Abstract
Study Design:
Systematic review and meta-analysis.
Objective:
Current surgical management of degenerative spondylolisthesis (DS) involves decompression of the spinal canal followed by fusion with or without interbody. The additional functional and operative benefits derived from interbody inclusion has yet to be thoroughly established with a number of recent studies producing conflicting results. Thus, we aim to compare the functional and operative outcomes after fusion against interbody fusion in the treatment of DS.
Methods:
This systematic review of the literature comparing posterolateral fusion (PLF) and posterior lumbar interbody fusion (PLIF) outcomes in the treatment of DS was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Electronic searches of 6 databases yielded 386 articles from database inception to July 2016, which were screening against established criteria for inclusion into this study.
Results:
A total of 6 studies, satisfied criteria and reported outcomes for 721 patients. Fusion alone was performed in 458 (63.5%) patients and interbody fusion was performed in 263 (36.5%) patients. Functional outcomes Oswestry Disability Index (P = .29) and visual analog scale (P = .13) were not statistically different between the 2 approaches. Furthermore, there was no significant inferiority between fusion alone and with interbody in terms of the operative outcomes of blood loss (P = .38), reoperation rate (P = .66), hospital stay (P = .96), complication rate (P = .78), or fusion rate (P = .15).
Conclusions:
There was no statistically significant difference in functional and operative outcomes following fusion alone versus with interbody. Additional subgroup analysis of intrinsic DS features in future large, prospective, randomized controlled trials will improve the validity of these findings.
Keywords: spondylolisthesis, degenerative spondylolisthesis, posterolateral fusion, interbody fusion, systematic review, spine
Introduction
Degenerative spondylolisthesis (DS) is the anterior slip of a superior vertebral body relative to the vertebra below, due to degenerative processes involving the vertebrae and intervertebral discs (IVDs). DS can result in instability, facet arthritis, spinal stenosis, or nerve root compression, leading to clinical symptoms such as lower back pain (LBP), radiculopathy, and neurogenic claudication.1 There are several conservative options for treatment, including physiotherapy, steroid injections, and so on. Where conservative management fails, surgery may be indicated. Currently, surgical management involves decompression only, which may potentially destabilize the spine, or decompression followed by fusion to prevent further destabilization.2,3 There is controversy whether decompression alone is sufficient or additional fusion (posterolateral fusion [PLF]) is required with 2 recent randomized trials showing diametrically opposite conclusions.4
There are a variety of techniques used for fusion for DS; however, the optimal approach remains controversial.5–7 PLF utilizes bone graft (autograft or allograft) laid over the posterolateral region (between transverse processes and over the intertransverse membrane and adjacent facet joint with or without instrumentation). PLF alone has been used widely for fusion in DS procedures.1 However, the addition of interbody fusion, through either posterior lumbar interbody fusion (PLIF) or transforaminal interbody fusion (TLIF), has several theoretical advantages.8–10 PLIF/TLIF fuses the anterior column, which bears the majority of weight, thus its addition can increase the rate of fusion and relieve strain from the PLF instrumentation whilst indirectly achieving foraminal decompression.8–10 Furthermore, as interbody fusion replaces the IVD with bone graft and/or a cage, LBP, which may be caused by the degenerating disc is alleviated.11,12 However, recent clinical studies comparing PLF against PLF with the addition of PLIF/TLIF has produced conflicting results.13–18 The present systematic review and meta-analysis aims to compare a range of functional and operative outcomes, including clinical improvement, fusion and complication rates for PLF versus PLF with the addition of PLIF/TLIF in the treatment of DS.
Methods
Literature Search Strategy
This study was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19 Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), American College of Physicians Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to July 2016. To achieve maximum sensitivity of the search strategy and identify all studies, we combined the terms: “degenerative,” “spondylolisthesis,” “interbody fusion,” and “posterolateral fusion,” as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection Criteria
Eligible comparative studies for the present systematic review and meta-analysis included those in which patient cohorts underwent PLF alone compared to PLF with PLIF or TLIF. Only studies including degenerative spondylolisthesis patients were included. Studies which included isthmic spondylolisthesis or had mixed populations were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data Extraction and Critical Appraisal
All data was extracted from article texts, tables, and figures. Two investigators independently reviewed each retrieved article (R.C., K.P.). Discrepancies between the 2 reviewers were resolved by discussion and consensus. Assessment of risk of bias for each selected study was performed according to the most updated Cochrane statement.
Statistical Analysis
The statistics have been described elsewhere.20–25 Briefly, the mean difference (MD) and relative risk (RR) were used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I 2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I 2 can be calculated as: I 2 = 100% × (Q – df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degrees of freedom. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. Sensitivity leave-one out analysis was also performed. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible because raw data was not available. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.3.2 (Cochrane Collaboration, Software Update, Oxford, UK).
Results
Identified Studies and Patient Characteristics
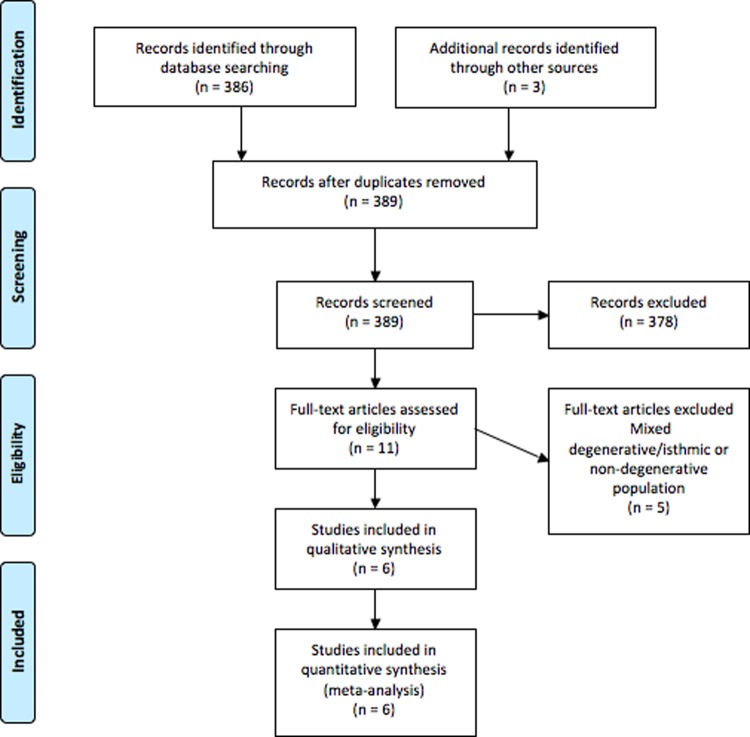
A total of 386 references were identified through searches of 6 electronic databases (Figure 1). After exclusion of duplicated and irrelevant references, 11 studies were assessed against the eligibility criteria. Six studies that satisfied the criteria were then analyzed.13–18 Interrater agreement was excellent for determining eligibility of titles and abstracts (κ = 0.92) and full-text studies (κ = 0.87). This included 721 patients who had undergone either a PLF alone or a PLF with additional interbody fusion. There were 2 prospective cohort studies13,18 and 4 retrospective cohort studies.14–17 The studies included focused only on patients treated for DS.13–18 The average age of patients was 64.6 years, with 59.9% (n = 432) females and 40.1% (n = 289) males.13–18 The studies reported a mean follow-up period of 41.4 months.13–18 The PLF alone group consisted of 63.5% (n = 458) of the patients, while 36.5% (n = 263) underwent additional interbody fusion. A summary of these study characteristics is presented in Table 1.13–18 The use of instrumentation and definition of instability as per study is summarized in Supplemental Table S1 (available in the online version of the article).
Figure 1.
PRISMA search strategy for the present systematic review and meta-analysis.
Table 1.
Study Characteristics.
| First Author, Year | Study Design | Period | Country | n | Female, n | Average Age (Years) | Follow-up Period (Months) | Indication | PLF, n | PLIF/TLIF, n | PLIF or TLIF | Functional Outcomes Reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdu, 2009 | Prospective cohort | 2000-2005 | USA | 356 | 244/356 | 64.4 | 48 | DS | 293 | 63 | Both | ODI, SF-36 BP, SF-36 PF |
| Fujimori, 2014 | Retrospective cohort | 2006-2011 | USA | 56 | 39/56 | 60.1 | 23 | DS | 32 | 24 | TLIF | ODI, VAS, SF-12 PCS, SF-12 MCS |
| Gottschalk, 2015 | Retrospective cohort | 2004-2012 | USA | 179 | 82/179 | 66.8 | 39 | DS | 68 | 111 | NR | ODI, SF-36 PCS, SF-36 MCS, VAS |
| Ha, 2008 | Retrospective cohort | 1992-2001 | Korea | 40 | 29/40 | 57.8 | 53 | DS | 21 | 19 | PLIF | ODI, VAS |
| Kuraishi, 2016 | Retrospective cohort | 2008-2010 | Japan | 31 | 13/31 | 69.5 | 32 | DS | 12 | 19 | PLIF | JOA |
| Sivaraman, 2015 | Prospective cohort | NR | UK | 59 | 25/59 | 66 | 24 | DS | 32 | 27 | PLIF | VAS, SF-12 PH, SF-12 MH |
Abbreviations: NR, no result; ODI, Oswestry Disability Index; SF-36 Short Form–36; BP, bodily pain; PF, physical function; QALY, quality-adjusted life year; VAS, visual analog scale; PCS, physical component score; MCS, mental component score; DDD, degenerative disc disease; DS, degenerative spondylolisthesis; JOA, Japanese Orthopaedic Association
Assessment of Clinical Outcomes
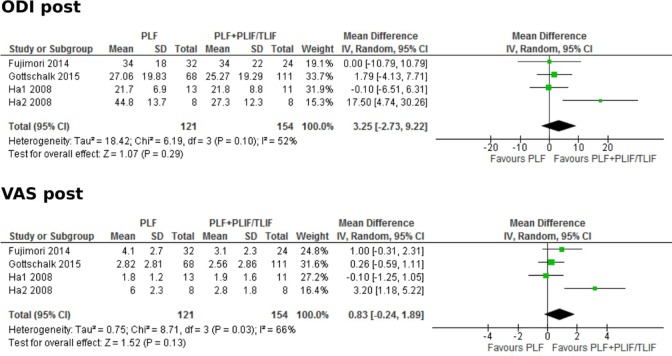
After pooling of results, Oswestry Disability Index (ODI) scores across articles that reported the standard deviation suggested a slight advantage in the PLF with PLIF/TLIF group (Figure 2).14–16 However, this was not statistically significant (MD, 3.25; 95% CI, −2.73 to 9.22; P = .29). Similarly, there was a nonstatistically significant advantage to the PLF with PLIF/TLIF group in the pooled results of postoperative visual analog scale (VAS) scores (MD, 0.83; 95% CI, −0.24 to 1.89; P = .13).14–16
Figure 2.
Forest plots comparing posterolateral fusion versus interbody fusion in terms of postoperative Oswestry Disasbility Index (ODI) and Visual Analog Scale (VAS).
Assessment of Postoperative Clinical Outcomes
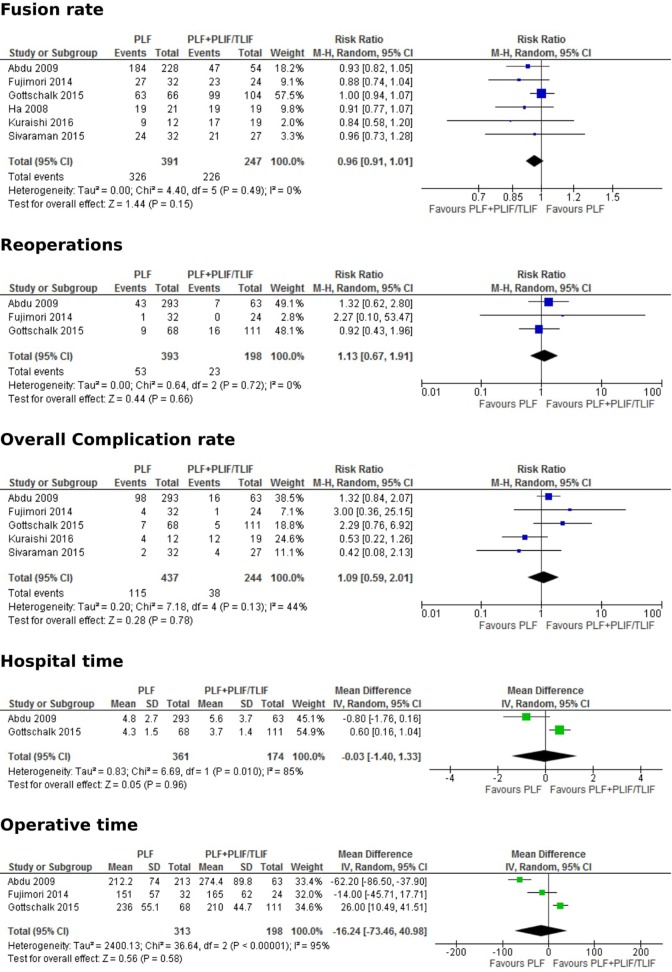
From fusion rates, which were reported in all 6 studies, there was shown to be a higher rate of fusion in the PLF with PLIF/TLIF group (Figure 3).13–18 However, this was not statistically significant (RR, 0.96; 95% CI, 0.91-1.01; P = .15). Similar results were seen in outcomes of reoperation rates (P = .66), hospital stay (P = .96), complication rate (P = .78), and blood loss (P = .38) (Figure 3). Operative time was lower in the PLF alone group; however, this was not statistically significant (MD, −16.24; 95% CI. −73.46 to 40.98; P = .58).13–15
Figure 3.
Forest plots comparing posterolateral fusion versus interbody fusion in terms of fusion rate, reoperations, overall complication rate, hospital time and operative time.
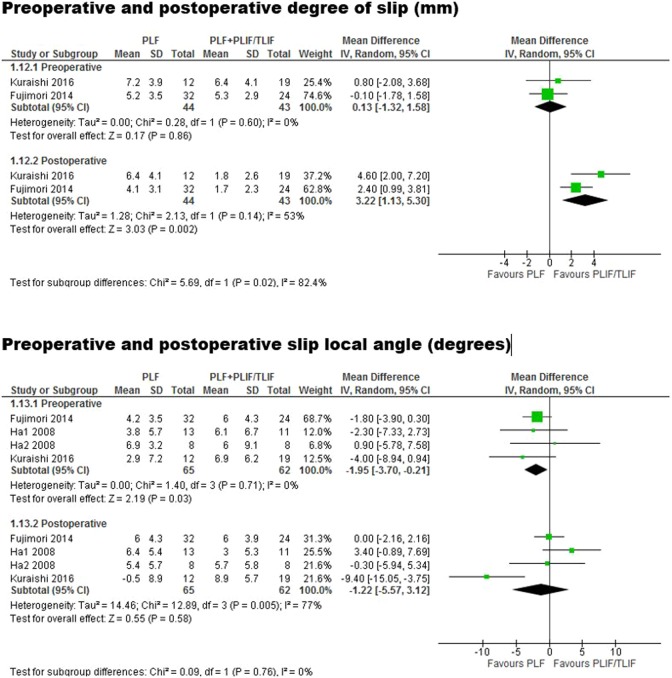
Assessment of Preoperative and Postoperative Degree of Slip
Preoperatively, there was no significant difference in the degree of slip (mm) between PLF versus PLIF/TLIF cohorts (weighted mean difference [WMD], 0.13; 95% CI, −1.32 to 1.58; I 2 = 0%; P = .86). Postoperative, the PLIF/TLIF cohort had a significantly lower degree of slip compared with PLF (WMD, 3.22; 95% CI, 1.13-5.30, I 2 = 53%; P = .002).
Regarding local angle, preoperatively this was smaller in the PLF group compared with PLIF/TLIF (WMD, −1.95; 95% CI, −3.70 to −0.21, I 2 = 0%, P = .03). However, after surgery, there was no significant difference between the 2 cohorts (WMD, −1.22; 95% CI, −5.57 to 3.12; I 2 = 77%; P = .58) (Figure 4).
Figure 4.
Forest plots comparing posterolateral fusion versus interbody fusion in terms of degree of slip and slip local angle.
Assessment of Bias Risk and Sensitivity Analysis
All included studies were evaluated for bias risk according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) criteria26 and the results are provided in Table 2. No articles presented significant risk of bias. Sensitivity analysis (leave-one out analysis) demonstrated no significant differences in the overall pooled effect size for complication rates, fusion rate, reoperations, postoperative ODI and postoperative VAS scores. There was only one exception, which was the removal of Gottschalk et al,15 which caused pooled results to show a marginally but significantly higher fusion rate in the PLIF/TLIF cohort versus PLF.
Table 2.
Assessment of the Quality of Included Studies by MOOSE Criteria.
| Abdu (2009) | Fujimori (2015) | Gottschalk (2015) | Ha (2008) | Kuraishi (2016) | Sivaraman (2015) | |
|---|---|---|---|---|---|---|
| Clear definition of study population | Yes | Yes | Yes | Yes | Yes | Yes |
| Clear definition of outcomes and outcome assessment | Yes | Yes | Yes | Yes | Yes | Yes |
| Independent assessment of outcome parameters | No | Yes | Yes | Yes | Unclear | Yes |
| Sufficient duration of follow-up | Yes | Yes | Yes | Yes | Yes | Yes |
| No selective loss during follow-up | Yes | Yes | Yes | Yes | Unclear | Yes |
| Important confounders and prognostic factors identified | Yes | Yes | Yes | Yes | Yes | No |
Discussion
DS frequently causes patients to undergo surgical decompression and fusion.2,3 However, the addition of interbody fusion to PLF has been debated and several retrospective cohort studies, prospective cohort studies and past meta-analyses have yielded conflicting results.13–18,27 Recently, a meta-analysis addressing the difference between PLF and PLF with additional PLIF/TLIF for DS was conducted by McAnany et al.27 However, 1 of the 5 studies included in this analysis involved 73% of patients with either degenerative disc disease, spinal stenosis, or failed back surgery. The remaining 27% of patients presented with spondylolisthesis, though it was not clarified if this was degenerative or isthmic. Thus, the outcome of the meta-analysis, which was focused on DS, was most likely affected by this inclusion. The current study addresses the question of whether additional interbody fusion is advantageous for the treatment of DS through an analysis of all available clinical and surgical data. In the current meta-analysis 6 studies, comprising 721 patients were identified and included. All patients had undergone decompression surgery followed by fusion through either a PLF alone (which was instrumented for the majority of patients) or a PLF combined with PLIF/TLIF13–18 and both their functional and operative outcomes were successfully compared.
Patient Outcomes
Similar patient outcomes have been reported by some authors between interbody and non-interbody fusions in DS treatment. Among a cohort of 56 patients produced by Fujimori et al,14 a similar improvement was seen as the mean postoperative ODI scores for the PLF and PLF with PLIF/TLIF groups were the same. Conversely, after dividing the cohort into stable and unstable groups based on the degree of slip, Ha et al16 found a significant difference between the fusion methods in the unstable group. This group had a higher average preoperative ODI than the stable group. Postoperatively though, this fell to 27.3 ± 12.3 in the PLF with interbody fusion group, but only to 44.8 ± 13.7 in the PLF alone group.16 A statistically significant advantage for the interbody group was also seen VAS scores.16 Thus, among patients with a greater degree of slip additional interbody fusion may be advantageous. However, this study is limited by its smaller sample size and a larger trial is required to confirm these findings. Also, there is controversy in defining instability in DS. Gottshalk et al15 also found postoperative ODI scores to be lower among the PLF with PLIF/TLIF group. Overall, postoperative ODI and VAS scores favored the PLF with PLIF/TLIF groups, though this was not statistically significant.
Operative Outcomes
The current study also analyzed variables such as complication, fusion, and subsequent surgery rates. Notably, the rate of fusion was found to be marginally higher in those who had additional interbody fusion.13–18 The addition of TLIF/PLIF fuses the anterior column as well as the posterior column, thus relieving the strain on posterior column structures, including the instrumentation involved in the PLF.8–10 Instrumentation failure could lead to a higher rate of reoperation.8–10 In a study comparing PLF with PLF and PLIF/TLIF for patients with either DS or isthmic spondylolisthesis, Macki et al28 found that all reoperations in the PLF group were due to instrumentation failure. However, in the current analysis focusing only on DS, there were similar rates of reoperation in both groups (RR, 1.13; 95% CI, 0.67-1.91; P = .66). Complications that may arise from spinal decompression and fusion include wound infection, deep venous thrombosis or hematoma.13–15,17,18,28–30 In the current analysis, the rates of complications were found to be similar in both groups.13–18 There was also no significant difference in the blood loss or length of hospitalization between each group. Finally, as would be expected, there was a longer operating time in the interbody fusion group, though this was not statistically significant and there was a high level of heterogeneity (I 2 = 95%).13–18
Degree of Slip and Instability
The definition of instability was broadly consistent among the included studies, defined as a slip >4 mm or local degree angle >10°. Our analysis demonstrated that despite baseline slip between similar between PLF and PLIF/TLIF groups, the interbody fusion group had a significantly lower slip postoperatively. Along the same lines, the PLIF/TLIF group had a more severe local degree angle slip compared with PLF at baseline, but postoperatively the groups were comparable. Collectively, these results suggest that interbody use may be efficacious in patients with a greater degree of slip.
Cost-Effectiveness
Currently, there is limited data with regard to the cost-effectiveness of each procedure. Gottshalk et al15 analyzed the direct surgical costs of undergoing additional interbody fusion, and found that it increased the costs. The cost increases ranged between $577 and $5276; however, this was not statistically significant.15 Conversely, Bydon et al31 analyzed the cost-effectiveness ratio of each group among a cohort of degenerative and isthmic spondylolisthesis patients. This was taken as the difference in cost between the interbody group and the PLF alone group, divided by the difference in quality-adjusted life year.31 They found that because of the higher reoperation rate in the PLF alone group, there were long-term cost savings in the group with additional interbody fusion.31 However, as the current analysis for DS showed there to be no difference in reoperation rates, this same conclusion cannot be substantiated.
Limitations
The current meta-analysis has several inherent limitations. The analysis was limited by the variety of measures that were inconsistently used across the included studies for reporting of variables such as patient outcomes. In this case, a variety of measures were used, including ODI, VAS, physical function, Japanese Orthopaedic Association (JOA) scores, or the presence and resolution of pain. Another major limitation is the assessment of instability in the included studies. The authors did not perform flexion-extension radiographs to assess degree of instability in patients with spondylolisthesis. Other radiographic parameters such as changes in disc height and facet angle were not reported.32 Other factors that have not been thoroughly tested and require further evidence include issues of facet angle and morphology, the presence of facet joint cyst with stenosis, degree of disc degeneration with canal stenosis, sagittal balance and the effect of prior intervention at the index level.33 Furthermore, a critical parameter in evaluating the superior approach is cost-effectiveness. However, this was inconsistently reported across the included studies and could not be analyzed due to the limited data. There is also heterogeneity across the studies owing to nonstandardized approaches in aspects such as the selection of bone graft material, varying operative, rehabilitation and hospitalization protocols and nonstandardized operative techniques. With regards to the degree of spondylolisthesis and instability, this was heterogeneous and poorly defined by the included studies. Future studies should focus specifically on the relative effectiveness of these 2 surgical approaches based on the degree and extent of slip and spondylolisthesis. Explicitly, the inherent patient selection bias is a product of poor or limited randomization. Finally, several of the included studies were retrospectively conducted, and were thus subject to bias.
Conclusion
Through the large sample size obtained by the pooling of results, the current data suggests that PLF with additional PLIF/TLIF does not have a significant effect on operative outcomes such as complication rates, reoperation rates, blood loss or hospitalization time. While there may be slight advantages in patient outcomes such as ODI and VAS as well as rates of fusion, results from the currently available literature indicate them to not be statistically significant. To confirm the current findings, a large randomized controlled trial in fusion techniques stratified according to degree of instability is required.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online data supplements are available at http://journals.sagepub.com/doi/suppl/10.1177/2192568217701103.
References
- 1. Koreckij TD, Fischgrund JS. Degenerative spondylolisthesis. J Spinal Disord Tech. 2015;28:236–241. doi:10.1097/BSD.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 2. Schroeder GD, Kepler CK, Kurd MF, et al. Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine (Phila Pa 1976). 2015;40:E1161–E1166. doi:10.1097/BRS.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 3. Steiger F, Becker HJ, Standaert CJ, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J. 2014;23:945–973. doi:10.1007/s00586-013-3144-3. [DOI] [PubMed] [Google Scholar]
- 4. Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–1434. doi:10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 5. Kleinstueck F, Fekete T, Mannion A, et al. To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J. 2012;21:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu VM, Kerezoudis P, Gilder HE, et al. Minimally invasive surgery versus open surgery spinal fusion for spondylolisthesis: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2017;42:E177–E185. doi:10.1097/BRS.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 7. Rao PJ, Ghent F, Phan K, Lee K, Reddy R, Mobbs RJ. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci. 2015;22:1619–1624. doi:10.1016/j.jocn.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 8. Fleischer GD, Hart D, Ferrara LA, Freeman AL, Avidano EE. Biomechanical effect of transforaminal lumbar interbody fusion and axial interbody threaded rod on range of motion and S1 screw loading in a destabilized L5-S1 spondylolisthesis model. Spine (Phila Pa 1976). 2014;39:E82–E88. doi:10.1097/BRS.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 9. McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976). 2005;30:S60–S65. [DOI] [PubMed] [Google Scholar]
- 10. Suk SI, Lee CK, Kim WJ, Lee JH, Cho KJ, Kim HG. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 1997;22:210–219. [DOI] [PubMed] [Google Scholar]
- 11. Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine (Phila Pa 1976). 2000;25:853–857. [DOI] [PubMed] [Google Scholar]
- 12. Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1995;20:356–361. [DOI] [PubMed] [Google Scholar]
- 13. Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? Four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2009;34:2351–2360. doi:10.1097/BRS.0b013e3181b8a829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujimori T, Le H, Schairer W, Berven S, Qamirani E, Hu S. Does transforaminal lumbar interbody fusion have advantages over posterolateral lumbar fusion for degenerative spondylolisthesis. Global Spine J. 2015;5:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottschalk MB, Premkumar A, Sweeney K, et al. Posterolateral lumbar arthrodesis with and without interbody arthrodesis for L4-L5 degenerative spondylolisthesis: a comparative value analysis. Spine (Phila Pa 1976). 2015;40:917–925. doi:10.1097/BRS.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 16. Ha KY, Na KH, Shin JH, Kim KW. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2008;21:229–234. doi:10.1097/BSD.0b013e3180eaa202. [DOI] [PubMed] [Google Scholar]
- 17. Kuraishi S, Takahashi J, Mukaiyama K, et al. Comparison of clinical and radiological results of posterolateral fusion and posterior lumbar interbody fusion in the treatment of L4 degenerative lumbar spondylolisthesis. Asian Spine J. 2016;10:143–152. doi:10.4184/asj.2016.10.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sivaraman A, Altaf F, Jalgaonkar A, et al. Prospective study of posterior lumbar interbody fusion with either interbody graft or interbody cage in the treatment of degenerative spondylolisthesis. J Spinal Disord Tech. 2015;28:E467–E471. doi:10.1097/BSD.0b013e31829baac1. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phan K, Scherman DB, Xu J, Leung V, Virk S, Mobbs RJ. Laminectomy and fusion vs laminoplasty for multi-level cervical myelopathy: a systematic review and meta-analysis. Eur Spine J. 2017;26:94–103. doi:10.1007/s00586-016-4671-5. [DOI] [PubMed] [Google Scholar]
- 21. Phan K, Leung V, Scherman DB, Tan AR, Rao PJ, Mobbs RJ. Bilateral versus unilateral instrumentation in spinal surgery: systematic review and trial sequential analysis of prospective studies. J Clin Neurosci. 2016;30:15–23. doi:10.1016/j.jocn.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 22. Phan K, Hogan JA, Mobbs RJ. Cost-utility of minimally invasive versus open transforaminal lumbar interbody fusion: systematic review and economic evaluation. Eur Spine J. 2015;24:2503–2513. doi:10.1007/s00586-015-4126-4. [DOI] [PubMed] [Google Scholar]
- 23. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion—systematic review and meta-analysis. Br J Neurosurg. 2015;29:705–711. doi:10.3109/02688697.2015.1036838. [DOI] [PubMed] [Google Scholar]
- 24. Phan K, Rao PJ, Kam AC, Mobbs RJ. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J. 2015;24:1017–1030. doi:10.1007/s00586-015-3903-4. [DOI] [PubMed] [Google Scholar]
- 25. Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg. 2015;1:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 27. McAnany SJ, Baird EO, Qureshi SA, Hecht AC, Heller JG, Anderson PA. Posterolateral fusion versus interbody fusion for degenerative spondylolisthesis: a systematic review and meta-analysis. 2016;41:E1408–E1414. doi:10.1097/BRS.0000000000001638. [DOI] [PubMed] [Google Scholar]
- 28. Macki M, Bydon M, Weingart R, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. doi:10.1016/j.clineuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 29. Høy K, Bünger C, Niederman B, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J. 2013;22:2022–2029. doi:10.1007/s00586-013-2760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, Tang H, Li Z, Zhang Q, Shi Z. Outcome of posterior lumbar interbody fusion versus posterolateral fusion in lumbar degenerative disease. J Clin Neurosci. 2011;18:780–783. doi:10.1016/j.jocn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 31. Bydon M, Macki M, Abt NB, et al. The cost-effectiveness of interbody fusions versus posterolateral fusions in 137 patients with lumbar spondylolisthesis. Spine J. 2015;15:492–498. doi:10.1016/j.spinee.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 32. Joaquim AF. Point of view: a randomized, controlled trial of fusion surgery for lumbar spinal stenosis—lessons learnt and practical considerations. J Spine Surg. 2016;2:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mobbs RJ, Phan K. Surgery for spinal stenosis: more thought, less metal? J Spine Surg. 2016;2:87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.