Abstract
Study Design:
Biomechanical study.
Objective:
Recently, a posterior concave periapical distraction device for fusionless scoliosis correction was introduced. The goal of this study was to quantify the effect of the periapical distraction device on spinal range of motion (ROM) in comparison with traditional rigid pedicle screw-rod instrumentation.
Methods:
Using a spinal motion simulator, 6 human spines were loaded with 4 N m and 6 porcine spines with 2 N m to induce flexion-extension (FE), lateral bending (LB), and axial rotation (AR). ROM was measured in 3 conditions: untreated, periapical distraction device, and rigid pedicle screw-rod instrumentation.
Results:
The periapical distraction device caused a significant (P < .05) decrease in ROM of FE (human, −40.0% and porcine, −55.9%) and LB (human, −18.2% and porcine, −17.9%) as compared to the untreated spine, while ROM of AR remained unaffected. In comparison, rigid instrumentation caused a significantly (P < .05) larger decrease in ROM of FE (human, −80.9% and porcine, −94.0%), LB (human, −75.0% and porcine, −92.2%), and AR (human, −71.3% and porcine, −86.9%).
Conclusions:
Although no destructive forces were applied, no device failures were observed. Spinal ROM was significantly less constrained by the periapical distraction device compared to rigid pedicle screw-rod instrumentation. Therefore, provided that scoliosis correction is achieved, a more physiological spinal motion is expected after scoliosis correction with the posterior concave periapical distraction device.
Keywords: in vitro biomechanics, nonfusion, ApiFix, adolescent idiopathic scoliosis, less invasive scoliosis surgery
Introduction
Adolescent idiopathic scoliosis (AIS) affects 1-3% of children between 10 and 16 years of age.1 In progressive scoliosis, a good correction of the deformity can be achieved with current surgical techniques, but the postoperative spinal motion is significantly diminished due to the fusion of the spine.2 Moreover, patients who underwent fusion surgery report a lower physical function as compared to nonscoliotic controls at long-term follow-up, which could be attributed to the invasive surgery and stiffening of the spine.3 To overcome these negative consequences of extensive fusion procedures, recent research has been aimed at developing techniques that allow scoliosis correction without fusion.4,5
A new less-invasive and potentially fusionless scoliosis correction system is a posterior concave periapical distraction device (the ApiFix system, ApiFix Ltd, Misgav, Israel), which spans 3 to 4 motion segments (Figure 1). The device is a unilateral construct that connects 2 periapical pedicle screws through polyaxial mobile ball-and-socket joints with a rod. The device is inserted on the concave side of the curve. The rod includes a ratchet that can elongate when the patient performs exercises after surgery, especially with side bending toward the convexity. By side bending, the ratchet allows elongation of the rod and thereby corrects the scoliotic curve (Figure 1). The ratchet does not allow for shortening when the patient stands upright again and thus it preserves the correction. The poly-axial joints allow the pedicle screws to have a range of motion (ROM) of 30° (15° per pedicle screw) in flexion, extension, and axial rotation (Figure 2). Floman et al6 recently demonstrated the effectiveness of the periapical distraction device by reducing the Cobb angle from 43°-53° to 22°-33° in a small case series. The authors stated that the new device is a less rigid construct than traditional rigid pedicle screw-rod instrumentation and as a result, preserves a more physiologic spinal ROM after treatment with this device.6
Figure 1.
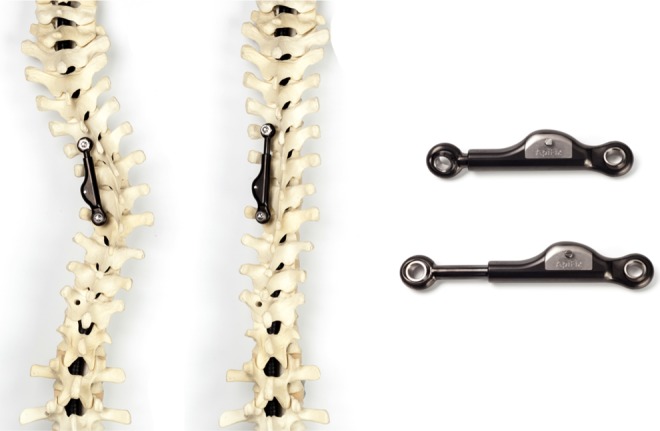
Left: The posterior concave periapical nonfusion distraction device for scoliosis correction (the ApiFix system, ApiFix Ltd, Misgav, Israel). The device consists of a unilateral construct that connects 2 periapical pedicle screws through mobile polyaxial ball-and-socket joints with a rod with ratchet mechanism. Right: The device in detail. The device is inserted on the concave side of the curve. The rod includes a ratchet and can elongate as the patient is required to perform exercises after surgery. In theory, by side bending the ratchet allows elongation of the rod and thereby corrects the scoliotic curve.
Figure 2.
The posterior concave periapical nonfusion distraction device with polyaxial pedicle screw-rod joints. The polyaxial joints allow the pedicle screws to have a range of motion (ROM) of 30° in flexion, extension, and axial rotation (15° per pedicle screw).
Nevertheless, it is unknown whether spinal motion is indeed preserved after treatment with this new device. This is important, because rigid spinal instrumentation has been shown to fail in long term if fusion is not achieved and spinal loads are transferred to the instrumentation.7 Also, when spinal flexibility remains intact after treatment with this new device, this could have beneficial effects on the postoperative physical function in comparison to traditional long fusion surgery. Before large clinical studies are started with this fusionless scoliosis correction device, it should be analyzed whether spinal motion is indeed minimally constrained to prevent the transfer of loads to the implant. Therefore, the goal of this study was to analyze the effect of the posterior concave periapical nonfusion distraction device on the in vitro biomechanics of the spine.
Materials and Methods
Prior to the study, research agreements were signed by all parties to ensure the rights of academic freedom of the VU University Medical Center and consequently an independent publication of the research results.
Specimens and Specimen Preparation
Seventeen thoracic spines were harvested from fresh frozen (−20°C) human cadavers. After radiographic evaluation and exclusion of specimens with bridging osteophytes or collapsed intervertebral disc spaces, 6 human thoracic spines (mean age 79.2 years, range 65-86 years) were included. Additionally, we included 6 thoracic spines of immature domestic pigs (mean weight 72 kg, range 64-80 kg) obtained from a local abattoir. This allowed for a better comparison with the flexible spine of a young adolescent. In the human spine, the morphology of the facet joint alters below T12-L1. This change in facet orientation, and as a result a change in biomechanics, in porcine spines already occurs more proximally at T10-T11.8 To allow for a comparison between the human and porcine specimens, we used the levels T5-T12 of the human specimens and T3-T10 of the porcine specimens.
Testing Conditions
The spines were tested in 3 phases: untreated (for baseline analysis), instrumented with the (unilateral) posterior concave periapical nonfusion distraction device and finally, after bilateral segmental rigid pedicle screw-rod rod instrumentation (Figure 3). The pedicle screws of the periapical distraction were implanted unilaterally at levels T7 and T10 in the human specimens and at levels T5 and T8 in the porcine specimens.
Figure 3.

Biomechanical testing sequence with an untreated human cadaveric thoracic spine (left), after instrumentation with the posterior concave periapical nonfusion distraction device (middle), and after rigid pedicle screw-rod instrumentation (right).
The internal ratchet of the periapical distraction device can be set in 3 positions: 1-way, locked and unlocked, with the latter 2 positions aiming to prevent and correct, respectively, any adverse overcorrection if needed. For this study, we set the ratchet in the locked position, thus fixing the rod length. We consider that the locked ratchet position represents the end situation after rod elongation and scoliosis correction, where the system effectively functions as a unilateral posterior rigid rod with a mobile connection to the polyaxial screws.
In half of the specimens the implant was placed on the right side and in the other half of the specimens on the left side, to exclude a possible effect of implant location. In the final third test the periapical distraction device was replaced by a bilateral segmental pedicle screw-rod system (Medtronic, CD Horizon Legacy Spinal System, Minneapolis, MN, USA) across the same motion segments as the periapical distraction device (T7, T8, T9, and T10 in the human specimens and at levels T5, T6, T7, and T8 in the porcine specimens) (Figure 3). Proximal and distal to the instrumented segments there were 2 untreated adjacent spinal motion segments (ie, 2 intervertebral discs). For the third phase of testing with the rigid instrumentation, the pedicle screw holes in the proximal and distal instrumented vertebra of the investigated periapical distraction device were reused by implanting pedicle screws with a 1 mm larger diameter and 2 to 5 mm greater length (depending on the size of the vertebral body) to ensure adequate bone purchase of the screws using the same pedicle screw holes. No screw loosening was observed during or after testing based on manual inspection of the instrumentation and analysis of the load displacement curves.
Biomechanical Testing
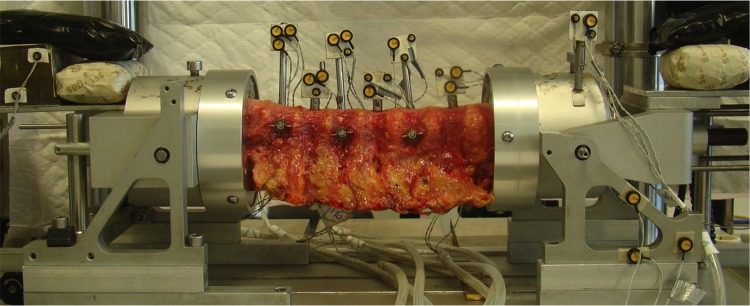
The test setup and analysis has been described and validated previously.9-14 Briefly, before testing, a compressive axial preload of 250 N was applied for 1 hour to obtain physiological conditions in the intervertebral disc.9 Throughout testing, no compressive load was applied to prevent buckling of the spine.15 The spinal segments were placed horizontally in a custom made spinal motion simulator (Figure 4) in which pure moments in flexion-extension (FE), lateral bending (LB), and axial rotation (AR) can be applied to the spine using a hydraulic materials testing machine (Instron, model 8872; Instron and IST, Norwood, MA, USA). This spinal motion simulator allows for the biomechanical analysis of long, multisegment, spinal specimens.
Figure 4.
The experimental setup is shown with a human cadaveric thoracic spinal specimen positioned in the spinal motion simulator. The proximal and distal vertebrae were potted in a casting-mold and partially buried in a low melting point (48°C) bismuth alloy (Cerrolow-147; 48.0% bismuth, 25.6% lead, 12.0% tin, 9.6% cadmium, and 4.0% indium). The proximal and distal vertebrae were fixed securely into the alloy by adding screws into the vertebral body. All articulating parts were kept free. During the experiment, the spinal specimens were kept moist with 0.9% saline spray. A materials testing machine applied loads to the setup to induce flexion-extension, lateral bending and axial rotation. The markers with the LEDs were rigidly fixed to the vertebrae. An Optotrak camera registered the movement of the infrared-emitting LEDs.
The human spinal segments were tested at up to −4 and +4 N m and the porcine spinal segments at up to a moment of −2 and +2 N m to allow comparison with previous work.8 Each movement direction was tested for 3 subsequent cycles. The third cycle was used for analysis. During loading, the displacement of 3 infrared-emitting LEDs, rigidly attached to each of the vertebral bodies, was recorded by an optoelectronic 3-dimensional movement registration system (Optotrak 3020, Northern Digital Inc, Waterloo, Ontario, Canada). A custom Matlab (Mathworks Inc, Natick, MA, USA) program was used to calculate the load-displacement curves and ROM of the loaded and coupled directions (rotation about an axis with additional motions about 1 or 2 other axes16) of the bridged and adjacent levels.
Statistical Analysis
Effects of type of instrumentation were tested separately for the untreated cranial motion segment, bridged motion segments (average of 3 bridged spinal motion segments), and for the untreated caudal motion segment, using a 1-way repeated-measure analysis of variance with the surgical condition as the within-subject factor. Multiple comparisons based on Bonferroni-corrected paired t tests were applied for analysis among testing conditions. P values less than .05 were considered statistically significant. SPSS for Mac version 23.0 (IBM Corp, Armonk, NY, USA) was used for statistical analyses.
Results
No device failures were observed during testing.
Bridged Segments
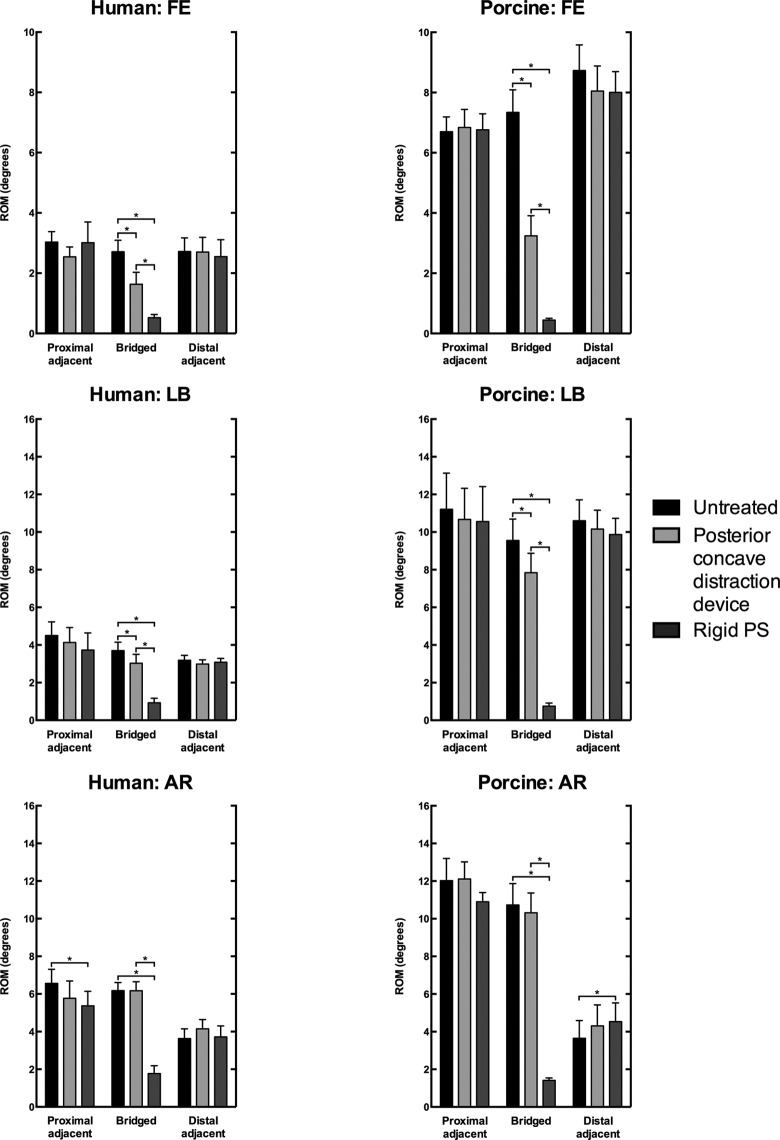
In the loaded direction, the (locked) periapical distraction device resulted in a significant decrease in ROM of FE (human, −40.0%, −1.1°, P = .009; porcine, −55.9%, −4.1°, P < .001) and LB (human, −18.2%, −0.7°, P = .020; porcine, −17.9%, −1.7°, P = .007) of the bridged motion segments as compared with the untreated segments (Tables 1 and 2; Figure 5). No significant alteration in the ROM of AR of the bridged motion segments was observed.
Table 1.
Human Spines: Change in Range of Motion (ROM) in Flexion-Extension (FE), Lateral Bending (LB), and Axial Rotation (AR), With Mean and SD Expressed as Percent (%).a
| ROM (% Change) | Posterior Concave Distraction Device vs Untreated | Rigid PS vs Untreated | Rigid PS vs Posterior Concave Distraction Device | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Mean | SD | P | Mean | SD | P | Mean | SD | P |
| FE | |||||||||
| Proximal adjacent | −16.1 | 23.3 | .454 | −0.8 | 33.9 | 1.000 | 18.3 | 50.6 | 1.000 |
| Bridged | −40.0 | 18.0 | .009 | −80.9 | 27.3 | .002 | −68.2 | 45.1 | .042 |
| Distal adjacent | −0.6 | 12.1 | 1.000 | −6.3 | 21.8 | 1.000 | −5.7 | 11.4 | .816 |
| LB | |||||||||
| Proximal adjacent | −8.2 | 10.1 | .313 | −17.1 | 22.4 | .362 | −9.7 | 30.0 | 1.000 |
| Bridged | −18.2 | 10.0 | .020 | −75.0 | 19.1 | .001 | −69.4 | 20.5 | .001 |
| Distal adjacent | −6.0 | 6.3 | .199 | −3.2 | 11.9 | 1.000 | 3.0 | 14.1 | 1.000 |
| AR | |||||||||
| Proximal adjacent | −12.2 | 12.9 | .208 | −18.1 | 8.7 | .011 | −6.8 | 22.0 | 1.000 |
| Bridged | −0.1 | 2.6 | 1.000 | −71.3 | 17.2 | <.001 | −71.3 | 18.0 | .001 |
| Distal adjacent | 14.3 | 10.2 | .056 | 2.5 | 23.4 | 1.000 | −10.3 | 22.9 | .966 |
aData of the 3 bridged motion segments is averaged. Boldfaced values indicate statistical significance (P < .05). Rigid PS: bilateral rigid pedicle screw-rod instrumentation.
Table 2.
Porcine Spines: Change in Range of Motion (ROM) in Flexion-Extension (FE), Lateral Bending (LB), and Axial Rotation (AR), With Mean and SD Expressed as Percent (%).a
| ROM (% Change) | Posterior Concave Distraction Device vs Untreated | Rigid PS vs Untreated | Rigid PS vs Posterior Concave Distraction Device | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Porcine | Mean | SD | P | Mean | SD | P | Mean | SD | P |
| FE | |||||||||
| Proximal adjacent | 2.1 | 7.4 | 1.000 | 1.0 | 6.3 | 1.000 | −1.0 | 2.8 | 1.000 |
| Bridged | −55.9 | 7.1 | <.001 | −94.0 | 23.0 | .001 | −86.4 | 46.6 | .018 |
| Distal adjacent | −7.7 | 5.5 | .056 | −8.3 | 7.1 | .105 | −0.6 | 6.4 | 1.000 |
| LB | |||||||||
| Proximal adjacent | −4.7 | 6.9 | .455 | −5.8 | 3.9 | .045 | −1.1 | 6.8 | 1.000 |
| Bridged | −17.9 | 7.7 | .007 | −92.2 | 26.2 | .001 | −90.4 | 28.0 | .002 |
| Distal adjacent | −4.1 | 4.8 | .268 | −6.8 | 7.7 | .244 | −2.8 | 6.4 | .970 |
| AR | |||||||||
| Proximal adjacent | 0.7 | 10.4 | 1.000 | −9.4 | 22.1 | 1.000 | −10.0 | 14.6 | .459 |
| Bridged | −3.9 | 3.3 | .104 | −86.9 | 23.2 | .001 | −86.3 | 21.8 | .001 |
| Distal adjacent | 17.9 | 18.2 | .183 | 23.9 | 15.4 | .038 | 5.1 | 17.3 | 1.000 |
aData of the 3 bridged motion segments is averaged. Boldfaced values indicate statistical significance (P < .05). Rigid PS: bilateral rigid pedicle screw-rod instrumentation.
Figure 5.
Range of motion (ROM) of the cranial adjacent, bridged, and caudal adjacent motion segments of the human spines under 4 N m loading and porcine spines under 2 N m loading in flexion-extension (FE), lateral bending (LB), and axial rotation (AR) of the untreated spine, after posterior concave periapical nonfusion distraction device, and bilateral rigid pedicle screw-rod (rigid PS) instrumentation (mean ± SD). Data of the 3 bridged motion segments is averaged. Asterisks (*) indicate statistical significance (P < .05).
Rigid pedicle screw-rod instrumentation caused a larger and significant decrease in ROM of FE (human, −80.9%, −2.2°, P = .002; porcine, −94.0%, −6.9°, P = .001), LB (human, −75.0%, −2.77°, P = .001; porcine, −92.2%, −8.8°, P = .001), and AR (human, −71.3%, −4.4°, P < .001; porcine, −86.9%, −9.3°, P = .001) of the bridged motion segments as compared with the untreated spine (Tables 1 and 2; Figure 5).
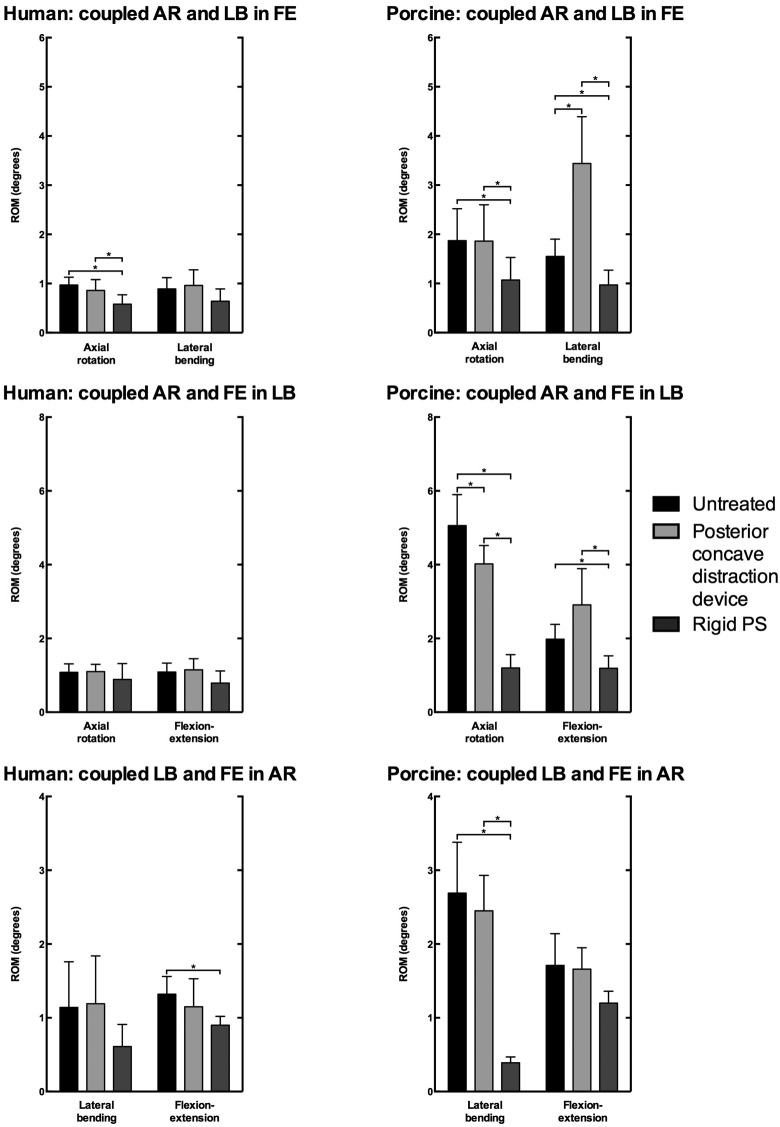
In the human specimens, coupled motions of the bridged motion segments were not significantly affected after instrumentation with the periapical distraction device (Table 3; Figure 6). In the porcine specimens, ROM of coupled LB during FE loading increased (+122.7%, +1.9°, P = .007), while ROM of coupled AR during LB decreased (−20.5%, −1.0°, P = .009) after instrumentation with the periapical distraction device (Table 4; Figure 6).
Table 3.
Human Spines: Change in Coupled Range of Motion (ROM) in Flexion-Extension (FE), Lateral Bending (LB), and Axial Rotation (AR), With Mean and SD Expressed as Percent (%).a
| Coupled ROM (% Change) | Posterior Concave Distraction Device vs Untreated | Rigid PS vs Untreated | Rigid PS vs Posterior Concave Distraction Device | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Mean | SD | P | Mean | SD | P | Mean | SD | P |
| FE | |||||||||
| Coupled AR | −11.5 | 24.6 | .908 | −39.7 | 16.4 | .006 | −31.9 | 19.9 | .033 |
| Coupled LB | 8.0 | 35.7 | 1.000 | −28.1 | 31.1 | .234 | −33.4 | 27.7 | .095 |
| LB | |||||||||
| Coupled AR | 1.1 | 17.5 | 1.000 | −18.1 | 26.6 | .467 | −19.0 | 32.4 | .628 |
| Coupled FE | 5.7 | 30.1 | 1.000 | −27.6 | 27.3 | .169 | −31.5 | 31.2 | .170 |
| AR | |||||||||
| Coupled LB | 4.6 | 11.4 | 1.000 | −46.6 | 61.3 | .366 | −48.9 | 62.0 | .334 |
| Coupled FE | −13.3 | 12.1 | .130 | −31.9 | 19.6 | .031 | −21.5 | 31.8 | .476 |
aData of the 3 bridged motion segments is averaged. Boldface values indicate statistical significance (P < .05). Rigid PS: bilateral rigid pedicle screw-rod instrumentation.
Figure 6.
Coupled range of motion (ROM) of the cranial adjacent, bridged, and caudal adjacent motion segments of the human spines under 4 N m loading and porcine spines under 2 N m loading in flexion-extension (FE), lateral bending (LB), and axial rotation (AR) of the untreated spine, and after posterior concave periapical nonfusion distraction device and bilateral rigid pedicle screw-rod (rigid PS) instrumentation (mean ± SD). Data of the 3 bridged motion segments is averaged. Asterisks (*) indicate statistical significance (P < .05).
Table 4.
Porcine Spines: Change in Coupled Range of Motion (ROM) in Flexion-Extension (FE), Lateral Bending (LB), and Axial Rotation (AR), With Mean and SD Expressed as Percent (%).a
| Coupled ROM (% Change) | Posterior Concave Distraction Device vs Untreated | Rigid PS vs Untreated | Rigid PS vs Posterior Concave Distraction Device | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Porcine | Mean | SD | P | Mean | SD | P | Mean | SD | P |
| FE | |||||||||
| Coupled AR | −0.5 | 21.0 | 1.000 | −42.5 | 23.1 | .019 | −42.2 | 33.6 | .083 |
| Coupled LB | 122.7 | 53.4 | .007 | −37.0 | 25.0 | .045 | −71.7 | 28.5 | .005 |
| LB | |||||||||
| Coupled AR | −20.5 | 9.4 | .009 | −76.2 | 15.9 | <.001 | −70.0 | 12.6 | <.001 |
| Coupled FE | 46.5 | 44.4 | .151 | −39.9 | 23.5 | .026 | −59.0 | 32.3 | .020 |
| AR | |||||||||
| Coupled LB | −9.0 | 12.4 | .407 | −85.6 | 23.1 | .001 | −84.2 | 17.2 | <.001 |
| Coupled FE | −2.8 | 22.7 | 1.000 | −29.7 | 31.8 | .212 | −27.7 | 26.4 | .151 |
aData of the 3 bridged motion segments is averaged. Boldfaced values indicate statistical significance (P < .05). Rigid PS: bilateral rigid pedicle screw-rod instrumentation.
Rigid pedicle screw-rod instrumentation caused a significant decrease in ROM of coupled AR (−39.7%, −0.39°, P = .006) during FE loading, and conversely, in ROM of coupled FE (−31.9%, −0.42°, P = .031) during AR loading in the human spines of the bridged motion segments (Table 3; Figure 6). In the porcine spines, rigid pedicle screw-rod instrumentation caused a significant decrease in all coupled ROM except ROM of coupled FE during AR loading (Table 4; Figure 6).
Adjacent Segments
Other than a decrease in ROM of AR (−18.1%, −1.19°, P = .011) of the proximal adjacent motion segment in the human specimens and an increase in ROM of AR (+23.9%, +0.88°, P = .038) of the distal adjacent segment in the porcine specimens after rigid pedicle screw-rod instrumentation, no significant adjacent segment effects were observed (Tables 1 and 2; Figure 5).
Discussion
Recently, a posterior concave periapical distraction device was presented for fusionless surgical correction of adolescent idiopathic scoliosis.6 This device is designed to maintain spinal motion by using mobile poly axial pedicle screw-rod joints. In fusion surgery, rigid spinal instrumentation has been shown to fail if fusion is not achieved and spinal loads are transferred to the instrumentation.7 A spinal implant designed for fusionless scoliosis correction should therefore minimally constrain spinal motion to prevent the transfer of loads to the implant, thereby reducing the risk of implant failure. The present in vitro biomechanical study was designed to determine the effect of the new periapical distraction device on spinal range of motion in comparison to traditional rigid pedicle screw-rod instrumentation.
This study shows that, across the bridged motion segments, the periapical distraction device roughly halved spinal ROM of FE (human −40.0% and porcine −55.9%), while LB was only slightly affected (human −18.2% and porcine −17.9%) and AR was unaffected. In comparison, the rigid pedicle screw-rod instrumentation resulted in a far larger decrease in ROM of FE (human −80.9% and porcine −94.0%), LB (human −75.0% and porcine −92.2%), and AR (human −71.3% and porcine −86.9%).
The periapical distraction device was tested with the internal ratchet set in “locked” position. This ratchet position prevented rod elongation with each subsequent load cycle (each spine was loaded for 3 cycles) and thus prevented the creation of a scoliotic deformity while testing. In this mode, the periapical distraction device has a fixed length and functions as a unilateral posterior tether (ie, lengthening or shortening was not possible) attached to the pedicle screws with mobile ball and socket joints with large degrees of freedom. This is the situation that would occur after correction of the deformity. The fixed length of the device explains the decrease in ROM of FE after instrumentation of the spine. The decrease in ROM of LB (human, −18.2%, −0.7°; porcine, −17.9%, −1.7°) due to the periapical distraction device was less than expected. With the ratchet of the device set in “locked” position, a larger decrease in LB was anticipated. The relatively mild reduction in ROM may be explained by the phenomenon of coupled motion. The thoracic spine has been shown to exhibit significant coupled motion under LB loading.17 It appears that the mobile polyaxial rod-screw joint design of the periapical distraction device allows coupled AR and FE during LB loading of the bridged segments in a magnitude comparable to the untreated spine with only minor changes (Figure 6). We speculate that, because of this, the instrumented spinal segments can find the path of motion with the least resistance (ie, a combination of LB, AR, and FE) to allow most of the LB without requiring a change in length of the periapical distraction device. Combined with the unilateral rod design, which is less rigid than a bilateral rod system, this may explain why there was only a relatively small and less than expected decrease of ROM of LB.
Although a causal relationship has yet to be established, adjacent segment degeneration after spinal fusion has been attributed to altered motion and disruption of the anatomy of the adjacent level.18 In this study, the periapical distraction device did not significantly affect adjacent segment motion. In contrast, after rigid pedicle screw-rod instrumentation the ROM of AR decreased by 18.1% in the cranial adjacent segment of the human spines and increased by 23.9% in the caudal adjacent segment of the porcine spines. This could possibly be the result of an altered spinal alignment due to the instrumentation, although care was taken to customize the rod curvature to each individual specimen.19 Also, proximal adjacent segment biomechanics can be affected by damage of spinal ligaments and joint capsules of the proximal facet joint due to pedicle screw placement.20,21
Other options for fusionless surgery for adolescent idiopathic scoliosis such as vertebral body stapling or anterior spinal tethering have been investigated.4,5 Current research on the use of vertebral body stapling shows a wide range of results of the effects on the ROM of FE (11% decrease to no decrease), LB (4% to 40% decrease) and AR (6% to 60% decrease) and therefore it is difficult to conclude if and how stapling alters postoperative spinal ROM.22-24
We took great care to study the implant in vitro in a simulated end situation after rod elongation and scoliosis correction to evaluate possible safety concerns regarding its motion preserving properties. However, this and other preclinical studies examining new treatment options for AIS are limited due to the unavailability of cadaveric AIS specimens or an animal model that combines all characteristics of AIS (ie, a lateral deviation, rotation and alterations of the sagittal profile). Thus, the effectiveness of the periapical distraction device in correcting scoliosis could not be tested. The disadvantage of human cadaveric specimens is their high age and associated state of degeneration. Therefore, we excluded specimens with bridging osteophytes or collapsed intervertebral disc spaces. Additionally, we also included thoracic spines from immature pigs, which have previously been shown to be good biomechanical models for the human spine and are more flexible to represent the adolescent spine.8,25
In conclusion, although its effectiveness in scoliosis correction has still only been demonstrated in a small case series, this study demonstrates that the spinal ROM of the bridged segments after spinal instrumentation with the novel posterior concave periapical non-fusion distraction device is partially diminished and adjacent segment biomechanics were not significantly altered.6 Consequently, a more physiological spinal ROM is expected after scoliosis correction with the periapical distraction device as compared with rigid spinal instrumentation. Thus, it is expected that the spinal flexibility of patients treated with this device is not significantly altered, which could have beneficial effects on the post-operative physical function in comparison with traditional long fusion surgery. Also, the risk of implant failure is deemed low as implant loads are expected to be minimal. Although no destructive forces were applied to the specimens in this study, no device failures were seen. We do not know how the implant will function over time in vivo with continued loading and motion. However, while clinically important, failure testing was beyond the scope of this study. Thus, patients treated with this new device should have regular and prolonged follow-up.
Acknowledgments
A research grant and implants were provided to the research institution by ApiFix Ltd (Misgav, Israel). Medtronic Netherlands (Heerlen, The Netherlands) provided implants to the research institution. The authors did not receive any personal financial or in-kind support; all support was transferred directly to the research institute.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A research grant and implants were provided to the research institution by ApiFix Ltd (Misgav, Israel).
References
- 1. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371:1527–1537. [DOI] [PubMed] [Google Scholar]
- 2. Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: a case-control study. Spine. 2006;31:275–283. [DOI] [PubMed] [Google Scholar]
- 3. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aronsson DD, Stokes IA. Nonfusion treatment of adolescent idiopathic scoliosis by growth modulation and remodeling. J Pediatr Orthop. 2011;31(1 suppl):S99–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu JM, Shen JX. Advances in nonfusion techniques for the treatment of scoliosis in children. Orthop Surg. 2010;2:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Floman Y, Burnei G, Gavriliu S, et al. Surgical management of moderate adolescent idiopathic scoliosis with ApiFix®: a short peri-apical fixation followed by post-operative curve reduction with exercises. Scoliosis. 2015;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss HR, Goodall D. Rate of complications in scoliosis surgery—a systematic review of the Pub Med literature. Scoliosis. 2008;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Busscher I, Van der Veen AJ, Van Dieën JH, Kingma I, Verkerke GJ, Veldhuizen AG. In vitro biomechanical characteristics of the spine: a comparison between human and porcine spinal segments. Spine. 2010;35:E35–E42. [DOI] [PubMed] [Google Scholar]
- 9. Busscher I, van Dieën JH, Kingma I, Van der Veen AJ, Verkerke GJ, Veldhuizen AG. Biomechanical characteristics of different regions of the human spine: an in vitro study on multilevel spinal segments. Spine. 2009;34:2858–2864. [DOI] [PubMed] [Google Scholar]
- 10. Bisschop A, Kingma I, Bleys RL, et al. Effects of repetitive movement on range of motion and stiffness around the neutral orientation of the human lumbar spine. J Biomech. 2013;46:187–191. [DOI] [PubMed] [Google Scholar]
- 11. van Engelen SJPM, Ellenbroek MHM, Van Royen BJ, De Boer A, Van Dieën JH. Validation of vibration testing for the assessment of the mechanical properties of human lumbar motion segments. J Biomech. 2012;45:1753–1758. [DOI] [PubMed] [Google Scholar]
- 12. van Engelen SJPM, Bisschop A, Smit TH, Van Royen BJ, Van Dieën JH. The effect of neighboring segments on the measurement of segmental stiffness in the intact lumbar spine. Spine J. 2015;15:1302–1309. [DOI] [PubMed] [Google Scholar]
- 13. Bisschop A, van Engelen SJPM, Kingma I, et al. Single level lumbar laminectomy alters segmental biomechanical behavior without affecting adjacent segments. Clin Biomech. 2014;29:912–917. [DOI] [PubMed] [Google Scholar]
- 14. Bisschop A, Holewijn RM, Kingma I, et al. The effects of single-level instrumented lumbar laminectomy on adjacent spinal biomechanics. Global Spine J. 2015;5:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willems JM, Jull GA, Ng J-KF. An in vivo study of the primary and coupled rotations of the thoracic spine. Clin Biomech. 1996;11:311–316. [DOI] [PubMed] [Google Scholar]
- 17. Mannen EM, Anderson JT, Arnold PM, Friis EA. Mechanical analysis of the human cadaveric thoracic spine with intact rib cage. J Biomech. 2015;48:2060–2066. [DOI] [PubMed] [Google Scholar]
- 18. Helgeson MD, Bevevino AJ, Hilibrand AS. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013;13:342–351. [DOI] [PubMed] [Google Scholar]
- 19. Chen WJ, Lai PL, Tai CL, Chen LH, Niu CC. The effect of sagittal alignment on adjacent joint mobility after lumbar instrumentation—a biomechanical study of lumbar vertebrae in a porcine model. Clin Biomech. 2004;19:763–768. [DOI] [PubMed] [Google Scholar]
- 20. Anderson AL, McIff TE, Asher MA, Burton DC, Glattes RC. The effect of posterior thoracic spine anatomical structures on motion segment flexion stiffness. Spine. 2009;34:441–446. [DOI] [PubMed] [Google Scholar]
- 21. Volkheimer D, Malakoutian M, Oxland TR, Wilke HJ. Limitations of current in vitro test protocols for investigation of instrumented adjacent segment biomechanics: critical analysis of the literature. Eur Spine J. 2015;24:1882–1892. [DOI] [PubMed] [Google Scholar]
- 22. Shillington MP, Labrom RD, Askin GN, Adam CJ. A biomechanical investigation of vertebral staples for fusionless scoliosis correction. Clin Biomech. 2011;26:445–451. [DOI] [PubMed] [Google Scholar]
- 23. Glaser DA, Nandipati C, Nunn TN, Farnsworth CL, Newton PO. Biomechanics of two fusionless scoliosis correction techniques—rigid staple vs. flexible tether. Paper presented at: Orthopaedic Research Society Annual Meeting; January 13-16, 2011; Long Beach, CA Abstract number 827. [Google Scholar]
- 24. Coombs MT, Glos DL, Wall EJ, Kim J, Bylski-Austrow DI.Biomechanics of spinal hemiepiphysiodesis for fusionless scoliosis treatment using titanium implant. Spine. 2013;38: E1454–E1460. [DOI] [PubMed] [Google Scholar]
- 25. Wilke HJ, Geppert J, Kienle A. Biomechanical in vitro evaluation of the complete porcine spine in comparison with data of the human spine. Eur Spine J. 2011;20:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]