Abstract
Study Design:
Retrospective analysis.
Objective:
The purpose of this study is to determine the incidence, impact, and risk factors for short-term postoperative complications following elective adult spinal deformity (ASD) surgery.
Methods:
Current Procedural Terminology codes were used to query the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) for adults who underwent spinal deformity surgery from 2010 to 2014. Patients were separated into groups of those with and without complications. Univariate analysis and multivariate logistic regression were used to assess the impact of patient characteristics and operative features on postoperative outcomes.
Results:
In total, 5803 patients were identified as having undergone ASD surgery in the NSQIP database. The average patient age was 59.5 (±13.5) years, 59.0% were female, and 81.1% were of Caucasian race. The mean body mass index was 29.5(±6.6), with 41.9% of patients having a body mass index of 30 or higher. The most common comorbidities were hypertension requiring medication (54.5%), chronic obstructive pulmonary disease (4.9%), and bleeding disorders (1.2%). Nearly a half of the ASD patients had an operative time >4 hours. The posterior fusion approach was more common (56.9%) than an anterior one (39.6%). The mean total relative value unit was 73.4 (±28.8). Based on multivariate analyses, several patient and operative characteristics were found to be predictive of morbidity.
Conclusion:
Surgical correction of ASD is associated with substantial risk of intraoperative and postoperative complications. Preoperative and intraoperative variables were associated with increased morbidity and mortality. This data may assist in developing future quality improvement activities and saving costs through measurable improvement in patient safety.
Keywords: adult spinal deformity, spinal fusion, arthrodesis, complications, outcomes, National Surgical Quality Improvement Project (NSQIP)
Introduction
Adult spinal deformity (ASD) is a serious medical condition that continues to affect a growing number of patients every year.1–3 Reports estimate the prevalence of ASD to range from 1.4% to 32%, with the majority presenting with significant back pain and worsening loss of function.3–8 Recent advances in perioperative medical management, instrumentation, and surgical technique have contributed to the increased utilization of surgical fusion in ASD patients.2,6,9–12 Complications associated with ASD are not insignificant, ranging from 10% to 40%, and costly.8,13–18 Therefore, a thorough assessment of the factors that may adversely influence the patient’s clinical course after ASD surgery is warranted.
There are a number of studies that report on the risks associated with complications in this population. Among the largest to date, the Scoliosis Research Society report on morbidity and mortality of adult scoliosis surgery indicated complication rates to be significantly higher when osteotomies, revision procedures, and combined anterior/posterior approaches were used.17 A number of other studies have found that higher rates of overall complication in ASD surgery are associated with advanced age, smoking, osteoporosis, long fusions, and extensive blood loss.6,19–21 Although these studies provide valuable insight, these published works have been criticized for a lack of functional outcome data, relying on self-reported data or limited sample sizes, and using varying definitions of clinical outcomes. This has led to a significant amount of heterogeneity in the literature and has limited the provider’s ability to make meaningful quality assessment and improvement plans.
To address these concerns, the current study leverages data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), which is a nationally validated, risk-adjusted database with standardized quality improvement measurements. The specific purposes of this study are to (a) identify the incidence and impact of adverse events as defined by NSQIP and (b) determine the significant patient and clinical risk factors for 30-day morbidity and mortality.
Materials and Methods
Data Source
The ACS NSQIP database is a multicenter registry that is widely used nationwide. With more than 150 patient variables collected, the database provides high-dimensional clinical data, which includes preoperative risk factors, intraoperative variables, and 30-day postoperative outcomes for patients undergoing major surgical procedures in either the inpatient or outpatient setting. At each participating site, highly trained surgical clinical reviewers prospectively collect data directly from patients’ medical records. Additionally, a random sampling of cases is included from each participating hospital. To ensure high interrater reliability (IRR), ACS NSQIP continuously audits their data. Per NSQIP, the IRR Audit disagreement rate of 5% or less is acceptable. Audit reports have revealed an overall disagreement rate of approximately 2%.22
Data Collection
The current study retrospectively reviewed all adult patients (≥18 years) who underwent spinal fusion for deformity using the following Current Procedural Terminology (CPT) codes: 22 800, 22 802, 22 804, 22 808, 22 810, 22 812, 22 818, and 22 819. CPT codes 22 843, 22 844, 22 846, or 22 847 were also included to capture long, multi-level fusion constructs. Patients with CPT codes 22 842 and 22 845 were included if they had an International Classification of Diseases, Ninth Edition, diagnosis for spinal deformity (including 737.1, 737.2, 737.3, 737.4, 737.8, and 737.9; see Supplemental Table S1, available in the online version of the article). Cases with missing preoperative data; wound class of 2, 3, or 4; an open wound on their body; current sepsis; current pneumonia; prior surgeries within 30 days; cases requiring cardiopulmonary resuscitation prior to surgery; emergency cases; or any patient undergoing a nonelective procedure were excluded from the analysis. Additionally, patients with diagnoses relating to cervical spine, trauma or injury to spine, or neoplasm of spine were excluded in order to focus on elective spinal deformity cases.
Independent Variables
Independent variables included patient demographics, medical comorbidities, operative data, and postoperative outcomes. Several comorbidities included pulmonary (history of severe chronic obstructive pulmonary disease or ventilator dependence during the 48 hours preceding surgery), renal (dialysis or acute renal failure), and cardiac (history of congestive heart failure within 30 days before admission or hypertension requiring medication) systems. Using the list of CPT codes described in the work by Martin et al, patients were additionally categorized according to the type of surgical procedure performed.23 Additional procedure subanalyses of fusion length and approach were included as well (Supplemental Table S1, available in the online version of the article).
Outcome Variables
The primary outcome variables in this study were early morbidity and mortality. Morbidity was defined as the development of one or more complications in the postoperative period after spinal fusion in ASD patients. A list of subtype complications for morbidity can be found in Table 1. To assess the impact of complications on other 30-day clinical outcomes, the rates of hospital stay in the acute care setting >30 days after surgery, unplanned reoperation, and unplanned readmission were assessed for those with and without any morbidity. The unplanned reoperation was defined as an unplanned return to the operating room of any surgical facility or hospital for a surgical procedure related to either the index or concurrent procedure performed.
Table 1.
Frequency of Complication in Elective ASD Surgery (Total Cases = 5803; 2010-2014).
| Complication | Number | Rate |
|---|---|---|
| Total patients with ≥1 complication | 1843 | 31.8% |
| Mortality | 29 | 0.5% |
| Morbidity (patients with ≥1 complication) | 1814 | 31.3% |
| Total patients with ≥1 wound complication | 140 | 2.4% |
| Superficial SSI | 62 | 1.1% |
| Deep incisional SSI | 49 | 0.8% |
| Organ/space SSI | 17 | 0.3% |
| Wound disruption | 28 | 0.5% |
| Pneumonia | 91 | 1.6% |
| Unplanned intubation | 54 | 0.9% |
| Pulmonary embolism | 59 | 1.0% |
| On ventilator >48 hours | 42 | 0.7% |
| Progressive renal insufficiency | 9 | 0.2% |
| Acute renal failure | 48 | 0.8% |
| Urinary tract infection | 93 | 1.6% |
| Stroke/CVA occurrences | 5 | 0.1% |
| Peripheral nerve injury | 6 | 0.1% |
| Cardiac arrest requiring CPR | 17 | 0.3% |
| Myocardial infarction | 488 | 8.4% |
| Intra-/postoperative blood transfusion | 1568 | 27.0% |
| Graft/prosthesis/flap failure | 5 | 0.1% |
| DVT/PE | 105 | 1.8% |
| Systemic sepsis | 76 | 1.3% |
| Septic shock | 24 | 0.4% |
| Other outcomes | ||
| Unplanned readmissions (2011-2012), N = 5339 | 331 | 6.2% |
| Unplanned reoperations (2011-2014), N = 5339 | 216 | 4.0% |
| Hospital stay (acute care setting) >30 days | 21 | 0.4% |
Abbreviations: ASD, adult spinal deformity; SSI, surgical site infection; CVA, cardiovascular accident; CPR, cardiopulmonary resuscitation; DVT, deep vein thrombosis; PE, pulmonary embolism.
Statistical Analysis
Patients were divided into cohorts of those with and without the outcomes of interest. Both univariate and multivariate analyses were performed to study the influence of patient demographics, comorbidities, operative conditions, and other clinical parameters on complications. In the univariate analyses, student t tests and χ2 tests were used for continuous and categorical variables, respectively. A P value less than .05 was defined as significant. Factors that demonstrated a P value less than .2 in the univariate analysis were subsequently assessed in the multivariate analysis. A stepwise multivariate logistic regression was fitted for the presence or absence of any morbidity or mortality. The odds ratios (ORs) and 95% confidence intervals (CIs) were reported for the final multivariate models. The overall quality of the model was assessed by calculating the C-statistic and the calibration of the Homer-Lemeshow goodness-of-fit test. SAS software (Version 9.3, SAS Institute Inc, Cary, NC) was used for all statistical analyses.
Results
Between 2010 and 2014, 5803 patients were identified as having undergone ASD surgery in the NSQIP database. The average patient age was 59.5 (±13.5) years, 59.0% were female, and 81.1% were of Caucasian race. The mean body mass index was 29.5 (±6.6), with 41.9% of patients having a body mass index of 30 or higher. The most common comorbidities were hypertension requiring medication (54.5%), chronic obstructive pulmonary disease (4.9%), and bleeding disorders (1.2%). Nearly a half of the ASD patients had an operative time >4 hours. The posterior fusion approach was more common (56.9%) than the anterior one (39.6%). The mean total relative value unit was 73.4 ± 28.8 (Table 3).
Table 3.
Univariate Analysis of Morbidity for ASD Surgery.a
| Characteristic | Total (N = 5803) | No Morbidity (N = 3989) | Morbidity (N = 1814) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| <51 | 21.6% | 24.1% | 16.2% | <.0001 |
| 51-60 | 26.0% | 28.1% | 21.3% | |
| 61-70 | 31.7% | 30.4% | 34.6% | |
| 71-80 | 17.5% | 14.8% | 23.4% | |
| >80 | 3.2% | 2.5% | 4.5% | |
| Female | 59.0% | 56.9% | 63.6% | <.0001 |
| Race | ||||
| African American | 7.6% | 8.1% | 6.5% | .001 |
| Caucasian | 81.1% | 81.2% | 81.1% | |
| Hispanic | 3.8% | 4.0% | 3.3% | |
| Otherb | 7.5% | 6.7% | 9.2% | |
| BMI, kg/m2 | ||||
| Nonobese | 58.1% | 58.2% | 57.9% | .288 |
| Obese class I (30.0-34.9) | 23.8% | 23.9% | 23.7% | |
| Obese class II (35.0-39.9) | 11.3% | 11.6% | 10.8% | |
| Obese class III (≥40) | 6.7% | 6.3% | 7.6% | |
| ASA 3 or 4 | 53.2% | 48.5% | 63.5% | <.0001 |
| Diabetes | ||||
| Nondiabetic | 85.4% | 85.9% | 84.2% | .243 |
| Insulin dependent | 4.7% | 4.5% | 5.0% | |
| Insulin independent | 9.9% | 9.6% | 10.8% | |
| Dyspnea | 6.0% | 5.5% | 7.0% | .027 |
| Dependent functional health status prior to surgery | 4.6% | 3.6% | 6.8% | <.0001 |
| Steroid use | 3.8% | 3.4% | 4.5% | .044 |
| Recent weight loss | 0.2% | 0.2% | 0.3% | .309 |
| Comorbidities | ||||
| Ventilator dependent | 0.2% | 0.1% | 0.4% | .001 |
| COPD | 4.9% | 5.1% | 4.6% | .402 |
| CHF | 0.4% | 0.3% | 0.6% | .123 |
| Hypertension requiring medication | 54.5% | 52.3% | 59.3% | <.0001 |
| Renal failure | 0.1% | 0.1% | 0.2% | .508 |
| Dialysis use | 0.3% | 0.2% | 0.6% | .022 |
| Bleeding disorder | 1.2% | 0.9% | 1.8% | .004 |
| Preoperative blood transfusion | 0.2% | 0.1% | 0.6% | <.0001 |
| Operative variables | ||||
| Operative time >4 hours | 47.9% | 34.3% | 77.7% | <.0001 |
| Total RVU, mean (SD) | 73.4 (28.8) | 68.5 (25.9) | 84.1 (31.8) | <.0001 |
| Inpatient | 96.4% | 95.0% | 99.4% | <.0001 |
| Procedure length | ||||
| Long | 63.0% | 60.7% | 68.1% | <.0001 |
| Short (≤3 levels) | 37.0% | 39.3% | 31.9% | |
| Procedure approach | ||||
| Anterior | 39.6% | 51.9% | 12.5% | <.0001 |
| Posterior | 56.7% | 45.2% | 81.9% | |
| Combined | 3.7% | 2.8% | 5.6% | |
| Procedure subtypes | ||||
| Insertion of intervertebral device | 41.5% | 45.4% | 33.1% | <.0001 |
| Osteotomy | 14.4% | 7.1% | 30.6% | <.0001 |
| Bone grafting | 69.8% | 70.4% | 68.4% | .113 |
| Fusion to pelvis | 8.3% | 3.2% | 19.6% | <.0001 |
Abbreviations: ASD, adult spinal deformity; BMI, body mass index; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; RVU, relative value unit.
aBoldface indicates P < .05.
bOther race includes Native Hawaiian, Pacific Islander, Asian, American Indian, or Alaska Native.
The overall rates of morbidity and mortality were 31.3% and 0.5%, respectively. The incidences for other patient outcomes included an unplanned readmission rate of 6.2%, unplanned reoperation rate of 4.0%, and extended hospital stay rate of 0.4% (Table 1). The ASD morbidity rate has trended downward over the last several years from 37.9% (2011) to 28.0% (2014). Similarly, the mortality rate has trended downward from 1.7% to 0.4% (Figure 1). The top 5 most common subgroups of morbidity were intra-/postoperative blood transfusions (27.0%), myocardial infarction (8.4%), wound complication (2.4%), deep vein thrombosis/pulmonary embolism (1.8%), and urinary tract infection (1.6%; Table 1).
Figure 1.
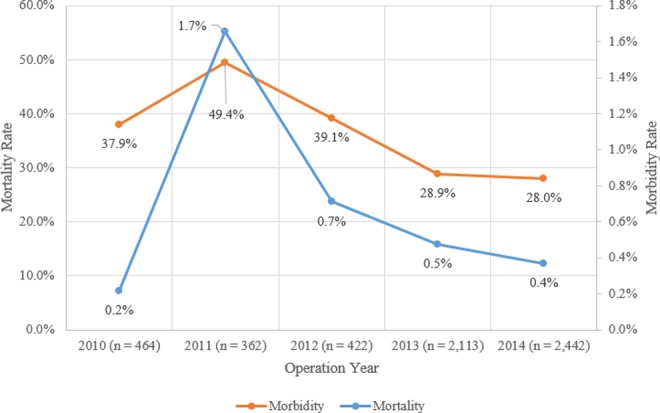
Morbidity and mortality versus operation year.
Postoperative morbidity was associated with increased rates of death (0.5%, P < .0001), hospital stay in the acute care setting >30 days (0.3%, P < .0001), unplanned reoperation (2.8%, P < .0001), and unplanned readmission (3.9%, P < .0001; Table 2). Univariate analysis identified multiple risk factors for morbidity and mortality that were assessed in the multivariate testing (Table 3 and 4).
Table 2.
Morbidity and Associated Patient Outcomes.a
| 30-Day Postoperative Complications | No Morbidity (N = 3989) | Morbidity (N = 1814) | P |
|---|---|---|---|
| Hospital stay (acute care setting) >30 days | 0.1% | 0.3% | <.0001 |
| Death | 0.03% | 0.5% | <.0001 |
| Unplanned reoperations (2011-2014); N = 5339 | 1.2% | 2.8% | <.0001 |
| Unplanned readmissions (2011-2014); N = 5339 | 2.3% | 3.9% | <.0001 |
aBoldface indicates significant P < .05.
Table 4.
Univariate Analysis of Mortality for ASD Surgery.a
| Characteristic | Total (N = 5803) | No Mortality (N = 5775) | Mortality (N = 29) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| <51 | 21.6% | 21.7% | 6.9% | .157 |
| 51-60 | 26.0% | 26.0% | 20.7% | |
| 61-70 | 31.7% | 31.7% | 37.9% | |
| 71-80 | 17.5% | 17.5% | 31.0% | |
| >80 | 3.2% | 3.2% | 3.5% | |
| Female | 59.0% | 48.3% | .240 | |
| Race | ||||
| African American | 7.6% | 7.6% | 3.5% | .212 |
| Caucasian | 81.1% | 81.2% | 75.9% | |
| Hispanic | 3.8% | 3.8% | 3.5% | |
| Otherb | 7.5% | 7.4% | 17.2% | |
| BMI, kg/m2 | ||||
| Nonobese | 58.1% | 55.2% | 58.1% | .840 |
| Obese class I (30.0-34.9) | 23.8% | 23.8% | 27.6% | |
| Obese class II (35.0-39.9) | 11.3% | 11.3% | 13.8% | |
| Obese class III (≥40) | 6.7% | 6.7% | 3.5% | |
| ASA 3 or 4 | 53.2% | 52.9% | 96.6% | <.0001 |
| Diabetes | ||||
| Nondiabetic | 85.4% | 85.5% | 69.0% | .028 |
| Insulin dependent | 4.7% | 4.7% | 6.9% | |
| Insulin independent | 9.9% | 9.8% | 24.1% | |
| Dyspnea | 6.0% | 6.0% | 3.5% | .564 |
| Dependent functional Health status prior to surgery | 4.6% | 4.5% | 13.8% | .012 |
| Steroid use | 3.8% | 3.8% | 6.9% | .376 |
| Recent weight loss | 0.2% | 0.2% | 3.5% | <.0001 |
| Comorbidities | ||||
| Ventilator dependent | 0.2% | 0.2% | 3.5% | <.0001 |
| COPD | 4.9% | 4.9% | 13.8% | .027 |
| CHF | 0.4% | 0.4% | 3.5% | .011 |
| Hypertension requiring medication | 54.5% | 54.4% | 69.0% | .117 |
| Renal failure | 0.1% | 0.1% | 0.0% | .851 |
| Dialysis Use | 0.3% | 0.3% | 3.5% | .004 |
| Bleeding disorder | 1.2% | 1.2% | 0.0% | .551 |
| Preoperative blood transfusion | 0.2% | 0.2% | 0.0% | .798 |
| Operative variables | ||||
| Operative time >4 hours | 47.9% | 47.8% | 72.4% | .008 |
| Total RVU, mean (SD) | 73.4 (28.8) | 73.3 (28.7) | 93.0 (33.8) | .0002 |
| Inpatient | 96.4% | 96.4% | 100.0% | .294 |
| Procedure length | ||||
| Long | 63.0% | 62.9% | 93.1% | .001 |
| Short (≤3 levels) | 37.0% | 6.9% | 37.1% | |
| Procedure approach | ||||
| Anterior | 39.6% | 39.7% | 20.7% | .042 |
| Posterior | 56.7% | 56.6% | 79.3% | |
| Combined | 3.7% | 3.7% | 0.0% | |
| Procedure subtypes | ||||
| Insertion of intervertebral device | 41.5% | 41.6% | 37.9% | .693 |
| Osteotomy | 14.4% | 14.4% | 17.2% | .665 |
| Bone grafting | 69.8% | 69.8% | 58.6% | .190 |
| Fusion to pelvis | 8.3% | 8.3% | 17.2% | .0805 |
Abbreviations: ASD, adult spinal deformity; BMI, body mass index; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; RVU, relative value unit.
aBoldface indicates significant at P < .05.
bOther race includes Native Hawaiian, Pacific Islander, Asian, American Indian, or Alaska Native.
The multivariate analysis showed that female sex (OR = 1.3, P = .0001), long fusion (OR = 1.3, P = .002), posterior fusion (OR = 3.6, P < .0001), combined approach (OR = 3.3, P < .0001), pelvic fusion (OR = 1.9, P < .0001), osteotomy (OR = 2.1, P < .0001), inpatient (OR = 3.5, P = .0003), age >60 (P < .0001), insulin-independent diabetes (OR = 1.3, P = .040), dependent functional status (OR = 1.9, P < .0001), bleeding disorder (OR = 2.2, P = .008), American Society of Anesthesiologists (ASA) class ≥3 (OR = 1.2, P = .003), and operative time >4 hours (OR = 3.5, P < .0001) were statistically significant predictors of morbidity (Table 5). The model had good discrimination and calibration with a C-statistic of .83 and a Hosmer-Lemeshow goodness-of-fit test P value equal to .276. Given the small number of deaths, the variable adjustment with multivariable analysis was unable to be statistically supported.
Table 5.
Independent Risk Factors for Morbidity Identified by Multivariate Logistic Regression.a
| Effect | Estimate | 95% Confidence Limits | P |
|---|---|---|---|
| Total RVU | 1.0 | 1.0−1.0 | .001 |
| Female | 1.3 | 1.1−1.5 | .0001 |
| Fusion length: long vs short (≤3 levels) | 1.3 | 1.1−1.5 | .002 |
| Fusion approach | |||
| Combined vs anterior | 3.3 | 2.3−4.7 | <.0001 |
| Posterior vs anterior | 3.6 | 3.0−4.3 | <.0001 |
| Other surgery | |||
| Pelvic fusion | 1.9 | 1.5−2.5 | <.0001 |
| Osteotomy | 2.1 | 1.7−2.6 | <.0001 |
| Inpatient vs outpatient | 3.5 | 1.8−7.0 | .0003 |
| Age (Reference: ≤50 years) | |||
| 51−60 | 1.2 | 1.0−1.5 | .092 |
| 61−70 | 1.5 | 1.2−1.8 | <.0001 |
| 71−80 | 1.9 | 1.5−2.4 | <.0001 |
| >80 | 2.6 | 1.8−3.9 | <.0001 |
| Diabetes (Reference: None) | |||
| Insulin-dependent | 1.3 | 1.0−1.8 | .074 |
| Insulin-independent | 1.3 | 1.0−1.6 | .040 |
| Dependent functional status | 1.9 | 1.4−2.5 | <.0001 |
| Bleeding disorder | 2.2 | 1.2−3.8 | .008 |
| ASA ≥3 | 1.2 | 1.1−1.4 | .003 |
| Operative time >4 hours | 3.5 | 3.0−4.0 | <.0001 |
Abbreviations: RVU, relative value unit; ASD, adult spinal deformity.
aBoldface indicates P < .05.
Discussion
Surgical correction for ASD is a challenging procedure that is known to carry substantial risk for perioperative complications, many of which culminate into major patient and financial consequences.8,16–18 As such, it is relevant to provide a thorough analysis of what may predict poor outcomes in this population. The current study leveraged the unique capability of the NSQIP database to examine the incidence, impact, and risk factors for 30-day complications in ASD surgery. This national surgical sample provided the power to study short-term complications at a scale that was not possible in prior works that relied on single-surgeon and single-center case series.6,16,19–21 Furthermore, the NSQIP permitted the use of functional outcome data, including 30-day morbidity and mortality, at a level of detail that is not available in other registries.24
In this study, the incidence of morbidity and mortality for ASD surgery was 31.3% and 0.5%, respectively. These rates are in agreement with prior works on adult scoliosis surgery.2,17,25,26 Furthermore, the current study demonstrated a noticeable peak for both morbidity and mortality in 2011 (Figure 1). When investigating the subset complications by year, pulmonary complications, venous thromboembolism, and blood transfusion rates were highest in 2011. However, the NSQIP does not state any alteration of the data collection methodology that might reflect the changes observed during this year. It is important to note that the peak observed in the morbidity graph is within 1 standard deviation of the mean morbidity rate, which suggests that the morbidity peak is a common cause variation. The high mortality rate observed in 2011 may be an artifact of the relatively fewer number of patients prior to 2013. Additionally, the study’s results confirmed that morbidity was associated with a higher incidence of other postoperative adverse outcomes, including unplanned readmission and reoperations. These findings suggest that an increasing understanding of the risks for morbidity can be pivotal for improving other surgical outcomes in these patients.
This study identified several independent risk factors for the development of morbidity within 30 days of surgery. Many of these factors, including age, ASA ≥3, dependent functional status, operative time >4 hours, and bleeding disorder have been highlighted in previous works.27–29 In this analysis, operative time >4 hours was associated with the highest risk for morbidity (OR = 3.5, P < .0001). Extended procedural time is often reflective of case complexity and is known to be associated with increased exposure to bacteria.30 In a prior work, Daley et al used NSQIP to study 104 632 cases, including spine, and demonstrated that the risk of complications increased with every additional hour of operative time and markedly increased after 2 hours in the operating room.31 Other research have reported that serum concentrations of antibiotics, which is typically administered at the time of anesthesia induction, rapidly decreases to less than therapeutic levels during the course of surgery.32,33 In future strategies aimed at reducing the risk of complications, particularly wound infections, it may be advantageous to consider the pharmacokinetics of antibiotic treatment as well as expeditious surgical technique.
Spinal fusion is known to be among the top procedures most associated with blood loss.34 Since blood transfusions are known to carry a potential risk for immunological reactions and infections, attention toward hematological deficiencies in the preoperative period is important.35 In our study, nearly 27.0% of patients required at least one unit of packed or whole red blood cells given from the surgical start time up to and including 72 hours postoperatively. Interestingly, patients with a bleeding disorder (OR = 2.2, P = .008) were significantly more likely to have a morbid outcome. These results suggest that preoperative hematological status must be closely monitored prior to ASD surgery. Potential perioperative interventions to limit blood loss may include better patient positioning, reduced operative time, and minimally invasive surgery when possible.36
Given the paucity of literature that compares approach-related consequences of spinal fusion surgery, there continues to be debate about which fusion approach (anterior vs posterior) may be superior. For instance, Pradhan et al investigated 122 patients and found that an anterior approach to single-level lumbar fusion was associated with less morbidity than the posterolateral approach.37 However, a retrospective analysis by Geck et al analyzed 62 patients and found that patients who were treated with a posterior lumbar instrumentation and fusion had better outcomes and shorter length of stay than those who received an anterior approach.38 The current analysis demonstrates that either combined or posterior fusions was associated with more than a 3-fold increased risk for postoperative morbidity compared to anterior fusions. In comparison to the posterior approach, anterior fusion is argued to have less blood loss, no disturbance of paraspinal muscles, improved access to the disk space, lower risk of neurologic injury, and shorter operating times.39–42 Studies on this topic have also found that the use of instrumentation, fusion to pelvis, and other procedures with increased complexity are associated with a higher risk of infection.23,43,44 Both fusion to pelvis and osteotomies were statistically significant in the multivariate analyses in our series.
The present study identified that both non–insulin-dependent diabetes and insulin-dependent diabetes to be significantly associated with morbidity and mortality; however, only non–insulin-dependent diabetes was found to be an independent risk factor for early complications after ASD. Given its pervasive impact on multiple organ systems (eg, cardiac, renal, nervous), diabetes is known to adversely affect outcomes in several surgical specialties. This is likely due to the increased hematological and wound healing abnormalities associated with diabetes, which puts these patients at greater risk for postsurgical morbidity.45–47
Patient safety is especially important in ASD surgery as the complication rates are high and deaths still occur. Research suggests that hospitals may be able to more meaningfully improve outcomes by utilizing tools that leverage the patient’s unique circumstances.48 Alternatively, literature has demonstrated the utility of intraoperative checklists in order to avoid adverse events in surgery.49 Although it is clear that avoiding complications is important, it may be equally important to focus on improving patient care once complications have occurred.50 For example, the Surviving Sepsis Campaign, an initiative designed to enhance early identification and management of patients with suspected sepsis, has been credited for achieving significant reductions in mortality. Timely recognition of adverse events, effective postoperative management, as well as preoperative risk assessments will be essential for reducing death and morbidity in ASD surgery.
There are several limitations to this study that must be appreciated. NSQIP is designed to capture a wide variety of surgical operations; therefore, the variables are generic in nature and limit a more in-depth analysis on specific conditions within spine research. Due to privacy and resource constraints, certain patient-specific factors were not accounted for in the NSQIP database. The lack of data can hinder the ability to detect important confounding variables and reduce the quality of regression models. Although this is a nationwide sample, data comes from quality-seeking institutions; therefore, it is possible that a disparity in outcomes exists at other institutions not in ACS-NSQIP.26 As such, this limitation may affect the generalizability of the study’s conclusion across the nation.51 Given the limited nature of the NSQIP database, this study focuses only on the short-term complications; however, it is equally important to consider the long-term ramifications of ASD surgery. Finally, this study’s findings generate hypotheses about how ASD outcomes might be improved, yet the challenge remains how to implement these findings and others into best practices effectively across different patient populations. More research needs to be conducted to further understand the nature and clinical utility of these connections between the identified risk factors and outcomes.
Despite these shortcomings, the results of this study may guide the development of future strategies aimed at improving the quality of care, and perhaps assist providers in efficiently allocating resources where impact may be greatest needed in ASD patients.
Supplementary Material
Footnotes
Authors’ Note: This study was qualified as exempt by the Mount Sinai Hospital Institutional Review Board. The article does not contain information about medical device(s)/drug(s).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online supplemental materials are available at http://journals.sagepub.com/doi/suppl/10.1177/2192568217699384.
References
- 1. Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–948. [DOI] [PubMed] [Google Scholar]
- 2. Yadla S, Maltenfort MG, Ratliff JK, Harrop JS. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus. 2010;28(3):E3. [DOI] [PubMed] [Google Scholar]
- 3. Youssef JA, Orndorff DO, Patty CA, et al. Current status of adult spinal deformity. Global Spine J. 2013;3:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kostuik JP, Bentivoglio J. The incidence of low back pain in adult scoliosis. Acta Orthop Belg. 1981;47:548–559. [PubMed] [Google Scholar]
- 5. Perennou D, Marcelli C, Herisson C, Simon L. Adult lumbar scoliosis. Epidemiologic aspects in a low-back pain population. Spine (Phila Pa 1976). 1994;19:123–128. [DOI] [PubMed] [Google Scholar]
- 6. Bradford DS, Tay BK, Hu SS. Adult scoliosis: surgical indications, operative management, complications, and outcomes. Spine (Phila Pa 1976). 1999;24:2617–2629. [DOI] [PubMed] [Google Scholar]
- 7. Jackson RP, Simmons EH, Stripinis D. Incidence and severity of back pain in adult idiopathic scoliosis. Spine (Phila Pa 1976). 1983;8:749–756. [DOI] [PubMed] [Google Scholar]
- 8. Ong KL, Auerbach JD, Lau E, Schmier J, Ochoa JA. Perioperative outcomes, complications, and costs associated with lumbar spinal fusion in older patients with spinal stenosis and spondylolisthesis. Neurosurg Focus. 2014;36(6):E5. [DOI] [PubMed] [Google Scholar]
- 9. Cowan JA, Jr, Dimick JB, Wainess R, Upchurch GR, Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59:15–20. [DOI] [PubMed] [Google Scholar]
- 10. Paulus MC, Kalantar SB, Radcliff K. Cost and value of spinal deformity surgery. Spine (Phila Pa 1976). 2014;39:388–393. [DOI] [PubMed] [Google Scholar]
- 11. Ali RM, Boachie-Adjei O, Rawlins BA. Functional and radiographic outcomes after surgery for adult scoliosis using third-generation instrumentation techniques. Spine (Phila Pa 1976). 2003;28:1163–1169. [DOI] [PubMed] [Google Scholar]
- 12. Hu SS, Berven SH. Preparing the adult deformity patient for spinal surgery. Spine (Phila Pa 1976). 2006;31(19 suppl):S126–S131. [DOI] [PubMed] [Google Scholar]
- 13. Smith JS, Shaffrey CI, Glassman SD, et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa 1976). 2011;36:817–824. [DOI] [PubMed] [Google Scholar]
- 14. Dickson JH, Mirkovic S, Noble PC, Nalty T, Erwin WD. Results of operative treatment of idiopathic scoliosis in adults. J Bone Joint Surg Am. 1995;77:513–523. [DOI] [PubMed] [Google Scholar]
- 15. Cho KJ, Suk SI, Park SR, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2007;32:2232–2237. [DOI] [PubMed] [Google Scholar]
- 16. Bhagat S, Vozar V, Lutchman L, Crawford RJ, Rai AS. Morbidity and mortality in adult spinal deformity surgery: Norwich Spinal Unit experience. Eur Spine J. 2013;22(suppl 1):S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sansur CA, Smith JS, Coe JD, et al. Scoliosis research society morbidity and mortality of adult scoliosis surgery. Spine (Phila Pa 1976). 2011;36:E593–E597. [DOI] [PubMed] [Google Scholar]
- 18. Glassman SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976). 2007;32:2764–2770. [DOI] [PubMed] [Google Scholar]
- 19. Edwards CC, 2nd, Bridwell KH, Patel A, Rinella AS, Berra A, Lenke LG. Long adult deformity fusions to L5 and the sacrum. A matched cohort analysis. Spine (Phila Pa 1976). 2004;29:1996–2005. [DOI] [PubMed] [Google Scholar]
- 20. Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976). 1995;20:1592–1599. [DOI] [PubMed] [Google Scholar]
- 21. McDonnell MF, Glassman SD, Dimar JR, 2nd, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78:839–847. [DOI] [PubMed] [Google Scholar]
- 22. American College of Surgeons. User guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Accessed March 11, 2017.
- 23. Martin CT, Pugely AJ, Gao Y, Ilgenfritz RM, Weinstein SL. Incidence and risk factors for early wound complications after spinal arthrodesis in children: analysis of 30-day follow-up data from the ACS-NSQIP. Spine (Phila Pa 1976). 2014;39:1463–1470. [DOI] [PubMed] [Google Scholar]
- 24. Enomoto LM, Hollenbeak CS, Bhayani NH, Dillon PW, Gusani NJ. Measuring surgical quality: a national clinical registry versus administrative claims data. J Gastrointest Surg. 2014;18:1416–1422. [DOI] [PubMed] [Google Scholar]
- 25. Schoenfeld AJ, Carey PA, Cleveland AW, 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13:1171–1179. [DOI] [PubMed] [Google Scholar]
- 26. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. [DOI] [PubMed] [Google Scholar]
- 27. Carreon LY, Puno RM, Dimar JR, 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85:2089–2092. [DOI] [PubMed] [Google Scholar]
- 28. Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34:1869–1872. [DOI] [PubMed] [Google Scholar]
- 29. Tang H, Zhu J, Ji F, Wang S, Xie Y, Fei H. Risk factors for postoperative complication after spinal fusion and instrumentation in degenerative lumbar scoliosis patients. J Orthop Surg Res. 2014;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaudhary SB, Vives MJ, Basra SK, Reiter MF. Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med. 2007;30:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg. 2015;220:550–558. [DOI] [PubMed] [Google Scholar]
- 32. Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg. 1996;131:1165–1171. [DOI] [PubMed] [Google Scholar]
- 33. Polly DW, Jr, Meter JJ, Brueckner R, Asplund L, van Dam BE. The effect of intraoperative blood loss on serum cefazolin level in patients undergoing instrumented spinal fusion. A prospective, controlled study. Spine (Phila Pa 1976). 1996;21:2363–2367. [DOI] [PubMed] [Google Scholar]
- 34. Segal JB, Guallar E, Powe NR. Autologous blood transfusion in the United States: clinical and nonclinical determinants of use. Transfusion. 2001;41:1539–1547. [DOI] [PubMed] [Google Scholar]
- 35. Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. 2015;84:780–785. [DOI] [PubMed] [Google Scholar]
- 36. Stirling A. Patient safety in adult spinal deformity surgery. J Trauma Orthop. 2014;2(4):48–49. [Google Scholar]
- 37. Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002;15:355–361. [DOI] [PubMed] [Google Scholar]
- 38. Geck MJ, Rinella A, Hawthorne D, et al. Comparison of surgical treatment in Lenke 5C adolescent idiopathic scoliosis: anterior dual rod versus posterior pedicle fixation surgery: a comparison of two practices. Spine (Phila Pa 1976). 2009;34:1942–1951. [DOI] [PubMed] [Google Scholar]
- 39. Burke PJ. Anterior lumbar interbody fusion. Radiol Technol. 2001;72:423–430. [PubMed] [Google Scholar]
- 40. Strube P, Hoff E, Hartwig T, Perka CF, Gross C, Putzier M. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J Spinal Disord Tech. 2012;25:362–369. [DOI] [PubMed] [Google Scholar]
- 41. Kim JS, Kim DH, Lee SH, et al. Comparison study of the instrumented circumferential fusion with instrumented anterior lumbar interbody fusion as a surgical procedure for adult low-grade isthmic spondylolisthesis. World Neurosurg. 2010;73:565–571. [DOI] [PubMed] [Google Scholar]
- 42. Greenough CG, Taylor LJ, Fraser RD. Anterior lumbar fusion: results, assessment techniques and prognostic factors. Eur Spine J. 1994;3:225–230. [DOI] [PubMed] [Google Scholar]
- 43. Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976). 2006;31:2329–2336. [DOI] [PubMed] [Google Scholar]
- 44. Smorgick Y, Park DK, Baker KC, et al. Single- versus multilevel fusion for single-level degenerative spondylolisthesis and multilevel lumbar stenosis: four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2013;38:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wukich DK. Diabetes and its negative impact on outcomes in orthopaedic surgery. World J Orthop. 2015;6:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin C, Kim JY, Hsu WK. Impact of insulin dependence on lumbar surgery outcomes: an NSQIP analysis of 51,277 patients. Spine (Phila Pa 1976). 2016;41:E687–E693. [DOI] [PubMed] [Google Scholar]
- 47. Jones RL, Peterson CM. Hematologic alterations in diabetes mellitus. Am J Med. 1981;70:339–352. [DOI] [PubMed] [Google Scholar]
- 48. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44:251–267. [DOI] [PubMed] [Google Scholar]
- 49. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. [DOI] [PubMed] [Google Scholar]
- 50. Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. [DOI] [PubMed] [Google Scholar]
- 51. ACS NSQIP hospitals significantly improve outcomes over time. Bull Am Coll Surg. 2015;100(5):64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


