Abstract
Study Design:
Cross-sectional survey study.
Objective:
To determine what are the most commonly used graft materials in anterior cervical discectomy and fusion and whether the choice of graft is affected by surgeon’s training, years in practice, geographic location, practice setting, or surgeon’s perceived difficulty in achieving fusion.
Methods:
A 23-question survey was sent out to 5334 surgeons using the Global AO Spine database. Response data was then stratified into surgeon training, years of practice, practice type, and global region.
Results:
Overall, surgeons believe that graft selection affects fusion rates (89.3% of surgeons) and affects time to fusion (86.0% of surgeons). The use of a cage is currently the most common structural graft component used worldwide at 64.1%. Of surgeons that use cages, the PEEK Cage makes up 84%. North American surgeons differ from this global trend and use composite allograft more commonly. The choice to add a nonstructural graft component was reported at 74%. This result was similar for performing multilevel fusions at 72.8%. The selection of nonstructural graft material depends on whether the type of surgery is considered simple or complex. Most surgeons are not satisfied with available literature comparing effectiveness of grafts but believed that there was sufficient evidence to support the use of their chosen graft.
Conclusion:
Almost all surgeons believe that fusion is important to anterior cervical discectomy and fusion surgery outcomes and that most surgeons believe graft choice affects fusion. However, this survey indicates that there is great variability in the type of graft material used by spine surgeons across the world.
Keywords: ACDF, graft, AO Spine members, survey
Introduction
Anterior cervical discectomy and fusion (ACDF) is a common and well-accepted surgical procedure utilized for treating cervical spine disease.1 There is a wide variety of graft material available with varying cost, availability, and potential safety and efficacy. However, there is no literature reporting the frequency of graft materials used for ACDF by surgeons across the world.
The first well-described graft material used for ACDF surgery was the use of autograft iliac bone graft.2 Many variations of the graft geometry were studied including the Cloward dowel, Smith-Robinson horseshoe, Simmons-Bhallia keystone, and Simmons-Badgley onlay strut.3 Common autograft locations include iliac crest and fibula donor sites. Although autograft materials are widely used, they are also associated with several morbidities associated with secondary surgical sites causing increased pain and risk of infection from graft site harvest.4 Minor complications of using iliac bone graft include superficial infections, superficial seromas, and minor hematomas. Major complications include herniation of abdominal contents, deep infections, vascular injuries, neurologic injuries, deep hematoma formation, and iliac wing fractures.5
Allograft grafts offer many advantages over autograft including decreased surgical time and decreased donor site morbidity, but at the same time, they increase the risk of disease transmission when using donor bone.6 Common allograft material include cortical, cancellous, and composite. No statistical significance in clinical outcome was measured comparing autograft versus allograft for single-level or multilevel ACDF surgery.7,8
Cages or interbody fusion devices are a newer category of devices used for ACDF surgery. Three primary materials including carbon fiber reinforced polymers, titanium, and polyetheretherketone (PEEK) make up most of the current designs with PEEK cage as the most common.9 A study by Lied and colleagues concluded similar clinical outcomes with regard to return to work comparing PEEK cage to autologous iliac crest graft.10 Cage material can be combined with nonstructural graft material including bone marrow aspirate,11 platelet-rich plasma,12 bone morphogenetic protein,13 and other synthetic material. Long-term efficacy and safety of this class of graft material is not well documented to date.
A small survey study questioned spine surgeons on the importance of fusion in ACDF and 91.3% responded that they felt fusion was necessary.14 This is contradictory to a 1998 randomized prospective study that concluded fusion is unnecessary for single-level disease.15
Fusion rates relative to graft choice in ACDF is an area of discussion when selecting the appropriate graft. Previous data reported fusion rates in ACDF of 91% for allograft and autograft and cage construct yielded a 97% fusion rate.
The purpose of our study was to determine the most commonly used graft materials in ACDF, whether this choice is affected by a given surgeon’s training, practice setting, geographic region, number of years in practice, and perceived difficulty in achieving fusion for a particular case. In addition, we want to determine what surgeons perceive to be the gold standard for ACDF surgery. Also, we want to determine whether surgeons feel that choice of graft materials is important for outcome and whether surgeons feel that there is sufficiently good comparative data in the literature comparing the most common grafts.
Methods
We developed a 23-question questionnaire (see the appendix) to assess this gap in information by determining the current ACDF graft use, type of graft used for simple and challenging cases, the perceived effectiveness for currently used graft, perceived gold standard, depth of current literature, and safety data. A web-based questionnaire was emailed to 5334 surgeons using the AO International Global Spine database. Questions were asked about the use of “structural” versus “nonstructural” graft materials. The graft choices were asked in the context of simple (easy to get fusion) versus challenging (harder to get fusion). The choices for structural graft were categorized into 5 choices: allograft, autograft, cage, synthetic, and other. Allograft was subdivided further into composite (cortical cancellous), cortical, and dense cancellous. Autograft was subdivided into fibula and iliac crest. Cages were subdivided into PEEK and metallic. The choices for nonstructural graft were allograft, autograft, BMA, DBM, platelet, synthetic, rhBMP2, and nothing.
Demographic data enabled us to stratify the response data into surgeon training, years of practice, practice type, and global region. To further characterize type of ACDF grafts, we classified common graft materials into structural and nonstructural components.
Data analysis was performed utilizing Microsoft Excel spreadsheets to create tables and graphs. Most data gathered was displayed using descriptive statistics including counts, percentages, and plots.
Results
Surgeon Demographics
The surgeon demographic data is presented in Table 1. In total, 599 surgeons responded (11% of total) to the ACDF graft questionnaire. Of the responding participants, two thirds were orthopedic surgeons and one third neurosurgery surgeons. More than half (59%) of the responding surgeons work in academia and the remaining at nonacademic centers. Surgeons practicing between 5 and 14 years make up the largest portion of respondents followed by surgeons working more than 15 years. The lowest respondents were surgeons practicing less than 5 years. Regionally, the highest percentage of respondents was from the Asia Pacific (29%) region and the lowest was from Africa/Middle East. The highest numbers of surgeons responding were from Asia Pacific, Europe, Latin America, and then North America.
Table 1.
Surgeon Demographic Data.
| n | % | |
|---|---|---|
| Overall | ||
| Sent to | 5334 | |
| Completed | 599 | 11% |
| Specialty | ||
| Neurosurgery | 201 | 34% |
| Orthopedics | 396 | 66% |
| Practice setting | ||
| Academic | 355 | 59% |
| Nonacademic | 244 | 41% |
| Years of practice | ||
| <5 years | 140 | 23% |
| 5-14 years | 261 | 44% |
| ≥15 years | 198 | 33% |
| Region | ||
| Europe | 165 | 28% |
| North America | 65 | 11% |
| Africa/Middle East | 59 | 10% |
| Asia Pacific | 175 | 29% |
| Latin America | 133 | 22% |
Most Common Structural Component Used in Simple ACDF
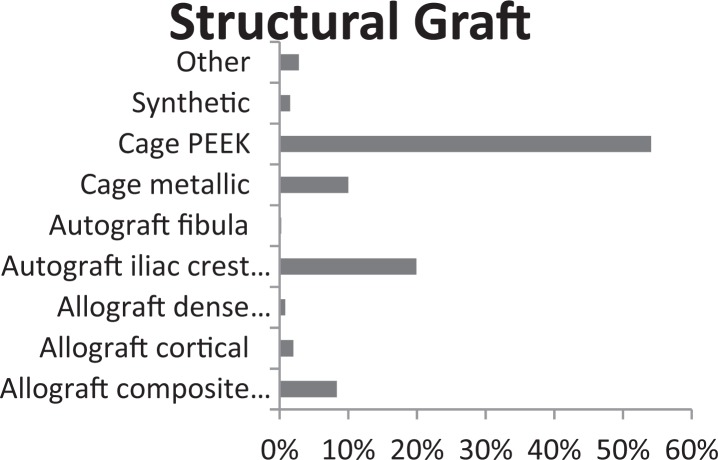
The most commonly used structural graft is cage (64.1%), and PEEK cage makes up 84% of all cages used worldwide (Figure 1). The next most common are autograft iliac crest tricortical bone (20%) and allograft composite graft (8%).
Figure 1.
Most common structural graft material over all demographic data.
In Table 2, the most notable difference between orthopedic (26.3%) and neurological surgery (8.0%) was the use of iliac crest autograft. Orthopedic surgeons use iliac crest more than 3 times as much as neurosurgeons. Academic when compared to nonacademic and years of practice have similar trends with respect to structural component. North America utilizes allograft composite material at 49.2%. Cage material is the most common in the remaining regions.
Table 2.
Demographic Data: Most Common Structural Component.
| Structural Graft Usage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allograft | Autograft | Cage | |||||||
| Composite | Cortical | Dense Cancellous | Fibula | Iliac Crest | PEEK | Metallic | Synthetic | Other | |
| Specialty | |||||||||
| Neurosurgery | 12 (6.0) | 7 (3.5) | 2 (1.0) | 0 (0.0) | 16 (8.0) | 132 (65.7) | 16 (8.0) | 4 (2.0) | 12 (6.0) |
| Orthopedics | 38 (9.6) | 5 (1.3) | 3 (0.8) | 1 (0.3) | 103 (26.2) | 191 (48.5) | 44 (11.2) | 4 (1.0) | 5 (1.3) |
| Practice setting | |||||||||
| Academic | 24 (10.5) | 10 (4.4) | 2 (0.9) | 0 (0) | 51 (22.3) | 110 (48.0) | 23 (10) | 3 (1.3) | 6 (2.6) |
| Nonacademic | 26 (7.1) | 2 (0.5) | 3 (0.8) | 1 (0.3) | 68 (18.6) | 211 (57.8) | 37 (10.1) | 6 (1.6) | 11 (3.0) |
| Years of practice | |||||||||
| <5 years | 23 (12.6) | 6 (3.3) | 1 (0.5) | 0 (0.0) | 47 (25.7) | 83 (45.4) | 15 (8.2) | 4 (2.2) | 4 (2.2) |
| 5-14 years | 14 (6.4) | 5 (2.3) | 1 (0.5) | 1 (0.5) | 41 (18.7) | 128 (58.4) | 18 (8.2) | 5 (2.3) | 6 (2.7) |
| ≥15 years | 13 (6.7) | 1 (0.5) | 3 (1.5) | 0 (0) | 31 (16.0) | 112 (57.5) | 27 (13.9) | 0 (0) | 7 (3.6) |
| Region | |||||||||
| Europe | 3 (1.8) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 19 (11.4) | 102 (61.4) | 22 (13.3) | 6 (3.6) | 12 (7.2) |
| North America | 32 (49.2) | 9 (13.8) | 2 (3.1) | 0 (0.0) | 0 (0.0) | 18 (27.7) | 2 (3.1) | 2 (3.1) | 0 (0) |
| Africa/Middle East | 2 (3.4) | 0 (0) | 1 (1.7) | 0 (0) | 13 (22.4) | 33 (56.9) | 6 (10.3) | 0 (0) | 3 (5.2) |
| Asia Pacific | 5 (2.9) | 2 (1.1) | 0 (0) | 0 (0) | 67 (38.5) | 83 (47.7) | 15 (8.6) | 1 (0.6) | 1 (0.6) |
| Latin America | 8 (6.0) | 1 (0.8) | 0 (0) | 1 (0.8) | 20 (15.0) | 87 (65.4) | 15 (11.3) | 0 (0) | 1 (0.8) |
Most Common Nonstructural Component Used in Simple ACDF
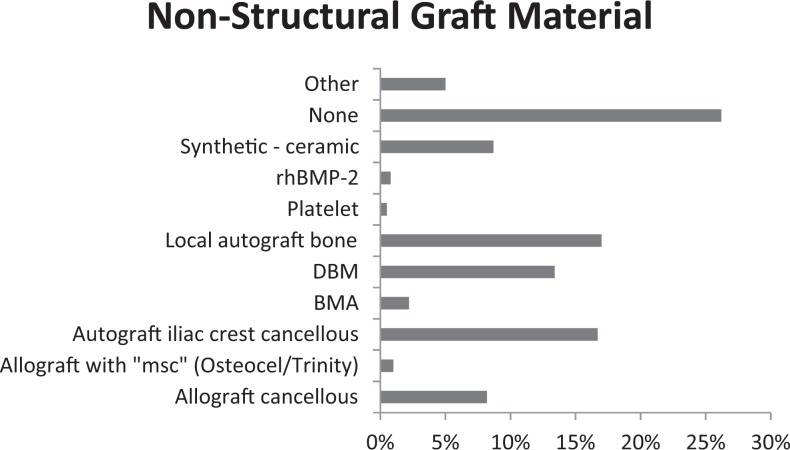
The use of a nonstructural component in ACDF appears to be of wide diversity. Worldwide, 74% of surgeons use a nonstructural component and 26% use no component. The choice is widespread, however, among those choosing a nonstructural component. In Figure 2, the most common nonstructural component was local autograft (17%), autograft iliac crest cancellous (17%), DBM (13%), allograft cancellous (8%), and ceramic (9%). The least used was platelets.
Figure 2.
Nonstructural graft material for single-level ACDF surgery.
Demographic data in Table 3 demonstrates orthopedic surgeons (22.1%) are more likely to use iliac crest cancellous component compared to neurosurgeons (6.5%). Neurosurgeons (12.9%) use synthetic ceramic material nearly twice as likely as orthopedic surgeons (6.6%). Practice setting did not reveal any major difference in preference. Duration of practice reveals surgeons practicing more than 15 years chose to use a graft (77.9%) compared to duration of practice between 5 and 14 years (74.3%) and those practicing less than 5 years use a nonstructural graft (68.3%).
Table 3.
Demographic Data: Most Common Non-Structural Component.
| Simple Nonstructural Component | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allograft | ||||||||||
| Cancellous | Osteocel Trinity | Iliac Crest Cancellous | Autograft (Local) | BMA | DBM | None | Platelet | Synthetic (Ceramic) | rhBMP2 | |
| Specialty | ||||||||||
| Neurosurgery | 15 (7.5) | 4 (2.0) | 13 (6.5) | 35 (17.4) | 6 (3.0) | 31 (15.4) | 49 (24.4) | 2 (1.0) | 26 (12.9) | 3 (1.5) |
| Orthopedics | 33 (8.4) | 2 (0.5) | 87 (22.1) | 67 (17.0) | 6 (1.5) | 49 (12.4) | 108 (27.4) | 1 (0.3) | 26 (6.6) | 2 (0.5) |
| Practice setting | ||||||||||
| Academic | 12 (5.2) | 1 (0.4) | 28 (12.2) | 44 (19.2) | 6 (2.6) | 38 (16.6) | 72 (31.4) | 2 (0.9) | 17 (7.4) | 2 (0.9) |
| Nonacademic | 36 (9.9) | 5 (1.4) | 72 (19.7) | 57 (15.6) | 7 (1.9) | 42 (11.5) | 85 (23.3) | 1 (0.3) | 34 (9.3) | 3 (0.8) |
| Years of practice | ||||||||||
| <5 years | 14 (7.7) | 1 (0.5) | 31 (16.9) | 36 (19.7) | 4 (2.2) | 23 (12.6) | 58 (31.7) | 1 (0.5) | 7 (3.8) | 1 (0.5) |
| 5-14 years | 18 (8.3) | 1 (0.5) | 43 (19.7) | 35 (16.1) | 4 (1.8) | 25 (11.5) | 56 (25.7) | 2 (0.9) | 21 (9.6) | 2 (0.9) |
| ≥15 years | 16 (8.2) | 4 (2.1) | 26 (13.3) | 31 (15.9) | 5 (2.6) | 32 (16.4) | 43 (22.1) | 0 (0) | 24 (12.3) | 2 (1.0) |
| Region | ||||||||||
| Europe | 12 (7.3) | 2 (1.2) | 11 (6.7) | 27 (16.4) | 3 (1.8) | 21 (12.7) | 50 (30.3) | 0 (0) | 21 (12.7) | 2 (1.2) |
| North America | 3 (4.6) | 1 (1.5) | 4 (6.2) | 17 (26.2) | 1 (1.5) | 17 (26.2) | 21 (32.3) | 0 (0) | 0 (0) | 0 (0) |
| Africa/Middle East | 7 (12.1) | 2 (3.4) | 10 (17.2) | 11 (19.0) | 4 (6.9) | 2 (3.4) | 11 (19.0) | 2 (3.4) | 6 (10.3) | 6 (10.3) |
| Asia Pacific | 12 (6.9) | 0 (0) | 32 (18.3) | 30 (17.1) | 2 (1.1) | 20 (11.4) | 58 (33.1) | 1 (0.6) | 13 (7.4) | 13 (7.4) |
| Latin America | 14 (10.5) | 1 (0.8) | 43 (32.3) | 17 (12.8) | 3 (2.3) | 20 (15) | 17 (!2.8) | 0 (0) | 12 (9.0) | 12 (9.0) |
Most Common Structural Component Used in Complex ACDF
The most common structural component for multilevel ACDF surgery is a cage at nearly 62%. The most common type of cage is PEEK at 68%, which can be seen in Figure 3. When comparing structural components for multilevel versus single-level ACDF, PEEK cage was by far the most common choice for both types of ACDF surgery. However, for the second choice, metallic cages are more common for multilevel and iliac crest tricortical is more common for single-level ACDF surgeries.
Figure 3.
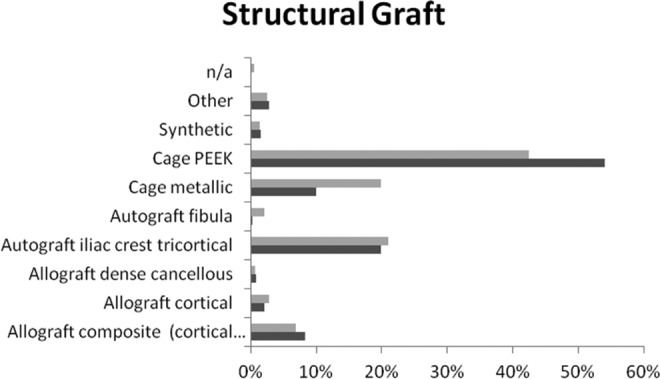
Structural component preferences in surgeons performing multilevel (grey) versus single-level (black) ACDF surgery.
In Table 4, orthopedic surgeons (24.4%) were more inclined to use iliac crest compared to neurosurgery (14.4%). Years of practice seemed to have a direct relationship with the use of PEEK cage. The more years of experience relate to increased use of peek cage. On the other hand, there exists an inverse relationship between years of experience with the use of iliac crest. When comparing North America to other AO Spine regions, it appears that North America uses allograft composite as their primary selection. The remainder of the regions selects a PEEK cage as the most popular use.
Table 4.
Demographic Data on Nonstructural Multilevel Component.
| Multilevel Structural Component | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allograft | Autograft | Cage | |||||||
| Composite | Cortical | Dense Cancellous | Fibula | Iliac Crest | PEEK | Metallic | Synthetic | Other | |
| Specialty | |||||||||
| Neurosurgery | 11 (5.5) | 9 (4.5) | 0 (0) | 0 (0) | 29 (14.4) | 106 (52.7) | 32 (15.9) | 2 (1.0) | 12 (6.0) |
| Orthopedics | 30 (7.6) | 8 (2.0) | 4 (1.0) | 12 (3.1) | 96 (24.4) | 148 (37.7) | 87 (22.1) | 5 (1.3) | 3 (0.8) |
| Practice setting | |||||||||
| Academic | 20 (8.8) | 9 (3.9) | 2 (0.9) | 5 (2.2) | 53 (23.2) | 88 (38.6) | 43 (18.9) | 3 (1.3) | 5 (2.2) |
| Nonacademic | 21 (5.8) | 8 (2.2) | 2 (0.5) | 7 (1.9 | 71 (19.5) | 165 (45.2) | 76 (20.8) | 5 (1.4) | 10 (2.7) |
| Years of practice | |||||||||
| <5 years | 19 (10.4) | 9 (4.9) | 2 (1.1) | 4 (2.2) | 44 (24.0) | 66 (36.1) | 33 (18) | 4 (2.2) | 2 (1.1) |
| 5-14 years | 10 (4.6) | 6 (2.8) | 1 (0.5) | 6 (2.8) | 48 (22.0) | 98 (45.0) | 41 (18.8) | 2 (0.9) | 6 (2.8) |
| ≥15 years | 12 (6.2) | 2 (1.0) | 1 (0.5) | 2 (1.0) | 33 (17.0) | 90 (46.4) | 45 (23.2) | 2 (1.0) | 7 (3.6) |
| Region | |||||||||
| Europe | 6 (3.6) | 3 (1.8) | 2 (12) | 1 (0.6) | 24 (14.5) | 80 (48.2) | 36 (21.7) | 4 (2.4) | 10 (6.0) |
| North America | 24 (37.5) | 10 (15.6) | 0 (0) | 1 (1.6) | 6 (9.4) | 14 (21.9) | 6 (9.4) | 2 (3.1) | 1 (1.6) |
| Africa/Middle East | 2 (3.5) | 0 (0) | 2 (3.5) | 1 (1.8) | 10 (17.5) | 32 (56.1) | 8 (14.0) | 0 (0) | 2 (3.5) |
| Asia Pacific | 5 (2.9) | 3 (1.7) | 0 (0) | 8 (4.6) | 62 (35.4) | 64 (36.6) | 30 (17.1) | 2 (1.1) | 1 (0.6) |
| Latin America | 4 (3.0) | 1 (0.8) | 0 (0) | 1 (0.8) | 23 (17.3) | 64 (48.1) | 39 (29.3) | 0 (0) | 1 (0.8) |
Most Common Nonstructural Component Used in Complex ACDF
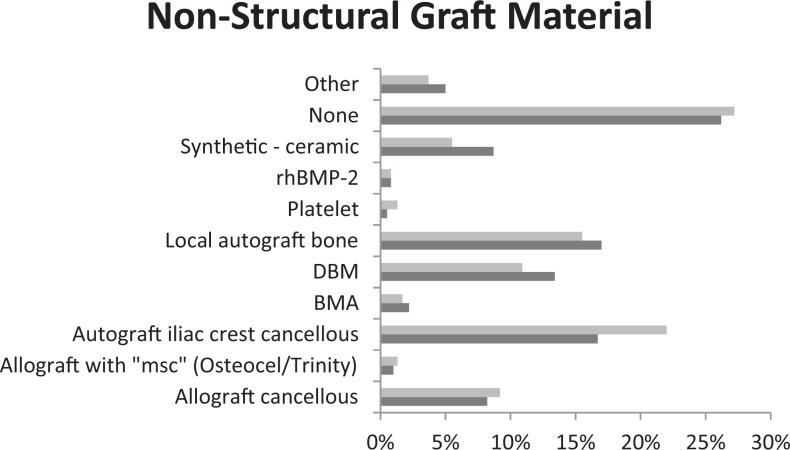
Overall, surgeons performing multilevel ACDFs use a nonstructural component 72.8% of the time compared to 27.2% who use only a structural construct. The most common nonstructural component is autograft iliac crest cancellous at 22% used in multilevel surgery. As seen in Figure 4, the preference for single-level ACDF is shared between local bone autograft and autograft iliac crest cancellous.
Figure 4.
Nonstructural component preferences in surgeons performing multilevel (grey) versus single-level (black) ACDF surgery.
Demographic data in Table 5 reveals the following data. Orthopedic surgeons are more likely to use iliac crest cancellous compared to neurosurgery. Academic centers are less likely to use nonstructural graft material than nonacademic centers. Less experienced surgeons are less likely to use a nonstructural component when compared to older surgeons. North America and Europe use local autograft most frequently while Africa/Middle East, Asia Pacific, and Latin America use allograft (iliac crest).
Table 5.
Demographic Data on Nonstructural Component Used in Multilevel ACDF Surgery.
| Multilevel Nonstructural Component | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allograft | Autograft (Local) | BMA | DBM | None | Platelet | Synthetic (Ceramic) | rhBMP2 | |||
| Cancellous | Osteocel Trinity | Iliac Crest Cancellous | ||||||||
| Specialty | ||||||||||
| Neurosurgery | 19 (9.5) | 4 (2.0) | 22 (11.1) | 35 (17.6) | 3 (1.5) | 30 (15.1) | 49 (24.6) | 6 (3.0) | 18 (9.0) | 1 (0.5) |
| Orthopedics | 35 (8.9) | 4 (1.0) | 110 (28.0) | 58 (14.8) | 6 (1.5) | 35 (8.9) | 114 (29) | 2 (0.5) | 15 (3.8) | 4 (1.0) |
| Practice setting | ||||||||||
| Academic | 17 (7.5) | 2 (0.9) | 39 (17.2) | 39 (17.2) | 4 (1.8) | 29 (12.8) | 72 (31.7) | 7 (3.1) | 12 (5.3) | 1 (0.4) |
| Nonacademic | 37 (10.2) | 6 (1.6) | 93 (25.5) | 53 (14.6) | 6 (1.6) | 36 (9.9) | 91 (25.0) | 1 (0.3) | 20 (5.5) | 4 (1.1) |
| Years of practice | ||||||||||
| <5 years | 15 (8.2) | 4 (2.2) | 40 (22.0) | 29 (15.9) | 3 (1.6) | 19 (10.4) | 62 (34.1) | 1 (0.5) | 2 (1.1) | 0 (0) |
| 5-14 years | 17 (7.9) | 2 (0.9) | 49 (22.7) | 30 (13.9) | 3 (1.4) | 21 (9.7) | 56 (25.6) | 6 (2.8) | 21 (9.7) | 3 (1.4) |
| ≥15 years | 22 (11.3) | 2 (1.0) | 43 (22.1) | 34 (17.4) | 4 (2.1) | 25 (12.8) | 45 (23.1) | 1 (0.5) | 10 (5.1) | 2 (1.0) |
| Region | ||||||||||
| Europe | 18 (10.9) | 2 (1.2) | 22 (13.3) | 29 (17.6) | 2 (1.2) | 17 (10.3) | 45 (27.3) | 2 (1.2) | 16 (9.7) | 1 (0.6) |
| North America | 4 (6.3) | 4 (6.3) | 6 (9.4) | 14 (21.9) | 1 (1.6) | 12 (18.8) | 19 (29.7) | 0 (0) | 1 (1.6) | 1 (1.6) |
| Africa/Middle East | 10 (17.5) | 0 (0) | 13 (22.8) | 8 (14.0) | 2 (3.5) | 2 (3.5) | 15 (26.3) | 3 (5.3) | 3 (5.3) | 1 (1.8) |
| Asia Pacific | 8 (4.6) | 1 (0.6) | 42 (24.1) | 28 (16.1) | 4 (2.3) | 18 (10.3) | 59 (33.9) | 2 (1.1) | 7 (4.0) | 0 (0) |
| Latin America | 14 (10.5) | 1 (0.8) | 49 (36.8) | 14 (10.5) | 1 (0.8) | 16 (12.0) | 25 (18.8) | 1 (0.8) | 6 (4.5) | 2 (1.5) |
Importance of Fusion
Overall, 94.6% all surgeons who responded stated fusion is important to successful outcomes in ACDF fusion. This is uniformly agreed on with respect to specialty, practice setting, years of practice, and region.
Graft’s Impact on Rate of Fusion and Time to Fusion
Graft material is strongly perceived to affect important measures of ACDF fusion, fusion rate, and time to fusion. Overall, 89.3% view grafts affect fusion rates and 86.0% affect time to fusion as seen in Table 6. Orthopedic surgeons believe that grafts affect fusion parameters more than neurosurgeons. Type of practice setting and years of practice have very similar response rates. The region a surgeon is from changes the perception grafts have on fusion. Latin America perceives that graft affects fusion rate at the highest percentage, 94.6%, and Europe the lowest, 83.7%. Additionally, Latin America views time to fusion the highest, 88.7%, and North America the lowest, 12.3%.
Table 6.
Graft Impact on Fusion Rate and Time to Fusion.
| Graft Impact: Fusion Rate | Graft Impact: Time to Fusion | |||
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Yes, n (%) | No, n (%) | |
| Specialty | ||||
| Neurosurgery | 168 (84.8) | 30 (15.2) | 168 (84.1) | 32 (15.9) |
| Orthopedics | 359 (91.6) | 33 (8.4) | 341 (87.0) | 51 (13.0) |
| Practice setting | ||||
| Academic | 205 (90.3) | 22, (9.7) | 203 (89.0) | 25 (11) |
| Nonacademic | 322 (89.0) | 40 (11.0) | 306 (84.1) | 58 (15.9) |
| Years of practice | ||||
| <5 years | 165 (90.2) | 18 (9.8) | 148 (81.3) | 34 (18.7) |
| 5-14 years | 195 (90.7) | 20 (9.3) | 198 (90.8) | 20 (9.2) |
| ≥15 years | 168 (87.0) | 25 (13.0) | 165 (85.1) | 29 (14.9) |
| Region | ||||
| Europe | 139 (83.7) | 27 (16.3) | 135 (81.3) | 31 (18.7) |
| North America | 59 (90.8) | 6 (9.2) | 57 (87.7) | 8 (12.3) |
| Africa/Middle East | 50 (89.3) | 6 (10.7) | 49 (86.0) | 8 (14.0) |
| Asia Pacific | 157 (90.2) | 17 (9.8) | 152 (87.9) | 21 (12.1) |
| Latin America | 123 (94.6) | 7 (5.4) | 118 (88.7) | 15 (11.3) |
| Overall | 528 (89.3) | 63 (10.7) | 511 (86.0) | 83 (14.0) |
Surgeon Satisfaction With Current Literature
Overall, 63.1% of surgeons believe there is adequate evidence in support of graft material they use. A total of 54.5% of surgeons are satisfied with comparative effectiveness data available on bone graft materials for bone graft of cervical spine. A total of 66.7% of surgeons do not believe risk factors for nonunions and poor clinical outcomes are sufficiently well described.
Gold Standard for Fusion
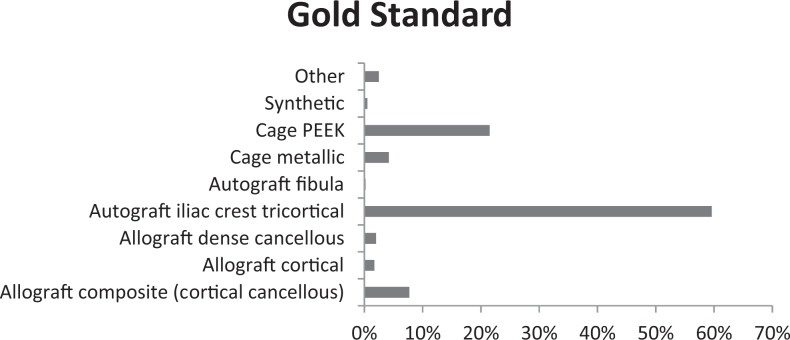
Autograft tricortical iliac crest graft was most often chosen as the gold standard for choice of graft material for ACDF, as shown in Figure 5. This was reported at 60% from all surgeons. However, PEEK cage was chosen as gold standard by 22% of surgeons and allograft composite grafts were chosen by 8% of surgeons.
Figure 5.
Gold standard to compare other graft types.
Safety
Sixty-one percent of respondents believe there is similar safety among choices of the most common current graft material.
Discussion
Our data indicates that there is great variation in the structural and nonstructural graft materials used by surgeons. By far the most common structural graft used was cage (64%). Nearly 74% of surgeons use some sort of nonstructural graft. The choice of graft does not seem to change much from the “simple” case to the “challenging” case. Based on the results, it appears that there is a large variance in graft choice throughout the world. Furthermore, the majority of surgeons feel that comparative data regarding the efficacy and safety of graft material is necessary.
Interestingly, the “gold standard” most often chosen by surgeons was autograft iliac crest tricortical bone; however, most surgeons surveyed used cages instead. This suggest that many surgeons feel that their graft of choice is not the gold standard, but they still use cages, especially PEEK cages instead. This is most certainly not a cost consideration as there is no implant cost associated with using cages. Rather, it must be some other issue such as convenience or avoidance of graft harvest donor site morbidity.
In our study, we confirmed that 94.6% of spine surgeons believe fusion is important to ACDF, which is similar to the reported 91.3% from a study of Greek spine surgeons.14 Graft choice is believed to be important in fusion, and our data supports this as well. Given that graft likely plays a significant role in fusion and surgeons have not clearly defined a best in class, we likely require more evidence to determine this. For example, in previous studies allograft, autograft, and cage constructs all yielded greater than 90% fusion rates; however, there is a clear trend that cage constructs appear to be most popular in ACDF surgery. This significant variability in surgeon practice and gap in the literature indicates an important opportunity to help guide surgeon practice with prospective well-conducted comparative studies.
Appendix
Surgeon Questionnaire (ACDF Survey)
| 1. What is your specialty? |
| 2. How many years have you been in practice? |
| 3. How many ACDFs do you perform per year? |
| 4. Location of practice by AOSpine Region: |
| 5. What is your practice setting? |
| 6. What is the most common structural bone graft or cage material you use for ACDFs? |
| 7. Along with the structural graft above, do you use additional nonstructural graft material to supplement the structural graft? |
| 8. What is the most common structural bone graft or cage material you use for ACDF in multilevel or challenging host cases? |
| 9. Along with the structural graft above for ACDF in multilevel or challenging host cases, do you use additional nonstructural graft material to supplement the structural graft? |
| 10. What is the plate or cage that you most commonly use for your ACDFs? |
| 11. Do you think that achieving fusion is important in ACDF? |
| 12a. Do you think that graft material impacts fusion rate in ACDF? |
| 12b. Do you think that graft material impacts fusion rate in a clinically meaningful way? |
| 13a. Do you think that graft material impacts time to fusion? |
| 13b. Do you think that graft material impacts time to fusion in a clinically meaningful way? |
| 14. If a supplemental graft material enhanced your current fusion rate by 5% without adverse effects, what is a reasonable amount of money that the hospital should pay per case for the material? |
| 15. In your opinion, what is the most commonly used graft material in the most typical ACDF in the United States? |
| 16. In your opinion, what is the gold standard for graft material in the most typical ACDF? |
| 17. Are you satisfied with the level of evidence available in support of the graft material you use for ACDF? |
| 18. Are you satisfied with the comparative effectiveness data available on bone graft materials for the cervical spine? |
| 19. Do you feel that the risk factors for non-unions and poor clinical outcomes are sufficiently well-defined by the literature? |
| 20. Have you changed your graft choice within the last year? |
| 21. Do you think that the most commonly used graft materials in ACDFs have similar efficacy? |
| 22. Do you think that the most commonly used graft materials in ACDFs have similar safety? |
| 23. Why do you use the graft materials you most commonly use in ACDF? |
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976). 2007;32:2310–2317. [DOI] [PubMed] [Google Scholar]
- 2. Robinson RA. Fusions of the cervical spine. J Bone Joint Surg Am. 1959;41-A:1–6. [PubMed] [Google Scholar]
- 3. Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18:449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28:134–139. [DOI] [PubMed] [Google Scholar]
- 5. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–309. [DOI] [PubMed] [Google Scholar]
- 6. Wigfield CC, Nelson RJ. Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine (Phila Pa 1976). 2001;26:687–694. [DOI] [PubMed] [Google Scholar]
- 7. Samartzis D, Shen FH, Goldberg EJ, An HS. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976). 2005;30:1756–1761. [DOI] [PubMed] [Google Scholar]
- 8. Samartzis D, Shen FH, Matthews DK, Yoon ST, Goldberg EJ, An HS. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451–459. [DOI] [PubMed] [Google Scholar]
- 9. Chong E, Pelletier MH, Mobbs RJ, Walsh WR. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review. BMC Musculoskelet Disord. 2015;16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lied B, Roenning PA, Sundseth J, Helseth E. Anterior cervical discectomy with fusion in patients with cervical disc degeneration: a prospective outcome study of 258 patients (181 fused with autologous bone graft and 77 fused with a PEEK cage). BMC Surg. 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoueir P, Oh BC, DiRisio DJ, Wang MY. Multilevel anterior cervical fusion using a collagen-hydroxyapatite matrix with iliac crest bone marrow aspirate: an 18-month follow-up study. Neurosurgery. 2007;61:963–970. [DOI] [PubMed] [Google Scholar]
- 12. Feiz-Erfan I, Harrigan M, Sonntag VK, Harrington TR. Effect of autologous platelet gel on early and late graft fusion in anterior cervical spine surgery. J Neurosurg Spine. 2007;7:496–502. [DOI] [PubMed] [Google Scholar]
- 13. Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976). 2003;28:1219–1224. [DOI] [PubMed] [Google Scholar]
- 14. Spanos SL, Siasios ID, Dimopoulos VG, Fountas KN. Anterior cervical discectomy and fusion: practice patterns among greek spinal surgeons. J Clin Med Res. 2016;8:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savolainen S, Rinne J, Hernesniemi J. A prospective randomized study of anterior single-level cervical disc operations with long-term follow-up: surgical fusion is unnecessary. Neurosurgery. 1998;43:51–55. [DOI] [PubMed] [Google Scholar]