Abstract.
This study aimed to estimate the organ dose reduction potential for organ-dose-based tube current modulated (ODM) thoracic computed tomography (CT) with a wide dose reduction arc. Twenty-one computational anthropomorphic phantoms (XCAT) were used to create a virtual patient population with clinical anatomic variations. The phantoms were created based on patient images with normal anatomy (age range: 27 to 66 years, weight range: 52.0 to 105.8 kg). For each phantom, two breast tissue compositions were simulated: and (glandular-to-adipose ratio). A validated Monte Carlo program (PENELOPE, Universitat de Barcelona, Spain) was used to estimate the organ dose for standard tube current modulation (TCM) (SmartmA, GE Healthcare) and ODM (GE Healthcare) for a commercial CT scanner (Revolution, GE Healthcare) using a typical clinical thoracic CT protocol. Both organ dose and -to-organ dose conversion coefficients ( factors) were compared between TCM and ODM. ODM significantly reduced all radiosensitive organ doses (). The breast dose was reduced by . For factors, organs in the anterior region (e.g., thyroid and stomach) exhibited substantial decreases, and the medial, distributed, and posterior region saw either an increase of less than 5% or no significant change. ODM significantly reduced organ doses especially for radiosensitive superficial anterior organs such as the breasts.
Keywords: organ dose, tube current modulation, organ dose based tube current modulation, computed tomography, tube current modulation
1. Introduction
Computed tomography (CT) usage has grown substantially in the past decades, and CT is now widely used for the diagnosis of a broad range of diseases. In 2016, million CT exams were performed in the United States.1,2 Consequently, the growing number of CT procedures has raised concerns about population-based radiation risk.3 To reduce unnecessary radiation dose, manufacturers, researchers, and healthcare providers in the CT community have been developing dose optimization techniques.4
The breast is particularly vulnerable to radiation-induced cancer.5,6 In thoracic CT, the breasts are directly exposed to x-rays despite not being diagnostically relevant.7–10 To reduce breast dose while maintaining the image quality for diagnostically relevant organs such as lungs and heart, the organ-based tube current modulation (OTCM) technique is typically used.11,12 OTCM reduces the tube current (mA) by 75% to 80% over a 120-deg arc anterior of the patients (referred to as the dose-reduction zone) and the reduced mAs was evenly divided and added to the remaining projections within each rotation (referred to as the dose-increase zone) to maintain the same (X-CARE, Siemens Healthcare, Forchheim, Germany). However, the breasts can be located beyond the 120-deg arc, and on average, extend to the dose-increase zone, compromising the overall breast dose reduction from TCM to OTCM.13 Moreover, most of the breast tissue in the dose-increase zone is the outer quadrant, in which most breast carcinomas first develop.14–16 Additionally, the posterior or distributed radiosensitive organs see dose increase with OTCM. Notably, the bone-marrow and kidney doses can increase by 10% and 7%, respectively.
Recently, a new organ-dose-based tube current modulation (ODM) (GE Healthcare) technique was introduced, in which the mA, based on TCM, is reduced by 40% over about a 180 deg arc anterior to the patients without a corresponding increase posteriorly. ODM compared to TCM has been shown to reduce both the breast dose as well as dose to other organs; for example, the spine dose was reduced by 6% compared to TCM.17 Prior studies have included only a small patient population (10 females) with six organ types considered.17
The purpose of this study was to extend the evaluation of the dose reduction potential of ODM under a presentation of clinical population variations (21 anthropomorphic phantoms; XCAT) with 44 explicitly modeled radiosensitive organs. A recent commercially available clinical CT scanner (Revolution, GE Healthcare) was modeled and the organ doses were simulated and compared between TCM and ODM. The -to-organ dose conversion coefficients ( factors) were derived and compared between TCM and ODM to isolate the effect of total photon flux.
2. Materials and Methods
2.1. Computational Phantoms
Twenty-one female four-dimensional extended cardiac-torso (XCAT) computational phantoms were used in this work (Fig. 1). The phantoms were developed based on normal female patient chest and abdomen-pelvic or chest-abdomen-pelvic CT images acquired at Duke University.18 The patients were selected within the age range of 27 to 66 years and weight range of 52.0 to 105.8 kg to provide variable and clinically relevant anatomy, and their age and weight values were assigned to corresponding XCAT phantoms. Figure 2 shows the BMI versus age for this patient cohort. The phantoms were voxelized at a resolution of 3.45 mm for the Monte Carlo simulations.19
Fig. 1.
Frontal view of the female XCAT phantoms.
Fig. 2.
BMI of the 21 patients modeled in this study.
For Monte Carlo simulations, each organ was assigned one of four materials explicitly described in previous publications: soft tissue, lung, skeleton, and breast (composed of 50% glandular and 50% adipose tissue).20 Additionally, in this study, we also simulated (glandular-to-adipose ratio) breasts to represent the clinical average.21,22 The dose of the breasts was compared to that of the breasts.
2.2. CT Models
In our previous study, we developed a Monte Carlo program to simulate the radiation transport in CT scanners.20 The simulation package utilized PENELOPE as a subroutine to track the energy loss of photons (PENELOPE, version 2006; Universitat de Barcelona, Barcelona, Spain)23,24 and was validated using physical cylindrical and anthropomorphic phantoms.20
In this study, the PENELOPE-based Monte Carlo simulation package was used to simulate organ dose. A commercial CT scanner (Revolution CT; GE Healthcare, Waukesha, Wisconsin) was simulated with explicitly modeled scanner geometry, x-ray source spectrum, and bowtie filtering with parameters provided by the manufacturer. A typical chest CT protocol was simulated using 120 kVp, a pitch factor of 0.5, a rotation time of 0.35 s, and a 40-mm collimation. The tube current profile was determined individually for each phantom as shown in the next paragraph. The cross-phantom averaged mAs (tube current × rotation time) was and for TCM and ODM scans, respectively. The value was derived as a function of -per-mAs, where the -per-mAs value was determined by Monte Carlo simulations using a 32-cm-diameter CTDI phantom. The cross-phantom averaged was and for TCM and ODM scans, respectively.
The standard attenuation-based tube current profile (referred to as ) (SmartmA; GE Healthcare, Waukesha, Wisconsin) was generated for each phantom. This simulated real time mA calculation based on AP and LAT radiographies.19 The line integral of attenuation coefficients from the source to the detector was calculated for each ray .19 The maximum of a projection was used to calculate this projection’s body attenuation .19 The values at AP and LAT directions are proportional to body attenuation as
| (1) |
where is a fixed mA. at other projection angles were calculated through sinusoidal interpolation, using and as minimum and maximum values of one rotation, respectively. The mAs were truncated to 700 mA according to system mA limits.
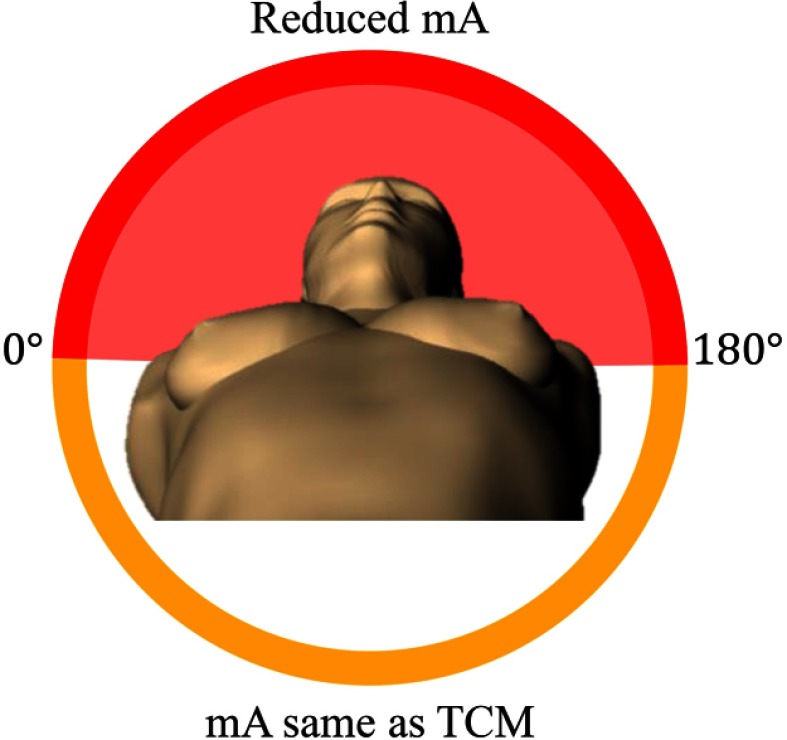
The organ-dose-based TCM profile (referred to as ) (GE Healthcare, Waukesha, Wisconsin) was generated by reducing the by 40% for projection angles within the dose reduction zone with the at other angles kept constant. As shown in Fig. 3, the dose reduction zone was defined as anterior to the patient. Per communication with the manufacturer and illustrated by Gandhi et al.,17 the transition of between the dose reduction zone and the unreduced dose zone (mA bump down and bump up) was modeled as a linear gradual change. The gradual change was modeled to take 5% of the rotation time starting at the transition. The and profiles for a sampled scan length from a sample phantom are shown in Fig. 4.
Fig. 3.
Illustration of the dose reduction zone (red) for organ-dose-based tube current modulation.
Fig. 4.
Standard attenuation-based tube current modulation profile () and organ-dose-based tube current modulation profile () for a sampled scan length of a sample XCAT phantom. The shaded region represents the dose reduction zone of ODM.
2.3. Organ Dose Estimation
Each scan simulation initiated and tracked x-ray photons to achieve organ dose () measurements with 1% or less relative error for each organ dose (except for ovaries and vagina doses, which had 3% or less relative error). The energy deposited within each organ was tallied to calculate organ dose. The breast dose from and breasts was obtained by separate simulations. Organ dose differences were calculated between ODM and TCM. Since ODM decreased mAs (and consequently ) by 20% compared to TCM,25 in order to isolate the influence of and compare the net effect of modulation, the organ dose was normalized by to derive the so-called factors (). The effect of breast mass on ODM breast dose reduction was analyzed. The correlation coefficients were calculated between breast factors reduction and breast mass. Further, the phantoms were evenly divided into three groups according to breast mass (group 1: ; group 2: ; group 3: ). The group means of breast dose reduction were compared by one-way analysis of variance.
The organs were classified into anterior, medial or distributed organs, and posterior organs depending on the location with regard to the aorta and inferior vena cava.
The effective dose was calculated as the sum of organ dose multiplied by the respective tissue weighting factors (ICRP 103). To include all radiosensitive organs specified by ICRP 103, the doses for organs not explicitly modeled were approximated by neighboring organ doses. Specifically, the salivary glands, extrathoracic region, and oral mucosa doses were approximated by the larynx-pharynx dose. The lymphatic nodes and muscle doses were approximated by the body dose, which included both fat and muscle tissues. These five tissue types exhibited small contributions to the effective dose due to low tissue weighting factors (ICRP 103).
The effective dose attributed to each organ was estimated by the product of organ dose and effective dose weighting coefficients (ICRP 103), averaged across patients, and normalized by TCM effective dose as
| (2) |
where , , is an organ dose is the ICRP 103 effective dose weighting coefficient, is the total number of phantoms, and is the TCM effective dose. This method was adapted from a previous study.25
3. Results
3.1. Breast Geometry Versus Dose Reduction Zone
Across the 21 phantoms, the average breast volume for both breasts (combined) was . On average, of the breast volume was within the dose reduction zone. In 16 phantoms, 100% of the breast was within the dose reduction zone, whereas for the remaining five phantoms, 0.3% to 22% of the breast lay outside the dose reduction zone.
The angle of breast extension was calculated individually for each phantom. The left and right breast ranges were and , respectively. No statistically significant difference was found between the left and right breast extension angular ranges (). The breasts extension angle of both breasts (combined) ranged from 148 deg to 209 deg for all phantoms.
3.2. Breast Dose
Figure 5 shows the breast dose and factors of the and breasts corresponding to the TCM and ODM scans. Figure 6 shows dose distribution plots from a sample XCAT patient (transverse plane) corresponding to TCM, ODM, and ODM with the same as TCM. The average breast dose was and for TCM and ODM, respectively. Compared to TCM, ODM showed a reduction in breast dose and a reduction in factors.
Fig. 5.
(a) Breast dose and (b) factors for TCM and ODM scans.
Fig. 6.

Dose distribution plots of (a) TCM, (b) organ-dose-based TCM (ODM) scheme, and (c) ODM with same as in (a) for a sample XCAT phantom.
In terms of breast composition, breasts showed lower dose compared to the breasts in both TCM and ODM scans. The percentage of breast dose reduction by ODM was similar for and breasts. Due to this similarity, only simulations with breasts were presented in the remainder of the study.
3.3. Breast Dose Reduction Versus Breast Size
No significant correlation was found between the reduction of breast dose and breast size (, ) or the reduction of breast factor and breast size (, ). No statistical significant difference was found among the three groups of different breast sizes for the reduction of breast dose () or breast factors ().
3.4. Other Organ Doses
Figure 7(a) shows the cross-patient average of organ dose differences between ODM and TCM. Compared to TCM, ODM significantly reduced all radiosensitive organ doses (all, ). As expected, this dose reduction was more prominent in anterior organs than in posterior organs. For anterior organs, ODM reduced organ doses by more than 20%. Notably, thyroid and thymus doses were reduced by 28% and 26%, respectively. For posterior organs such as kidneys and spleen, the organ doses were reduced by 15% to 16%. The medial/distributed organs showed dose reduction between that of anterior and posterior organs, with an across-patient average dose reduction range of 15% to 20%.
Fig. 7.
Cross-patient averaged (a) organ dose and (b) -normalized-organ dose coefficients ( factors) differences simulated by ODM versus TCM. Negative numbers indicate that dose reduced with ODM compared to TCM.
Figure 7(b) shows the cross-patient averaged differences of factors between the ODM and TCM scans. The anterior organ factors significantly decreased by 5% or more (all, ). The medial/distributed organs showed factors reduced by 2% (all, ), except the skin dose, which remained almost constant (0.1% reduction). All posterior organ factors showed dose increases of 2% to 4% (all, ).
Compared to TCM, ODM reduced the effective dose by . When normalized by dose length product (i.e., , DLP), the reduction was .
Figure 8 shows the product of organ dose and effective dose weighting factor divided by TCM effective dose. Only the organs with greater than 2% are shown in descending order. For TCM scans, one quarter of the effective dose was attributed to the lungs, which was the highest of all organs considered. Compared to TCM, ODM reduced the by . The breasts had the most prominent reduction due to ODM by . ODM reduced by when ODM had the same as TCM. Other radiosensitive organs not shown in Fig. 6 consisted of a total contribution of 10.5%, 10.2%, and 8.3% for TCM, ODM with the same , and ODM scans, respectively.
Fig. 8.
Contribution of effective dose, as a percentage of TCM effective dose under TCM, ODM (same as TCM), and ODM.
4. Discussion
In this study, we evaluated a commercially available organ-dose-based tube current modulation technique (ODM), which has a dose reduction zone of , with the reduced tube current not further compensated by increasing the mA in posterior projections. We found that ODM on average covered of the breasts tissue volume within the dose reduction zone. ODM showed significant dose reductions compared to TCM: the breast dose reduced by 30%, and other organ doses decreased by 10% to 30%. When the same was used, ODM reduced breast dose by 15%, and all other anterior organ doses by 5% to 12%.
In this study, ODM significantly reduced the effective dose by and DLP-normalized effective dose by . This result was consistent with an anthropomorphic physical phantom study, by which the effective dose was reduced by 5.3% using matched by Dixon et al.25 The authors further reported the effective dose reduction attributed to each organ, with a high fraction of the overall effective dose reduction attributed to the breasts. The effective dose was less influenced by the posterior organs, despite seeing increased organ doses, because most of the radiosensitive organs susceptible to the dose variations are located anteriorly.25
Besides ODM, OTCM is also a commercially available radiosensitive-organ-dose optimization technique. OTCM reduces the tube current (mA) by 75% to 80% over a 120-deg arc anterior to the patients, thus, compared to ODM, OTCM has a higher reduction of mA with narrower angular range. Directly comparing ODM and OTCM may be challenging17 because these techniques are based on different standard TCM techniques. OTCM is based on longitudinal (-) TCM while ODM is based on angular and longitudinal TCM. Moreover, the standard TCM could use different modulation strengths. Comparing ODM/OTCM with the corresponding vendor’s standard TCM with the same , ODM and OTCM reduced the breast dose on average by 15% and 19%, respectively.26 In terms of image quality, reported in physical phantom studies, neither OTCM nor ODM show any detrimental effect, compared to standard TCM. The advantage of ODM is that it has a wider angular dose reduction range, so that most of the breast tissue is within the dose reduction zone. OTCM may potentially be used with a breast positioning technique that constrains most of the breast tissue within the dose reduction zone. In a previous simulation study, we evaluated the dose reduction potential of a sports bra with padded foam.27 OTCM with this breast positioning technique showed decreased breast dose by compared to standard TCM. However, a clinical study in follow up to the simulation is required to evaluate the comfort and cost of this technique along with the effect on image quality.
The effect on image quality due to ODM was not evaluated in this study. Theoretically, ODM reduced 40% of mA across anterior arc and resulted in a reduction of 20% of , and consequently, an 11.8% increase in noise.25 Compared to a fixed mA, ODM has shown increased noise by 14% and 1.6% with and without matched , respectively.25 When same was used, Dixon et al. did not observe changes in noise texture using phantom studies.25 In terms of image uniformity, the ODM technique showed less than 1 HU difference in absolute CT number compared to fixed mA.25 With reduced mA, a potential artifact with ODM could be introduced by photon starvation especially in the shoulder region or for patients with metal implants in clinical cases,25 which will be evaluated in future work.
The detailed behavior of mA modulation is complex and proprietary.28 In this study, the mA was generated based on a ray-tracing program and refined by predictable physical features. For example, the maximum mA was constrained by protocol’s maximum mA limitation. The between the dose reduction and unchanged zones was modeled using a gradual change. However, other mA behaviors were not characterized. For example, Li et al. observed that the mA was relatively high at the transition between the lung and abdominal regions from studies conducted by Khatonabadi et al.19,29 Li et al. also reported that the mA may not strictly follow the attenuation of the patients at the beginning stages of the scan.19,29 With these complexities, a separate study is warranted to model the actual TCM profile.
Unlike factors in fixed mA, which can be generalized to other CT systems, factors under TCM are less generalizable and are dependent on the TCM scheme. In this study, the factors were used to isolate the influence of (thus, total photon flux) between TCM and ODM, and thereby evaluate the net effect of modulation on dose reduction. Additionally, factors differences between ODM and TCM represent dose differences with more similar image quality. Using physical phantoms, Dixon et al. reported that ODM with the same as TCM showed a slight noise increase of , whereas ODM without adjusting increased noise by .25
There are several limitations in this study. First, all analyses were performed considering only one scanner and tube current modulation scheme. Prior studies have evaluated the effect of OTCM on other scanners (e.g., Optima, GE Healthcare)25 and other organ-based TCM schemes (e.g., XCARE, Siemens Healthcare).11,30,31 Second, the glandular tissue distribution was not explicitly modeled and, in our simulation, it was assumed to be uniformly distributed in the breast. Glandular tissue is sensitive to radiation-induced cancer.32 Estimating glandular dose reduction potential using ODM is warranted in future work. Finally, the image quality was not evaluated in this study. Generally, using ODM results in noise increases of more than 10%.25 Clinical studies are required to evaluate the effect of increased noise on the diagnostic outcome of the scans.
5. Conclusion
In this study, we evaluated the organ dose reduction potential with organ-dose-based tube current modulation with a wide dose reduction angle. This angular range was sufficient to cover the whole breast extension for a majority of the patients. This technique shows strong potential in reducing organ dose for most of the radiosensitive organs.
Acknowledgments
The authors would like to thank Dominic J. Crotty for valuable discussions about the organ-dose-based tube current modulation technique and Maria Parker for helpful advice in editing this manuscript.
Biographies
Wanyi Fu is a PhD student at Duke University. She received her MS degree from Duke University in 2016. She received her BE degree from the University of Minnesota, Twin Cities, and Beijing Jiaotong University in 2014. Her research focuses on CT dose and image quality. She is a member of SPIE.
Gregory M. Sturgeon is a mechanical engineer specializing in geometric and computational modeling of anatomical structures with particular interest in deformable breast models, texture synthesis, and cardiac modeling.
Greeshma Agasthya, PhD, is a biomedical engineer with research expertise in medical imaging, modeling, and simulations. She has completed three plus years of postdoctoral research in breast tomosynthesis, CT, and radiography. She received her MS and PhD degrees from Duke University in 2013. She is interested in developing and optimizing the next-generation of imaging systems, clinical and translational research, and virtual clinical trials.
William Paul Segars, PhD, is an associate professor of radiology and biomedical engineering and a member of the Carl E. Ravin Advanced Imaging Laboratories (RAILabs) at Duke University, Durham, North Carolina, USA. He received his PhD in biomedical engineering from the University of North Carolina in 2001. He is among the leaders in the development of simulation tools for medical imaging research where he has applied state-of-the-art computer graphics techniques to develop realistic anatomical and physiological models.
Anuj J. Kapadia, PhD, is an assistant professor of radiology, physics, and medical physics at Duke University. He also serves as the director of graduate studies at Duke Medical Physics Graduate Program. His research interests include x-ray and neutron scatter imaging, dosimetry analysis from clinical and preclinical imaging systems, and Monte-Carlo simulation development for assessment of image quality, safety, and system performance evaluation. He is a member of SPIE.
Ehsan Samei, PhD, DABR, FAAPM, FSPIE, FAIMBE, is a tenured professor at Duke University, where he serves as the director of Duke Medical Physics Graduate Program and the Clinical Imaging Physics Program. His interests include clinically relevant metrology of imaging quality and safety for optimum interpretive and quantitative performance. He strives to bridge the gap between scientific scholarship and clinical practice by meaningful realization of translational research and the actualization of clinical processes that are informed by scientific evidence.
Disclosures
E.S. has grant support unrelated to this study from Siemens Medical Solutions and GE Healthcare. Other authors have no relevant conflicts of interest to disclose.
References
- 1.IMV, CT Market Outlook Report IMV Medical Information Division, Des Plaines, Illinois: (2014). [Google Scholar]
- 2.Evens R. G., Mettler F., “National CT use and radiation exposure: United States 1983,” Am. J. Roentgenol. 144(5), 1077–1081 (1985). 10.2214/ajr.144.5.1077 [DOI] [PubMed] [Google Scholar]
- 3.Brenner D. J., Hall E. J., “Computed tomography—an increasing source of radiation exposure,” N. Engl. J. Med. 357(22), 2277–2284 (2007). 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 4.McCollough C. H., et al. , “Achieving routine submillisievert CT scanning: report from the summit on management of radiation dose in CT,” Radiology 264(2), 567–580 (2012). 10.1148/radiol.12112265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentin J., The 2007 Recommendations of the International Commission on Radiological Protection, Elsevier, Oxford: (2007). [Google Scholar]
- 6.Angel E., et al. , “Dose to radiosensitive organs during routine chest CT: effects of tube current modulation,” Am. J. Roentgenol. 193(5), 1340–1345 (2009). 10.2214/AJR.09.2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricke B. L., et al. , “In-plane bismuth breast shields for pediatric CT: effects on radiation dose and image quality using experimental and clinical data,” Am. J. Roentgenol. 180(2), 407–411 (2003). 10.2214/ajr.180.2.1800407 [DOI] [PubMed] [Google Scholar]
- 8.Mc Laughlin D., Mooney R., “Dose reduction to radiosensitive tissues in CT. Do commercially available shields meet the users’ needs?” Clin. Radiol. 59(5), 446–450 (2004). 10.1016/j.crad.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 9.Kubo T., et al. , “Radiation dose reduction in chest CT: a review,” Am. J. Roentgenol. 190(2), 335–343 (2008). 10.2214/AJR.07.2556 [DOI] [PubMed] [Google Scholar]
- 10.Raissaki M., et al. , “Eye-lens bismuth shielding in paediatric head CT: artefact evaluation and reduction,” Pediatr. Radiol. 40(11), 1748–1754 (2010). 10.1007/s00247-010-1715-6 [DOI] [PubMed] [Google Scholar]
- 11.Duan X., et al. , “Dose reduction to anterior surfaces with organ-based tube-current modulation: evaluation of performance in a phantom study,” Am. J. Roentgenol. 197(3), 689–695 (2011). 10.2214/AJR.10.6061 [DOI] [PubMed] [Google Scholar]
- 12.Samei E., “Pros and cons of organ shielding for CT imaging,” Pediatr. Radiol. 44(3), 495–500 (2014). 10.1007/s00247-014-3084-z [DOI] [PubMed] [Google Scholar]
- 13.Euler A., et al. , “Organ-based tube current modulation in a clinical context: dose reduction may be largely overestimated in breast tissue,” Eur. Radiol. 26(8), 2656–2662 (2015). 10.1007/s00330-015-4085-5 [DOI] [PubMed] [Google Scholar]
- 14.Taylor S., et al. , “Organ-based tube current modulation: are women’s breasts positioned in the reduced-dose zone?” Radiology 274(1), 260–266 (2014). 10.1148/radiol.14140694 [DOI] [PubMed] [Google Scholar]
- 15.Lee A. H. S., “Why is carcinoma of the breast more frequent in the upper outer quadrant? A case series based on needle core biopsy diagnoses,” Breast 14(2), 151–152 (2005). 10.1016/j.breast.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Hurt K. J., et al. , The Johns Hopkins Manual of Gynecology and Obstetrics, Lippincott Williams & Wilkins, Philadelphia, Pennsylvania: (2012). [Google Scholar]
- 17.Gandhi D., et al. , “Technical note: phantom study to evaluate the dose and image quality effects of a computed tomography organ-based tube current modulation technique,” Med. Phys. 42(11), 6572–6578 (2015). 10.1118/1.4933197 [DOI] [PubMed] [Google Scholar]
- 18.Segars W. P., et al. , “Population of anatomically variable 4D XCAT adult phantoms for imaging research and optimization,” Med. Phys. 40(4), 043701 (2013). 10.1118/1.4794178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Segars W. P., Samei E., “The impact on CT dose of the variability in tube current modulation technology: a theoretical investigation,” Phys. Med. Biol. 59(16), 4525–4548 (2014). 10.1088/0031-9155/59/16/4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., et al. , “Patient-specific radiation dose and cancer risk estimation in CT: Part I. Development and validation of a Monte Carlo program,” Med. Phys. 38(1), 397–407 (2011). 10.1118/1.3515839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaffe M., et al. , “The myth of the 50-50 breast,” Med. Phys. 36(12), 5437–5443 (2009). 10.1118/1.3250863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerstein R., et al. , “Absorbed radiation dose in mammography 1,” Radiology 130(2), 485–491 (1979). 10.1148/130.2.485 [DOI] [PubMed] [Google Scholar]
- 23.Baro J., et al. , “PENELOPE: an algorithm for Monte Carlo simulation of the penetration and energy loss of electrons and positrons in matter,” Nucl. Instrum. Methods Phys. Res. Sect. B 100(1), 31–46 (1995). 10.1016/0168-583X(95)00349-5 [DOI] [Google Scholar]
- 24.Sempau J., et al. , “Experimental benchmarks of the Monte Carlo code PENELOPE,” Nucl. Instrum. Methods Phys. Res. Sect. B 207(2), 107–123 (2003). 10.1016/S0168-583X(03)00453-1 [DOI] [Google Scholar]
- 25.Dixon M. T., et al. , “An evaluation of organ dose modulation on a GE optima CT660-computed tomography scanner,” J. Appl. Clin. Med. Phys. 17(3), 380–391 (2016). 10.1120/jacmp.v17i3.5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu W., et al. , “CT breast dose reduction with the use of breast positioning and organ-based tube current modulation,” Med. Phys. 44(2), 665–678 (2017). 10.1002/mp.12076 [DOI] [PubMed] [Google Scholar]
- 27.Fu W., et al. , “Estimation of breast dose saving potential using a breast positioning technique for organ-based tube current modulated CT,” Proc. SPIE 9783, 97833C (2016). 10.1117/12.2217239 [DOI] [Google Scholar]
- 28.Khatonabadi M., et al. , “A comparison of methods to estimate organ doses in CT when utilizing approximations to the tube current modulation function,” Med. Phys. 39(8), 5212–5228 (2012). 10.1118/1.4736807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatonabadi M., et al. , “The feasibility of a regional CTDIvol to estimate organ dose from tube current modulated CT exams,” Med. Phys. 40(5), 051903 (2013). 10.1118/1.4798561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., et al. , “Radiation dose reduction to the breast in thoracic CT: comparison of bismuth shielding, organ-based tube current modulation, and use of a globally decreased tube current,” Med. Phys. 38(11), 6084–6092 (2011). 10.1118/1.3651489 [DOI] [PubMed] [Google Scholar]
- 31.Lungren M. P., et al. , “Radiation dose estimations to the thorax using organ-based dose modulation,” Am. J. Roentgenol. 199(1), W65–W73 (2012). 10.2214/AJR.11.7798 [DOI] [PubMed] [Google Scholar]
- 32.Ronckers C. M., Erdmann C. A., Land C. E., “Radiation and breast cancer: a review of current evidence,” Breast Cancer Res. 7(1), 21 (2004). 10.1186/bcr970 [DOI] [PMC free article] [PubMed] [Google Scholar]