Abstract
Neuropathological studies often use autopsy brain tissue as controls to evaluate changes in protein or RNA levels in several diseases. In mesial temporal lobe epilepsy (MTLE), several genes are up or down regulated throughout the epileptogenic and chronic stages of the disease. Given that postmortem changes in several gene transcripts could impact the detection of changes in case-control studies, we evaluated the effect of using autopsy specimens with different postmortem intervals (PMI) on differential gene expression of the Pilocarpine (PILO)induced Status Epilepticus (SE) of MTLE. For this, we selected six genes (Gfap, Ppia, Gad65, Gad67, Npy, and Tnf-α) whose expression patterns in the hippocampus of PILO-injected rats are well known. Initially, we compared hippocampal expression of naïve rats whose hippocampi were harvested immediately after death (0h-PMI) with those harvested at 6h postmortem interval (6h-PMI): Npy and Ppia transcripts increased and Tnf-α transcripts decreased in the 6h-PMI group (p<0.05). We then investigated if these PMI-related changes in gene expression have the potential to adulterate or mask RT-qPCR results obtained with PILO-injected rats euthanized at acute or chronic phases. In the acute group, Npy transcript was significantly higher when compared with 0h-PMI rats, whereas Ppia transcript was lower than 6h-PMI group. When we used epileptic rats (chronic group), the RT-qPCR results showed higher Tnf-α only when compared to 6h-PMI group. In conclusion, our study demonstrates that PMI influences gene transcription and can mask changes in gene transcription seen during epileptogenesis in the PILO-SE model. Thus, to avoid erroneous conclusions, we strongly recommend that researchers account for changes in postmortem gene expression in their experimental design.
Introduction
Mesial temporal lobe epilepsy (MTLE) is a chronic disease characterized by spontaneous and recurrent seizures (SRS) [1]. The molecular mechanisms underlying its pathogenesis have been widely investigated by differential gene expression approaches using both human data and animal models [2–9]. However, despite advances in knowledge of genes implicated in the epileptogenic process, these studies have generated some unexpected findings related to unpredictable variability sources in experimental design. In differential gene expression studies, two types of variation can affect the results: i) biological variation among individuals, such as age, gender, ethnicity, body mass index (BMI), lifestyle, and other individual characteristics [10–14]; ii) technical variation due to sample processing, such as pipetting errors, reverse transcription efficiency, RNA quality and other [15,16]. Several strategies can be adopted to minimize these factors, including the use of numerous biological replicates in matched-pairs design for biological variances and the use of suitable normalizers for technical variations [17–20]. Recognition of these confounding effects is, therefore, essential for a robust experimental design.
Investigations of gene expression in patients with MTLE are compromised by the absence of a well-matched control group. Brain tissue collected after death is typically used for comparison in case-control gene expression studies. Indeed, most studies are based on differences in gene expression between biopsies from MTLE patients and autopsy samples from non-epileptic individuals [21,22]. However, human postmortem tissue shows a high degree of biological variance [23,24] which may influence the results obtained by quantitative gene expression analysis. Indeed, it is well known that both antemortem (fever, hypoxia-ischemia, and acidosis) and postmortem (postmortem interval—PMI, brain or cerebrospinal fluid pH changes) factors can influence the production of gene transcripts [25–29].
For a long time, it was assumed that all transcripts from postmortem tissue degraded to the same degree. Consequently, this could be controlled in gene expression analysis by adopting a suitable normalization strategy [30,31]. This assumption was challenged by observing that a subgroup of mammalian mRNA transcripts with the AUUUA motif in the 3′ untranslated region are particularly susceptible to PMI-related degradation [32]. Since then, a growing body of evidence has indicated that selective gene expression or uneven half-lives of transcripts are characteristic of postmortem human tissues [29,33,34]. Part of this variation is attributable to hypoxic stresses occurring after somatic death that result in altered gene expression profiles, including up-regulation of certain genes (hypoxic inducible factor—HIF [23,35], cytoskeleton-related genes [29], Hsp70 [36,37], Bag1 [38], Gapdh [31]) and down-regulation of serine protease inhibitors [29,39], Nos3 and related genes [40]. Even under conditions of minimal biological variance (e.g. animal model studies), the specific cause of death can influence transcription quantities of certain genes [41,42]. Such specific transcript profiles associated with postmortem tissue raise important questions about the use of autopsy specimens as control tissue for differential gene expression studies.
Here, we evaluate the use of autopsy specimens as controls for differential gene expression in epileptogenesis. To control for antemortem effects and to minimize biological variance, we used the pilocarpine (PILO) model of MTLE. This model has been widely used to study the pathogenesis of temporal lobe epilepsy and to evaluate potential antiepileptogenic drugs [43,44]. Specifically, we selected six genes (Gfap, Ppia, Gad65, Gad67, Npy, and Tnf-α), whose expression patterns in the hippocampus of PILO-injected rats have been previously documented. We first used naïve rats to compare hippocampi harvested immediately after death (0h-PMI) with those harvested 6h after death (6h-PMI), similar to the typical postmortem interval used in human case-control studies in Brazil. We then examined the influence of different PMIs on real-time quantitative RT-PCR (RT-qPCR) results obtained from the hippocampi of PILO-injected rats.
Materials and methods
Animals
Experiments were conducted on male Wistar rats (200–280 g, n = 24) from the main breeding stock of the Federal University of Alagoas: 12 naïve individuals and 12 individuals submitted to an epilepsy induction protocol. Rats were kept at 22°C in groups of four per cage with free access to food and water, on a 12-h light/dark cycle (lights on at 06:00 am). All animal experiments were performed under a protocol approved by the Research Ethics Committee of the Federal University of Alagoas (Permit number: 27/2015) and were consistent with the International guidelines for the ethical use of animals, such as those from the Society for Neuroscience. The research staff monitored the rats’ health throughout the experimental period as described previously [16]. No animals presented clinical/behavioral signals of pain or unexpected distress used as humane endpoint criteria for euthanasia.
Naïve rats were divided into two groups: i. Rats submitted to autopsies that resemble the typical human autopsy process in which brain tissue is viable for case-control studies in Brazil. Briefly, after the death by a guillotine, the bodies were stored at room temperature (25°C) and humidity (58%) for 6 h (6h-PMI group, n = 6); ii. Control group: rats submitted to hippocampi collection immediately after the death by a guillotine (0h-PMI group, n = 6).
PILO-injected rats were also divided into two groups: i. Animals euthanized 24h after Status Epilepticus (SE) termination (acute group, n = 6), and; ii. Animals euthanized 11 weeks after SE (chronic group, n = 6). All these animals were submitted to hippocampi collection immediately after the death by guillotine.
SE induction
Rats were injected intraperitoneally (ip) with lithium chloride (127 mg/kg, Sigma) followed by PILO (30 mg/kg, Sigma) after 18 h. To counteract peripheral cholinergic effects, scopolamine butyl-bromide (1 mg/kg, Sigma) was administered 30 min before the administration of PILO. All animals developed SE, which was defined as self-sustained behavioral seizures or intermittent seizures of less than 5 minutes. Animals were kept in SE for 90 min before seizure interruption with diazepam (5 mg/kg; ip). For the chronic group, from the third day animals were individually placed in acrylic cages and their behavior was videotaped for up to 8 hours per day over 11 weeks. All the videos were analyzed by two independent observers, and the severity of spontaneous recurrent seizures (SRS) was classified according to Racine scale [45]. All chronic animals showed two or more SRS with severity scores equal or greater than 3 (S1 Table).
RNA extraction
Hippocampi were isolated on an ice-chilled plate and immediately frozen and stored at -80°C until RNA extraction. Total RNA was purified from left hippocampus using Trizol reagent (Invitrogen, CA, USA), following the manufacturer’s protocol. RNA integrity was estimated by analysis of the ratio of 28S to 18S ribosomal RNAs after electrophoresis in 1% agarose gel. All samples from the autopsy groups showed 28S/18S ratios ranging at 1.9 to 2.2 indicating an intact RNA.
RT-qPCR
RT-qPCR was used to quantify the expression of Gfap, Gad65, Gad67, Npy, Ppia, and Tnf-α. Total RNA was treated with DNase I (Ambion, TX, USA) for 30 min to avoid genomic DNA amplification, and cDNA was generated from 1μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. Once reverse-transcription was complete, samples were diluted (10X) in TE (Tris 10mM, pH 7.4; EDTA 0.1mM, pH 8,0) and stored at –80°C until further analysis. RT-qPCR was carried out on a StepOnePlus PCR System (Applied Biosystems)—all primer sequences and characteristics are listed in Table 1. Reactions were performed in a 12μL volume containing cDNA (2μL), 0.2–0.6μM each of specific forward (F) and reverse (R) primers, and 6μl Power Syber® Green PCR Master Mix (Applied Biosystem, CA, USA). The amplification protocol was as follows: initial 10min denaturation and 40 cycles of 95°C for 15s and 60°C for 1min. To ensure specificity of the PCR amplicon we performed a melting curve analysis, ranging from 60°C to 95°C, with temperature increases in steps of 0.5°C every 10 s. All primers showed an RT-qPCR efficiency ranging from 90 to 110%, as assessed by a standard curve based on a 5 points serial dilution of pooled cDNA (1:20; 1:40; 1:80; 1:160 and 1:320). Relative fold change was determined by the 2-ΔΔCt method [46]. PCR amplification confirmed the absence of contamination in the absence of cDNA. Each assay was performed in triplicate, and mean values were used for further analysis. All the target gene expression was normalized to Actb, as validated in the current study for 0h and 6h PMI; previously in epileptogenesis [17] and used for autopsy-derived brain tissue [47].
Table 1. Primer sequences and amplification summary.
| Gene | Symbol | Reference | 5'-3' sequence | Amplicon length (pb) |
|---|---|---|---|---|
| Glutamate decarboxylase 2 | Gad65/Gad2 | NM_012563.1 | F-CAATGTTCGGCTCTCCTGGT | 120 |
| R-CTTGTCTCCCGTGTCATAGG | ||||
| Glutamate decarboxylase 1 | Gad67/Gad1 | NM_017007.1 | F-ATCCTAATACTACCAACCTGC | 55 |
| R-GCTACGCCACACCAAGTATCA | ||||
| Glial fibrillary acidic protein | Gfap | NM_017009.2 | F-AACCGCATCACCATTCCTGT | 123 |
| R-CATCTCCACCGTCTTTACCAC | ||||
| Neuropeptide Y | Npy | NM_012614.2 | F-GCTCTGCGACACTACATCAATC | 147 |
| R-CCATCACCACATGGAAGGGT | ||||
| Peptidylprolyl isomerase A (cyclophilin A) | Ppia | NM_017101.1 | F- GGTCCTGGCATCTTGTCCAT | 134 |
| R-GCCTTCTTTCACCTTCCCAA | ||||
| Tumor necrosis factor | Tnf | NM_012675.3 | F-GCTCCCTCTCATCAGTTCCA | 106 |
| R-CTCCGCTTGGTGGTTTGCTA | ||||
| Actin beta | Actb | NM_031144.3 | F-AGCCTTCCTTCCTGGGTA | 92 |
| R-GAGGTCTTTACGGATGTCAAC |
Selection of reference gene
Five commonly used reference genes Beta-actin (Actb), Beta-2-microglobulin (B2m), Glyceraldehyde-3-phospate dehydrogenase (Gapdh), Beta-glucuronidase (Gusb) and Beta-tubulin (Tubb2a) were selected and their expression measured in the hippocampus of Wistar rats at two different postmortem intervals (0h and 6h). The primer sequences and characteristics are available in Marques et al [17]. We assessed the stability of candidate reference genes using two commonly and publicly available programs named geNorm and NormFinder. For this, Ct values were converted into relative quantities via the delta-Ct method using the sample with the lowest Ct as calibrator, accordling with the 2DCt method [48]. By using the geNorm, we also estimated the minimal number of genes required to calculate a robust normalization factor.
Statistical analysis
Statistics were performed using GraphPad Prism 5.00 (GraphPad Software, Inc. San Diego, CA, USA). All datasets were assessed for normal distribution by Kolmogorov-Smirnov test. Unpaired t-test with Welch´s correction test were used to the comparisons between 0h-PMI and 6h-PMI, to identify if a longer time between death and collection would impact gene expression in otherwise naïve animals. An one-way analysis of variance (ANOVA) with Bonferroni Test was used to test whether the difference between groups regarding postmortem interval could produce false positive (i.e., detection of changes when they do not exist) or false negative (i.e., obscure true changes from being detected) results when these control groups were compared with epileptic rats in the acute or chronic periods. Mean differences were considered statistically significant when P<0.05, and the results were presented graphically as mean and standard error of mean.
Results
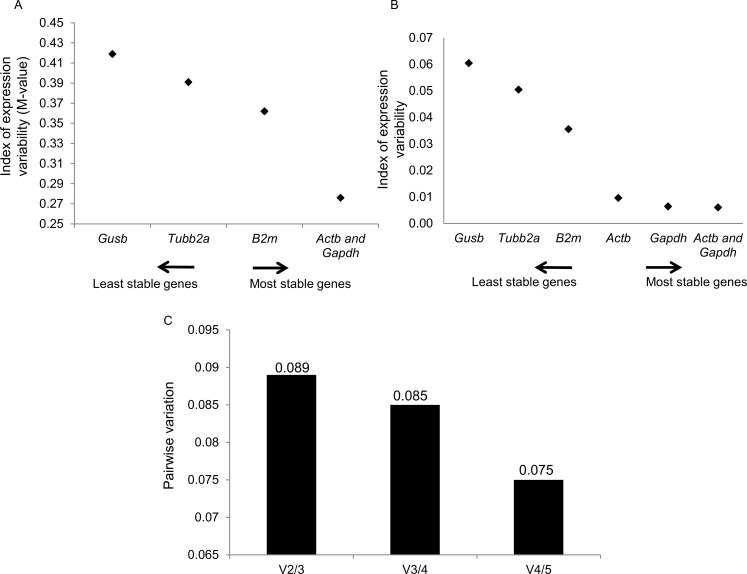
Initially, we evaluated expression stability of the candidate reference genes in hippocampus samples harvest at 0h or 6h after death, using geNorm and Normfinder softwares. The average expression stability values (M values) of the reference genes are displayed in Fig 1A. All the genes presented high expression stability, with the M values varying between 0.27 (Actb and Gapdh) and 0.41 (Gusb). The pairwise variation V2/3 was 0.089 (Fig 1C); thus, the Actb/Gapdh genes were indicated as the optimal pair to provide normalization of gene expression at the different postmortem intervals tested. Results of NormFinder analysis are shown in Fig 1B. Also, Gapdh and Gusb appeared, as the most and the least stable genes (stability value of 0.006 and 0.06), respectively. The best combination of reference genes indicated was Actb/Gapdh. These data sets are comparable with those obtained using geNorm, with slight differences in the ranking order of the most stable genes. In the current study, we used Actb as normalizer since this gene was pointed as an optimal reference gene for PILO-induced epileptogenesis [17]. Conversely, Gapdh was not considered a good reference gene for expression analysis of both epileptogenesis induced by kainate and chronic phase in the PILO model [49–51]. By using Actb as normalizer, we found no statistically significant differences in Gusb, Tubb2a, B2m and Gapdh transcript levels between the two PMI groups (S1 Fig).
Fig 1. Selection of the most suitable reference genes for 0h and 6h PMI in the hippocampus of Naïve rats.
Expression stability measurements for the 5 reference genes calculated by geNorm (A) and NormFinder (B). The x-axis from left to right indicates the ranking of the genes according to their expression stability; lower values indicate higher expression stability. C) Determination of the optimal number of reference genes for normalization by geNorm. The Software calculates the normalization factor from at least two genes at which the variable V defines the pair-wise variation between two sequential normalization factors.
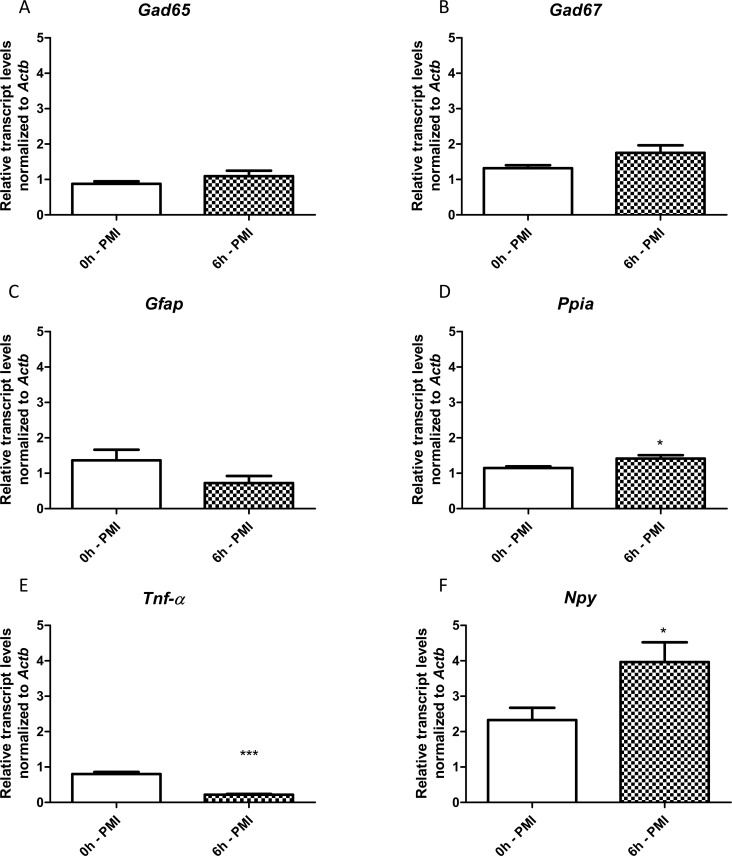
Following, we compared the transcript levels from hippocampi of naïve rats harvested 6 h after death (6h-PMI) with those collected immediately after death (0h-PMI). We found no differences in the Gad65, Gad67 and Gfap transcripts between the two PMI groups (Fig 2A–2C). However, Npy and Ppia transcripts were significantly higher, and Tnf-α were significantly lower in the 6h-PMI group (Fig 2D–2F).
Fig 2. Hippocampal relative expression of Gad65, Gad67, Gfap, Tnf-α, Npy and Ppia genes comparing 0h and 6h of postmortem intervals.
Values are mean ± SEM, n = 6 per group, Unpaired t-test with Welch´s correction, *p< 0.05 and ***p<0.001.
We then investigated if these differences in PMI-related gene expression could affect RT-qPCR results obtained from the hippocampi of PILO-injected rats. For this, we used hippocampi from 0h-PMI and 6h-PMI as controls for a case-control gene expression study.
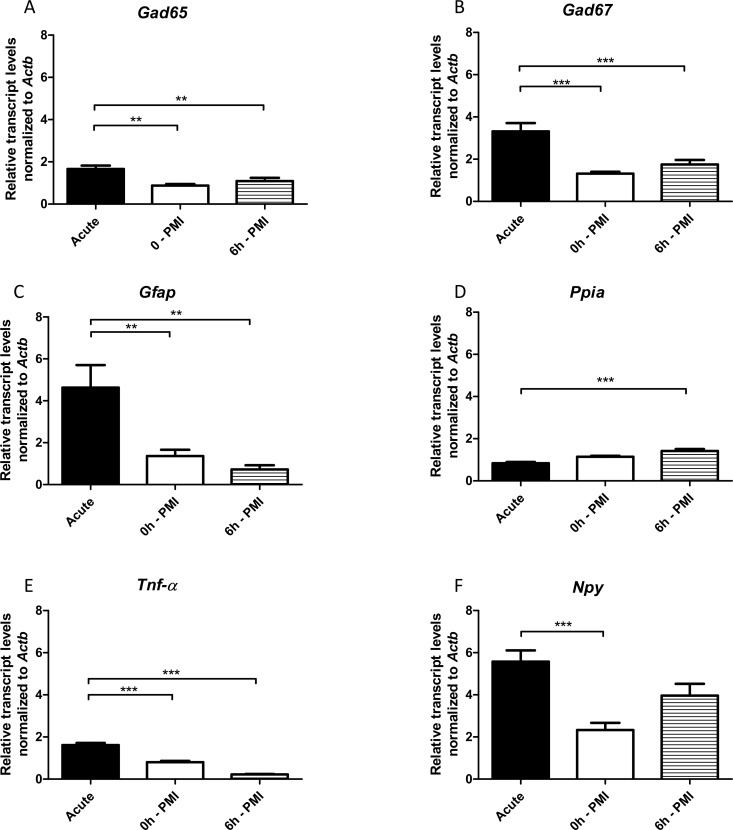
Initially, our experimental group contained hippocampi harvested in the acute phase of epileptogenesis (24 h after SE blockade). We detected higher transcript levels of Gad65, Gad67, Gfap, and Tnf-α in the hippocampi of PILO-injected rats when compared with either the 0h-PMI or 6h-PMI control groups (Fig 3A–3C and 3E). Ppia transcripts were significantly lower in the acute SE rats, compared with 6h-PMI control group (Fig 3D), whereas Npy transcripts were significantly higher in the acute group only when compared with 0h-PMI rats (Fig 3F).
Fig 3. Hippocampal relative expression of Gad65, Gad67, Gfap, Tnf-α, Npy and Ppia genes in acute-SE group compared with 0h and 6h postmortem intervals.
Values are mean ± SEM, n = 6 per group, ANOVA with Bonferroni post hoc, *p< 0.05, **p<0.01, ***p<0.001.
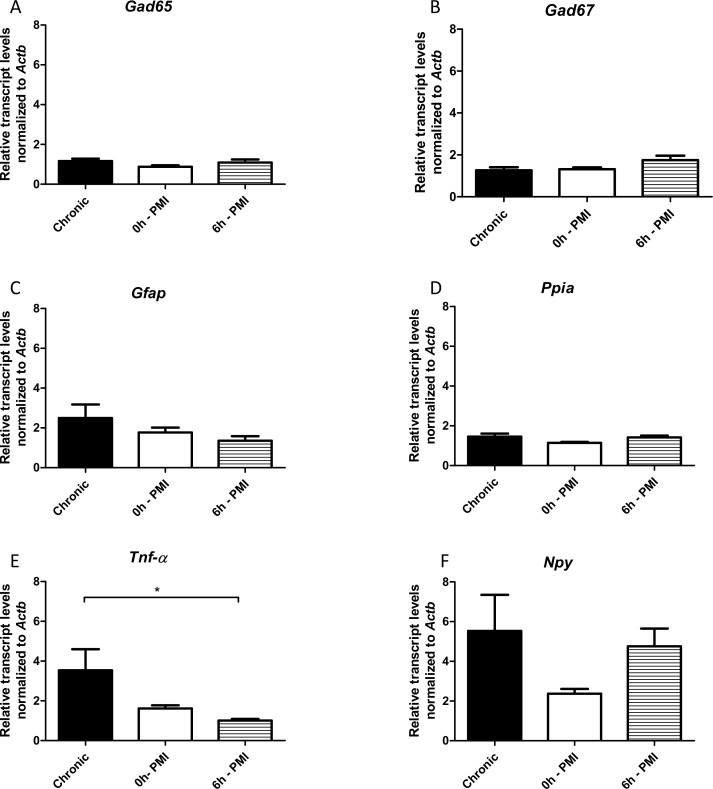
Finally, we performed the same comparisons using chronic epileptic rats (with SRS) as the experimental group. The Tnf-α transcripts were higher in chronic group only when compared with 6h-PMI control. There were no significant differences between chronic animals and the control groups for the remaining genes (Fig 4).
Fig 4. Hippocampal relative expression of Gad65, Gad67, Gfap, Tnf-α, Npy and Ppia genes in chronic group compared with 0h and 6h postmortem intervals.
Values are mean ± SEM, n = 6, ANOVA with Bonferroni post hoc, *p< 0.05.
Discussion
Several factors can cause selective fluctuations in gene transcript amounts in tissues during the postmortem interval [11,23,39]. This knowledge casts doubt on the utility and credibility of using autopsy-derived tissue as controls in gene expression analysis. To the best of our knowledge, this is the first study that has systematically investigated whether postmortem interval is a significant source of variability for differential gene expression analysis of epileptogenesis. Our initial analysis showed that only Npy, Ppia and Tnf-α transcript levels (out of the six genes tested) were significantly different between hippocampus of 6h-PMI and 0h-PMI rats. This indicates that, in hippocampal tissue, the variation in expression profile caused by autolysis is relatively restricted. These results are similar to those obtained with other tissues where the quantity of most transcripts was stable over the postmortem interval in comparison to samples harvested immediately after death [29,47,52,53]. The Tnf-α reduction in 6h-PMI group may reflect a selective post mortem degradation. Indeed, a subgroup of mammalian mRNA transcripts carrying the 3´UTR AUUUA motif, such as Tnf-α, is particularly vulnerable to PMI-related degradation [32]. Further studies, with more time points and investigating degradation pathways, should clarify the degradation pattern of Tnf-α. The increase in Npy transcripts in the 6h-PMI group could be due to the hypoxic stresses that occurs after somatic death. There are consistent reports about hypoxia-induced Npy expression in different vertebrate brain regions [54–56]. Moreover, other hypoxia-related genes have also been described as over-expressed during the postmortem interval [26]. On the other hand, the biological meaning of the increased Ppia transcripts in 6h-PMI group remains to be further clarified.
We then investigated whether our observed PMI-related changes in gene expression were sufficient to adulterate or even mask RT-qPCR results obtained with PILO-injected rats. As anticipated, the results for Gfap, Gad65 and Gad67 transcripts were the same for acute and chronic phases of epileptogenesis, regardless of the control group used (0h-PMI or 6h-PMI). Increased levels of Gfap [57,58], Gad65 [59] and Gad67 transcripts [59,60], and a decreased levels of Ppia in the hippocampi of rats harvested 24 hours after SE [61], as seen in our study, are well documented in the literature. However, results for Npy and Ppia transcripts for the acute phase of epileptogenesis were different depending on whether the 0h-PMI or 6h-PMI control groups were used for comparison. Previous studies have described that Npy is up-regulated after SE as a mechanism of counteracting the hyperexcitability underlying epileptic activity [62,63]. Here, we observed this Npy increase only when the 0h-PMI group was used as a control: the results were ‘masked’ in the comparisons using the 6h-PMI group, probably due to the up-regulation of Npy during the postmortem interval. Curiously, although Ppia levels are close between 0h- and 6h-PMI (Fig 2D), the decreased Ppia transcript levels in acute group were significant only when compared to 6h-PMI (Fig 3D). Previous studies in epilepsy models indicated Ppia as a potential reference gene [16,49]. The fact that the acute group has no difference from its time-matched control group (0h-PMI) further corroborate the use of Ppia as a reference gene for epilepsy, and shows that controls with a longer postmortem interval (6h-PMI) could create false results. Concerning Tnf-α, several studies have reported an SE-induced increase in mRNA and protein level [64,65]. We also observed a hippocampal Tnf-α increase of acute group rats in the comparisons with both 0h and 6h-PMI groups. However, the latter comparison resulted in a more prominent difference, probably due to the selective depletion of Tnf-α during the postmortem interval. The results on Npy, Ppia and Tnf-α indicate that researchers should be extremely cautious when interpreting RT-qPCR data generated from comparisons with postmortem hippocampi, particularly in investigations of the epileptogenic period or in the post-SE period.
In relation to comparisons using chronic epileptic rats (with SRS) as the experimental group, the RT-qPCR results were the same, regardless of the control group (0h- or 6h-PMI), for all genes but Tnf-α, which are only higher than 6h-PMI (Fig 4E). This difference is, as mentioned before, a result from Tnf-α susceptibility to postmortem degradation. In the chronic animals, Npy transcripts have a broader dispersion from the mean, if compared to acute SE rats. A probable reason for this higher variance and the resulting loss of difference seen in acute animals is the high variability in frequency and severity of SRS across individuals [66]. Thus, several additional parameters might exert a stronger influence on transcript levels, including temporal pattern and severity of seizures, interval between the last seizure and tissue collection and individual variation in epileptogenesis. Such biological variance could also explain why researchers have had problems with reproducibility in gene expression analysis using epileptic rats. For Gfap and Tnf-α, different experimental models have revealed a peak in the levels of RNA and protein in the hippocampus (and other brain structures) after seizures or SE, with a decline and return to control values weeks later [49,57,67–72], similar to our results. Conversely, some studies have reported that Gfap and Tnf-α levels remain high during the chronic phase [17,58,73–75]. These differences in the Gfap and Tnf-α expression profile in animal models are likely related to individual variations in the levels of astrogliosis and inflammation in the hippocampus during epileptogenesis. Indeed, one study indicated that half of the animals subjected to PILO-induced SE showed no reactive gliosis at all [76]. Additionally, data from patients with drug-resistant MTLE also suggests that the level of astrogliosis varies depending on the presence of hippocampal sclerosis, the degree of neuron loss, and the presence of psychiatric comorbidities [77–80].
Our study has some limitations that must be highlighted. First, as previously addressed, the higher standard deviations of transcripts in the chronic epilepsy groups could be related with differences in SRS frequency, severity or duration between animals. Another limitation of the present study is the evaluation of only two postmortem intervals (0h and 6h). Whereas brain tissue used in case-control human studies often come from 6h-PMI, it is not unusual to use tissue from other time points up to 10h-PMI. Thus, differences between our findings and other studies could be related to the PMI evaluated. Moreover, our study only evaluated a few gene transcripts, when several other genes could be differentially expressed with a longer postmortem interval. New studies with transcriptome approaches would be crucial for a larger picture of postmortem gene expression changes. Additionally, a change in gene transcript levels not always is associated with changes in protein levels, thus our data could not match studies that used immunohistochemistry/Western Blot analysis. Finally, it is also important to consider that further investigations including 6h PMI acute and chronic SE groups could contribute to the knowledge of how the PMI of an epileptic brain interferes on gene expression.
Conclusions
We clearly demonstrated that PMI can influence some gene transcript amounts and that this may be sufficient to mask changes the RT-qPCR results from PILO-injected rats. However, only a small number of genes (Tnf-α, Ppia and Npy) were differentially expressed at different postmortem intervals, and these PMI changes masked or adulterated the changes seen in the acute epilepsy period. Thus, even though we believe that it is valid to use hippocampi removed during autopsies to identify genes that are altered during disease pathogenesis, it is critical that researchers understand postmortem fluctuations in the expression of target genes and their potential influence on detecting disease-specific variations. As a cautionary note, we strongly recommend that researchers account for the possible influence of postmortem interval in their experimental design. Thus, as a first step, ones could use animal models to assess if PMI interferes with the expression of the studied genes and if this could affect the analysis of the epileptogenic process. For this, the experimental rationale used in the current study could be useful. Alternatively, a less desirable approach would be an ANCOVA evaluation with PMI as a covariate. However, this latter approach would require a larger control sample, to correct any individual variability not associated with PMI. Since procurement of control tissue for comparisons in gene expression analysis of human MTLE is an ongoing challenge, our study provides important information for analysis of gene transcription in postmortem hippocampi.
Supporting information
(DOCX)
Values are mean ± SEM, n = 6 per group.
(TIF)
Data Availability
All relevant data are within the paper.
Funding Statement
Funded by Fundação de Amparo a Pesquisa do Estado de São Paulo, grant numbers: 2016/17882-4 and 2015/20840-9, JPL, NG, MLP. Conselho de Desenvolvimento Científico e Tecnológico, grant numbers: 466995/2014-8 and 312161/2015-8, JPL, DLGG. Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL); DLG, JPLB, HCM. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); MAA, JPLB, HCM. Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPAHCRP). JPL, NG, MLP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Engel J. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26: 141–150. doi: 10.1016/S0920-1211(96)00043-5 [DOI] [PubMed] [Google Scholar]
- 2.Laurén HB, Lopez-Picon FR, Brandt AM, Rios-Rojas CJ, Holopainen IE. Transcriptome analysis of the hippocampal CA1 pyramidal cell region after kainic acid-induced status epilepticus in juvenile rats. PLoS One. 2010;5: e10733 doi: 10.1371/journal.pone.0010733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukasiuk K, Kontula L, Pitkänen A. cDNA profiling of epileptogenesis in the rat brain. Eur J Neurosci. 2003;17: 271–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/12542663 [DOI] [PubMed] [Google Scholar]
- 4.Pitkänen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. Elsevier Inc.; 2009;14: 16–25. doi: 10.1016/j.yebeh.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 5.Romcy-Pereira RN, Gitaí DLG, Gitaí LLG, Leite JP, Garcia-Cairasco N, Paçó-Larson ML. Genes e epilepsia II: expressão gênica diferencial. Rev Assoc Med Bras. 2008;54: 461–466. doi: 10.1590/S0104-42302008000500022 [DOI] [PubMed] [Google Scholar]
- 6.Gitaí DLG, Martinelli HN, Valente V, Pereira MGAG, Oliveira JAC, Elias CF, et al. Increased expression of GluR2-flip in the hippocampus of the wistar audiogenic rat strain after acute and kindled seizures. Hippocampus. 2010;20: 125–133. doi: 10.1002/hipo.20590 [DOI] [PubMed] [Google Scholar]
- 7.Gitaí DLG, Fachin AL, Mello SS, Elias CF, Bittencourt JC, Leite JP, et al. The non-coding RNA BC1 is down-regulated in the hippocampus of Wistar Audiogenic Rat (WAR) strain after audiogenic kindling. Brain Res. Elsevier B.V.; 2011;1367: 114–121. doi: 10.1016/j.brainres.2010.10.069 [DOI] [PubMed] [Google Scholar]
- 8.Hansen KF, Sakamoto K, Pelz C, Impey S, Obrietan K. Profiling status epilepticus-induced changes in hippocampal RNA expression using high-throughput RNA sequencing. Sci Rep. 2014;4: 6930 doi: 10.1038/srep06930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva A V, Scorza FA, et al. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11: 230 doi: 10.1186/1471-2164-11-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker DG, Whetzel AM, Serrano G, Sue LI, Lue LF, Beach TG. Characterization of RNA isolated from eighteen different human tissues: results from a rapid human autopsy program. Cell Tissue Bank. Springer Netherlands; 2016;17: 1–15. doi: 10.1007/s10561-016-9555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vennemann M, Koppelkamm A. Postmortem mRNA profiling II: Practical considerations. Forensic Sci Int. Elsevier Ireland Ltd; 2010;203: 76–82. doi: 10.1016/j.forsciint.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Opitz L, Salinas-Riester G, Grade M, Jung K, Jo P, Emons G, et al. Impact of RNA degradation on gene expression profiling. BMC Med Genomics. 2010;3: 36 doi: 10.1186/1755-8794-3-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preece P, Cairns NJ. Quantifying mRNA in postmortem human brain: Influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Mol Brain Res. 2003;118: 60–71. doi: 10.1016/S0169-328X(03)00337-1 [DOI] [PubMed] [Google Scholar]
- 14.Corton JC, Bushel PR, Fostel J, O&apos Lone RB. Sources of variance in baseline gene expression in the rodent liver. Mutat Res—Genet Toxicol Environ Mutagen. Elsevier B.V.; 2012;746: 104–112. doi: 10.1016/j.mrgentox.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15: 155–66. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2291693&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 16.Santos EA da S, Marques TEBS, Matos H de C, Leite JP, Garcia-Cairasco N, Paçó-Larson ML, et al. Diurnal Variation Has Effect on Differential Gene Expression Analysis in the Hippocampus of the Pilocarpine-Induced Model of Mesial Temporal Lobe Epilepsy. PLoS One. 2015;10: 1–20. doi: 10.1371/journal.pone.0141121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques TEBS, de Mendonça LR, Pereira MG, de Andrade TG, Garcia-Cairasco N, Paçó-Larson ML, et al. Validation of Suitable Reference Genes for Expression Studies in Different Pilocarpine-Induced Models of Mesial Temporal Lobe Epilepsy. PLoS One. 2013;8: 1–9. doi: 10.1371/journal.pone.0071892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Araújo MA, Marques TEBS, Taniele-Silva J, Souza FMDA, De Andrade TG, Garcia-Cairasco N, et al. Identification of endogenous reference genes for the analysis of microRNA expression in the hippocampus of the pilocarpine-induced model of mesial temporal lobe epilepsy. PLoS One. 2014;9: 1–7. doi: 10.1371/journal.pone.0100529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27: 126–139. doi: 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6: 279–84. doi: 10.1038/sj.gene.6364190 [DOI] [PubMed] [Google Scholar]
- 21.de Araújo M, Marques T, Octacílio-Silva S, Arroxelas-Silva C, Pereira MG, Peixoto-Santos JE, et al. Identification of microRNAs with Dysregulated Expression in Status Epilepticus Induced Epileptogenesis. PLoS One. 2016;11: 1–17. doi: 10.1371/journal.pone.0163855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thom M. Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014;40: 520–543. doi: 10.1111/nan.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durrenberger PF, Fernando S, Kashefi SN, Ferrer I, Hauw J-J, Seilhean D, et al. Effects of antemortem and postmortem variables on human brain mRNA quality: a BrainNet Europe study. J Neuropathol Exp Neurol. 2010;69: 70–81. doi: 10.1097/NEN.0b013e3181c7e32f [DOI] [PubMed] [Google Scholar]
- 24.Barton AJ, Pearson RC, Najlerahim A, Harrison PJ. Pre- and postmortem influences on brain RNA. J Neurochem. 1993;61: 1–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/7685811 [DOI] [PubMed] [Google Scholar]
- 25.Muciaccia B, Vico C, Aromatario M, Fazi F, Cecchi R. Molecular analysis of different classes of RNA molecules from formalin-fixed paraffin-embedded autoptic tissues: a pilot study. Int J Legal Med. 2015;129: 11–21. doi: 10.1007/s00414-014-1066-1 [DOI] [PubMed] [Google Scholar]
- 26.Huth A, Vennemann B, Fracasso T, Lutz-Bonengel S, Vennemann M. Apparent versus true gene expression changes of three hypoxia-related genes in autopsy derived tissue and the importance of normalisation. Int J Legal Med. 2013;127: 335–344. doi: 10.1007/s00414-012-0787-2 [DOI] [PubMed] [Google Scholar]
- 27.Abasolo N, Torrell H, Roig B, Moyano S, Vilella E, Martorell L. RT-qPCR study on post-mortem brain samples from patients with major psychiatric disorders: Reference genes and specimen characteristics. J Psychiatr Res. Elsevier Ltd; 2011;45: 1411–1418. doi: 10.1016/j.jpsychires.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 28.Lue L, Sue LI, Beach TG . Postmortem interval effect on RNA and gene expression in human brain tissue. 2011;12: 311–318. doi: 10.1007/s10561-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobue S, Sakata K, Sekijima Y, Qiao S, Murate T, Ichihara M. Characterization of gene expression pro fi ling of mouse tissues obtained during the postmortem interval. Exp Mol Pathol. Elsevier Inc.; 2016;100: 482–492. doi: 10.1016/j.yexmp.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26: 509–15. Available: http://www.ncbi.nlm.nih.gov/pubmed/15127793 [DOI] [PubMed] [Google Scholar]
- 31.Linden A Van Der, Blokker BM, Kap M, Weustink AC. Post-Mortem Tissue Biopsies Obtained at Minimally Invasive Autopsy: An RNA-Quality Analysis. PLoS One. 2014;9: 1–14. doi: 10.1371/journal.pone.0115675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catts VS, Catts SV, Fernandez HR, Taylor JM, Coulson EJ, Lutze-Mann LH. A microarray study of post-mortem mRNA degradation in mouse brain tissue. Mol Brain Res. 2005;138: 164–177. doi: 10.1016/j.molbrainres.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 33.El-Kashef N, Gomes I, Mercer-Chalmers-Bender K, Schneider PM, Rothschild MA, Juebner M. Validation of adequate endogenous reference genes for reverse transcription-qPCR studies in human post-mortem brain tissue of SIDS cases. Forensic Sci Med Pathol. 2015;11: 517–29. doi: 10.1007/s12024-015-9717-1 [DOI] [PubMed] [Google Scholar]
- 34.Lv Y, Ma K, Zhang H, He M, Zhang P, Shen Y, et al. A time course study demonstrating mRNA, microRNA, 18S rRNA, and U6 snRNA changes to estimate PMI in deceased rat’s spleen. J Forensic Sci. 2014;59: 1286–94. doi: 10.1111/1556-4029.12447 [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, Ishikawa T, Quan L, Michiue T, Zhu B-L, Maeda H. Postmortem quantitative mRNA analyses of death investigation in forensic pathology: an overview and prospects. Leg Med (Tokyo). 2009;11 Suppl 1: S43–5. doi: 10.1016/j.legalmed.2009.01.066 [DOI] [PubMed] [Google Scholar]
- 36.Kafel J, Baldinger L, Chabla JM, Hallas BH, Horowitz JM, Torres G. Blood content modulates the induction of heat shock proteins in the neurovascular network. Brain Res Bull. 2006;70: 304–11. doi: 10.1016/j.brainresbull.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 37.van der Weerd L, Lythgoe MF, Badin RA, Valentim LM, Akbar MT, de Belleroche JS, et al. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia—an MRI study. Exp Neurol. 2005;195: 257–66. doi: 10.1016/j.expneurol.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 38.Curcio C, Asheld JJ, Chabla JM, Ayubcha D, Hallas BH, Horowitz JM, et al. Expression profile of Bag 1 in the postmortem brain. J Chem Neuroanat. 2006;32: 191–5. doi: 10.1016/j.jchemneu.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 2011;12: 311–8. doi: 10.1007/s10561-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partemi S, Berne PM, Batlle M, Berruezo A, Mont L, Riuró H, et al. Analysis of mRNA from human heart tissue and putative applications in forensic molecular pathology. Forensic Sci Int. 2010;203: 99–105. doi: 10.1016/j.forsciint.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Ikematsu K, Tsuda R, Nakasono I. Gene response of mouse skin to pressure injury in the neck region. Leg Med (Tokyo). 2006;8: 128–31. doi: 10.1016/j.legalmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 42.Vennemann M, Koppelkamm A. MRNA profiling in forensic genetics I: Possibilities and limitations. Forensic Sci Int. Elsevier Ireland Ltd; 2010;203: 71–75. doi: 10.1016/j.forsciint.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 43.Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello a, et al. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem. 2014;21: 663–88. Available: http://www.ncbi.nlm.nih.gov/pubmed/24251566 doi: 10.2174/0929867320666131119152201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172: 143–57. doi: 10.1016/j.jneumeth.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32: 281–94. Available: http://www.ncbi.nlm.nih.gov/pubmed/4110397 [DOI] [PubMed] [Google Scholar]
- 46.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3: 1101–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/18546601 [DOI] [PubMed] [Google Scholar]
- 47.Castensson A, Emilsson L, Preece P, Jazin E. High-resolution quantification of specific mRNA levels in human brain autopsies and biopsies. Genome Res. 2000;10: 1219–1229. doi: 10.1101/gr.10.8.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25: 402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 49.Pernot F, Dorandeu F, Beaup C, Peinnequin A. Selection of reference genes for real-time quantitative reverse transcription-polymerase chain reaction in hippocampal structure in a murine model of temporal lobe epilepsy with focal seizures. J Neurosci Res. 2010;88: 1000–8. doi: 10.1002/jnr.22282 [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, Becker AJ. Activity-induced expression of common reference genes in individual cns neurons. Lab Invest. 2001;81: 913–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/11406652 [DOI] [PubMed] [Google Scholar]
- 51.Wierschke S, Gigout S, Horn P, Lehmann T-N, Dehnicke C, Bräuer AU, et al. Evaluating reference genes to normalize gene expression in human epileptogenic brain tissues. Biochem Biophys Res Commun. 2010;403: 385–90. doi: 10.1016/j.bbrc.2010.10.138 [DOI] [PubMed] [Google Scholar]
- 52.Cummings TJ, Strum JC, Yoon LW, Szymanski MH, Hulette CM. Recovery and expression of messenger RNA from postmortem human brain tissue. Mod Pathol. 2001;14: 1157–1161. doi: 10.1038/modpathol.3880451 [DOI] [PubMed] [Google Scholar]
- 53.Gupta S, Halushka MK, Hilton GM, Arking DE. Postmortem cardiac tissue maintains gene expression profile even after late harvesting. BMC Genomics. 2012;13: 26 doi: 10.1186/1471-2164-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali I, Bhargava S. Neuropeptide Y in the brain of Euphlyctis cyanophlyctis tadpoles responds to hypoxic stress. Gen Comp Endocrinol. 2016; doi: 10.1016/j.ygcen.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 55.Duszczyk M, Ziembowicz A, Gadamski R, Wieronska JM, Smialowska M, Lazarewicz JW. Changes in the NPY immunoreactivity in gerbil hippocampus after hypoxic and ischemic preconditioning. Neuropeptides. 2009;43: 31–9. doi: 10.1016/j.npep.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 56.Poncet L, Denoroy L, Dalmaz Y, Pequignot J-M, Jouvet M. Alteration in central and peripheral substance P- and neuropeptide Y-like immunoreactivity after chronic hypoxia in the rat. Brain Res. 1996;733: 64–72. doi: 10.1016/0006-8993(96)00539-2 [DOI] [PubMed] [Google Scholar]
- 57.Torre ER, Lothman E, Steward O. Glial response to neuronal activity: GFAP-mRNA and protein levels are transiently increased in the hippocampus after seizures. Brain Res. 1993;631: 256–64. Available: http://www.ncbi.nlm.nih.gov/pubmed/8131053 [DOI] [PubMed] [Google Scholar]
- 58.Xu Z, Xue T, Zhang Z, Wang X, Xu P, Zhang J, et al. Role of signal transducer and activator of transcription-3 in up-regulation of GFAP after epilepsy. Neurochem Res. 2011;36: 2208–15. doi: 10.1007/s11064-011-0576-1 [DOI] [PubMed] [Google Scholar]
- 59.Freichel C, Potschka H, Ebert U, Brandt C, Löscher W. Acute changes in the neuronal expression of GABA and glutamate decarboxylase isoforms in the rat piriform cortex following status epilepticus. Neuroscience. 2006;141: 2177–2194. doi: 10.1016/j.neuroscience.2006.05.040 [DOI] [PubMed] [Google Scholar]
- 60.Szabó G, Kartarova Z, Hoertnagl B, Somogyi R, Sperk G. Differential regulation of adult and embryonic glutamate decarboxylases in rat dentate granule cells after kainate-induced limbic seizures. Neuroscience. 2000;100: 287–95. Available: http://www.ncbi.nlm.nih.gov/pubmed/11008167 [DOI] [PubMed] [Google Scholar]
- 61.Lo W-Y, Tsai F-J, Liu C-H, Tang N-Y, Su S-Y, Lin S-Z, et al. Uncaria rhynchophylla upregulates the expression of MIF and cyclophilin A in kainic acid-induced epilepsy rats: A proteomic analysis. Am J Chin Med. 2010;38: 745–59. doi: 10.1142/S0192415X10008214 [DOI] [PubMed] [Google Scholar]
- 62.Goto EM, Silva M de P, Perosa SR, Argañaraz GA, Pesquero JB, Cavalheiro ÉA, et al. Akt pathway activation and increased neuropeptide Y mRNA expression in the rat hippocampus: Implications for seizure blockade. Neuropeptides. 2010;44: 169–176. doi: 10.1016/j.npep.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 63.Silva AP, Xapelli S, Pinheiro PS, Ferreira R, Lourenco J, Cristovao A, et al. Up-regulation of neuropeptide Y levels and modulation of glutamate release through neuropeptide Y receptors in the hippocampus of kainate-induced epileptic rats. J Neurochem. 2005;93: 163–170. doi: 10.1111/j.1471-4159.2004.03005.x [DOI] [PubMed] [Google Scholar]
- 64.Du X, Sun M, Yu Z, Chen H, Tian D, Xie M, et al. [Effects of cyclin dependent protein kinase inhibitor olomoucine on the neuronal apoptosis after status epilepticus: experiment with rats]. Zhonghua Yi Xue Za Zhi. 2007;87: 2025–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/17925171 [PubMed] [Google Scholar]
- 65.Kuteykin-Teplyakov K, Brandt C, Hoffmann K, Löscher W. Complex time-dependent alterations in the brain expression of different drug efflux transporter genes after status epilepticus. Epilepsia. 2009;50: 887–897. doi: 10.1111/j.1528-1167.2008.01916.x [DOI] [PubMed] [Google Scholar]
- 66.Dudek FE, Staley KJ. The Time Course and Circuit Mechanisms of Acquired Epileptogenesis In:Noebels JL, Avoli M, Rogawski MA et al. , editors.Jasper’s Basic Mechanisms of the Epilepsies. Bethesda: National Center for Biotechnology Information; 2012. pp.595–610. Available: http://www.ncbi.nlm.nih.gov/pubmed/22787656 [PubMed] [Google Scholar]
- 67.Holmberg KH, Patterson PH. Leukemia inhibitory factor is a key regulator of astrocytic, microglial and neuronal responses in a low-dose pilocarpine injury model. Brain Res. 2006;1075: 26–35. doi: 10.1016/j.brainres.2005.12.103 [DOI] [PubMed] [Google Scholar]
- 68.Pernot F, Heinrich C, Barbier L, Peinnequin A, Carpentier P, Dhote F, et al. Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia. 2011;52: 2315–25. doi: 10.1111/j.1528-1167.2011.03273.x [DOI] [PubMed] [Google Scholar]
- 69.Steward O, Torre ER, Phillips LL, Trimmer PA. The process of reinnervation in the dentate gyrus of adult rats: time course of increases in mRNA for glial fibrillary acidic protein. J Neurosci. 1990;10: 2373–84. Available: http://www.ncbi.nlm.nih.gov/pubmed/2376778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehtimäki KA, Peltola J, Koskikallio E, Keränen T, Honkaniemi J. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110: 253–60. Available: http://www.ncbi.nlm.nih.gov/pubmed/12591161 [DOI] [PubMed] [Google Scholar]
- 71.De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12: 2623–33. Available: http://www.ncbi.nlm.nih.gov/pubmed/10947836 [DOI] [PubMed] [Google Scholar]
- 72.Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, et al. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43 Suppl 5: 30–5. Available: http://www.ncbi.nlm.nih.gov/pubmed/12121291 [DOI] [PubMed] [Google Scholar]
- 73.Ozbas-Gerçeker F, Redeker S, Boer K, Ozgüç M, Saygi S, Dalkara T, et al. Serial analysis of gene expression in the hippocampus of patients with mesial temporal lobe epilepsy. Neuroscience. 2006;138: 457–74. doi: 10.1016/j.neuroscience.2005.11.043 [DOI] [PubMed] [Google Scholar]
- 74.Hammer J, Alvestad S, Osen KK, Skare Ø, Sonnewald U, Ottersen OP. Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia. 2008;56: 856–68. doi: 10.1002/glia.20659 [DOI] [PubMed] [Google Scholar]
- 75.Ashhab MU, Omran A, Kong H, Gan N, He F, Peng J, et al. Expressions of Tumor Necrosis Factor Alpha and MicroRNA-155 in Immature Rat Model of Status Epilepticus and Children with Mesial Temporal Lobe Epilepsy. J Mol Neurosci. 2013;51: 950–958. doi: 10.1007/s12031-013-0013-9 [DOI] [PubMed] [Google Scholar]
- 76.Garzillo CL, Mello LEAM. Characterization of reactive astrocytes in the chronic phase of the pilocarpine model of epilepsy. Epilepsia. 2002;43 Suppl 5: 107–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/12121303 [DOI] [PubMed] [Google Scholar]
- 77.Kandratavicius L, Peixoto-Santos J, Monteiro M, Scandiuzzi R, Carlotti C, Assirati J, et al. Mesial temporal lobe epilepsy with psychiatric comorbidities: a place for differential neuroinflammatory interplay. J Neuroinflammation. 2015;12: 38 doi: 10.1186/s12974-015-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peixoto-Santos JE, Velasco TR, Galvis-Alonso OY, Araujo D, Kandratavicius L, Assirati JA, et al. Temporal lobe epilepsy patients with severe hippocampal neuron loss but normal hippocampal volume: Extracellular matrix molecules are important for the maintenance of hippocampal volume. Epilepsia. 2015;56: 1562–1570. doi: 10.1111/epi.13082 [DOI] [PubMed] [Google Scholar]
- 79.Peixoto-Santos JE, Galvis-Alonso OY, Velasco TR, Kandratavicius L, Assirati JA, Carlotti CG, et al. Increased Metallothionein I/II Expression in Patients with Temporal Lobe Epilepsy. Sensi SL, editor. PLoS One. 2012;7: 1–11. doi: 10.1371/journal.pone.0044709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peixoto‐Santos JE, Kandratavicius L, Velasco TR, Assirati JA, Carlotti CG, Scandiuzzi RC, et al. Individual hippocampal subfield assessment indicates that matrix macromolecules and gliosis are key elements for the increased T2 relaxation time seen in temporal lobe epilepsy. Epilepsia. 2017;58: 149–159. doi: 10.1111/epi.13620. Epub 2016 Nov 18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Values are mean ± SEM, n = 6 per group.
(TIF)
Data Availability Statement
All relevant data are within the paper.