Abstract
Increasing evidence shows that maternal nutrition status has a vital effect on offspring susceptibility to obesity. MicroRNAs are related to lipid metabolism processes. This study aimed to evaluate whether maternal chromium restriction could affect miRNA expression involved in lipid metabolism in offspring. Weaning C57BL/6J mice born from mothers fed with normal control diet or chromium-restricted diet were fed for 13 weeks. The adipose miRNA expression profile was analyzed by miRNA array analysis. At 16 weeks old, pups from dams fed with chromium-restricted diet exhibit higher body weight, fat weight, and serum TC, TG levels. Six miRNAs were identified as upregulated in the RC group compared with the CC group, whereas eight miRNAs were lower than the threshold level set in the RC group. In the validated target genes of these differentially expressed miRNA, the MAPK signaling pathway serves an important role in the influence of early life chromium-restricted diet on lipid metabolism through miRNA. Long-term programming on various specific miRNA and MAPK signaling pathway may be involved in maternal chromium restriction in the adipose of female offspring.
Impact statement
For the first time, our study demonstrates important miRNA differences in the effect of maternal chromium restriction in offspring. These miRNAs may serve as “bridges” between the mother and the offspring by affecting the MAPK pathway.
Keywords: microRNA, lipid metabolism, developmental programming, MAPK pathway, chromium restriction, nutrition
Introduction
The nutrition status during the gestational and lactation period is a key time for both mothers and their offspring. Malnutrition during pregnancy could influent the mother’s health and change the offspring’s metabolic status.1,2 This association may be regulated epigenetically via various changes, including expression of microRNAs (miRNAs) and variations in their target genes. Alterations in miRNA expression change the metabolic plasticity and physiological processes in response to early environment conditions.3,4 MiRNA may represent a link between the prenatal, maternal nutrition and offspring metabolism status.
As an necessary element, chromium has important function on normal lipid metabolism.5 Chromium deficiency subjects exhibit elevated triglycerides (TG), total cholesterol (TC) and decreased high density lipoproteins (HDL-C).6 In many area, all around the world, the average chromium intake in adults does not meet the minimum suggested daily chromium intake (30 µg/day).7,8 Particularly, because of the enhance of metabolic stress and the reduction of absorption ratio, pregnant and elderly individuals have high risk of chromium deficiency.9,10,11 Feng et al.12,13 found that chromium supplementation reduced serum lipid levels in type 2 diabetic rodents. A recent study showed that maternal chromium restriction induced obesity in rodent pups likely due to increased oxidase stress.14 Our previous study highlighted the DNA methylation changes in MAPK signaling pathway and involved with lipid metabolism disorder in the mice pups from chromium-restricted mothers.15
Besides DNA methylation, miRNAs, which are small non-coding RNAs, represent a key governing factor in adipogenesis and lipid metabolism. For example, miR-33 controls cholesterol and lipid metabolism through regulating its target gene, sterol-regulatory element-binding protein (SREBP).16 MiR-34a is an important switch of hepatic lipid homeostasis.17 MiR-122 can also control cholesterol synthesis and lipoprotein secretion in the liver.18
In this study, we aimed to identify epigenetic mechanisms of fat metabolism dysregulation as a result of maternal chromium restriction using a mouse model.
Materials and methods
Animal treatments and diets
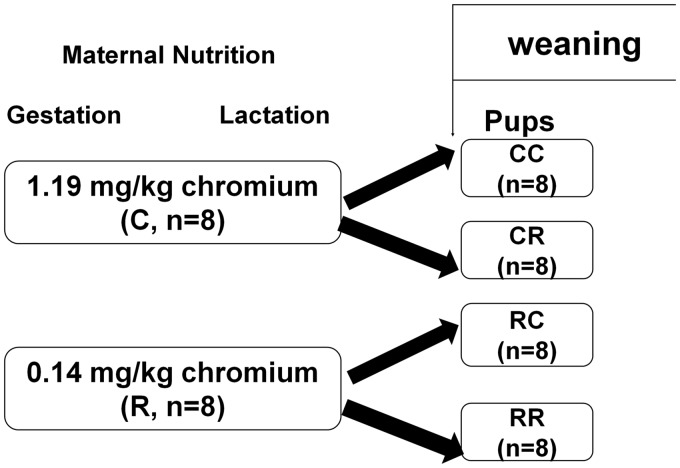
All animal experiment protocols were approved by the Animal Care Committee of Peking Union Medical Hospital (Permit Number: MC-07-6004), and all efforts were made to minimize suffering. Seven-week-old female and male C57BL mouse were obtained from Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China, SCXK-2013-0107). According to our previous paper,15 virgin female C57BL mice (18.2 ± 1.3 g, n = 16) were mated with male mice. Vaginal plug was checked to confirm the pregnancy. In gestation and lactation period, female mice were fed with either normal control diet (casein-based diet based on the American Institute of Nutrition AIN-93G diet) or chromium-restricted diet (only 90% reduction of chromium potassium sulfate from control diet, n = 8/group). The concentrations of chromium in the control diet and chromium-restriction diet are 1.19 and 0.14 mg chromium/kg diet, respectively (assessed by atomic absorption spectrometer (TAS986, Beijing Persee General Corporation, Beijing, China)). The integrant of AIN-93G diet is suitable for rodent normal growth and reproduction (including 1.19 mg chromium/kg diet). Ninety percent reduction of chromium content is the lowest chromium content in pregnant mice diet. All diets were produced by Research Diets (New Brunswick, NJ, USA). To make each pup get equal nutrition, new born pups were adjusted to three male and three female mice in each litter. At weaning (week 3), pups born from control dams were randomly assigned to normal control diet (CC) or chromium-restricted diet (CR); pups born from chromium-restricted diet dams were adopted by control (RC) and chromium-restriction diet (RR, n = 8/group, eight female pups from eight different litters). Because this study focused on the influence of prenatal chromium-restricted diet on offspring, RC group was designed to avoid the effect of offspring diet on the lipid metabolism. Because of the different phenotypic of maternal malnutrition on male and female offspring, this study exclusively focused on female offspring.19 At 16 weeks of age, female mice (n = 8/group) were sacrificed. The blood sample was taken from overnight fasting mice (anesthetized by ketamine 100 mg/kg i.p., Pharmacia and Upjohn Ltd, Crawley, UK). Pup adipose tissue was immediately collected and stored at −80℃ for further analysis. Figure 1 shows the experiment protocol.
Figure 1.
Schematic representation of the feeding protocol for different groups
Analysis of serum chromium levels
Serum was collected from the mothers at weaning and offspring at 16 weeks old to measure chromium levels. The serum chromium concentration was measured by atomic absorption spectrometer (Atomic Absorption Spectrophotometer, Hitachi, Japan) as previously described.15
Measurements of food intake and body weight
Body weight was recorded at birthday, 3 weeks and 16 weeks of age in offspring. Food intake was calculated at 16 weeks from pre-weighed food portions dispensed from the food hopper. Food intake measurements represent the average 24-h food intake calculated over the stated time period.
Measurement of adiposity index
At 16 weeks of age, mice were sacrificed, and fat pads were collected and weighed. The adiposity index (AI) was determined as follows.20

Measurement of serum biochemistry parameters
At 16 weeks of age, serum lipid levels were measured by an enzyme end-point method (Roche Diagnostics, GmbH, Mannheim, Germany).
RNA extraction and Reverse Transcription of miRNA
Total RNA isolated from fat in RC and CC groups was extracted by mirVana™ RNA Isolation Kit (Ambion, SanPaulo, SP, Brazil). cDNA was synthesized using TaqMan miRNA Reverse Transcription kit (Life Technologies, Rockville, MA, USA) as previously described.21
miRNA array hybridization
To elucidate the influence of maternal chromium restriction on miRNA expression in pup adipose, miRNA expression levels in RC and CC group were assessed by Affymetrix Multispecies miRNA 4.0 Array (Affymetrix, Santa Clara, CA, USA). This array fully covers all known rodent miRNA (557 unique miRNAs, based on miRBase version 20.0, released in Jun 2013, http://www.mirbse.org). After subtracting the background, the data were normalized using a LOWESS filter (Affymetrix, Santa Clara, CA, USA). A heat map was constructed by TIGR MeV (MultiExperiment Viewer) software (http://www.tm4.org/mev.html).22 The validated gene targets for differentially expressed miRNA by maternal chromium restriction in offspring liver were searched using the MiRTarBase Database version 6.0 (http://mirtarbase.mbc.nctu.edu.tw/, released Sep 2015).23
miRNA real-time PCR
To validate miRNAs regulated by chromium restriction in dams identified by arrays, we performed qPCR analysis of eight differentially expressed miRNAs. U6 was used as a reference control for normalization. Real-time PCR reactions were conducted with ABI 7700 (Applied Biosystems, Foster City, CA, USA) as previously described.21
miRNA target prediction
miRTarBase database 6.0 (mirtarbase.mbc.nctu.tw, released in Sep 2015) was used to seek the validated target genes of differentially expressed miRNAs.23
Real time PCR for target genes of differentially expressed miRNAs
For the validation of miRNA target genes in the MAPK pathway, real-time PCR experiments were performed using the SYBR Green method on ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) as previously described.21 Primers were obtained from Applied Biosystems (Supplementary Table S1). The signal of the housekeeping gene CypA (cytochrome P450 A) was used to normalize the result. Comparative Ct method was used to compare the relative quantification between different groups.
miRNA target genes pathway analysis
To fully interpret the biological meaning of target genes, we analyzed the gene ontology (GO) classification and Kyoto encyclopedia of genes and genomes (KEGG) pathways of the target genes, by using DAVID (Data-base for annotation, visualization and integrated discovery) software (http://david.abcc.ncifcrf.gov/).24 The miRNA and mRNA interaction was analyzed using Cytoscape software (http://www.cytoscape.org).25
Statistical analysis
All results are shown as the mean ± standard deviation (SD). Statistical analyses were calculated with two-way ANOVA followed by Tukey’s post hoc test, unpaired Student’s t test, and Fisher’s exact test. P < 0.05 was set as statistical significance. GraphPad Prism Software version 5.0 (San Diego, CA, USA) was used for data analysis.
Results
Mother
As expected, C57BL female mice fed a chromium-restriction diet exhibited lower serum chromium content compared with the normal control mice at weaning (0.32 ± 0.02 ng/mL vs. 0.92 ± 0.12 ng/mL, P < 0.01).
Offspring
Serum chromium
The CR (0.23 ± 0.03 ng/mL) and RR groups (0.17 ± 0.02 ng/mL) had lower serum chromium levels compared with the CC groups (0.83 ± 0.09 ng/mL, P < 0.01), while the RC group (0.79 ± 0.07 ng/mL) had comparable serum chromium level with the CC group (P > 0.05) at 16 weeks.
Body weight in offspring at birth, 3 weeks and 16 weeks
Birth and three-week-old weight of the offspring was comparable between the C and R groups. At 16 weeks of age, body weight in the RR (28.43 ± 3.21 g) and RC groups (26.37 ± 2.14 g) was increased compared with the CC group (20.37 ± 1.84 g, P < 0.05), while the CR group (21.26 ± 1.46 g) had similar body weight with the CC group (P > 0.05).
Food intake and fat pad weight
Consistent with the increased body weight, RR (6.71 ± 0.46 g) and RC (6.43 ± 0.36 g) offspring exhibited increased fat pad weight than that in CC group (4.63 ± 0.43 g, P < 0.05). Moreover, the CR group had increased fat pad weight (P < 0.05). However, the food intake was comparable among the CC, CR, RC, and RR groups.
Serum lipid profile levels
Serum TC levels were increased in the RC group compared with the CC group (1.83 ±0.15 mmol/L vs. 1.25 ± 0.08 mmol/L, P < 0.05), while the CR group (1.38 ± 0.11 mmol/L) and RR group (1.43 ±0.16 mmol/L) had similar TC levels with CC group (P < 0.05). Serum TG levels were increased in the RC (1.04 ± 0.08 mmol/L), CR (1.25 ± 0.06 mmol/L), and RR (1.21 ± 0.08 mmol/L) groups compared with the CC groups (0.57 ± 0.03 mmol/L, P < 0.05). Serum LDL-C and HDL-C levels were comparable among groups.
MiRNA expression profiles
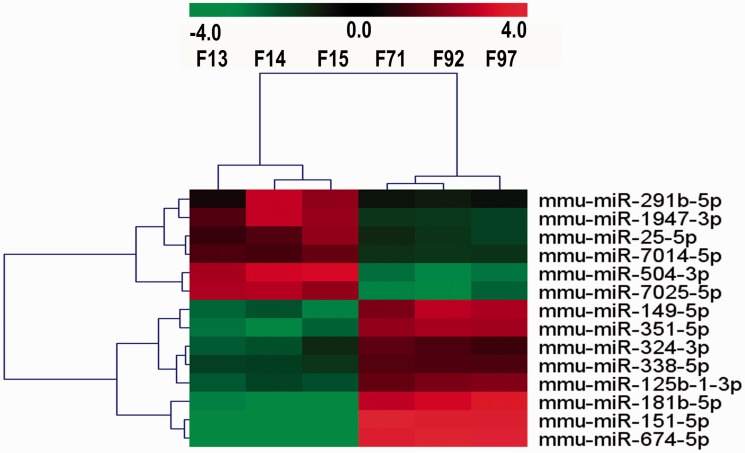
Differentially expressed miRNAs in the RC group were filtered by volcano plot, compared with CC group (fold change ≥ 2 and P-value<0.05). Six miRNAs (mmu-miR-25-5p, mmu-miR-291b-5p, mmu-miR-504-3p, mmu-miR-1947-3p, mmu-miR-7014-5p, and mmu-miR-7025-5p) were identified as upregulated (≥2-fold) in the RC group compared with the CC group, whereas eight miRNAs (mmu-miR-149-5p, mmu-miR-151-5p, mmu-miR-324-3p, mmu-miR-338-5p, mmu-miR-351-5p, mmu-miR-181b-5p, mmu-miR-125b-1-3p, and mmu-miR-674-5p) were less than the threshold level (0.5-fold) set in the RC group (Supplementary Table S2, Figure 2). Then, hierarchical clustering of the 14 miRNAs was performed. These differentially expressed miRNAs clearly distinguished the RC samples from the CC sample (Figure 3).
Figure 2.
Differentially expressed miRNAs identified in the microarray. Fourteen miRNA passed the volcano plot filtering. Volcano plots are a useful tool for visualizing differential expression patterns between two groups (RC vs. CC). The vertical lines correspond to 2-fold up-and down-regulation, and the horizontal line represents a P-value of 0.05. (A color version of this figure is available in the online journal.)
Figure 3.
MiRNA profiles differentiate RC group from CC group. Samples consist of three mice from each group. Both down-regulated (green) and up-regulated (red) miRNAs were identified in RC group. F13, F14, F15 belong to RC group; F71, F92, F97 belong to CC group
Validation of microarray results by real time PCR
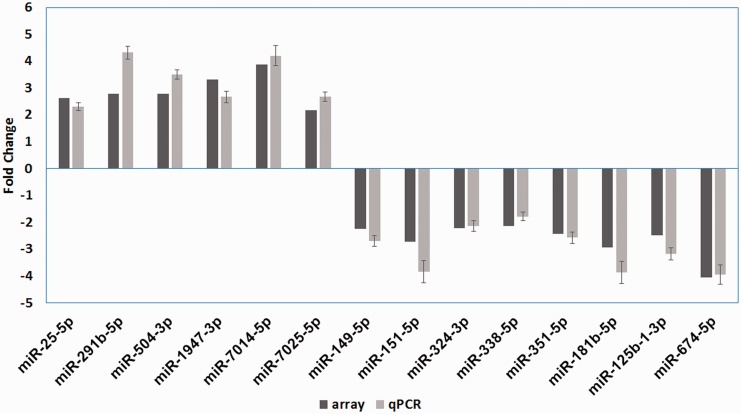
To confirm the array results, all differentially expressed (DE) miRNAs were subject to further validation using real-time PCR. Among the 14 miRNAs, all of them were differentially expressed between the RC and CC groups (P < 0.05, Figure 4). The results agreed with the array data.
Figure 4.
Differential miRNA expression in gene array and qPCR validation
Microarray-base gene ontology analysis of different expression miRNAs
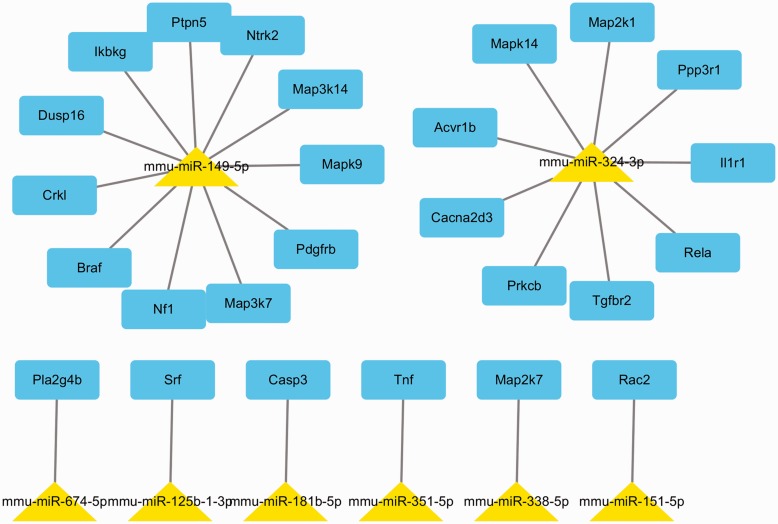
In miRTarBase database, only nine DE miRNAs have experimentally validated target genes. To explore the function of 9 DE miRNAs among the two groups, we obtained the 929 experimentally validated miRNA targets using miRNA database (Supplementary Table S3). Then, we studied the biological function of validated target genes. The validated miRNA targets were classified into several biological functions (Supplementary Table S4). Biological processes affected by targets genes include phosphorus metabolic, phosphate metabolic (Figure 5). Figure 6 presents an miRNA-mRNA interaction network using Cytoscape.
Figure 5.
MiRNA target significant GO biology process
Figure 6.
The network of target genes of the eight miRNAs involved in MAPK pathway. (A color version of this figure is available in the online journal.)
Microarray-based KEGG pathway analysis of different expression miRNAs
Using DAVID, we found the target genes of DE miRNAs clustered into the neurotrophin signaling pathway, pancreatic cancer, colorectal cancer, chronic myeloid leukemia, ErbB signaling pathway, MAPK signaling pathway, chemokine signaling pathway, axon guidance, and apoptosis (P < 0.0001, Supplementary Table S5).
DE miRNAs affect the MAPK pathway
Among the involved pathways, we identified eight DE miRNAs affected the MAPK pathway (Supplementary Table S5). In particular, numerous target genes of miR-149-5p and miR-324-3p are in the MAPK pathway.
Real time PCR
The expression of mitogen-activated protein kinase 7 (Map3k7), mitogen-activated protein kinase 14 (Mapk14), activating transcription factor 2 (Atf2) and peroxisome proliferator-activated receptor gamma (Pparg) increased in the RC group compared with the CC group (P < 0.01, Figure 7).
Figure 7.
Q-PCR result of target genes of DE miRNAs and downstream molecular in MAPK pathway. **P < 0.01 vs. CC group
Discussion
Although body weight at birth and three weeks from mice born to chromium-restricted mothers did not change, in this study, the body weight of pup mice from chromium-restricted mothers increased at 16 week of age. Other maternal undernutrition animal model also reported this result. Maternal vitamin B12 deficiency reduced pup birth weight. However, pup body weight increased after three months of age.26
In this study, chromium restriction (CR group) could increase the fat pad in mice. Tinkov et al.27 found that high-fat fed rats have lower chromium content in adipose tissue. And significant inverse correlation was observed for the chromium in adipose tissue and adipose tissue mass. In addition, maternal chromium restriction (RC group) increased irreversibly the fat pad weight of offspring at 16 weeks. Venu et al.28 reported that maternal Mg restriction increased pup body adipose percentage, and reduced lean body and fat-free mass at postnatal 90 days. Berends et al.29 found that pups from protein-restricted diet mothers had higher adipocytes at 22 days and 3 months. Maternal zinc deficiency increased body adipose ratio in offspring at six months of age.30
Moreover, chromium restriction (CR group) increased serum TG level in mice. Previous studies showed that chromium supplementation had beneficial effect on serum TG in T2DM patients,9,31,32 hyperlipidemic subjects33 and in high-sugar diet rodents.34 Importantly, maternal chromium restriction (RC group) increased irreversibly serum TC and TG at 16 weeks. Venu et al.28 found that maternal Mg restriction increased pup plasma TC levels compared with control pups at 90 days. Bringhenti et al.35 found that low-protein diet increase serum TC levels in offspring at 3 month.
By using miRNA array, this study showed that maternal chromium restriction (RC group vs. CC group) could downregulate miR-149 in offspring adipose. Mohamed et al.36 found miR-149 was highly downregulated in skeletal muscle of obese mice. Poly(ADP-ribose) polymerase-2 (PARP-2) is a validated target gene of miR-149. MiR-149 inhibits the expression of PARP-2, thus reducing the mitochondrial function and biogenesis by inhibiting the sirtuin-1 (SIRT-1)/peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) pathway. In miR-149-overexpressing myotubes, mitochondria respiration with complex 1-linked substrates was significantly increased.36
Moreover, maternal chromium restriction downregulates miR-338-5p in offspring adipose. High-fat diet mice, pre-diabetic (6 weeks of age) and diabetic (14–20 weeks of age) db/db mice have increased miR-338-3p levels in islets.37,38 Reduced miR-338-3p levels are related to compensatory β cell mass hypertrophy.
In addition, maternal chromium restriction downregulates miR-181b-5p in offspring adipose. The miR-181 family consists of miR-181a, miR-181b, miR-181c, and miR-181d. MiR-181a, miR-181b, and miR-181-d levels are low in obese subject monocytes. Weight loss normalizes their expression to the normal levels.39
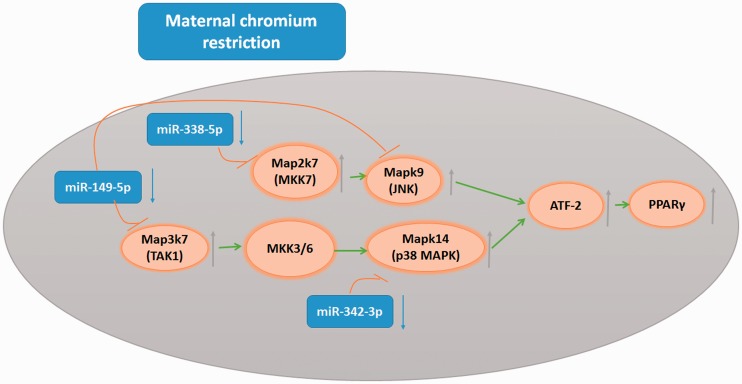
Interestingly, miRNA target gene pathway enrichment analysis showed that the MAPK pathway was highly ranked. We found that maternal chromium restriction downregulated miR-149-5p, miR-338-5p and miR-324-3p in offspring adipose. Their targets include Map3k7, Mapk9, Map2k7, and Mapk14, which all belong to the MAPK pathway.
The mitogen-activated protein kinase (MAPK) family consists of the following three main members: extracellular signal-regulated protein kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 kinases. Upon several different signals stimulation, MAPK kinases (mitogen-activated protein kinase 3/6 (MKK3/6)) can activate P38MAPK.40 Upon stimulation, MKK3 or MKK6 is activated by upstream MAPKK kinase (TGFβ-activated kinase 1 (TAK1)).41 Increasing evidence has revealed TAK1-P38MAPK pathway.42,43 P38MAPK and TAK1 are encoded by the Mapk14 and Map3k7 genes in mice, respectively. Interestingly, Mapk14 and Map3k7 are target genes of miR-324-3p and miR-149-5p. P38MAPK and JNK activate PPARγ2 through activating transcription factor-2 (ATF-2).44,45 PPARγ2 is an important mediator in adipocyte differentiation.46 In this study, we also found that the expression of Atp2 and PPARγ2 in pup adipose tissue was enhanced by maternal chromium restriction. A previous study found that bisphenol A (BPA) can increase red lipid droplets and TG levels in 3T3-L1 cells through activating p38/MAPK.47 A P38 inhibitor reduces obesity in mice fed a high-fat diet.44 Our results shows that maternal chromium restriction downregulates miR-324-3p, miR-338-5p and miR-149-5p, thus upregulates Map3k7, Map2k7, and Mapk14, and activates the p38MAPK pathway and JNK pathway. There effects disrupt lipid metabolism (Figure 8).
Figure 8.
MicroRNAs involved in the effect of maternal chromium restriction in offspring. Red blunted arrows represent inhibition; green arrows represent activation. Map3k7: mitogen-activated protein kinase 7; Mspk14: mitogen-activated protein kinase 14; Atf2: activating transcription factor 2; Pparg: peroxisome proliferator activated receptor gamma; MKK3/6: mitogen-activated protein kinase 3/6; TAK1: TGFβ-activated kinase 1; ATF-2: activating transcription factor-2; JNK: c-Jun N-terminal kinase
In conclusion, for the first time, our study demonstrates miR-149-5p, miR-338-5p and miR-324-3p reduced in female offspring explored to maternal chromium restriction. These miRNAs may serve as “bridges” between the mother and the offspring by affecting the MAPK pathway. However, these hypotheses need to be tested in future. These key miRNAs may aid in the identification of new therapies to treat the maternal undernutrition effect in offspring.
Supplementary Material
Acknowledgments
We are very grateful to Beijing Compass Biotechnology Company for excellent technical assistance with the microarray experiments. This work was supported by the grants from the National Natural Science Foundation of China (No. 81170736, 81570715), National Natural Science Foundation for Young Scholars of China (No. 81300649), China Scholarship Council foundation (201308110443), PUMC Youth Fund (33320140022) and Fundamental Research Funds for the Central Universities.
Authors’ contributions
XH X designed the experiments, contributed reagents and materials; QZ, JZ, TW, CJ Q, ZX W and XJ W conducted the experiments; MY, ML and FP analyzed the data; QZ wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991; 303: 1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998; 351: 173–7. [DOI] [PubMed] [Google Scholar]
- 3.Zucchi FC, Yao Y, Metz GA. The secret language of destiny: stress imprinting and transgenerational origins of disease. Front Genet 2012; 3: 96–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JE, Lee PR, Chen S, Li W, Fields RD. MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci U S A 2011; 108: 11650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Board FaN. Recommended dietary allowances, Washington, DC: National Academy Press, 2000. [Google Scholar]
- 6.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr 1998; 17: 548–55. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RA, Kozlovsky AS. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am J Clin Nutr 1985; 41: 1177–83. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA. Chromium in the prevention and control of diabetes. Diabetes Metab 2000; 26: 22–7. [PubMed] [Google Scholar]
- 9.Offenbacher EG, Pi-Sunyer FX. Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes 1980; 29: 919–25. [DOI] [PubMed] [Google Scholar]
- 10.Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J Clin Pharm Ther 2014; 39: 292–306. [DOI] [PubMed] [Google Scholar]
- 11.Lefavi RG, Anderson RA, Keith RE, Wilson GD, McMillan JL, Stone MH. Efficacy of chromium supplementation in athletes: emphasis on anabolism. Int J Sport Nutr 1992; 2: 111–22. [DOI] [PubMed] [Google Scholar]
- 12.Feng W, Zhao T, Mao G, Wang W, Feng Y, Li F, Zheng D, Wu H, Jin D, Yang L, Wu X. Type 2 diabetic rats on diet supplemented with chromium malate show improved glycometabolism, glycometabolism-related enzyme levels and lipid metabolism. PLoS One 2015; 10: e0125952–e0125952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng W, Mao G, Li Q, Wang W, Chen Y, Zhao T, Li F, Zou Y, Wu H, Yang L, Wu X. Effects of chromium malate on glycometabolism, glycometabolism-related enzyme levels and lipid metabolism in type 2 diabetic rats: a dose-response and curative effects study. J Diabetes Investig 2015; 6: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padmavathi IJ, Rao KR, Venu L, Ganeshan M, Kumar KA, Rao Ch N, Harishankar N, Ismail A, Raghunath M. Chronic maternal dietary chromium restriction modulates visceral adiposity: probable underlying mechanisms. Diabetes 2010; 59: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, Ping F, Wang Z, Qi C, Wang T, Wang X. Effects of maternal chromium restriction on the long-term programming in MAPK signaling pathway of lipid metabolism in mice. Nutrients 2016; 8: 488–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010; 328: 1566–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li WQ, Chen C, Xu MD, Guo J, Li YM, Xia QM, Liu HM, He J, Yu HY, Zhu L. The rno-miR-34 family is upregulated and targets ACSL1 in dimethylnitrosamine-induced hepatic fibrosis in rats. FEBS J 2011; 278: 1522–32. [DOI] [PubMed] [Google Scholar]
- 18.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012; 122: 2884–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction 2013; 145: R1–13. [DOI] [PubMed] [Google Scholar]
- 20.Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics 1996; 34: 389–98. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, Wang Z, Xiang H. Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PloS one 2013; 8: e79697–e79697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol 2006; 411: 134–93. [DOI] [PubMed] [Google Scholar]
- 23.Chou CH, Chang NW, Shrestha S, Hsu SD, Lin YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, Tsai TR, Ho SY, Jian TY, Wu HY, Chen PR, Lin NC, Huang HT, Yang TL, Pai CY, Tai CS, Chen WL, Huang CY, Liu CC, Weng SL, Liao KW, Hsu WL, Huang HD. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res 2016; 44: D239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 2003; 4: P3–P3. [PubMed] [Google Scholar]
- 25.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13: 2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar KA, Lalitha A, Pavithra D, Padmavathi IJ, Ganeshan M, Rao KR, Venu L, Balakrishna N, Shanker NH, Reddy SU, Chandak GR, Sengupta S, Raghunath M. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in Wistar rat offspring. J Nutr Biochem 2013; 24: 25–31. [DOI] [PubMed] [Google Scholar]
- 27.Tinkov AA, Popova EV, Polyakova VS, Kwan OV, Skalny AV, Nikonorov AA. Adipose tissue chromium and vanadium disbalance in high-fat fed Wistar rats. J Trace Elem Med Biol 2015; 29: 176–81. [DOI] [PubMed] [Google Scholar]
- 28.Venu L, Kishore YD, Raghunath M. Maternal and perinatal magnesium restriction predisposes rat pups to insulin resistance and glucose intolerance. J Nutrition 2005; 135: 1353–8. [DOI] [PubMed] [Google Scholar]
- 29.Berends LM, Fernandez-Twinn DS, Martin-Gronert MS, Cripps RL, Ozanne SE. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J Obes 2013; 37: 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padmavathi IJ, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, Raghunath M. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol 2009; 94: 761–9. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Agrawal RP, Choudhary M, Jain S, Goyal S, Agarwal V. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J Trace Elem Med Biol 2011; 25: 149–53. [DOI] [PubMed] [Google Scholar]
- 32.Lee NA, Reasner CA. Beneficial effect of chromium supplementation on serum triglyceride levels in NIDDM. Diabetes Care 1994; 17: 1449–52. [DOI] [PubMed] [Google Scholar]
- 33.Elwood JC, Nash DT, Streeten DH. Effect of high-chromium brewer's yeast on human serum lipids. J Am Coll Nutr 1982; 1: 263–74. [DOI] [PubMed] [Google Scholar]
- 34.Doddigarla Z, Ahmad J, Parwez I. Effect of chromium picolinate and melatonin either in single or in a combination in high carbohydrate diet-fed male Wistar rats. Biofactors 2016; 42: 106–14. [DOI] [PubMed] [Google Scholar]
- 35.Bringhenti I, Schultz A, Rachid T, Bomfim MA, Mandarim-de-Lacerda CA, Aguila MB. An early fish oil-enriched diet reverses biochemical, liver and adipose tissue alterations in male offspring from maternal protein restriction in mice. J Nutr Biochem 2011; 22: 1009–14. [DOI] [PubMed] [Google Scholar]
- 36.Mohamed JS, Hajira A, Pardo PS, Boriek AM. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1alpha network in skeletal muscle. Diabetes 2014; 63: 1546–59. [DOI] [PubMed] [Google Scholar]
- 37.Jacovetti C, Abderrahmani A, Parnaud G, Jonas JC, Peyot ML, Cornu M, Laybutt R, Meugnier E, Rome S, Thorens B, Prentki M, Bosco D, Regazzi R. MicroRNAs contribute to compensatory beta cell expansion during pregnancy and obesity. J Clin Invest 2012; 122: 3541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, Prentki M, Regazzi R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia 2013; 56: 2203–12. [DOI] [PubMed] [Google Scholar]
- 39.Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab 2012; 97: E1213–8. [DOI] [PubMed] [Google Scholar]
- 40.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 2010; 429: 403–17. [DOI] [PubMed] [Google Scholar]
- 41.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 2005; 19: 2668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biesemann N, Mendler L, Kostin S, Wietelmann A, Borchardt T, Braun T. Myostatin induces interstitial fibrosis in the heart via TAK1 and p38. Cell Tissue Res 2015; 361: 779–87. [DOI] [PubMed] [Google Scholar]
- 43.Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor alpha1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol 2005; 19: 1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maekawa T, Jin W, Ishii S. The role of ATF-2 family transcription factors in adipocyte differentiation: antiobesity effects of p38 inhibitors. Mol Cell Biol 2010; 30: 613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figuera-Losada M, LoGrasso PV. Enzyme kinetics and interaction studies for human JNK1beta1 and substrates activating transcription factor 2 (ATF2) and c-Jun N-terminal kinase (c-Jun). J Biol Chem 2012; 287: 13291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 2003; 14: 545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie X, Song J, Li G. MiR-21a-5p suppresses bisphenol A-induced pre-adipocyte differentiation by targeting map2k3 through MKK3/p38/MAPK. Biochem Biophys Res Commun 2016; 473: 140–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.