Abstract
Chronic liver injury of any etiology is the main trigger of fibrogenic responses and thought to be mediated by hepatic stellate cells. Herein, activating transcription factors like forkhead box f1 are described to stimulate pro-fibrogenic genes in hepatic stellate cells. By using a liver-specific siRNA delivery system (DBTC), we evaluated whether forkhead box f1 siRNA treatment exhibit beneficial effects in murine models of acute and chronic CCl4-induced liver injury. Systemic administration of DBTC-forkhead box f1 siRNA in mice was only sufficient to silence forkhead box f1 in acute CCl4 model, but was not able to attenuate liver injury as measured by liver enzymes and necrotic liver cell area. Therapeutic treatment of mice with DBTC-forkhead box f1 siRNA upon chronic CCl4 exposition failed to inhibit forkhead box f1 expression and hence lacked to diminish hepatic stellate cells activation or fibrosis development. As a conclusion, DBTC-forkhead box f1 siRNA reduced forkhead box f1 expression in a model of acute but not chronic toxic liver injury and showed no positive effects in either of these mice models.
Impact statement
As liver fibrosis is a worldwide health problem, antifibrotic therapeutic strategies are urgently needed. Therefore, further developments of new technologies including validation in different experimental models of liver disease are essential. Since activation of hepatic stellate cells is a key event upon liver injury, the activating transcription factor forkhead box f1 (Foxf1) represents a potential target gene. Previously, we evaluated Foxf1 silencing by a liver-specific siRNA delivery system (DBTC), exerting beneficial effects in cholestasis. The present study was designed to confirm the therapeutic potential of Foxf1 siRNA in models of acute and chronic CCl4-induced liver injury. DBTC-Foxf1 siRNA was only sufficient to silence Foxf1 in acute CCl4 model and did not ameliorate liver injury or fibrogenesis. This underlines the significance of the experimental model used. Each model displays specific characteristics in the pathogenic nature, time course and severity of fibrosis and the optimal time point for starting a therapy.
Keywords: Liver disease, lipoplex, mouse, gene silencing, liver fibrosis, hepatic stellate cells
Introduction
Beside metabolic, inflammatory, parasitic, viral and vascular stimuli, exposure to toxins or drugs such as pharmaceuticals, herbals, food, and supplements may also lead to hepatic damage.1 Depending on the duration of the insult, this damage may range from acute hepatic failure to chronic hepatic injury or fibrosis, which often progresses to cirrhosis.
The fibrogenic process is a complex reaction, accompanied by defined molecular and cellular changes. At the cellular level, activation and transformation of hepatic stellate cells (HSC) to pro-fibrogenic myofibroblasts as well as activation of portal fibroblasts are key events upon liver injury, which result in deposition of large amounts of extracellular matrix (ECM) components within the liver.2,3 Cell tracking methods using the Cre/loxP recombination system under control of HSC-specific promoters (GFAP, vimentin) allow new insights into molecular biological mechanisms of HSC activation and hence reveal new potentially therapeutic target genes.4,5 Thus, targeted manipulation of HSC by specific deletion/inhibition or overexpression of pro- and antifibrotic genes or even induction of apoptosis or deactivation of HSC6,7 are still major goals for the treatment of liver fibrosis. To date, different strategies have been developed to manipulate gene expression specifically in quiescent or even activated HSC, e.g. by using cell-specific promoters8,9 or siRNA.10
To translate the current knowledge of potential target genes and mechanisms into human therapy, the development of specific and effective delivery systems to the liver as well as drug targeting without evoking systemic side effects are urgently needed. For this purpose, different viral11 and non-viral10,12 systems coupled with ligands which specifically bind to receptors solely expressed on HSC, like PDGF-Rβ,13 p75 neurotrophin receptor11 or retinol-binding protein (RBP) receptor14 are qualified for a HSC-selective gene or drug delivery.15 In this way, various studies have focused on the inhibition of the activation or proliferation of HSC,10 promotion of HSC apoptosis16,17 or inhibition of ECM deposition.18
Previously, we have shown that the lipid-based system DBTC has the ability to deliver siRNA specifically into the liver, including HSC.10 Molecular events associated with HSC activation are attractive targets for antifibrotic therapy. Thus, we successfully used this siRNA delivery technique to inhibit the expression of the transcription factor forkhead box f1 (Foxf1), being exclusively expressed in HSC.19 Experiments in Foxf1 haploinsufficient animals discovered that Foxf1 functions as an “activating” transcription factor for HSC transdifferentiation, as livers of these mice showed reduced expression of collagen 1α and the HSC activation marker alpha smooth muscle actin (αSMA).20 In our previous study, we could demonstrate that Foxf1 silencing by DBTC siRNA formulations was accompanied with a defective HSC activation process in vitro and in vivo, effected proliferation and contractility of HSC as well as attenuated progression of cholestatic liver fibrosis.10 Based on this, in the here presented study, we additionally investigated the therapeutic potential of Foxf1 silencing in models of acute and chronic CCl4-induced liver injury.
Materials and methods
Foxf1 DBTC lipoplexes
DBTC is a liver-specific siRNA delivery system developed by Silence Therapeutics GmbH (Berlin). DBTC Foxf1 formulation is described in Abshagen et al.10
Mice
Male Balb/c mice (Charles River Laboratories) at an age of 12 to 16 weeks and a body weight of ∼30 g were kept on standard pellet food and water ad libitum with a 12 h day-and-night-cycle. Animals were anesthetized by an intraperitoneal injection of ketamine (90 mg/kg bw) and xylazine (25 mg/kg bw) and placed on a warming pad to maintain the body temperature at 37℃. After blood sampling for liver enzyme analysis, liver was fixed in 4% saline-buffered formalin or frozen at −80℃ for histological and mRNA analysis. All experiments were performed according to approved protocols and in compliance with the guidelines of the local government of Mecklenburg-Vorpommern (LALLF M-V/TSD/7221.3-11-062/12) and conducted in accordance with the German legislation on protection of animals and the National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’ (Institute of Laboratory Animal Resources, National Research Council).
Liver damage models and experimental groups
To induce acute liver damage, mice received a single-dose CCl4 intraperitoneally (1:20 in corn oil; 0.5 μl/g bw). Under inhalation anesthesia with isoflurane (1.5 vol%), mice were injected intravenously via the jugular vein with DBTC-Foxf1 siRNA (2.3 mg/kg bw; 0.1 ml/10 g body wt) (n = 9) 48 h prior to and 24 h after CCl4 administration. Control animals received equivalent volumes of DBTC-Luci siRNA (n = 8) or sucrose buffer (n = 5). Mice were sacrificed 72 h after CCl4 application.
Chronic liver injury was induced by multiple doses of CCl4 (1:4 in corn oil; 1.0 μl/g bw, ip) twice a week for a period of six weeks. After four weeks, mice were simultaneously treated with DBTC-Foxf1 siRNA (2.3 mg/kg bw; 0.1 ml/10 g body wt, iv) (n = 5) every 72 h until six weeks. Control animals received equivalent volumes of DBTC-Luci siRNA (n = 7) or sucrose buffer (n = 5). Mice were sacrificed six weeks after the first CCl4 application.
Hematological measurements and plasma enzyme levels
Animals were anesthetized and exsanguinated by puncture of the vena cava inferior. Red blood cell, white blood cell and blood platelets count, hemoglobin, and hematocrit were assessed with an automated cell counter (Sysmex KX-21; Sysmex Deutschland GmbH, Norderstedt, Germany). Plasma activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and glutamate dehydrogenase (GLDH) were measured spectrophotometrically as indicators of hepatocellular disintegration and necrosis.10
Histopathology and image analysis
Liver tissue samples were fixed in formalin for two to three days and embedded in paraffin. For routine examination and quantification of necrotic areas, 5 µm liver sections were stained with hematoxylin and eosin (H&E). All samples from a series of experiments were stained simultaneously and evaluated in a blinded manner. For histomorphometric analysis, images of 30 random low power fields (10 × magnification, Olympus BX 51, Hamburg, Germany) were acquired with a Color View II FW camera (Color View, Munich, Germany) and evaluated using an image analysis system (Adobe Photoshop). Necrotic areas were quantified as the percentage of the focal necrosis surface to the whole liver section area. The surfaces of large centrilobular veins and large portal tracts were excluded from this calculation.10
Immunohistochemistry
Five micrometer thick paraffin-embedded liver slices were immunostained for αSMA to determinate activated HSC and portal myofibroblasts as well as for collagen 1α to quantify fibrosis deposition. The slides were incubated with the appropriate primary antibodies anti-αSMA (1:600; ab5694, Abcam, Cambridge, UK) or anti-collagen 1α (1:200, 34710; Abcam, Cambridge, UK) overnight at 4℃. The secondary antibody goat-anti-rabbit-HRP (1:100, horse radish peroxidase; DO487, Dako Cytomation, Hamburg, Germany) was incubated for 1 h at RT. Signal detection was performed by using 3,3′-diaminobenzidine for collagen 1α (DAB, Dako) or permanent red for αSMA (Dako). Furthermore, nuclei were counterstained with hemalaun. αSMA-positive cells were counted in a blinded manner within 30 consecutive high power fields (HPF) (40 × objective, numerical aperture 0.65) and are given as cells/HPF. In analogy to the quantification of necrotic areas, fibrosis deposition was quantified as the percentage of collagen 1α-positive area to the whole liver section area using an image analysis system (Adobe Photoshop).
Quantitative Taqman RT-PCR analysis
After sacrifice of the mice, liver tissues were immediately dissected and instantly snap frozen in liquid nitrogen. Tissues were homogenized in a Mixer Mill MM 301 (Retsch GmbH, Haan, Germany) using tungsten carbide beads (Qiagen, Hilden, Germany). Total RNA from liver was isolated with the RNeasy Mini Kit including column genomic DNA digestion with RNase-free DNase Set (Qiagen Inc., Valencia, CA, USA). For qRT-PCR, 100 ng total RNA was reverse transcribed to cDNA with TaqMan Reverse Transcription Reagents (Applera GmbH, Darmstadt, Germany). The TaqMan RT-PCR reactions were carried out with an ABI PRISM 7700 Sequence Detector (Software: Sequence Detection System v1.6.3 (ABI)) or StepOnePlus™ Real Time PCR System (ABI) using a standard protocol for RT-PCR (48℃ 30 min, 95℃ 10 min, 40 cycles at 95℃ 15 s followed by 60℃ 1 min) with a concentration of 300 nM for the primers (Table 1) and 100 nM for the probe, as described previously.21 All data were calculated by using the comparative ddCt method and target gene expression values were normalized to the expression levels of β-actin. Target gene expression was compared with wildtype Balb/c liver tissue pool.
Table 1.
Primer sequences used for amplification
| Gene | Primer | Sequence (5′ to 3′) |
|---|---|---|
| Foxf1 | Forward | GCAGCCATACCTTCACCAAAAC |
| Reverse | ACATGCTGGGCGACTGTGA | |
| Probe | AGAACTGCAAGGCATCCCT CGGTATCAC | |
| collagen 1α | Forward | ACGCATGGCCAAGAAGACA |
| Reverse | AAGCATACCTCGGGTTTCCAC | |
| Probe | AGCTGCATACACAATGGC CTAAGGGTCC | |
| β-actin | Forward | GTTTGAGACCTTCAACACCCCA |
| Reverse | GACCAGAGGCATACAGGGACA | |
| Probe | CCATGTACGTAGCCATCC AGGCTGTG |
Statistical analysis
Results are presented as the mean of five to nine independent experiments ± SEM. Differences between the groups were analyzed using a one way ANOVA (Holm-Sidak method) or one way ANOVA on Ranks (Dunn’s method) followed by an appropriate post hoc comparison depending on the distribution of the data. Not normally distributed data are presented as box plots indicating the median, the interquartile range in form of a box, and the 10th and 90th percentiles as whiskers. Statistical significance was set at p < 0.05. Statistics were performed using the software package Sigma-Stat (Jandel Corporation, San Rafael, CA).
Results
Foxf1 silencing in acute liver injury model
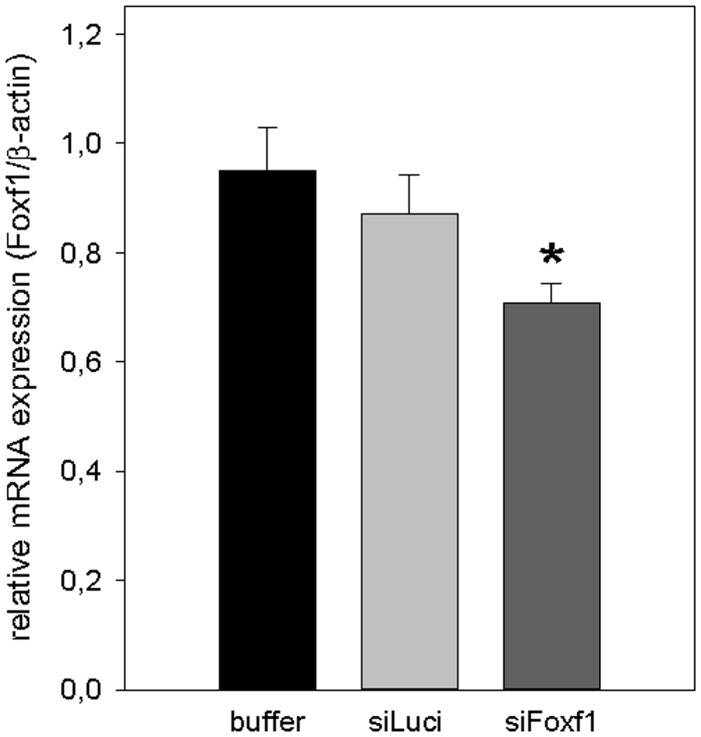
First, we analyzed the efficiency of gene silencing by DBTC lipoplexes in mice livers of the acute CCl4 model via qRT-PCR. Treatment of mice with DBTC-Foxf1 siRNA 48 h before and 24 h after the single administration of CCl4 resulted in a significantly reduced Foxf1 mRNA expression compared with buffer-treated mice (Figure 1).
Figure 1.
Validation of Foxf1-silencing by DBTC-siRNA in acute liver injury. Quantitative RT-PCR analysis of Foxf1 mRNA expression in liver tissue of buffer- and siRNA-treated animals 72 h after single CCl4 application (n = 5–9 per group). Values are given as means ± SEM. Significance of differences between the groups was tested by One way ANOVA (*p < 0.05 vs. buffer)
Acute liver injury
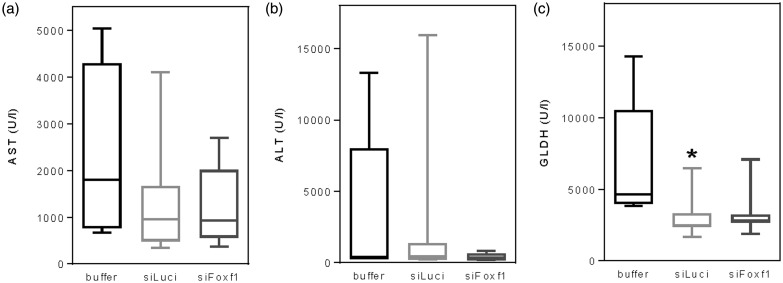
Single administration of CCl4 caused acute liver damage as indicated by strongly increased activities of liver enzymes in blood plasma (Figure 2), with no significant impact of Foxf1 silencing. Generally, values of all groups exhibit high variance.
Figure 2.
Analysis of plasma activities of aspartate aminotransferase (AST) (a), alanine aminotransferase (ALT) (b) and glutamate dehydrogenase (GLDH) (c) upon single CCl4 administration in buffer- and siRNA-treated mice (n = 5–9 per group). Data are presented as box plots indicating the median, the interquartile range in form of a box, and the 10th and 90th percentiles as whiskers. Significance of differences between the groups was tested by one way ANOVA (*p < 0.05 vs. buffer)
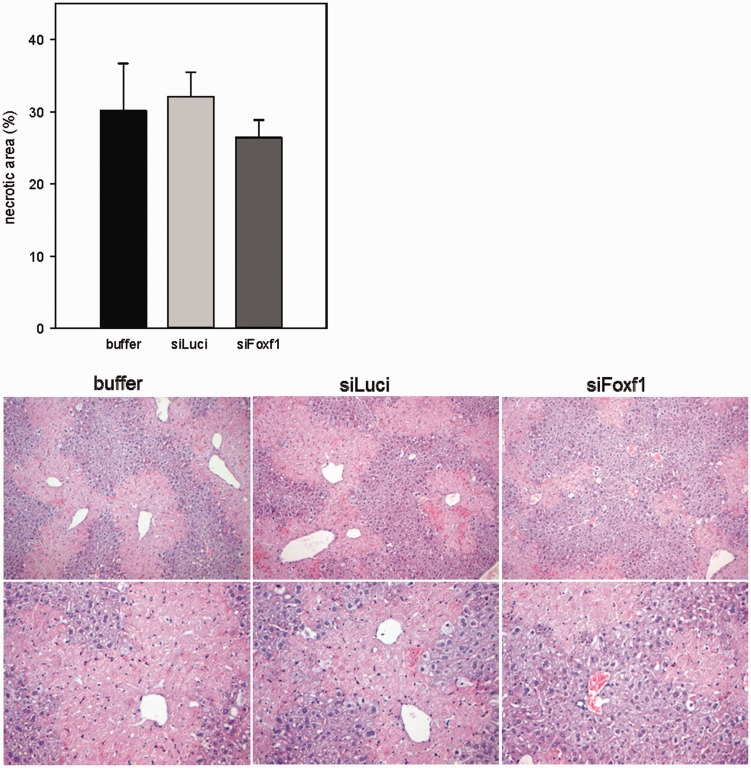
Additionally, mice of all groups showed a similarly high extent of necrotic cell death ranging between 27% and 32%, as analyzed by necrotic area in H&E stained liver sections (Figure 3).
Figure 3.
Quantification of necrotic area on H&E stained liver sections in buffer- and siRNA-treated mice with acute liver damage and representative images of H&E staining (original magnification 200 × (upper panel) and 400 × (lower panel)) (n = 5–9 per group). Values are given as means ± SEM (A color version of this figure is available in the online journal.)
Activation of HSC in acute liver injury model
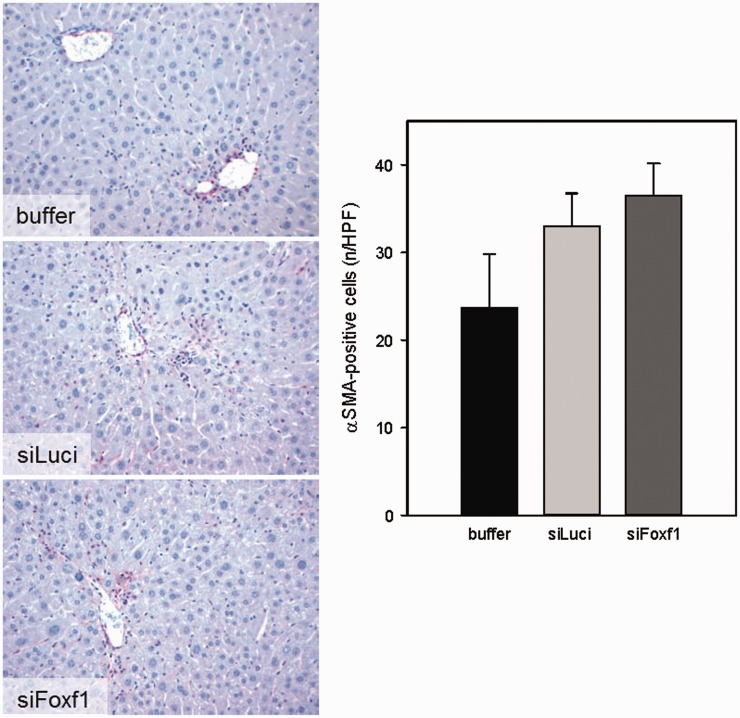
As Foxf1 in the liver is solely expressed by HSC, we investigated the effect of DBTC-Foxf1 siRNA on HSC activation by αSMA immunohistochemistry. Compared with animals treated with buffer or DBTC-Luci siRNA, treatment with DBTC-Foxf1 siRNA had no significant effect on the number of αSMA-positive cells, but even shared a slight increase (Figure 4).
Figure 4.
Representative images of immunohistochemical αSMA staining and quantification of αSMA-positive cells on liver sections of buffer- and siRNA-treated mice upon single CCl4 administration (original magnification 400×) (n = 5–9 per group). Values are given as means ± SEM (A color version of this figure is available in the online journal.)
Foxf1 silencing in chronic liver injury model
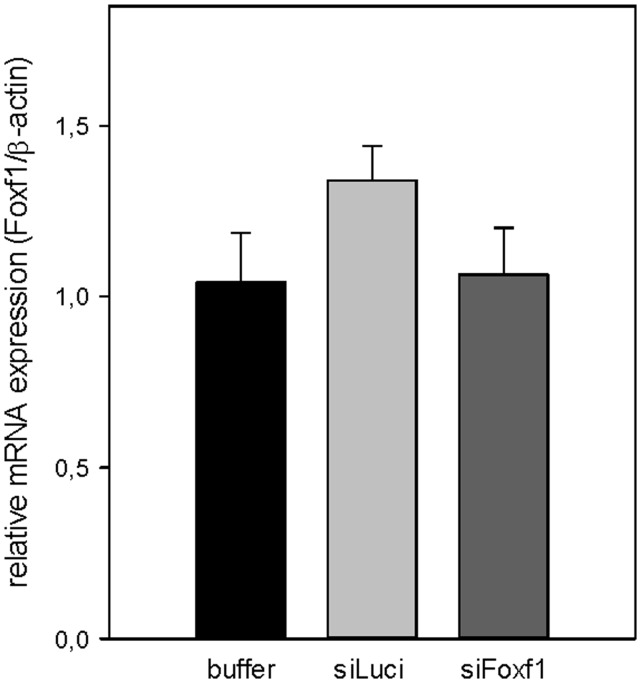
Analog to the acute CCl4 model, we validated the efficiency of Foxf1 gene silencing by repeated doses of DBTC lipoplexes (every 72 h, for two weeks) in mice livers, starting after four weeks upon chronic CCl4 exposition. Foxf1 mRNA expression was not reduced in livers of DBTC-Foxf1 siRNA-treated mice at six weeks after chronic CCl4 application (Figure 5).
Figure 5.
Validation of Foxf1-silencing by DBTC-siRNA in chronic liver injury. Quantitative RT-PCR analysis of Foxf1 mRNA expression in liver tissue of buffer- and siRNA-treated animals after chronic CCl4 application (n = 5–7 per group). Values are given as means ± SEM
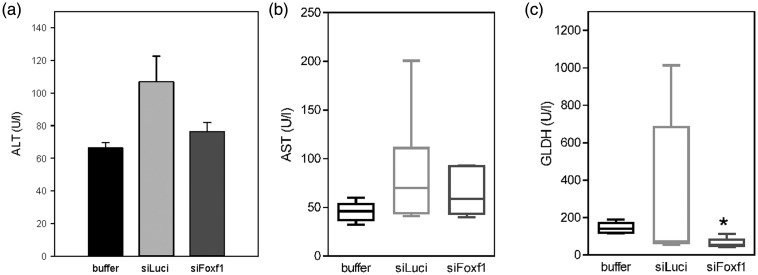
Chronic liver injury
In all experimental groups, we observed a moderate liver damage with significantly reduced GLDH values in Foxf1 siRNA-treated mice compared with buffer-treated animals (Figure 6).
Figure 6.
Analysis of plasma activities of aspartate aminotransferase (AST) (a), alanine aminotransferase (ALT) (b) and glutamate dehydrogenase (GLDH) (c) upon chronic CCl4 administration in buffer- and siRNA-treated mice (n = 5–7 per group). Values are given as means ± SEM (ALT) or as box plots indicating the median, the interquartile range in form of a box, and the 10th and 90th percentiles as whiskers (AST, GLDH). Significance of differences between the groups was tested by One way ANOVA on Ranks (*p < 0.05 vs. buffer)
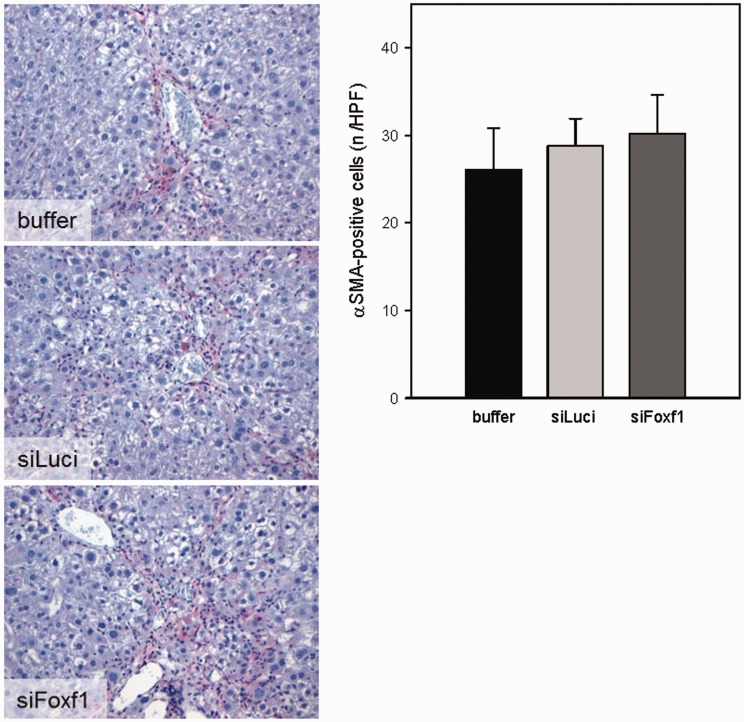
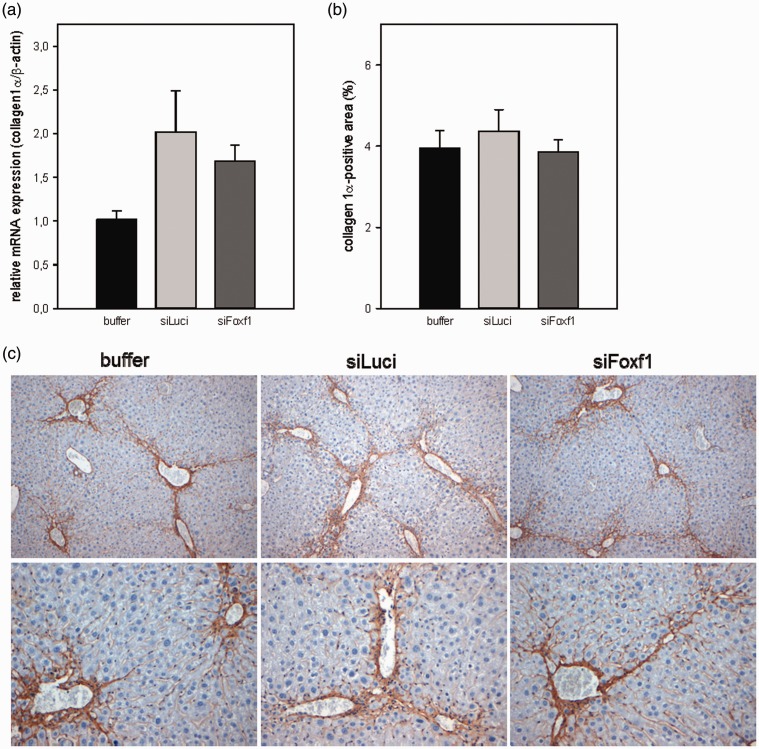
Liver fibrosis
Upon chronic CCl4 exposition mice developed bridging fibrosis with increased activation of HSC and substantial deposition of ECM, especially of collagen 1α. To verify whether Foxf1 siRNA treatment effects the progression of liver fibrosis, we immunohistochemically analyzed the activation of HSC by the activation marker αSMA and by collagen 1α deposition. Compared with animals treated with DBTC-Luci siRNA or buffer, treatment with DBTC-Foxf1 siRNA caused neither a change in the number of activated HSC (αSMA-positive cells, Figure 7) nor a change in amount of collagen 1α (Figure 8). Representative immunohistochemical images are displayed in Figure 8(c). The quantitative analysis of collagen 1α staining (Figure 8(b)) was further confirmed by RT-PCR analysis of collagen 1α expression, also showing no differences in all experimental groups (Figure 8(a)). Furthermore, in both liver injury models, no significant differences in systemic blood cell count (Table 2) were observed.
Figure 7.
Representative images of immunohistochemical αSMA staining and quantification of αSMA-positive cells on liver sections of buffer- and siRNA-treated mice upon chronic CCl4 administration (original magnification 400×) (n = 5–7 per group). Values are given as means ± SEM (A color version of this figure is available in the online journal.)
Figure 8.
Quantitative RT-PCR analysis of collagen 1α mRNA expression in liver tissue of buffer- and siRNA-treated animals after chronic CCl4 application (a). Quantitative data of collagen 1α-positive area (b) on appropriately stained liver sections of buffer- and siRNA-treated mice upon chronic CCl4 administration and representative images of immunohistochemical collagen 1α staining (original magnification 200 × (upper panel) and 400 × (lower panel)) (c). Values are given as means ± SEM (n = 5–7 per group) (A color version of this figure is available in the online journal.)
Table 2.
Analysis of systemic blood cell count of mice with CCl4 intoxication
| CCl4 model | Treatment | WBCa (109/l) | PLTb (109/l) | RBCc (1012/l) | HGBd (mmol/l) | HCTe (%) |
|---|---|---|---|---|---|---|
| Acute | Buffer | 6.5 ± 0.8 | 512 ± 116 | 9.1 ± 0.5 | 8.8 ± 0.4 | 46 ± 0 |
| siLuci | 7.9 ± 1.3 | 461 ± 56 | 8.4 ± 0.4 | 7.6 ± 0.7 | 43 ± 0 | |
| siFoxf1 | 6.3 ± 0.5 | 687 ± 77 | 8.3 ± 0.4 | 7.9 ± 0.4 | 42 ± 0 | |
| Chronic | Buffer | 6.3 ± 0.6 | 1302 ± 72 | 8.4 ± 0.1 | 7.5 ± 0.3 | 42 ± 0 |
| siLuci | 8.4 ± 2.9 | 980 ± 158 | 7.3 ± 0.7 | 6.8 ± 0.7 | 36 ± 1.0 | |
| siFoxf1 | 5.3 ± 0.5 | 658 ± 137* | 7.8 ± 0.3 | 7.2 ± 0.3 | 38 ± 1.0 |
Note: Values are given as means ± SEM. Significance of differences between the groups was tested by one way ANOVA (*p < 0.05 vs. buffer).
leukocytes.
bblood platelets.
cerythrocytes.
dhemoglobin.
ehematocrit.
Taken together, in acute and chronic models of CCl4 intoxication, the administration of DBTC-Foxf1 siRNA did not ameliorate liver injury or fibrogenesis.
Discussion
Numerous studies have investigated the mechanisms leading to liver fibrosis and subsequently developed strategies for antifibrotic therapies. However, no effective therapies in humans except antiviral treatment for chronic virus hepatitis patients22 exist so far. Therefore, further developments of new technologies including validation in different experimental models of liver disease are essential.
The present study served as a second, independent approach to confirm data obtained from a previous study using DBTC Foxf1 siRNA as a therapeutic approach in bile duct ligation (BDL)-induced cholestatic liver fibrosis. In the first study, we have demonstrated that the liver-specific siRNA delivery system DBTC is able to inhibit Foxf1 expression in HSC in vitro and in vivo, resulting in attenuated liver fibrosis.10
However, these positive effects of a Foxf1 siRNA treatment as observed in cholestatic liver injury10 could not be verified in toxin (CCl4)-induced acute and chronic liver injury models. This might be due to significant differences in the animal models used. Each model displays specific characteristics in the pathogenic nature, time course and severity of fibrosis and the optimal time point for starting a therapy.
Generally, in cholestatic and toxin-induced liver disease necrotic hepatocytes provoke a persistent inflammatory reaction.23 Finally, chronic inflammation and accumulation of ECM lead to loss of normal liver architecture by scar tissue. Periodic administration of CCl4 is a commonly used approach to induce toxin-mediated experimental liver fibrosis in mice and rats.24 A single CCl4 injection can not only be used to induce acute injury including induction of an inflammatory response but also as an attractive model of liver regeneration after toxic injury, as a strong regenerative response occurs 48 h after a single CCl4 administration.25
In accordance to our previous study, where silencing of Foxf1 could only be detected at the earliest observation time point after BDL,10 we also achieved a significantly reduced hepatic Foxf1 mRNA expression in the acute injury model after prophylactic administration of DBTC Foxf1 siRNA. Nevertheless, reduced Foxf1 expression had no significant impact on the extent of the acute liver injury and activation of HSC. Thus, as HSC are rather involved in chronic processes, Foxf1 may not serve as a suitable target gene in acute liver injury. Not knowing whether DBTC Foxf1 siRNA treatment lead to a transient Foxf1 gene silencing at the beginning of the therapy in the chronic CCl4 model, at the end of the observation period Foxf1 expression was unaffected upon repeated DBTC Foxf1 siRNA treatment. In contrast to the BDL model, where a strong fibrotic reaction originates from the portal fields,26 in the CCl4 model, massive centrilobular necrosis is present. This is due to the metabolic activation of CCl4 through p450 cytochromes that are highly expressed in hepatocytes surrounding the central vein. Whereas initially (acute model) high levels of plasma transaminases can be observed, transaminase levels decreased upon repeated CCl4 injections which reflects hepatocellular adaption and regeneration, with no differences between the groups. However, as indicated by reduced GLDH levels, during chronic injury necrotic cell death seems to be less in Foxf1 siRNA-treated mice.
While in the BDL model just partial bridges between portal tracts occur,27 it is known that in CCl4-treated mice histological development of fibrosis starts after four injections, while bridging fibrosis is present after eight injections.28 After four to six weeks, a robust and highly reproducible bridging fibrosis can be observed, first between central areas and secondly between central and portal areas. Importantly, depending on the time of the experimental end point in relation to the last CCl4 application, different pathophysiological features can be observed.29 While directly after the CCl4 application inflammation and necrosis occurs, more than three days after the last injection remodeling processes are prominent. Accordingly, in the acute model of liver injury, significant necrosis of about 30% of liver tissue was detected independent of the treatment. However, in our study of chronic CCl4 intoxication, we sacrificed the mice three days after the last CCl4 application, being in the pro-fibrogenic phase of the response. Thus, histologically and systemically (GLDH activity) no features of necrosis could be observed. Additionally, despite repeated doses of Foxf1 siRNA, we did not see any positive effect on the activation of HSC as well as the extent of ECM deposition, as shown by unchanged number of αSMA-positive cells and collagen 1α expression. Of most significance, compared to our previous study in BDL mice, which received siRNA prophylactically,10 in the chronic CCl4 model we used a therapeutic scheme and just started the treatment at week 4 after the first CCl4 application, i.e. an advanced stage of disease when bridging fibrosis is already present. Thus, as discussed before in our previous study, we strongly suggest that in fibrotic livers, the atypical liver architecture and insufficient blood supply contribute to an impaired distribution of systemically administrated DBTC formulations and thus cause a lack of transfection efficacy and positive effects. Regarding to the present study, it remains unclear whether Foxf1 is suitable as an antifibrotic target gene in chronic toxin-induced liver injury.
Acknowledgements
The authors kindly thank Dorothea Frenz, Berit Blendow, Maren Nerowski and Eva Lorbeer-Rehfeldt (Institute for Experimental Surgery, Rostock University Medical Center) for their excellent technical assistance.
Authors’ contributions
All authors were involved in the acquisition and interpretation of the results. KA and BV designed the research; TR and BG conducted the experiments; all authors analyzed the data; KA and BG wrote and edited the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair 2013; 6: 19–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008; 88: 125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J Hepatol 2015; 62(1 Suppl): S15–24. [DOI] [PubMed] [Google Scholar]
- 4.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 2010; 139: 987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012; 143: 1073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998; 102: 538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A 2012; 109: 9448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann J, Arias M, Van De Leur E, Gressner AM, Weiskirchen R. CSRP2, TIMP-1, and SM22alpha promoter fragments direct hepatic stellate cell-specific transgene expression in vitro, but not in vivo. Liver Int 2004; 24: 69–79. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita K, Iimuro Y, Fujimoto J, Inagaki Y, Namikawa K, Kiyama H, Nakajima Y, Otogawa K, Kawada N, Friedman SL, Ikeda K. Targeted and regulable expression of transgenes in hepatic stellate cells and myofibroblasts in culture and in vivo using an adenoviral Cre/loxP system to antagonise hepatic fibrosis. Gut 2007; 56: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abshagen K, Brensel M, Genz B, Roth K, Thomas M, Fehring V, Schaeper U, Vollmar B. Foxf1 siRNA delivery to hepatic stellate cells by DBTC lipoplex formulations ameliorates fibrosis in livers of bile duct ligated mice. Curr Gene Ther 2015; 15: 215–27. [DOI] [PubMed] [Google Scholar]
- 11.Reetz J, Genz B, Meier C, Kowtharapu BS, Timm F, Vollmar B, Herchenröder O, Abshagen K, Pützer BM. Development of adenoviral delivery systems to target hepatic stellate cells in vivo. PLoS One 2013; 8: e67091–e67091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan TL, Wang PW, Hung CF, Aljuffali IA, Dai YS, Fang JY. The impact of retinol loading and surface charge on the hepatic delivery of lipid nanoparticles. Colloids Surf B Biointerfaces 2016; 141: 584–94. [DOI] [PubMed] [Google Scholar]
- 13.Schoemaker MH, Rots MG, Beljaars L, Ypma AY, Jansen PL, Poelstra K, Moshage H, Haisma HJ. PDGF-receptor beta-targeted adenovirus redirects gene transfer from hepatocytes to activated stellate cells. Mol Pharm 2008; 5: 399–406. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 2008; 26: 431–42. [DOI] [PubMed] [Google Scholar]
- 15.Schon HT, Bartneck M, Borkham-Kamphorst E, Nattermann J, Lammers T, Tacke F, Weiskirchen R. Pharmacological intervention in hepatic stellate cell activation and hepatic fibrosis. Front Pharmacol 2016; 7: 33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KC, Park JH, Jeon JY, Kim SY, Kim JM, Lim CY, Lee TH, Kim HK, Lee HG, Kim SM, Kwon HJ, Suh JS, Kim SW, Choi SH. A new histone deacetylase inhibitor improves liver fibrosis in BDL rats through suppression of hepatic stellate cells. Br J Pharmacol 2014; 171: 4820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borkham-Kamphorst E, Steffen BT, Van de Leur E, Haas U, Tihaa L, Friedman SL, Weiskirchen R. CCN1/CYR61 overexpression in hepatic stellate cells induces ER stress-related apoptosis. Cell Signal 2016; 28: 34–42. [DOI] [PubMed] [Google Scholar]
- 18.Altrock E, Sens C, Wuerfel C, Vasel M, Kawelke N, Dooley S, Sottile J, Nakchbandi IA. Inhibition of fibronectin deposition improves experimental liver fibrosis. J Hepatol 2015; 62: 625–33. [DOI] [PubMed] [Google Scholar]
- 19.Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 2001; 128: 2397–406. [DOI] [PubMed] [Google Scholar]
- 20.Kalinichenko VV1, Bhattacharyya D, Zhou Y, Gusarova GA, Kim W, Shin B, Costa RH. Foxf1 +/- mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology 2003; 37: 107–17. [DOI] [PubMed] [Google Scholar]
- 21.Fehring V, Schaeper U, Ahrens K, Santel A, Keil O, Eisermann M, Giese K, Kaufmann J. Delivery of therapeutic siRNA to the lung endothelium via novel lipoplex formulation DACC. Mol Ther 2014; 22: 811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012; 56: 532–43. [DOI] [PubMed] [Google Scholar]
- 23.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115: 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mederacke I. Liver fibrosis – mouse models and relevance in human liver diseases. Z Gastroenterol 2013; 51: 55–62. [DOI] [PubMed] [Google Scholar]
- 25.Nevzorova YA, Bangen JM, Hu W, Haas U, Weiskirchen R, Gassler N, Huss S, Tacke F, Sicinski P, Trautwein C, Liedtke C. Cyclin E1 controls proliferation of hepatic stellate cells and is essential for liver fibrogenesis in mice. Hepatology 2012; 56: 1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abshagen K, König M, Hoppe A, Müller I, Ebert M, Weng H, Holzhütter HG, Zanger UM, Bode J, Vollmar B, Thomas M, Dooley S. Pathobiochemical signatures of cholestatic liver disease in bile duct ligated mice. BMC Syst Biol 2015; 9: 83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, Clavien PA. Characterization of time-related changes after experimental bile duct ligation. Br J Surg 2008; 95: 646–56. [DOI] [PubMed] [Google Scholar]
- 28.Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology 1983; 3: 112–20. [DOI] [PubMed] [Google Scholar]
- 29.Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol 2011; 25: 319–33. [DOI] [PubMed] [Google Scholar]