Abstract
Purpose
The present study compared laryngeal aerodynamic function of healthy older adults (HOA) to adults with Parkinson's disease (PD) while speaking at a comfortable and increased vocal intensity.
Method
Laryngeal aerodynamic measures (subglottal pressure, peak-to-peak flow, minimum flow, and open quotient [OQ]) were compared between HOAs and individuals with PD who had a diagnosis of hypophonia. Increased vocal intensity was elicited via monaurally presented multitalker background noise.
Results
At a comfortable speaking intensity, HOAs and individuals with PD produced comparable vocal intensity, rates of vocal fold closure, and minimum flow. HOAs used smaller OQs, higher subglottal pressure, and lower peak-to-peak flow than individuals with PD. Both groups increased speaking intensity when speaking in noise to the same degree. However, HOAs produced increased intensity with greater driving pressure, faster vocal fold closure rates, and smaller OQs than individuals with PD.
Conclusions
Monaural background noise elicited equivalent vocal intensity increases in HOAs and individuals with PD. Although both groups used laryngeal mechanisms as expected to increase sound pressure level, they used these mechanisms to different degrees. The HOAs appeared to have better control of the laryngeal mechanism to make changes to their vocal intensity.
Vincent and Velkoff (2010) reported that by the year 2030, individuals aged 65 and older will represent approximately 20% of the population. Furthermore, the National Institute of Neurological Disorders and Stroke (NINDS, 2013) approximates that 500,000 Americans are living with Parkinson's disease (PD). It is projected that more than 610,000 individuals will be affected by PD by 2030 (Dorsey et al., 2007). Aging of the general population and increased prevalence of PD motivate the need to understand how age, disease state, and disease progression affect underlying speech subsystems. With the aging of the general population, and with an increased prevalence of individuals with PD, the first purpose of the current study was to investigate whether healthy older adults (HOAs) and individuals with PD perform similarly with respect to laryngeal function during speech. Further, as hypophonia (perceptually quiet voice) is a concern in individuals with PD, the second purpose of the current study was to assess whether HOAs and adults with PD and hypophonia were able to utilize the same laryngeal adjustments when increasing sound pressure level (SPL).
Typically aging adults may experience qualitative voice changes as they age. One investigation of younger and older women reported that older women were no breathier than their younger peers (Gorham-Rowan & Laures-Gore, 2006). However, other studies described the voices of typically aging adults as having a breathy quality (Baken, 2005; Eadie, 2000; Gregory, Chandran, Lurie, & Sataloff, 2012; Linville, 2000, 2002, 2004; Ramig et al., 2000; Sauder, Roy, Tanner, Houtz, & Smith, 2010; Verdonck-de Leeuw & Mahieu, 2004). Further, older voices have been described by researchers as having reduced loudness (Goy, Fernandes, Pichora-Fuller, & van Lieshout, 2013; Gregory et al., 2012; Hodge, Colton, & Kelley, 2001; Linville, 2000; Zraick, Smith-Olinde, & Shotts, 2012). In sum, typically aging adults may experience a reduction in speech loudness, with a breathy vocal quality.
Individuals with PD experience some of the voice changes found in typically aging adults. Darley, Aronson, and Brown (1969b) outlined the perceptual characteristics of speech produced by individuals with PD, describing the characteristics with the term hypokinetic dysarthria. Darley, Aronson, and Brown (1969a) surmised that some of the observed speech characteristics in individuals with PD, including a breathy, harsh voice, could be due to rigidity in the muscles of the laryngeal system. In a study of 200 individuals with PD, Logemann, Fisher, Boshes, and Blonsky (1978) reported that 90% of people with PD experienced voice problems. The perceptual characteristic of breathiness was present in 15%, and hoarseness was present in 45% of their sample. Hypophonia, or perceptually quiet voice, is often present in individuals with PD (Dykstra, Adams, & Jog, 2012; Ho, Iansek, Marigliani, Bradshaw, & Gates, 1999; Holmes, Oates, Phyland, & Hughes, 2000; Kent & Kim, 2003; Logemann et al., 1978) and has been compared by Ho, Bradshaw, and Iansek (2008) as the laryngeal correlate to reduced limb movement (hypokinesia). Although the voices of typically aging adults and individuals with PD seem similar in qualitative description, little systematic objective data exist that compare the underlying laryngeal mechanisms reflected in their voices.
During speech, the laryngeal system regulates air flow (Smitheran & Hixon, 1981) and is an essential component in the production of a clear-sounding voice and normal regulation of vocal intensity (LaBlance, Steckol, & Cooper, 1991; Spencer, Yorkston, & Duffy, 2003). To produce voicing, the intrinsic muscles of the larynx must synergistically coordinate tone and tension to approximate the vocal folds to midline to allow for the build-up of subglottal pressure (Ps) and subsequent vocal fold vibration. In order to best assess laryngeal function during speech, evaluation of this valving mechanism is essential.
Laryngeal valving during speech has been studied using various invasive and noninvasive methods. Analysis of the acoustic signal is a noninvasive means of indirectly examining laryngeal function. In studies by H. M. Hanson (1997) and H. M. Hanson and Chuang (1999), the authors reported that select acoustic measures reflect glottal openness and timing. However, acoustic measures do not allow for calculation of Ps and glottal airflow and therefore do not allow for a more complex assessment of laryngeal function. Tracheal puncture with pressure transducer allows for accurate measure of Ps at its source but is highly invasive. Research has shown that indirect measures of laryngeal function through analysis of estimated subglottal air pressure (Löfqvist, Carlborg, & Kitzing, 1982; Smitheran & Hixon, 1981) and derived glottal airflow (Holmberg, Hillman, & Perkell, 1988; Stathopoulos & Sapienza, 1997) are effective, as well as substantially less invasive. Holmberg et al. (1988) and Stathopoulos and Sapienza (1997) reported successful inverse filtering of the glottal airflow waveform and used their measures to reflect vocal fold open time, extent of closure, and rate of closure. In a more recent study, Kobler, Hillman, Zeitels, and Kuo (1998) simultaneously collected videostroboscopic and aerodynamic data during voicing. The authors found that aerodynamic and videostroboscopic data are highly correlated. Laryngeal aerodynamic measures of interest in the present study include the estimate of Ps, the rate of vocal fold closure (maximum flow declination rate [MFDR]), the ratio of the time that the vocal folds are open in one cycle relative to the time of one cycle (open quotient [OQ]), translaryngeal airflow from maximal opening to maximal closing (peak-to-peak flow [PPFLow]), and the flow at the point of greatest glottal closure (minimum flow [MinFlow]; Holmberg et al. 1988; Stathopoulos & Sapienza, 1997). These laryngeal aerodynamic measures have been shown to provide reliable, noninvasive, quantitative assessments of laryngeal function during voicing.
Physiological adjustments necessary to increase SPL at the level of the larynx have long been substantiated and include increased driving pressure below the vocal folds, increased amplitude of vibration, increased speed of vocal fold closure, and increased closed time (Isshiki, 1964). Investigators have confirmed the accompanying aerodynamic changes due to increased SPL in a variety of speakers, including an increase in Ps (Holmberg et al., 1988; Melcon, Hoit, & Hixon, 1989; Stathopoulos & Sapienza, 1993), MFDR, PPFlow (Holmberg et al., 1988; Stathopoulos & Sapienza, 1993), and a decrease in OQ (Stathopoulos & Sapienza, 1993). In short, laryngeal physiological and concomitant aerodynamic adjustments occur to facilitate increased speaking SPL.
In adults with PD, studies have supported that many laryngeal aerodynamic adjustments to change SPL are consistent with those made by healthy younger and older adults. Researchers have found that measures of MFDR and Ps are similarly affected by increases in SPL in individuals with PD as in HOAs (Dromey, Ramig, & Johnson, 1995; Ramig & Dromey, 1996). OQ was found to nonsignificantly decrease in response to increased SPL (Dromey et al., 1995; Ramig & Dromey, 1996). However, no investigations have reported on PPFlow and MinFlow measures in individuals with PD relative to changes in SPL. It appears that individuals with PD produce adjustments in aerodynamics that reflect underlying laryngeal configurations similar to those used by HOAs, but more importantly, it is still unknown whether individuals with PD make laryngeal adjustments similar to those of HOAs when speaking at a higher vocal intensity. Comparison to a control group of HOAs would allow for differential understanding of the laryngeal aerodynamic mechanisms used by individuals with PD.
Aerodynamic studies show that laryngeal function can change due to typical aging, unrelated to pathology. Higgins and Saxman (1991) found that older adults produced voicing with significantly higher estimated Ps when compared to younger adults. This result was at odds with the results of Melcon et al. (1989) who found no significant difference in Ps across ages studied. Hodge et al. (2001) found that older participants produced voicing with the vocal folds open for a significantly longer portion of the cycle than their younger cohorts. These aerodynamic events reflect changes to voicing secondary to aging processes, such as perceptually quieter speech with a breathy quality (Goy et al., 2013; Gregory et al., 2012; Hodge et al., 2001; Linville, 2000, 2002, 2004; Ramig et al., 2000; Zraick et al., 2012).
Due to the fact that PD typically occurs in older adults, individuals with PD can experience decline in function related to disease, overlaying the functional changes in the larynx due to aging. Few studies report aerodynamic values in individuals with PD when compared to their healthy peers. One study by Jiang et al. (1999) investigated individuals with PD who had no reported problems with voice quality. Jiang et al. reported that individuals with PD produced voice with greater Ps than the HOAs at the same SPL. Further, Jiang et al. found no differences in translaryngeal flow values between the groups. Thus, they concluded that the increased Ps used by individuals with PD was due a greater level of resistance at the level of the larynx. Jiang et al. further surmised that, although not statistically analyzed, minFlow differed between the groups (higher in the PD group). The study did not report data related to rate of vocal fold closure or the amount of time that the vocal folds spent open during the vibratory cycle. Overall, it is clear that typical aging, as well as PD, can affect laryngeal aerodynamic measures reflecting altered laryngeal function. Although there are some studies comparing laryngeal aerodynamic function in HOA and individuals with PD, there are still questions related to rate and duration of vocal fold closure at baseline and while increasing SPL. Therefore, aerodynamic studies are needed to provide a systematic representation of glottal aerodynamic differences between the groups under conditions of vocal intensity alterations.
Study Aims and Hypotheses
The first purpose of the current study was to investigate whether HOAs and individuals with PD show similar laryngeal performance while speaking at a comfortable intensity. Due to the prevalence of hypophonia in individuals with PD and mixed reports on vocal loudness problems in typically aging adults, it was hypothesized that HOAs would produce higher SPLs when speaking at comfortable vocal intensity than adults with PD. On the basis of the prediction that HOAs will produce comfortable speech at higher SPLs than the adults with PD, it was hypothesized that HOAs would produce speech with higher Ps, MFDR, and PPFlow, smaller OQ, and lower MinFlow than the adults with PD.
The second purpose of this study was to assess whether HOAs and adults with PD utilize the same laryngeal adjustments when increasing SPL. On the basis of the reports of Adams and Lang (1992) and Adams et al. (2006) that individuals with PD increased SPL to the same degree as their healthy peers when speaking in noise, it was hypothesized that HOAs and adults with PD would respond to multitalker babble noise by increasing SPL to the same degree. It was further hypothesized that both groups would demonstrate concomitant changes to laryngeal function as reflected by higher Ps, MFDR, and PPFlow, smaller OQ, and lower MinFlow when speaking louder.
Method
Participants
Healthy, typically aging older adults and older adults with a diagnosis of PD were recruited for study. The HOA group consisted of 20 participants (mean 68.9 years; age range 60–81 years): 10 men and 10 women who were age-matched to the adults with PD. The PD group consisted of 42 participants (mean 70.17 years; age range 37–86 years): 34 men and eight women. Appendix A provides ages for each of the participants. The participants were required to meet the following criteria: (a) speaker of Standard American English; (b) no known history of neurological disease (aside from PD); (c) no known history of respiratory disease; (d) no history of chest, head, or neck surgery; (e) no smoking in last 5 years; (f) no bilateral use of hearing aids and functional hearing in at least one ear unaided; (g) in individuals with PD, a diagnosis of hypophonia from a speech-language pathologist or a self-report of hypophonia. Appendix B provides demographic data for the participants with PD including time since diagnosis, history of speech therapy, and medications.
Voice quality characteristics were assessed for each participant using a voice quality rating scale on the basis of the classic Mayo Clinic studies (Darley et al., 1969a, 1969b). The rating scale assessed characteristics typically observed in individuals with PD and included ratings of hypophonia, loudness decay, breathiness, and hoarseness (see Appendix A for ratings). HOAs were rated on the same qualitative scales. The hypophonia rating for individuals with PD was of particular importance because the present study targeted their capability to increase SPL. Forty of the 42 participants with PD were clinician-rated as hypophonic, and two of the 42 were self-rated as hypophonic. None of the HOAs were rated as hypophonic. The final inclusionary criterion was adequate performance in the data collection task. The participants were required to adequately produce a tight labial seal over the pitot tube placed between the lips to allow for accurate Ps estimation and produce enough adequate voicing for the airflow analyses. Any participants who were unable to complete the task in either the speech-in-quiet or speech-in-noise condition are marked with an “M” in Appendix B, and missing data are described in the measurements section below.
Equipment
Intraoral pressure was sensed using a pitot tube (1.6-mm inside diameter, 2.8-mm outside diameter, 0.58-mm wall thickness) with the fenestrated tip placed immediately posterior to the central incisors. The participants were instructed to close their lips tightly over the pitot tube when closing their mouth during speech. The pitot tube was seated in the left port of the circumferentially vented pneumotachograph mask, coupled to the PTL-1 Glottal Enterprises pressure transducer.
Oral airflow was collected using a circumferentially vented mask, with Glottal Enterprises PTW-1 flow transducer (Glottal Enterprises, Syracuse, NY) affixed in the right port of the mask. During data collection, the mask was held by the researcher to ensure an airtight seal between the circumferentially vented mask and face of the participant. A period of zero-flow was captured before and after the face mask was placed on the participant's face to account for potential drift of the airflow signal. The signals from the PTL-1 and PTW-1 transducers (Glottal Enterprises, Syracuse, NY) were amplified using the MS100-A2 (Glottal Enterprises, Syracuse, NY) amplifier and captured in LabChart (AD Instruments, Version 7.3.7, Colorado Springs, CO) at a 4 kHz sampling rate. The PTL-1 and PTW-1 Glottal Enterprises transducers were calibrated weekly using the MCU-4 (Glottal Enterprises, Syracuse, NY) pneumotachograph calibration unit.
In order to elicit the Lombard effect during speaking, the first generation SpeechVive device (further referred to as the Lombard-eliciting, or LE device) was used. The LE device sensed speech via an accelerometer that was placed on the thyroid lamina of the participant. In response to the onset of speech and a response from the accelerometer, multitalker babble noise (typically described as what can be heard in a noisy restaurant or cocktail party) was played via an open ear fitting into one of the participant's ears. The multitalker babble noise was calibrated to each individual, with the noise level adjusted to elicit an increase of 3–5 dB SPL above that found during a comfortable intensity level production of connected speech.
Speech Task
The current study used the use of a technique similar to that used by Löfqvist et al. (1982) and Smitheran and Hixon (1981) for estimating subglottal air pressure (Ps) from intraoral air pressure (PIO) and for estimating laryngeal airflow (V̇tl) from oral airflow. Participants produced the sentence “buy pop or pop a papa.” The sentence was designed to appropriately sense intraoral air pressure during the voiceless consonants and oral airflow during the vowels. A phrase was used, rather than a syllable train, because individuals with PD have difficulty with steady syllable repetition (Skodda, 2011). The sentence task is similar to a task used Södersten, Hertegård, and Hammarberg (1995) and meets criteria for reliable estimation of Ps (Demolin et al., 1997; Löfqvist et al., 1982).
Procedures
At the onset of data collection, each participant received an informed consent for review and signature and completed a health questionnaire, which was reviewed for completeness by the researcher. Each of the individuals with PD received a full audiometric hearing evaluation. Each of the HOA participants received a pure-tone hearing screening at the frequencies of 500, 1000, 2000, 4000, and 8000 Hz, with the signals presented at 35 dB SPL (American National Standards Institute; ANSI, 1969). The results of the hearing assessments provided the researchers with information regarding whether the participant had a “better ear.” If the participant had a better ear, the LE device was placed in that ear. If no better ear was identified in individuals with PD, the LE device was placed in the ear on the side of the body with better manual dexterity. If no better ear was identified in the HOAs, the LE device was placed in the right ear. Pure-tone averages are presented for the ear in which the device was placed in Appendix B.
Each participant was comfortably seated in an armchair. The speech tasks were first collected without the LE device present (speech-in-quiet condition). After the speech tasks were complete, the participant was instructed to speak on a topic of his or her choice so that the researchers could obtain a baseline SPL. A microphone coupled to an SPL meter was placed at the same height as, and at a distance of 12 in. from the participant's mouth. The display of the SPL meter, set on the slow weighting setting, was obscured from view of the participant. A baseline speaking SPL was determined by visually averaging the SPL meter readout. Two investigators observed the SPL meter and their judgments of the comfortable level agreed within 2 dB. Then, the open-fitting ear connector from the LE device was placed in the better ear of the participant and the accelerometer was affixed to the thyroid lamina using double-sided tape. The multitalker babble noise was adjusted as the participant continued to discuss a topic of his/her choice until the participant increased speaking SPL by 3–5 dB as observed by the same two researchers from the SPL meter. After the noise level was set, the participant completed the speech tasks again, this time with the device on (speech-in-noise condition).
For the speech task of interest in the current study, each participant was fitted with the appropriate size circumferentially vented pneumotachograph mask. The seal on each participant's face was assessed prior to each data collection trial by asking the client to exhale into the mask to check for leaks. If a leak was detected, the mask was reseated and the seal was reassessed. Once a seal was confirmed, each participant was instructed to produce the sentence task (as described above) on one breath, at a comfortable pitch and loudness, and in a slow and monotone manner. Production of the task was required to be produced in a slow manner to allow for pressure equalization of the intraoral to Ps during the voiceless plosive, and in a monotone manner to encourage steady-state vowel segments. The researcher provided a model and each participant practiced the task until the appropriate rate and manner of delivery was established. The participant performed the speech task until three trials of the task were adequately performed, with a maximum number of five repetitions to avoid fatigue.
Measurements
Ps
For a production to be included in the measurements, zero flow was required during bilabial closure, indicating adequate closure of the velopharyngeal port, as well as appropriate lip closure over the pitot tube. Measures of PIO were made at the peak of the oral pressure waveform for each [p] production in “pop” and “papa.” Measures of pharyngeal pressure (PPh) were made from oral pressure in the center of in the vowel segment between the target [p] pressure peaks. Ps was estimated using the formulas below (Smitheran & Hixon, 1981):
In the baseline analysis (speech-in-quiet group comparison), Ps data were included for 18 HOAs. Two HOAs did not occlude the tube adequately for estimation of Ps. Ps data from 32 individuals with PD were included. Five individuals with PD did not occlude the tube adequately for estimation of Ps and one individual with PD had too much hyperkinetic movement to collect pressure data. In the speech-in-quiet to speech-in-noise analysis, data had to be present for both conditions for the participant to be included. Ps data were included for 14 HOAs. Two HOAs did not occlude the tube adequately for estimation of Ps, and four HOA did not increase SPL across the conditions. Ps data were included for 14 individuals with PD. Ten people with PD did not occlude the tube adequately for estimation of Ps, five did not produce the syllable train with enough voicing to measure SPL, one individual with PD had too much hyperkinetic movement to collect pressure data, and 12 of the remaining individuals with PD did not increase SPL in the speech-in-noise condition.
Glottal Aerodynamic Measurements
The midpoint of each vowel segment in the “pop” productions and the first vowel in “papa” were examined for the following criteria before measurement: (a) a waveform that was steady and periodic with (b) a stable pitch trace, (c) a steady RMS trace, and (d) clear harmonics present in a narrow-band spectrogram. The middle portion of these vowel productions was inverse filtered (Rothenberg, 1973). Linear predictive coding in TF32 (Milenkovic, 2011) was used to determine the vowel resonances for use in inverse filtering of the airflow waveform. Measures made from the inverse-filtered waveform included PPFlow, OQ, and MinFlow. PPFlow was determined as the amount of flow that occurred from the point of maximal opening of the vocal folds (the peak of the inverse-filtered waveform) to the point of maximal closure of the vocal folds (the minimum trough of the inverse-filtered waveform; Holmberg et al., 1988). For calculation of OQ, first the point at 20% of peak-to-peak glottal airflow was determined. Then OQ was calculated at that 20% point as the amount of time that the vocal folds were open in the cycle divided by the duration of the entire cycle (Stathopoulos & Sapienza, 1993). MinFlow was determined as the amount of airflow present at the point of maximal closure of the vocal folds. MFDR was defined as the point of maximal rate of closure of the vocal folds along the closing portion of the airflow waveform. MFDR was determined from the greatest negative peak from the first derivative of the inverse-filtered airflow waveform (Stathopoulos & Sapienza, 1993).
In the baseline analysis (speech-in-quiet group comparison), airflow data were included for 19 HOAs. One HOA did not produce adequate voicing to estimate airflow parameters. Airflow data from 34 individuals with PD were included. Seven individuals with PD did not produce adequate voicing to estimate airflow parameters, and one had too much hyperkinetic movement to collect airflow data. In the speech-in-quiet to speech-in-noise analysis, data had to be present for both conditions for the participant to be included. Airflow data were included for 15 HOAs. One HOA did not produce adequate voicing to estimate the airflow parameters, and four HOAs did not increase SPL across the conditions. Airflow data were included for 17 individuals with PD. Nine did not produce the syllable train with enough voicing to estimate the airflow parameters, one had too much hyperkinetic movement to collect airflow data, and 15 of the remaining individuals with PD did not increase SPL in the speech-in-noise condition.
Statistical Analysis
Mixed-model analyses of variance (ANOVA) were computed in SAS, Version 9.3 (SAS Institute, Cary, NC). In the first analysis, group (PD and HOA) were the between-subjects factor and only data from the speech-in-quiet condition were included. This served to demonstrate baseline differences between the two groups. A second mixed-model ANOVA was computed with group (PD and HOA) as a between-subjects factor and condition (speech in quiet and speech in noise) as a within-subject factor. This analysis only included participants who increased SPL in the speech-in-noise condition (see Appendix B). If the interaction of group and condition was significant in the ANOVA, post hoc follow-up analyses were conducted (Tukey's honestly significant difference [HSD]). For this second analysis, our focus was on the changes from speech in quiet to speech in noise and group differences in the speech-in-noise condition. Both models included adjustments for repeated measures (with participant as the repeated factor). Significance was set at p < .05.
Before completing the ANOVAs, outlier analysis was conducted to reduce inclusion of data points that do not truly represent the sample. These outlier data points are considered to be due to algorithm failure to accurately track cycle-to-cycle boundaries and other time-based measures (Awan & Roy, 2006; Rabinov, Kreiman, Gerratt, & Bielamowicz, 1995; Roark, 2006), which can interfere with accurate inverse filtering of the glottal airflow waveform (Milenkovic, 1986). Outlier analysis was conducted prior to statistical analysis, following the model of Tukey (1977). Data outside of the doubled interquartile range (2 × IQR – the range encompassing the data from the first quartile, or 25th percentile to the third quartile, or 75th percentile) were excluded as outlier data points. For all dependent variables except MFDR, minimum acceptable values were not allowed to fall below 0 even if the 2 × IQR allowed these values. Because the data sets were different for the two analyses, the outlier analyses were conducted separately. For the off-condition-only data set, the outlier analysis lead to data points outside of 2 × IQR eliminated, as follows: SPL – no data points lost; Ps – no data points lost; MFDR – 4 data points lost (0.8% loss); OQ – no data points lost; PPFlow – 12 data points lost (2.5% loss); MinFlow – 49 data points lost (10% loss). For the off–on condition comparison data set, the outlier analysis led to data points outside of 2 × IQR eliminated, as follows: SPL – 6 data points lost (1% loss); Ps – 1 data point lost (0.1% data loss); MFDR – 20 data points lost (3.6% loss); OQ – no data points lost; PPFlow – 21 data points lost (3.8% loss); MinFlow – 48 data points lost (8.6% loss).
Both inter- and intrareliability measures were conducted on 10% of sessions. Intraclass correlation coefficient (ICC) analyses were conducted between the original and interreliability measurement, as well as between the original and intrareliability measurement, using SPSS Statistics, Version 21 (IBM SPSS Statistics for Windows, 2012). The ICC results range for interrater reliability was ICC = 0.823 to ICC = 0.984 (see Table 1). The ICC results range for intrarater reliability was ICC = 0.981 to ICC = 1.000 (see Table 1). These values indicate excellent inter- and intrameasurer reliability (Rosner, 2011).
Table 1.
Reliability statistics—Intraclass correlation coefficient (ICC).
| Dependent variable | Intermeasurer reliability | Intrameasurer reliability |
|---|---|---|
| SPL | 0.957 | 0.996 |
| Ps | 0.823 | 1.000 |
| MFDR | 0.964 | 0.992 |
| PPFlow | 0.969 | 0.996 |
| OQ | 0.984 | 0.981 |
| MinFlow | 0.955 | 0.986 |
Note. SPL = sound pressure level; Ps = estimated subglottal pressure; MFDR = maximum flow declination rate; PPFlow = peak-to-peak flow; OQ = open quotient; MinFlow = minimum flow.
Results
Speech-in-Quiet Analysis
SPL
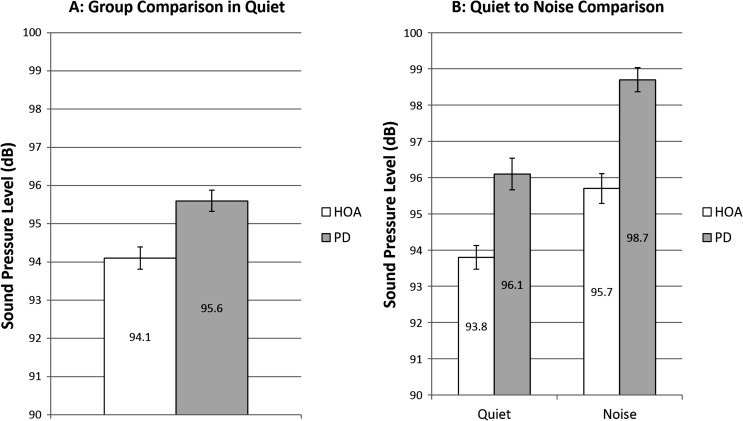
There was no significant main effect of group, F(1, 51) = 2.64, p = .110. Mean SPL in quiet for the HOA group was 94.1 dB (SE = 0.29 dB) and for the PD group was 95.6 dB (SE = 0.28 dB; see Figure 1).
Figure 1.
Sound pressure level. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of SPL at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise SPL comparisons of HOAs to individuals with PD and includes only those who increased SPL when speaking in noise. Error bars represent standard errors.
Ps
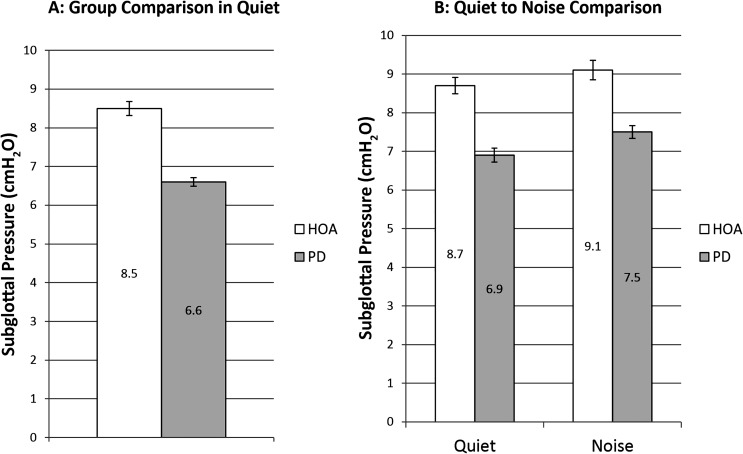
There was significant main effect of group, F(1, 48) = 9.82, p = .003; d = 0.89. HOA produced a significantly higher Ps in quiet (M = 8.5 cmH2O, SE = 0.18 cmH2O) than the individuals with PD (M = 6.6 cmH2O, SE = 0.11 cmH2O; see Figure 2).
Figure 2.
Subglottal pressure. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of Ps at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise Ps comparisons of HOAs to individuals with PD and includes only those who increased SPL when speaking in noise. Error bars represent standard errors.
MFDR
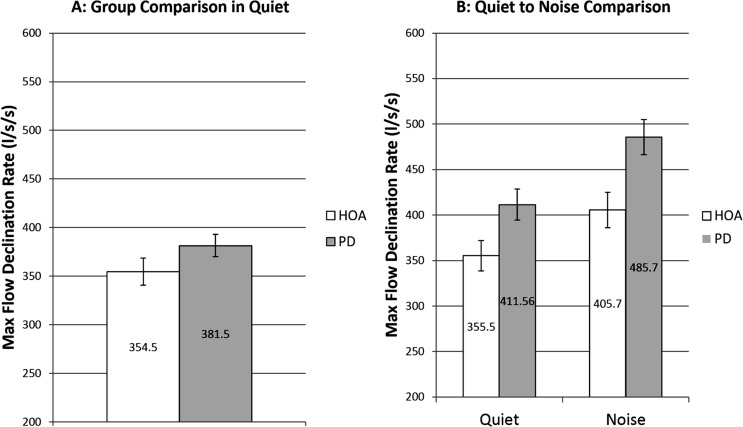
There was no significant main effect of group, F(1, 51) = .87, p = .354. Mean MFDR in quiet for the HOA group was 354.5 L/s/s (SE = 13.9 L/s/s) and for the PD group was 381.5 L/s/s (SE = 11.4 L/s/s; see Figure 3).
Figure 3.
Maximum flow declination rate. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of MFDR at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise MFDR comparisons of HOAs to individuals with PD and includes only those who increased SPL when speaking in noise. Error bars represent standard errors.
PPFlow
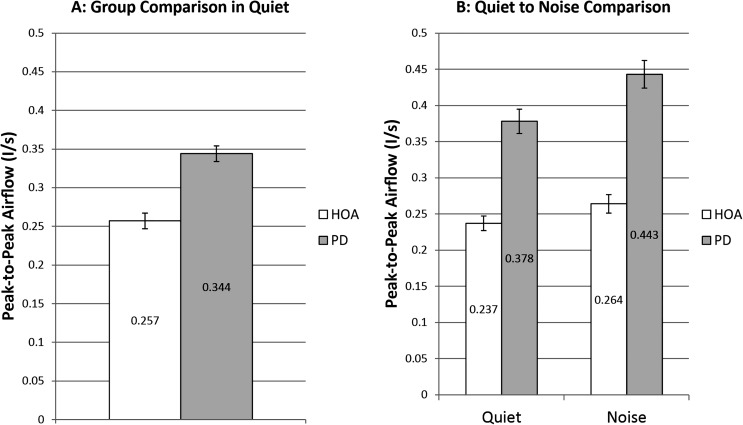
There was a significant main effect of group, F(1, 51) = 8.29, p = .006, d = 0.618. HOA produced a significantly lower PPFlow in quiet (M = 0.257 L/s, SE = 0.010 L/s) than the individuals with PD (M = 0.344 L/s, SE = 0.010 L/s; see Figure 4).
Figure 4.
Peak-to-peak glottal airflow. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of PPFlow at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise PPFlow comparisons of HOAs to individuals with PD and includes only those who increased SPL when speaking in noise. Error bars represent standard errors.
OQ
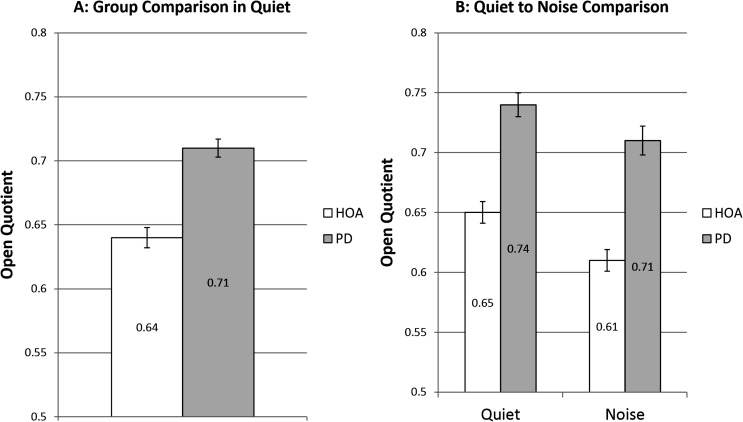
There was a significant main effect of group, F(1, 51) = 6.19, p = .016, d = 0.633. HOA produced a significantly lower OQ in quiet (M = 0.64, SE = .008) than the individuals with PD (M = 0.71, SE = 0.007; see Figure 5).
Figure 5.
Open quotient. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of OQ at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise OQ comparisons of HOAs to individuals with PD and includes only those who increased SPL when speaking in noise. Error bars represent standard errors.
MinFlow
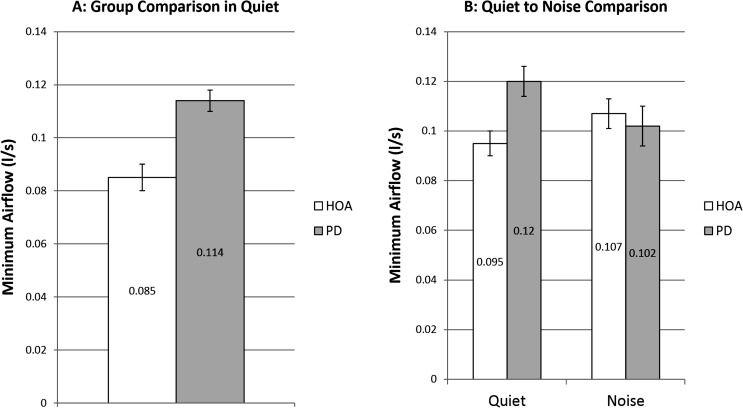
There was no significant main effect of group, F(1, 47) = 2.79, p = .102. Mean MinFlow in quiet for the HOA group was 0.085 L/s (SE = 0.005 L/s) and for the PD group was 0.114 L/s (SE = 0.004 L/s; see Figure 6).
Figure 6.
Minimum flow. White bars denote healthy older adults (HOAs). Gray bars denote individuals with Parkinson's disease (PD). The bar graph on the left represents comparison of minFlow at baseline speech-in-quiet across group. The bar graph on the right represents speech-in-quiet and speech-in-noise minFlow comparisons of HOAs to individuals with PD and included only those who increased SPL when speaking in noise. Error bars represent standard errors.
Speech-in-Quiet to Speech-in-Noise Comparison
SPL
There was a significant main effect of condition, F(1, 30) = 128.95, p < .001, d = 0.65, but there was no significant main effect of group, F(1, 30) = 3.56, p = .069, or interaction between group and condition, F(1, 30) = 0.06, p = .812. For the HOA group, speech-in-noise led to a significant 1.88 dB SPL gain over speech in quiet. For individuals with PD, speech in noise led to a significant 2.59 dB SPL gain over speech in quiet (see Figure 1).
Ps
There was significant main effect of group, F(1, 28) = 4.87, p = .036, d = 0.745, and condition, F(1, 27) = 15.29, p < .001, d = 0.186, but no significant interaction between group and condition, F(1, 27) = 0.00, p = .973. The HOA group significantly increased Ps 0.33 cmH2O from the speech-in-quiet to the speech-in-noise condition (see Figure 2). Ten HOA participants increased Ps. The PD group significantly increased Ps 0.58 cmH2O from the speech-in-quiet to the speech-in-noise condition (see Figure 2). Nine individuals with PD increased Ps. HOA had higher Ps (M = 9.1 cmH20, SE = 0.25 cmH20) than individuals with PD (M = 7.5 cmH20, SE = 0.17 cmH20) in the speech-in-noise condition.
MFDR
There was a significant main effect of condition, F(1, 28) = 84.65, p < .001, d = 0.307, but there no significant main effect of group, F(1, 30) = 1.37, p = .252, or interaction between group and condition, F(1, 28) = 1.18, p = .287. The HOA group significantly increased MFDR 50.19 L/s/s from the speech-in-quiet to the speech-in-noise condition (see Figure 3). Eleven HOA participants increased MFDR. Individuals with PD also significantly increased MFDR 75.17 L/s/s from the speech-in-quiet to the speech-in-noise condition (see Figure 3). Thirteen individuals with PD increased MFDR.
PPFlow
There were significant main effects of group, F(1, 30) = 8.05, p = .008, and condition, F(1, 28) = 69.55, p < .001, and a significant interaction between group and condition, F(1, 28) = 7.74, p = .010. The HOA group significantly increased PPFlow 0.27 L/s from the speech-in-quiet to the speech-in-noise condition (p < .001, d = 0.203; see Figure 4). Twelve HOA participants increased PPFlow. Individuals with PD significantly increased PPFlow 0.65 L/s from the speech-in-quiet to the speech-in-noise condition (p < .001, d = 0.315; see Figure 4). Twelve individuals with PD increased PPFlow. HOA produced significantly lower PPFlow in the speech-in-noise condition (M = 0.264 L/s, SE = 0.013 L/s) than the individuals with PD (M = 0.443 L/s, SE = 0.019 L/s; p = .021, d = 0.975; see Figure 4).
OQ
There were significant main effects of group, F(1, 30) = 7.01, p = .013, d = 0.843, and condition, F(1, 30) = 50.54, p < .001, d = 0.32, but no significant interaction between group and condition, F(1, 30) = 1.82, p = .188. The HOA group significantly decreased OQ 0.05 from the speech-in-quiet to the speech-in-noise condition (see Figure 5). Ten HOA participants decreased OQ. Individuals with PD significantly decreased OQ 0.03 from the speech-in-quiet to the speech-in-noise condition (see Figure 5). Nine individuals with PD decreased OQ. HOA had lower OQ (M = 0.61, SE = 0.009) than individuals with PD (M = 0.71, SE = 0.012) in the speech-in-noise condition.
MinFlow
There was no significant main effect of group, F(1, 29) = 0.00, p = .981, or condition, F(1, 27) = 0.06, p = .803, but there was a significant interaction between group and condition, F(1, 27) = 10.61, p = .003. The HOA group significantly increased MinFlow 0.12 L/s from the speech-in-quiet to the speech-in-noise condition (p = .013, d = 0.189; see Figure 6). Ten HOA participants increased MinFlow. Individuals with PD did not significantly alter MinFlow across the two conditions (p = .309; see Figure 6). There were no significant differences in MinFlow between the HOA and PD groups in the speech-in-noise condition (p = .974).
Discussion
There were two main objectives in the present study. The first was a comparison of laryngeal function between HOA and individuals with PD when they produced a speech task at a comfortable vocal intensity. The second was how the two groups responded to a monaural multitalker babble noise cue to increase SPL.
When speaking comfortably, in the speech-in-quiet condition, the two groups performed remarkably similarly with respect to SPL. The present results are contrary to the findings of some studies where individuals with PD were quieter than their healthy peers (Adams et al., 2006; Fox & Ramig, 1997; Hammer & Barlow, 2010; Ho et al., 1999; Quedas, Duprat, & Gasparini, 2007; Ramig, Sapir, Fox, & Countryman, 2001). In contrast, the present results are supported by a group of other studies that found no vocal intensity difference between individuals with PD and those who were aging normally (Canter, 1963; Darling & Huber, 2011; Dromey & Adams; 2000; Tjaden, Sussman, & Wilding, 2014; Tjaden & Wilding, 2004). This is an important finding in that behavioral treatment programs (i.e., Lee Silverman Voice Treatment; LSVT), as well as nonbehavioral stimulation programs (i.e., Lombard-eliciting), have been used with individuals who have PD and focus on treatment of hypophonia (Adams, Haralabous, Dykstra, Abrams, & Jog, 2005; Adams et al., 2006; Countryman & Ramig, 1993; Ramig, Bonitati, Lemke, & Horii, 1994; Ramig, Countryman, O'Brien, Hoehn, & Thompson, 1996; Ramig, Countryman, Thompson, & Horii, 1995; Stathopoulos et al., 2014). As all participants with PD were rated as hypophonic but spoke at a comparable intensity level as their healthy peers, it may be that other speech characteristics are influencing whether speakers with PD are perceived as hypophonic and warrants further study.
In addition, HOAs and individuals with PD used similar laryngeal mechanisms as evidenced by laryngeal aerodynamics. There was strong indication that both the HOAs and adults with PD showed similar rate of vocal fold return to midline (MFDR) and airflow at maximal vocal fold closure (MinFlow). However, the two groups differed in three key aspects: Ps, lateral displacement of the vocal folds (PPFlow), and the amount of time that the vocal folds remained open per cycle (OQ).
At comparable SPLs, the HOA group produced smaller OQs than the individuals with PD. The OQ mechanism is sensitive to muscular control, and therefore laryngeal adduction difficulties (Baker, Ramig, Luschei, & Smith, 1998; Hillman, Holmberg, Perkell, Walsh, & Vaughan, 1989; Hirano, Ohala, & Vennard, 1969; Zarzur, Duprat, Shinzato, & Eckley, 2007). The findings of the present study indicate that, within each cycle of vibration, individuals with PD spent significantly more time with the vocal folds open. Assuming that OQ is at least partially under muscular control (Isshiki, 1964, 1969; Titze, 1989), the vocal fold open time differences in the speakers with PD versus the HOAs could be explained by differences in laryngeal muscle function (Baker et al., 1998; Hillman et al., 1989; Hirano et al., 1969; Zarzur et al., 2007). Titze (1989) determined that midline bulging of the thyroarytenoid (TA) muscle contributes to medial closure of the vocal folds. In a 2007 electromyographic study of laryngeal muscles in individuals with PD and healthy older controls, Zarzur et al. observed what they considered evidence of “abnormal muscle firing” (p. 832) in the TA muscle while at rest in nearly three quarters of the individuals with PD. Less than one quarter of the older control participants in their study showed the same level of misfiring, suggesting evidence of hypertonicity in the individuals with PD. Baker et al. (1998) studied the electromyographic activity in the TA muscle of healthy younger and older adults, as well as in adults with PD. The activity in the TA in the group with PD was reduced when compared to the healthy older group while speaking at the same intensity. Stelzig, Hochhaus, Gall, and Henneberg (1999) found that almost half of their participants with PD were observed to have abnormal adductory and abductory laryngeal muscle function, as well as signs of bilateral vocal fold atrophy. Last, Hirano et al. (1969) observed that activation of the lateral cricoarytenoid (LCA) directly affects closure timing of the vocal folds. Atypical functioning of the LCA in individuals with PD could adversely affect closure timing of the vocal folds, potentially leading to longer vocal fold open times. Individuals with PD may experience atypical muscular function leading to decreased contact time, thereby adversely influencing OQ.
In conjunction with the vocal folds being open for more of the cycle (OQ), the individuals with PD also produced significantly lower Ps than their healthy peers. Choi, Ye, and Berke (1995) conducted a study of laryngeal muscle activation and Ps using excised canine larynges. Choi et al. found that stimulation of the LCA and interarytenoid muscles significantly affected measures of Ps. For instance, when stimulation to the LCA and interarytenoid muscles was increased, higher Ps was produced. Further, as noted in the previous paragraph, Stelzig et al. (1999) found atypical adductor laryngeal function in individuals with PD. As appropriate vocal fold closure is required in order to efficiently build Ps, it is possible that vocal fold openness for more of the cycle (OQ), coupled with atypical adductor muscle function, adversely affected the ability for individuals with PD to produce comparable Ps to their healthy peers.
In the speech-in-quiet condition, the individuals with PD produced significantly greater flow during the open phase (PPFlow) than the HOA group. Individuals with PD have been reported to experience vocal fold atrophy. Stelzig et al. (1999) reported that the individuals with PD in their study displayed bilateral vocal fold atrophy. Udaka et al. (2008) studied the effects of disuse atrophy on skeletal muscle performance and found that disuse atrophy led to a decrease in passive tension. With a loss in muscle fiber density due to atrophy, the tissue of the vocal folds would likely become more compliant to airflow displacing them from midline. Greater compliance resulting in farther displacement from midline would lead to higher PPFlow.
In summary, it appears that individuals with PD have anatomical changes that alter the laryngeal aerodynamics during comfortable level voicing. To be specific, increased PPFlow, increased OQ, and decreased Ps all result in less efficient vocal fold valving and aerodynamic mechanisms for voice production. These results demonstrate that for people with PD, the vocal mechanism is less efficient and making voice production more effortful at their self-selected comfortable intensity.
The second comparison of interest was the examination of the mechanisms supporting increased SPL in the HOAs and the individuals with PD when they were presented with monaural multitalker babble noise. It was hypothesized that the HOAs and individuals with PD would produce comparable increases in SPL when speaking in noise. The individuals with PD did significantly increase speaking SPL and increased it to the same extent as their healthy peers when speaking in the monaural multitalker noise. Both the HOA group and the PD group increased driving pressure to increase SPL, as demonstrated by significant increases in Ps; however, Ps remained statistically lower for individuals with PD as compared to HOA in the speech-in-noise condition. Both the HOAs and adults with PD decreased OQ to increase vocal intensity, but again OQ remained significantly higher in people with PD than in the HOA in the speech-in-noise condition.
The HOA group and the individuals with PD responded similarly with respect to changes in MFDR. The increased MFDR with increased vocal intensity findings are in agreement with several studies in young to middle age adults (Gauffin & Sundberg, 1989; Södersten et al., 1995; Stathopoulos & Sapienza, 1993; Sulter & Wit, 1996) and HOA (Hodge et al., 2001), as well as in studies of individuals with PD (Dromey et al., 1995; Ramig & Dromey 1996). The results of the present study indicate that individuals with PD produce similar adjustments to MFDR as HOAs when increasing SPL.
The likely impact of vocal fold atrophy on compliance and lateral vocal fold displacement in individuals with PD continued in the speech-in-noise condition. Although the PPFlow results for the HOA essentially are in agreement with those in young- and middle-aged adults studied by Holmberg et al. (1988) and Södersten et al. (1995), there are no studies reporting PPFlow in a HOA population or a population of individuals with PD when increasing their SPLs. Thus, the present study provides a baseline for comparison of future studies. Although both people with PD and HOA increased PPFlow in the speech-in-noise condition, the individuals with PD continued to produce higher PPFlow means than the HOA.
Taken together, the aerodynamic data presented here suggest that the laryngeal adjustments being made by people with PD to increase SPL mirror those in HOA in many respects. However, although both groups used laryngeal mechanisms as expected to increase SPL, they used these mechanisms to different degrees. The HOAs appeared to have better control of the laryngeal mechanism to make changes to their vocal intensity.
MinFlow, the amount of flow when the vocal folds are most closed, yielded surprising results. The individuals with PD produced higher, although not significantly higher, MinFlow than the HOA in the speech-in-quiet condition. However, when speaking in noise, the individuals with PD produced MinFlow values more in line with those produced by their healthy peers, driven by a significant increase in MinFlow in the HOA group, along with a nonstatistically significant decrease in MinFlow in the PD group. These results are partially aligned with one study (Sulter & Wit, 1996) and contradict other studies of typical speakers (Holmberg et al., 1988; Holmberg, Hillman, Perkell, Guiod, & Goldman, 1995; Södersten et al., 1995). However, none of these studies examined MinFlow strictly in HOAs. Sulter and Wit examined MinFlow in young- and middle-aged adults across intensity conditions. The authors found that women experienced no change in MinFlow when increasing SPL but found that men significantly increased MinFlow when increasing SPL. Further, it was surmised by Sulter and Wit that even those with typical glottal closure may experience an increase in posterior opening, and therefore increased tendency to glottal leak, due in part to an increase in Ps with SPL increase could lead to these results. There are no previous reports of changes to MinFlow with increased SPL in individuals with PD; therefore, the current study provides our first look at this aerodynamic event. When considering the anatomical configuration related to MinFlow, it is important to consider insufficient vocal fold approximation, or increased glottal gap, with age. In her study of elderly women, Linville (1992) hypothesized that glottal gap could be due to laryngeal muscle changes with age. Pontes, Yamasaki, and Behlau (2006) reported that vocal fold bowing resulting in glottal gap was observed in 36% of the women and 68% of the older men in their study. Behrman, Abramson, and Myssiorek (2001) found that 60% of individuals with a diagnosis of presbylarnygeal dysphonia (voice problems due to an aged larynx) presented with glottal gaps. Second, individuals with PD often present with increased glottal gap (Blumin, Pcolinsky, & Atkins, 2004; D. G. Hanson, Gerratt, & Ward, 1984; Midi et al., 2008). The present findings show individuals with PD utilize glottal approximation at maximal closure to the same degree as their healthy peers when increasing intensity, but that the mechanisms for changing MinFlow with increasing SPL may differ in people with PD as compared to HOA. Due to the lack of significant findings here, more research is warranted to clarify the changes to vocal fold closure and MinFlow during voicing at both comfortable and high intensity speaking conditions.
Several potential limitations exist in the present study. First, the measures of interest require a specific sequence of sounds produced in a specific manner in order to obtain accurate estimates of subglottal air pressure and oral airflow measurements. It may be argued whether the task required for making the measures is a natural speech task. It should be noted that the syllable train task that is typically used for these measures was modified in the current study to a phrase in order to improve applicability to natural speech. Second, during the design phase of the study, the speech task used was not intended for respiratory kinematic analysis. Therefore, as quantitative measures could not be made, respiratory adjustment can only be presumed. Last, as the study assessed laryngeal function, stroboscopic assessment could have contributed to the comprehensive observation of laryngeal configuration in all participants. That being said, Kobler et al. (1998) showed a high correlation between aerodynamic and stroboscopic assessment, and the aerodynamic assessment holds a strong advantage by providing quantifiable data.
Summary
The individuals with PD in the present study performed comparably to their healthy peers at a comfortable vocal intensity. The two groups produced similar SPLs, as well as similar MFDR, and MinFlow reflecting somewhat comparable laryngeal function. The major differences between the two groups while speaking comfortably were that the speakers with PD produced significantly higher OQs, indicating less efficient vocal fold closure patterns, likely affecting their ability to build Ps. Further, the individuals with PD showed possible evidence of increased vocal fold compliance with higher PPFlow values. It is important to note that both the HOAs and the adults with PD successfully increased their vocal intensity when speaking in noise. At the higher vocal intensities, the HOAs improved laryngeal function as did the adults with PD; they all decreased OQ and increased MFDR, PPFlow, and Ps. The MinFlow of individuals with PD aligned more closely with their healthy peers when speaking in noise. However, the HOAs produced speech in noise with more laryngeal efficiency as evidenced by the greater changes seen in the HOA group as compared to the PD group. The results of the present study show that individuals with PD respond like their healthy peers to speaking in noise, demonstrating the importance of continued exploration of multitalker babble as a cue for increasing speaking SPL in individuals with PD as well as further investigation of the use of this specific monaural presentation of noise to elicit the Lombard effect. Further, the use of the technique shows promise as a treatment option to automatically increase speaking intensity in adults with PD and hypophonia.
Acknowledgments
This research was supported by Grant No. 5R01DC9409 funded by the National Institutes of Health, the National Institute on Deafness and Other Communication Disorders and a pilot grant from the Indiana Clinical and Translational Sciences Institute (Grant No. TR000006) funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, or Indiana CTSI. We would also like to thank James Jones and Kirk Foster from the Biomedical Engineering Department at Purdue University for the technical expertise in the design and development of the SpeechVive.
Appendix A
Voice quality table
| Participant | Age | Increase SPL in noise | Hypophonic | Loudness decay | Breathy | Hoarse |
|---|---|---|---|---|---|---|
| F01 | 78 | N | Moderately | Present | Moderately | Moderately |
| F02 | 74 | Y | Moderately | Present | Mildly | Mildly |
| F03 a | 76 | Y | Normal | Present | Normal | Mildly |
| F04 | 81 | M | Mildly | Not present | Normal | Moderately severe |
| F05 | 61 | Y | Mildly | Present | Normal | Mildly/moderately |
| F06 | 83 | Y | Mildly | Not present | Normal | Mildly |
| F07 | 76 | N | Moderately | Present | Normal | Mildly |
| F08 | 76 | N | Moderately Severe | Present | Normal | Mildly |
| FC09 | 69 | Y | Normal | Not present | Normal | Normal |
| FC10 | 72 | Y | Normal | Not present | Normal | Normal |
| FC11 | 65 | Y | Normal | Not present | Normal | Normal |
| FC12 | 69 | Y | Normal | Not present | Mildly | Normal |
| FC13 | 81 | M | Normal | Not present | Mildly | Mildly |
| FC14 | 72 | Y | Normal | Not present | Mildly | Mildly |
| FC15 | 64 | Y | Normal | Not present | Normal | Normal |
| FC16 | 62 | N | Normal | Not present | Normal | Normal |
| FC17 | 64 | Y | Normal | Not present | Mildly | Moderately |
| FC18 | 61 | Y | Normal | Not present | Normal | Normal |
| M01 | 68 | Y | Mildly | Not present | Normal | Normal |
| M02 | 79 | M | Mildly | Not present | Mildly | Mildly |
| M03 | 47 | N | Mildly | Not present | Normal | Mildly |
| M04 | 68 | N | Mildly | Not present | Mildly | Normal |
| M05 | 76 | N | Moderately | Present | Moderately | Mildly |
| M06 | 66 | N | Moderately | Present | Moderately severe | Moderately |
| M07 | 81 | N | Mildly | Did not assess | Mildly | Mildly |
| M08 | 68 | Y | Moderately | Present | Mildly | Mildly |
| M09 | 74 | N | Normal | Present | Normal | Mildly |
| M10 | 69 | N | Mildly | Present | Normal | Mildly/moderately |
| M11 | 60 | Y | Moderately | Present | Moderately severe | Moderately |
| M12 | 71 | Y | Mildly | Present | Mildly | Moderately |
| M13 a | 62 | N | Mildly | Did not assess | Mildly | Mildly |
| M14 | 72 | Y | Moderately | Present | Mildly | Moderately |
| M15 | 86 | Y | Mildly | Did not assess | Mildly | Normal |
| M16 | 69 | N | Moderately | Present | Moderately | Mildly |
| M17 | 74 | N | Moderately | Present | Normal | Moderately |
| M18 | 73 | M | Moderately | Present | Mildly/moderately | Moderately |
| M19 | 37 | M | Severely | Present | Mildly | Normal |
| M20 | 71 | M | Moderately | Present | Normal | Moderately |
| M21 a | 73 | Y | Moderately | Present | Normal | Normal |
| M22 | 73 | M | Mildly | Present | Normal | Mildly |
| M23 | 67 | Y | Moderately | Present | Moderately | Mildly |
| M24 | 59 | N | Mildly | Not present | Normal | Mildly |
| M25 | 74 | Y | Mildly | Present | Normal | Mildly |
| M26 | 81 | Y | Mildly | Present | Mildly | Moderately |
| M27 | 62 | N | Moderately | Present | Moderately | Mildly |
| M28 | 59 | M | Mildly | Not present | Normal | Mildly |
| M29 | 69 | M | Mildly | Not present | Normal | Mildly |
| M30 | 73 | N | Moderately | Present | Moderately | Mildly |
| M31 | 81 | N | Mildly | Present | Normal | Moderately |
| M32 a | 56 | Y | Mildly | Present | Mildly | Moderately |
| M33 | 76 | Y | Mildly | Not present | Moderately | Moderately |
| M34 | 68 | Y | Mildly | Not present | Mildly | Mildly |
| MC35 | 61 | Y | Normal | Not present | Mildly | Mildly |
| MC36 | 69 | Y | Normal | Not present | Mildly | Mildly |
| MC37 | 63 | N | Normal | Not present | Normal | Mildly |
| MC38 | 76 | N | Normal | Not present | Normal | Mildly |
| MC39 | 72 | Y | Normal | Not present | Mildly | Mildly |
| MC40 | 78 | Y | Normal | Not present | Normal | Normal |
| MC41 | 77 | Y | Normal | Not present | Mildly | Mildly |
| MC42 | 66 | Y | Normal | Not present | Normal | Mildly |
| MC43 | 76 | N | Normal | Not present | Normal | Mildly |
| MC44 | 60 | Y | Normal | Not present | Normal | Normal |
Note. The Darley, Aronson, & Brown-based (1969b) rating scale assessed characteristics typically observed in individuals with Parkinson's disease and pertinent to the present study along with participant age and whether the participant's sound pressure level increased in the speech-in-noise condition. Participants F01-F10 and M01-M34 have a diagnosis of PD. Participants FC09-FC18 and MC35-MC44 are neurologically normal. SPL = sound pressure level; Y = yes; N = no; M = data missing due to measurement issues in one of the conditions.
Indicates placement of deep-brain stimulation (DBS) device.
Appendix B
Participant demographic information for the individuals with Parkinson's disease (PD) group
| Participant | Years since diagnosis | PTA | PD-related drugs | Previous behavioral therapy |
|---|---|---|---|---|
| F01 | 10 | 23 | Sinemet, Mirapex, Zelapar | None |
| F02 | 13 | 33 | None | None |
| F03 a | 5 | 35 | Requip, Stalevo, | None; DBS |
| F04 | 4 | 37 | Carbidopa-Levodopa | None |
| F05 | 7 | 8 | Mirapex, Stalevo | None |
| F06 | 29 | 35 | Mirapex, Sinemet | Speech therapy |
| F07 | 0 | 17 | Carbidopa-Levodopa | Speech therapy |
| F08 | 1 | 27 | Carbidopa-Levodopa | Speech therapy |
| M01 | 3 | 22 | Azilect Tablet, Carbidopa-Levodopa, Mirapex | LSVT |
| M02 | 6 | 23 | None | LSVT |
| M03 | 21 | 8 | Sinemet | LSVT |
| M04 | 11 | 17 | Sinemet, Mirapex, ER | None |
| M05 | 5 | 25 | Carbidopa-Levodopa, Mirapex | None |
| M06 | 6 | 12 | Amantadine, Azilect | Speech therapy |
| M07 | 5 | 33 | Azilect, Carbidopa-Levodopa | None |
| M08 | 1 | 10 | Azilect, Carbidopa-Levodopa | None |
| M09 | 3 | 48 | Carbidopa-Levodopa | Swallowing |
| M10 | 5 | 12 | Amantadine, Mirapex | LSVT |
| M11 | 1 | 8 | Mirapex | None |
| M12 | 6 | 53 | Amantadine, Mirapex | None |
| M13 a | 13 | 18 | Amantadine, Comtan, Sinemet, Sinemet CR | None |
| M14 | 12 | 28 | Sinemet, Tasmar | Speech therapy, LSVT |
| M15 | 2 | 28 | Carbidopa-Levodopa, Lodosyn | None |
| M16 | 1 | 17 | Amantadine, Ropinerole | None |
| M17 | 6 | 12 | Sinemet | Speech therapy |
| M18 | 5 | 28 | Sinemet | Speech therapy, LSVT |
| M19 | 11 | 12 | Amantadine, Carbidopa-Levodopa | Speech therapy, LSVT |
| M20 | 14 | 27 | Requip XL, Sinemet, Stalevo | None |
| M21 a | 6 | 37 | Mirapex, Selegiline | Speech therapy, LSVT |
| M22 | 14 | 12 | Mirapex, Selegiline | Speech therapy, LSVT |
| M23 | 3 | 32 | Requip XL | None |
| M24 | 6 | 22 | Artane, Azilect, Requip XL, Symmetrel | None |
| M25 | 2 | 20 | Requip XL | None |
| M26 | 6 | 23 | Artane, Mirapex | None |
| M27 | 12 | 20 | Mirapex, Stalevo | Speech therapy, LSVT |
| M28 | 18 | 8 | Requip XL, Stalevo | None |
| M29 | 3 | 8 | Requip XL, Stalevo | None |
| M30 | 3 | 25 | Selegiline | None |
| M31 | 7 | 28 | Sinemet | None |
| M32 a | 7 | 10 | Stalevo | Speech therapy |
| M33 | 3 | 15 | Requip, Sinemet | Speech therapy, LSVT |
| M34 | 5 | 12 | Requip, Sinemet | None |
Note. Participant demographic information for the individuals with PD: sex (F = female; M = male), years since diagnosis at the time of the study, pure-tone average (PTA was 500, 1000, and 2000 Hz) in the ear in which the SpeechVive was placed, medications related to PD, and previous behavioral therapy for speech/voice. LSVT = Lee Silverman Voice Treatment.
Indicates placement of deep-brain stimulation (DBS) device.
Funding Statement
This research was supported by Grant No. 5R01DC9409 funded by the National Institutes of Health, the National Institute on Deafness and Other Communication Disorders and a pilot grant from the Indiana Clinical and Translational Sciences Institute (Grant No. TR000006) funded by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
References
- Adams S., Haralabous O., Dykstra A., Abrams K., & Jog M. (2005). Effects of multi-talker background noise on the intensity of spoken sentences in Parkinson's disease. Canadian Acoustics, 33(3), 94–95. [Google Scholar]
- Adams S., & Lang A. E. (1992). Can the Lombard effect be used to improve low voice intensity in Parkinson's disease? European Journal of Disorders of Communication, 27, 121–127. [DOI] [PubMed] [Google Scholar]
- Adams S., Moon B., Dykstra A., Abrams K., Jenkins M., & Jog M. (2006). Effects of multitalker noise on conversational speech intensity in Parkinson's disease. Journal of Medical Speech-Language Pathology, 14, 221–228. [Google Scholar]
- American National Standards Institute. (1969). Methods for the calculation of the articulation index. ANSI (S3.5-1969). New York: Author. [Google Scholar]
- Awan S. N., & Roy N. (2006). Toward the development of an objective index of dysphonia severity: A four-factor acoustic model. Clinical Linguistics & Phonetics, 20, 35–49. [DOI] [PubMed] [Google Scholar]
- Baken R. J. (2005). The aged voice: A new hypothesis. Journal of Voice, 19, 317–325. [DOI] [PubMed] [Google Scholar]
- Baker K. K., Ramig L. O., Luschei E. S., & Smith M. E. (1998). Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology, 51, 1592–1598. [DOI] [PubMed] [Google Scholar]
- Behrman A., Abramson A. L., & Myssiorek D. (2001). A comparison of radiation-induced and presbylaryngeal dysphonia. Otolaryngology—Head and Neck Surgery, 125, 193–200. [DOI] [PubMed] [Google Scholar]
- Blumin J. H., Pcolinsky D. E., & Atkins J. P. (2004). Laryngeal findings in advanced Parkinson's disease. Annals of Otology, Rhinology & Laryngology, 113, 253–258. [DOI] [PubMed] [Google Scholar]
- Canter G. J. (1963). Speech characteristics of patients with Parkinson's disease: I. Intensity, pitch and duration. Journal of Speech and Hearing Disorders, 28, 221–229. [DOI] [PubMed] [Google Scholar]
- Choi H. S., Ye M., & Berke G. S. (1995). Function of the interarytenoid (IA) muscle in phonation: In vivo laryngeal model. Yonsei Medical Journal, 36, 58–67. [DOI] [PubMed] [Google Scholar]
- Countryman S., & Ramig L. O. (1993). Effects of intensive voice therapy on voice deficits associated with bilateral thalamotomy in Parkinson's disease: A case study. Journal of Medical Speech-Language Pathology, 1, 233–250. [Google Scholar]
- Darley F. L., Aronson A. E., & Brown J. R. (1969a). Clusters of deviant speech dimensions in the dysarthrias. Journal of Speech and Hearing Disorders, 12, 462–496. [DOI] [PubMed] [Google Scholar]
- Darley F. L., Aronson A. E., & Brown J. R. (1969b). Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Disorders, 12, 246–269. [DOI] [PubMed] [Google Scholar]
- Darling M., & Huber J. E. (2011). Changes to articulatory kinematics in response to loudness cues in individuals with Parkinson's disease. Journal of Speech, Language, and Hearing Research, 54, 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demolin D., Giovanni A., Hassid S., Heim C., Lecuit V., & Soquet A. (1997). Direct and indirect measurements of subglottal pressure. Larynx-1997, 69–72.
- Dorsey E. R., Constantinescu R., Thompson J. P., Biglan K. M., Holloway R. G., Kieburtz K., … Tanner C. M. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 68, 384–386. [DOI] [PubMed] [Google Scholar]
- Dromey C., & Adams S. (2000). Loudness perception and hypophonia in Parkinson's disease. Journal of Medical Speech-Language Pathology, 8, 255–259. [Google Scholar]
- Dromey C., Ramig L. O., & Johnson A. B. (1995). Phonatory and articulatory changes associated with increased vocal intensity in Parkinson's disease: A case study. Journal of Speech and Hearing Research, 38, 751–764. [DOI] [PubMed] [Google Scholar]
- Dykstra A. D., Adams S. G., & Jog M. (2012). The effects of background noise on the speech intensity of individuals with hypophonia associated with Parkinson's disease. Journal of Medical Speech-Language Pathology, 20(3), 19–30.26157329 [Google Scholar]
- Eadie T. L. (2000). Characteristics of the aging female voice. Journal of Speech-Language Pathology and Audiology, 24, 162–179. [Google Scholar]
- Fox C. M., & Ramig L. O. (1997). Vocal sound pressure level and self-perception of speech and voice in men and women with idiopathic Parkinson disease. American Journal of Speech-Language Pathology, 6, 85–94. [Google Scholar]
- Gauffin J., & Sundberg J. (1989). Spectral correlates of glottal voice source waveform characteristics. Journal of Speech and Hearing Disorders, 32, 556–565. [DOI] [PubMed] [Google Scholar]
- Gorham-Rowan M. M., & Laures-Gore J. (2006). Acoustic-perceptual correlates of voice quality in elderly men and women. Journal of Communication Disorders, 39, 171–184. [DOI] [PubMed] [Google Scholar]
- Goy H., Fernandes D. N., Pichora-Fuller M. K., & van Lieshout P. (2013). Normative voice data for younger and older adults. Journal of Voice, 27, 545–555. [DOI] [PubMed] [Google Scholar]
- Gregory N. D., Chandran S., Lurie D., & Sataloff R. T. (2012). Voice disorders in the elderly. Journal of Voice, 26, 254–258. [DOI] [PubMed] [Google Scholar]
- Hammer M. J., & Barlow S. M. (2010). Laryngeal somatosensory deficits in Parkinson's disease: Implications for speech respiratory and phonatory control. Experimental Brain Research, 201, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. G., Gerratt B. R., & Ward P. H. (1984). Cinegraphic observations of laryngeal function in Parkinson's disease. Laryngoscope, 94, 348–353. [DOI] [PubMed] [Google Scholar]
- Hanson H. M. (1997). Glottal characteristics of female speakers: Acoustic correlates. The Journal of the Acoustical Society of America, 101, 466–481. [DOI] [PubMed] [Google Scholar]
- Hanson H. M., & Chuang E. S. (1999). Glottal characteristics of male speakers: Acoustic correlates and comparison with female data. The Journal of the Acoustical Society of America, 106, 1064–1077. [DOI] [PubMed] [Google Scholar]
- Higgins M. B., & Saxman J. H. (1991). A comparison of selected phonatory behaviors of healthy aged and young adults. Journal of Speech and Hearing Research, 34, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Holmberg E. B., Perkell J. S., Walsh M., & Vaughan C. (1989). Objective assessment of vocal hyperfunction: An experimental framework and initial results. Journal of Speech and Hearing Disorders, 32, 373–392. [DOI] [PubMed] [Google Scholar]
- Hirano M., Ohala J., & Vennard W. (1969). The function of laryngeal muscles in regulating fundamental frequency and intensity of phonation. Journal of Speech and Hearing Disorders, 12, 616–628. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Bradshaw J. L., & Iansek R. (2008). For better or worse: The effect of levodopa on speech in Parkinson's disease. Movement Disorders, 23, 574–580. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Iansek R., Marigliani C., Bradshaw J. L., & Gates S. (1999). Speech impairment in a large sample of patients with Parkinson's disease. Behavioural Neurology, 11, 131–137. [PubMed] [Google Scholar]
- Hodge F. S., Colton R. H., & Kelley R. T. (2001). Vocal intensity characteristics in normal and elderly speakers. Journal of Voice, 15, 503–511. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Hillman R. E., & Perkell J. S. (1988). Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America, 84, 511–529. [DOI] [PubMed] [Google Scholar]
- Holmberg E. B., Hillman R. E., Perkell J. S., Guiod P. C., & Goldman S. L. (1995). Comparisons among aerodynamic, electroglottographic, and acoustic spectral measures of female voice. Journal of Speech and Hearing Research, 38, 1212–1223. [DOI] [PubMed] [Google Scholar]
- Holmes R. J., Oates J. M., Phyland D. J., & Hughes A. J. (2000). Voice characteristics in the progression of Parkinson's disease. International Journal of Communication Disorders, 35, 407–418. [DOI] [PubMed] [Google Scholar]
- IBM SPSS Statistics for Windows (Version 21.0) [Computer software]. (2012). Armonk, NY: IBM Corp. [Google Scholar]
- Isshiki N. (1964). Regulatory mechanism of voice intensity variation. Journal of Speech and Hearing Disorders, 7, 17–29. [DOI] [PubMed] [Google Scholar]
- Isshiki N. (1969). Remarks on mechanism for vocal intensity variation. Journal of Speech and Hearing Disorders, 12, 669–672. [Google Scholar]
- Jiang J., O'Mara T., Chen H. J., Stern J. I., Vlagos D., & Hanson D. (1999). Aerodynamic measurements of patients with Parkinson's disease. Journal of Voice, 13, 583–591. [DOI] [PubMed] [Google Scholar]
- Kent R. D., & Kim Y. J. (2003). Toward an acoustic typology of motor speech disorders. Clinical Linguistics & Phonetics, 17, 427–445. [DOI] [PubMed] [Google Scholar]
- Kobler J. B., Hillman R. E., Zeitels S. M., & Kuo J. (1998). Assessment of vocal function using simultaneous aerodynamic and calibrated videostroboscopic measures. Annals of Otology, Rhinology, and Laryngology, 107, 477–485. [DOI] [PubMed] [Google Scholar]
- LaBlance G. R., Steckol K. F., & Cooper M. H. (1991). Advances in non-invasive measures of vocal acoustics. Ear, Nose, & Throat Journal, 70, 678–684. [PubMed] [Google Scholar]
- Linville S. E. (1992). Glottal gap configurations in two age groups of women. Journal of Speech and Hearing Research, 35, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Linville S. E. (2000). The aging voice. In Kent R. E. & Ball M. J. (Eds.), Voice quality measurement (pp. 359–376). San Diego, CA: Singular. [Google Scholar]
- Linville S. E. (2002). Source characteristics of aged voice assessed from long-term average spectra. Journal of Voice, 16, 472–479. [DOI] [PubMed] [Google Scholar]
- Linville S. E. (2004, October). The aging voice. ASHA Leader, 9(19), 12–21. [Google Scholar]
- Löfqvist A., Carlborg B., & Kitzing P. (1982). Initial validation of an indirect measure of subglottal pressure during vowels. The Journal of the Acoustical Society of America, 72, 633–635. [DOI] [PubMed] [Google Scholar]
- Logemann J. A., Fisher H. B., Boshes B., & Blonsky B. (1978). Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders, 43, 47–57. [DOI] [PubMed] [Google Scholar]
- Melcon M. C., Hoit J. D., & Hixon T. J. (1989). Age and laryngeal airway resistance during vowel production. Journal of Speech and Hearing Disorders, 54, 282–286. [DOI] [PubMed] [Google Scholar]
- Midi I., Dogan M., Koseoglu M., Can G., Sehitoglu M. A., & Gunal D. I. (2008). Voice abnormalities and their relation with motor dysfunction in Parkinson's disease. Acta Neurologica Scandinavica, 117, 26–34. [DOI] [PubMed] [Google Scholar]
- Milenkovic P. (1986). Glottal inverse filtering by joint estimation of an AR system with a linear input model. IEEE Transactions on Acoustics, Speech, and Signal Processing, 34(1), 28–42. [Google Scholar]
- Milenkovic P. (2011). Time-frequency analysis (TF32). Madison, WI: University of Wisconsin-Madison. [Google Scholar]
- National Institute of Neurological Disorders and Stroke, Office of Communications and Public Liaison. (2013). Parkinson's Disease: Hope Through Research. Retrieved from http://www.ninds.nih.gov/disorders/parkinsons_disease/detail_parkinsons_disease.htm
- Pontes P., Yamasaki R., & Behlau M. (2006). Morphological and functional aspects of the senile larynx. Folia Phoniatrica et Logopaedica, 58, 151–158. [DOI] [PubMed] [Google Scholar]
- Quedas A., Duprat A., & Gasparini G. (2007). Lombard's effect's implication in intensity, fundamental frequency and stability on the voice of individuals with Parkinson's disease. Brazilian Journal of Otorhinolaryngology, 73, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinov C. R., Kreiman J., Gerratt B. R., & Bielamowicz S. (1995). Comparing reliability of perceptual ratings of roughness and acoustic measures of jitter. Journal of Speech and Hearing Research, 38, 26–32. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., Bonitati C. M., Lemke J. H., & Horii Y. (1994). Voice treatment for patients with Parkinson's disease: Development of an approach and preliminary efficacy data. Journal of Medical Speech-Language Pathology, 2, 191–209. [Google Scholar]
- Ramig L. O., Countryman S., O'Brien C., Hoehn M., & Thompson L. (1996). Intensive speech treatment for patients with Parkinson's disease: Short- and long-term comparison of two techniques. Neurology, 47, 1496–1504. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., Countryman S., Thompson L. L., & Horii Y. (1995). Comparison of two forms of intensive speech treatment for Parkinson disease. Journal of Speech, Language, and Hearing Research, 38(6), 1232–1251. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., & Dromey C. (1996). Aerodynamic mechanisms underlying treatment-related changes in vocal intensity in patients with Parkinson disease. Journal of Speech and Hearing Research, 39, 798–807. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., Gray S., Baker K., Corbin-Lewis K., Buder E., Luschei E., … Smith M. (2000). The aging voice: A review, treatment data and familial and genetic perspectives. Folia Phoniatrica et Logopaedica, 53, 252–265. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., Sapir S., Fox C., & Countryman S. (2001). Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson's disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders, 16, 79–83. [DOI] [PubMed] [Google Scholar]
- Roark R. M. (2006). Frequency and voice: Perspectives in the time domain. Journal of Voice, 20, 325–354. [DOI] [PubMed] [Google Scholar]
- Rosner B. (Ed.). (2011). Multisample inference. In Fundamentals of biostatistics. Boston, MA: Brooks/Cole. [Google Scholar]
- Rothenberg M. (1973). A new inverse filtering technique for deriving the glottal air flow waveform during voicing. The Journal of the Acoustical Society of America, 53, 1632–1645. [DOI] [PubMed] [Google Scholar]
- Sauder C., Roy N., Tanner K., Houtz D. R., & Smith M. E. (2010). Vocal function exercises for presbylaryngis: A multidimensional assessment of treatment outcomes. Annals of Otology, Rhinology, and Laryngology, 119, 460–467. [DOI] [PubMed] [Google Scholar]
- Skodda S. (2011). Aspects of speech rate and regularity in Parkinson's disease. Journal of the Neurological Sciences, 310, 231–236. [DOI] [PubMed] [Google Scholar]
- Smitheran J. R., & Hixon T. J. (1981). A clinical method for estimating laryngeal airway resistance during vowel production. Journal of Speech and Hearing Disorders, 46, 138–146. [DOI] [PubMed] [Google Scholar]
- Södersten M., Hertegård S., & Hammarberg B. (1995). Glottal closure, transglottal airflow, and voice quality in healthy middle-aged women. Journal of Voice, 9, 182–197. [DOI] [PubMed] [Google Scholar]
- Spencer K. A., Yorkston K. M., & Duffy J. R. (2003). Behavioral management of respiratory- phonatory dysfunction from dysarthria: A flowchart for guidance in clinical decision making. Journal of Medical Speech-Language Pathology, 11, xxxix–lxi. [Google Scholar]
- Stathopoulos E. T., Huber J. E., Richardson K., Kamphaus J., DeCiccio D., Darling M., … Sussman J. E. (2014). Increased vocal intensity due to the Lombard effect in speakers with Parkinson's disease: Simultaneous laryngeal and respiratory strategies. Journal of Communication Disorders, 48, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos E. T., & Sapienza C. (1993). Respiratory and laryngeal function of women and men during vocal intensity variation. Journal of Speech and Hearing Research, 36, 64–75. [DOI] [PubMed] [Google Scholar]
- Stathopoulos E. T., & Sapienza C. (1997). Developmental changes in laryngeal and respiratory function with variations in sound pressure level. Journal of Speech, Language, and Hearing Research, 40, 595–614. [DOI] [PubMed] [Google Scholar]
- Stelzig Y., Hochhaus W., Gall V., & Henneberg G. A. (1999). Laryngeal manifestations in patients with Parkinson disease. Laryngorhinootologie, 78, 544–551. [DOI] [PubMed] [Google Scholar]
- Sulter A. M., & Wit H. P. (1996). Glottal volume velocity waveform characteristics in subjects with and without vocal training, related to gender, sound intensity, fundamental frequency, and age. The Journal of the Acoustical Society of America, 100, 3360–3373. [DOI] [PubMed] [Google Scholar]
- Titze I. R. (1989). Physiologic and acoustic differences between male and female voices. The Journal of the Acoustical Society of America, 85, 1699–1707. [DOI] [PubMed] [Google Scholar]
- Tjaden K., Sussman J. E., & Wilding G. E. (2014). Impact of clear, loud, and slow speech on scaled intelligibility and speech severity in Parkinson's disease and multiple sclerosis. Journal of Speech, Language, and Hearing Research, 57, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., & Wilding G. E. (2004). Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research, 47, 766–783. [DOI] [PubMed] [Google Scholar]
- Tukey J. W. (1977). Exploratory data analysis. Reading, MA: Addison-Wesley. [Google Scholar]
- Udaka J., Ohmori S., Terui T., Ohtsuki I., Ishiwata S.-I., Kurihara S., & Fukuda N. (2008). Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. The Journal of General Physiology, 131, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonck-de Leeuw I. M., & Mahieu H. F. (2004). Vocal aging and the impact on daily life: A longitudinal study. Journal of Voice, 18, 193–202. [DOI] [PubMed] [Google Scholar]
- Vincent G. K., & Velkoff V. A. (2010). The next four decades the older population in the United States: 2010 to 2050. (U.S. Department of Commerce Economics and Statistics Administration Publication No. P25-1138. Washington, DC: U.S. Census Bureau. [Google Scholar]
- Zarzur A. P., Duprat A. C., Shinzato G., & Eckley C. A. (2007). Laryngeal electromyography in adults with Parkinson's disease and voice complaints. Laryngoscope, 117, 831–834. [DOI] [PubMed] [Google Scholar]
- Zraick R. I., Smith-Olinde L., & Shotts L. L. (2012). Adult normative data for the KayPENTAX Phonatory Aerodynamic System Model 6600. Journal of Voice, 26, 164–176. [DOI] [PubMed] [Google Scholar]