advantageous, because it additionally allows catalyst recycling.
Importantly, PHIP medium must be biocompatible, i.e. aqueous. The first observation of heterogeneous PHIP effects in aqueous medium was reported in 2010[30] utilizing supported metal catalysts. Recently a similar observation (indicating the possibility of heterogeneous PHIP formation in water) was made using ligand-capped platinum nanoparticles.[31,32] Although the transfer of polarization from nascent parahydrogen protons to 13C nuclei was accomplished at P13C = ~0.013% level,[31] subsequent applications of this system face serious challenges, because (i) separation of HP solution from 2 nm size nanoparticles is required, (ii) very low conversion levels were achieved (ca. 0.03% after 15 seconds of chemical reaction),[31] and (iii) the reaction product 2-hydroxyethyl propionate (HEP) has no biomedical relevance beyond angiographic applications.[18]
Recently, an efficient procedure of polarization transfer from nascent parahydrogen protons to 13C using side arm hydrogenation (SAH) approach in combination with adiabatic magnetic field cycling was introduced. It was exemplified with a range of esters bearing an unsaturated alcoholic or carboxylic moiety, including vinyl acetate which yielded HP ethyl acetate-1-13C upon hydrogenation and magnetic field cycling.[33,34] However, these previous studies employed homogeneous catalyst in organic medium, which is incompatible with the long-term goal of in-human translation of PHIP-SAH, which otherwise paves a straightforward and scalable route to efficient 13C hyperpolarization of many biomolecules including 13C-pyruvate.[33–35] Moreover, recent results based on the vinyl acetate heterogeneous hydrogenation in the gas phase with subsequent dissolution in aqueous phases and hydrolysis resulting in hyperpolarized ethanol and non polarized acetic acid mixture production allow to produce pure, biocompatible and catalyst free hyperpolarized fluid.[36]
In this work, we demonstrate that both previous shortcomings of PHIP-SAH can be resolved. Specifically, 2-3 mm beads of TiO2 with supported rhodium nanoparticles were utilized for the aqueous phase heterogeneous hydrogenation of vinyl acetate-1-13C with p-H2, followed by polarization transfer to 13C nuclei by means of magnetic field cycling.[33–35]
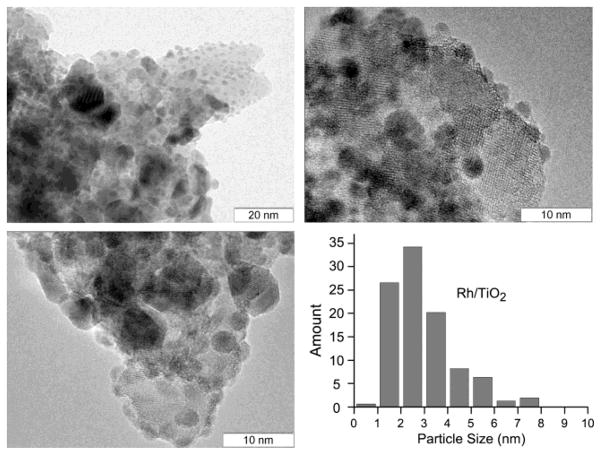
Recently it was shown that decreasing the size of metal nanoparticles leads to the increase in the pairwise p-H2 addition level,[9,36] and therefore, the catalyst under investigation should have the smallest possible metal nanoparticles. However, large amounts of metal introduced upon impregnation of a porous support often lead to larger particle sizes, which are disadvantageous in terms of PHIP efficiency. Therefore, in the preparation of the 23.2 wt% Rh/TiO2 catalyst an aqueous rhodium nitrate solution was used to obtain relatively small metal particles even at the high rhodium loading. Rh was chosen as an active component, because it usually provides the highest PHIP signal enhancements compared to other metals.[37] The catalyst was characterized in detail by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) techniques (Figures 1 and S2). The average particle size of the 23.2 wt% Rh/TiO2 catalyst was estimated from TEM data as ca. 3 nm, which is favorable for the observation of PHIP effects.[38] Therefore, this catalyst was used for heterogeneous hydrogenation of vinyl acetate in aqueous medium with subsequent polarization transfer from parahydrogen-derived protons to 13C nuclei and 13C NMR spectra detection (Figure 3).
Figure 1.
TEM images and metal particles size distribution for 23.2 wt% Rh/TiO2 catalyst obtained via wet precipitation from aqueous rhodium nitrate solution. The average size of metal particles is ca. 3 nm.
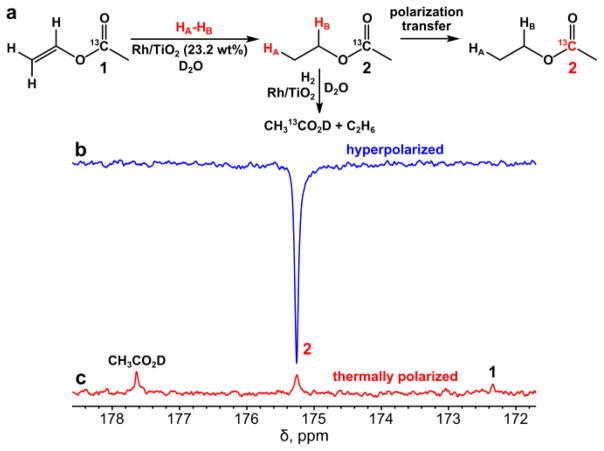
Figure 3.
(a) Reaction scheme of vinyl acetate-1-13C heterogeneous hydrogenation with parahydrogen (p-H2) over 23.2 wt% Rh/TiO2 catalyst in D2O solution with subsequent polarization transfer from protons to 13C nuclei, (b) 13C NMR spectrum of HP ethyl acetate-1-13C, and (c) corresponding 13C spectrum of thermally polarized sample after waiting for a time period longer than 5·T1. All spectra were acquired with 1 signal scan. NMR tube with the hydrogenation products was placed in a magnetic shield after termination of hydrogen bubbling. The 13C signal enhancement (ε13C) is ~14, %P13C ~ 0.011%.
The catalyst’s activity for vinyl acetate hydrogenation is expected to be lower in the aqueous medium (vs. that previously shown in organic solvents[39]), and the increase in the total available metal active centers by using 23.2 wt% Rh/TiO2 catalyst was intended to compensate for this activity loss. Indeed, it was found that 20 seconds of hydrogen bubbling in our setup (~0.5 mL of 0.08 M vinyl acetate-1-13C solution, ~7.1 atm p-H2 pressure at 150 standard cubic centimeters per minute (sccm) flow rate) was sufficient to reach more than 90% conversion level of vinyl acetate to the reaction products (Figures 3 and S3). 13C signal enhancement of ~14 was observed under these conditions (see Figure 3).
Importantly, the hyperpolarized 13C NMR signal was detected both when hydrogenation was carried out directly inside the magnetic shield and when the NMR tube with the hydrogenation products was briefly placed in the shield after termination of hydrogen bubbling. The latter approach generally resulted in greater 13C polarization values. The size of catalyst beads and the Rh loading can be potentially further optimized to maximize chemical conversion efficiently and the degree of pairwise addition, i.e. to maximize the overall yield of 13C hyperpolarization.
Figure 3 also shows that in case of polarization transfer only the 13C NMR signal from hyperpolarized ethyl acetate can be detected. However, in the 13C NMR spectrum of the thermally polarized reaction products (Figure 3c), the equilibrium signals of 13C-labeled unreacted vinyl acetate, ethyl acetate and acetic acid can be seen. Therefore, the formation of acetic acid by hydrogenolysis of ethyl acetate or via direct hydrogenation of vinyl acetate can be easily verified via 13C NMR. Along with the acetic acid the ethanol also is formed as the byproduct of the vinyl acetate heterogeneous hydrogenation with the estimated concentration of approximately 4 mM over supported rhodium catalyst, and the corresponding 1H NMR spectra before and after reaction are shown in Figure S3.
It should also be emphasized that the HET-PHIP 13C signal enhancement (ε13C) of ~14 achieved here is a factor of ~130 lower than that obtained under similar conditions using homogeneous Rh catalyst in methanol-d4.[35] However, it should be noted that hydrogenation duration was 20 s in this study vs. 10 s in the previous report,[35] and additional T1 relaxation losses have contributed to lower ε13C observed here (13C T1 of carboxyl 13C sites is generally on the order of 30-60 seconds in vitro[21,22,33–35] and in vivo,[10,41] and T1 of protons at low magnetic fields is generally on the order of 5–10 seconds[42,43]). It is likely that optimization of the catalyst structure and reaction conditions are the potential routes for major improvement for the presented approach.
The stability of the catalyst under reactive conditions is also important, and XPS investigations of the catalyst before and after the reaction were carried out (see SI for details). It was successfully confirmed that the catalyst did not undergo any changes after the reaction, and Rh nanoparticles are present at the surface of titania only in the metallic state (Figure S2).
Glöggler et al.[31] utilized ~2 nm beads that will likely be challenging to separate fast. In contrast, the 2–3 mm catalytically active beads used here can be easily separated, e.g. via simple decantation of hyperpolarized aqueous solution from the catalyst. For instance, Figure S4b shows that even during hydrogenation the beads reside at the bottom of the NMR tube. Alternatively, if the average beads are decreased down to 0.1 mm in diameter, the catalyst suspension (Figure S4a) can be potentially filtered.
The ability to produce hyperpolarized compounds in water using solid catalysts opens up new possibilities for the production of catalyst-free aqueous solutions of nontoxic hyperpolarized contrast agents. Furthermore, polarization transfer from parahydrogen-derived protons to heteronuclei via magnetic field cycling increases the lifetime of hyperpolarization of the produced compounds and minimizes signal background. All these factors are of paramount importance for in vivo applications.[18] Therefore, the first observation of 13C hyperpolarized molecules formed via heterogeneous hydrogenation of vinyl acetate with p-H2 over supported rhodium nanoparticles is a major step forward to biomedical applications of HET-PHIP approach, and to HET-PHIP-SAH in particular, because the latter can be used to hyperpolarize a wide range of biomolecules.[33–35]
In conclusion, we have presented a new approach to produce pure (from catalyst) aqueous solutions of a biomolecule carrying 13C hyperpolarization. It is based on the heterogeneous side arm hydrogenation (SAH) of a suitable precursor molecule with p-H2 over supported metal catalysts with subsequent or simultaneous polarization transfer from parahydrogen-derived protons to 13C nuclei via magnetic field cycling. The ~90% conversion in the heterogeneous hydrogenation reaction in water achieved here (which is comparable to the conversion achieved by homogenous PHIP hyperpolarization[23] for in vivo 13C imaging[24]) can be further increased by increasing p-H2 pressure and flow rate, and by increasing the amount of catalytically active sites on the catalyst surface. We believe that combination of aqueous phase heterogeneous catalysis by supported metals with HET-PHIP-SAH and polarization transfer approach offers a powerful tool for the production of hyperpolarized biocompatible contrast agents for MRI, because the demonstrated method is scalable and not demanding instrumentationally.
Experimental Section
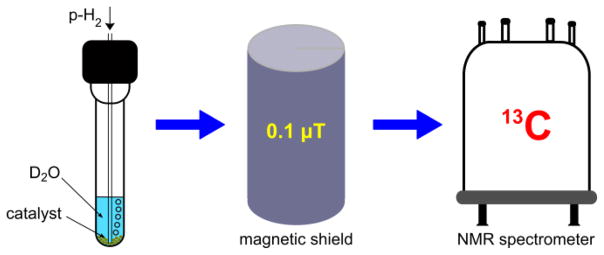
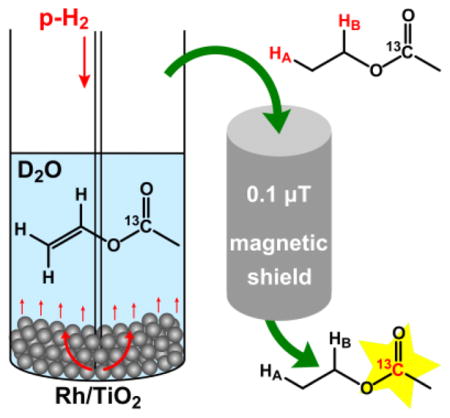
For the liquid phase heterogeneous hydrogenation 23.2% Rh/TiO2 solid catalyst was placed at the bottom of a 5 mm high-pressure medium-wall NMR tube which was then filled with the solution of 13C-labeled vinyl acetate in D2O. After that the NMR tube was pressurized up to ~7.1 atm of p-H2 pressure and heated to 90 °C, and then p-H2 was bubbled through the solution for the period of 20 seconds at the flow rate of 150 sccm. Note the samples were not degassed before reaction and hyperpolarization process. As soon as bubbling was stopped, the NMR tube was transferred from the Earth’s magnetic field to the very low magnetic field (~0.1 μT) inside the magnetic shield and then adiabatically transferred to the 9.4 T high field of the NMR spectrometer, where 13C NMR spectra were acquired (Figures 2, 3). The detailed experimental setup is presented in Figure S1. The procedures for catalysts preparation, hydrogenation and XPS experiments, and additional NMR spectra are given in SI.
Figure 2.
Experimental procedure for 13C hyperpolarization and NMR spectra detection via PHIP-SAH and magnetic field cycling.
Supplementary Material
Acknowledgments
OGS and IVK acknowledge the grant from the Russian Science Foundation (14-13-00445) for the support of heterogeneous hydrogenation experiments, KVK thanks president’s grant MK-4498.2016.3 and RFBR grant 14-03-93183 MCX_a for high field NMR experiments; ITC team thank FASO Russia project # 0333-2014-0001 for basic funding. BIC team thank RSF grant # 14-23-00146 for the support of catalysts characterization by XPS and TEM methods, LMK thanks FASO # 0303-2015-0010 for the support of catalysts preparation. The US team thanks NIH 1R21EB018014 and 1R21EB020323, NSF CHE-1416268 and CHE-1416432, DOD CDMRP W81XWH-12-1-0159/BC112431, and W81XWH-15-1-0271, and ExxonMobil Research and Engineering Company Knowledge Build.
References
- 1.Nikolaou P, Goodson BM, Chekmenev EY. Chem Eur J. 2015;21:3156–3166. doi: 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardenkjaer-Larsen J-H, Boebinger GS, Comment A, Duckett S, Edison AS, Engelke F, Griesinger C, Griffin RG, Hilty C, Maeda H, et al. Angew Chem Int Ed. 2015;54:9162–9185. doi: 10.1002/anie.201410653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardenkjaer-Larsen JH. J Magn Reson. 2016;264:3–12. doi: 10.1016/j.jmr.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Comment A. J Magn Reson. 2016;264:39–48. doi: 10.1016/j.jmr.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Comment A, Merritt ME. Biochemistry. 2014;53:7333–7357. doi: 10.1021/bi501225t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindle KM. J Am Chem Soc. 2015;137:6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]
- 7.Goodson BM. J Magn Reson. 2002;155:157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]
- 8.Green RA, Adams RW, Duckett SB, Mewis RE, Williamson DC, Green GGR. Prog Nucl Magn Reson Spectrosc. 2012;67:1–48. doi: 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Kovtunov KV, Zhivonitko VV, Skovpin IV, Barskiy DA, Koptyug IV. Top Curr Chem. 2013;338:123–180. doi: 10.1007/128_2012_371. [DOI] [PubMed] [Google Scholar]
- 10.Ardenkjær-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, et al. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brindle KM, Bohndiek SE, Gallagher FA, Kettunen MI. Magn Reson Med. 2011;66:505–519. doi: 10.1002/mrm.22999. [DOI] [PubMed] [Google Scholar]
- 13.Brindle K. Nat Rev Cancer. 2008;8:94–107. doi: 10.1038/nrc2289. [DOI] [PubMed] [Google Scholar]
- 14.Golman K, in’t Zandt R, Thaning M. Proc Natl Acad Sci U S A. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- 16.Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. J Am Chem Soc. 1987;109:8089–8091. [Google Scholar]
- 17.Pravica MG, Weitekamp DP. Chem Phys Lett. 1988;145:255–258. [Google Scholar]
- 18.Golman K, Axelsson O, Jóhannesson H, Månsson S, Olofsson C, Petersson JS. Magn Reson Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 19.Hövener JB, Chekmenev EY, Harris KC, Perman WH, Tran TT, Ross BD, Bhattacharya P. Magn Reson Mater Phys Biol Med. 2009;22:123–134. doi: 10.1007/s10334-008-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman M, Jóhannesson H, Axelsson O, Karlsson M. C R Chim. 2006;9:357–363. [Google Scholar]
- 21.Chekmenev EY, Hövener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. J Am Chem Soc. 2008;130:4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bünger R, Ross BD. NMR Biomed. 2011;24:1023–1028. doi: 10.1002/nbm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. Anal Chem. 2014;86:5601–5605. doi: 10.1021/ac500952z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffey AM, Shchepin RV, Truong ML, Wilkens K, Pham W, Chekmenev EY. Anal Chem. 2016;88:8279–8288. doi: 10.1021/acs.analchem.6b02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. J Am Chem Soc. 2012;134:934–943. doi: 10.1021/ja2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Magn Reson Mater Phys Biol Med. 2005;18:245–256. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 27.Kovtunov KV, Beck IE, Bukhtiyarov VI, Koptyug IV. Angew Chem Int Ed. 2008;47:1492–1495. doi: 10.1002/anie.200704881. [DOI] [PubMed] [Google Scholar]
- 28.Balu AM, Duckett SB, Luque R. Dalton Trans. 2009:5074–5076. doi: 10.1039/b906449d. [DOI] [PubMed] [Google Scholar]
- 29.Irfan M, Eshuis N, Spannring P, Tessari M, Feiters MC, Rutjes FPJT. J Phys Chem C. 2014;118:13313–13319. [Google Scholar]
- 30.Koptyug IV, Zhivonitko VV, Kovtunov KV. ChemPhysChem. 2010;11:3086–3088. doi: 10.1002/cphc.201000407. [DOI] [PubMed] [Google Scholar]
- 31.Glöggler S, Grunfeld AM, Ertas YN, Mccormick J, Wagner S, Schleker PPM, Bouchard LS. Angew Chem Int Ed. 2015;54:2452–2456. doi: 10.1002/anie.201409027. [DOI] [PubMed] [Google Scholar]
- 32.Glöggler S, Grunfeld AM, Ertas YN, McCormick J, Wagner S, Bouchard LS. Chem Commun. 2016;52:605–608. doi: 10.1039/c5cc08648e. [DOI] [PubMed] [Google Scholar]
- 33.Reineri F, Boi T, Aime S. Nat Commun. 2015;6:5858. doi: 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- 34.Cavallari E, Carrera C, Boi T, Aime S, Reineri F. J Phys Chem B. 2015;119:10035–10041. doi: 10.1021/acs.jpcb.5b06222. [DOI] [PubMed] [Google Scholar]
- 35.Shchepin RV, Barskiy DA, Coffey AM, Manzanera Esteve IV, Chekmenev EY. Angew Chem Int Ed. 2016;55:6071–6074. doi: 10.1002/anie.201600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salnikov OG, Kovtunov KV, Koptyug IV. Sci Rep. 2015;5:13930. doi: 10.1038/srep13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corma A, Salnikov OG, Barskiy DA, Kovtunov KV, Koptyug IV. Chem Eur J. 2015;21:7012–7015. doi: 10.1002/chem.201406664. [DOI] [PubMed] [Google Scholar]
- 38.Kovtunov KV, Barskiy DA, Coffey AM, Truong ML, Salnikov OG, Khudorozhkov AK, Inozemtseva EA, Prosvirin IP, Bukhtiyarov VI, Waddell KW, et al. Chem Eur J. 2014;20:11636–11639. doi: 10.1002/chem.201403604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ananikov VP, Khemchyan LL, Ivanova YV, Bukhtiyarov VI, Sorokin AM, Prosvirin IP, Vatsadze SZ, Medved’ko AV, Nuriev VN, Dilman AD, et al. Russ Chem Rev. 2014;83:885–985. [Google Scholar]
- 40.Kovtunov KV, Barskiy DA, Salnikov OG, Shchepin RV, Coffey AM, Kovtunova LM, Bukhtiyarov VI, Koptyug IV, Chekmenev EY. RSC Adv. 2016;6:69728–69732. doi: 10.1039/C6RA15808K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurd RE, Yen YF, Mayer D, Chen A, Wilson D, Kohler S, Bok R, Vigneron D, Kurhanewicz J, Tropp J, Spielman D, Pfefferbaum A. Magn Reson Med. 2010;63:1137–1143. doi: 10.1002/mrm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. J Phys Chem Lett. 2012;3:3281–3285. doi: 10.1021/jz301389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Truong ML, Coffey AM, Waddell KW, Chekmenev EY. ChemPhysChem. 2014;15:4100–4107. doi: 10.1002/cphc.201402607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.