Significance
Self-organization in biological systems spans from molecular to ecosystem levels. In plant populations and communities, only a primitive mode of self-organization has been described, which involves changes in demography (survival of individuals affected by the availability of resources). Here, we show that sunflower plants cultivated in high-density stands perceive light signals from their immediate neighbors, adopt alternate positions of their single stem along the crop row, and collectively increase production per unit land area. This process is a case where a communicative sensory network leads to self-organization in plants, without changes in demography. Agronomic and genetic adaptations will be necessary to capture the increased oil production of self-organized stands.
Keywords: crop yield, shade avoidance, self-organization, stand density, phytochrome
Abstract
Here, we show a unique crop response to intraspecific interference, whereby neighboring sunflower plants in a row avoid each other by growing toward a more favorable light environment and collectively increase production per unit land area. In high-density stands, a given plant inclined toward one side of the interrow space, and the immediate neighbors inclined in the opposite direction. This process started early as an incipient inclination of pioneer plants, and the arrangement propagated gradually as a “wave” of alternate inclination that persisted until maturity. Measurements and experimental manipulation of light spectral composition indicate that these responses are mediated by changes in the red/far-red ratio of the light, which is perceived by phytochrome. Cellular automata simulations reproduced the patterns of stem inclination in field experiments, supporting the proposition of self-organization of stand structure. Under high crop population densities (10 and 14 plants per m2), as yet unachievable in commercial farms with current hybrids due to lodging and diseases, self-organized crops yielded between 19 and 47% more oil than crops forced to remain erect.
Global food supply needs to increase by 70% to adequately feed the expected population of 9.2 billion for 2050 (1). Opportunities for expansion of agriculture into new areas are limited, and in some cases, this expansion may compromise environmentally fragile landscapes (2). A more promising approach is to improve yield per unit area, which requires a combination of improved varieties, better agronomic practices, and exploiting the synergies between varieties and agronomy (3, 4).
Progress in yield of maize in the United States over the last 70 y has been partially related to hybrids with improved tolerance to high stand density (5). There is no similar evidence for sunflower, but in experiments where disease and lodging have been prevented, oil yield increased from 2 to 3 t⋅ha−1 when plant density increased from 5 (the standard in commercial crops) to 14 plants per m2 (6). Increasing stand density reduces yield per plant, in part due to enhanced competition for resources between neighbors (7–9). Theory and empirical evidence show that high yield per unit area is associated with a less competitive plant (10–13). In addition to competing for resources, neighbor plants generate sensory signals. For instance, mutual shading in dense stands reduces the activity of photosensory receptors such as phytochromes and cryptochromes (14, 15). Light absorption by photosynthetic pigments reduces the red-light (R)/far-red-light (FR) ratio perceived by phytochromes and the blue-light irradiance perceived by cryptochromes. The simulation of shade signals can, by itself, generate reductions in yield of spaced sunflower (16) or wheat (17) plants.
Self-organized systems are widespread in nature, and feature order emerging from locally interacting components of the system, often initiated by random change that is amplified by positive feedback (18–22). Self-organization in biology has been described at levels of organization from molecular to ecosystem (22). At the organ level in plants, for example, the frequency distribution of seeds per fruit can be explained by simple rules of resource flow into ovules, random processes, and hormone-mediated positive feedback (21). At the population and community level, self-organization can lead to plant arrangements in rings, lines, labyrinths, or bands (23–25). This self-organization process results from the balance between facilitation and competition that alters resource availability, plant survival, and demography (26). These resource- and survival-driven processes are relatively primitive forms of self-organization compared with the dynamic signal communication-driven processes of social insects; for example, ants and bees can select among alternative pathways to food through coordinated behavior that emerges from chemical communication networks (27). Here, we show that field-grown sunflower plants self-organize in high-density stands, in a process involving light signal-mediated shifts in stem angles, with no changes in plant survival, and that this self-organization process increases oil yield per unit area.
Results
Sunflower Plants Incline Away from Neighbors in High-Density Stands.
Stands of sunflower were grown in east (E)–west (W)-oriented rows spaced at 0.70 m in the field at a density typical of commercial crops (5.1 plants per m2) and compared with two higher densities (10 and 14 plants per m2). At the higher densities, plants adopted an alternate spatial arrangement, whereby a given plant inclined toward the north (N) interrow space, and the immediate neighbors inclined in the opposite direction. At maturity, capitulae were ∼0.35 m away from the vertical plane passing through the row axis, and the angle of the stem from the vertical was ∼10° (Fig. 1A). This effect was not apparent in low-density stands (5.1 plants per m2; Fig. 1 A and B). Row orientation had no impact (P > 0.30) on the arrangement of high-density stands (14 plants per m2). At the floral-bud stage, 70 ± 7% (mean ± SEM) alternately inclined plants were recorded in N–south (S) rows compared with 77 ± 3% in E–W rows [experiment (Exp.) 2, Table S1].
Fig. 1.
Density-dependent pattern of alternate plant inclination in sunflower stands. (A) Alternate plant inclination at high plant densities. Stands grown at 5.1 or 14 plants per m2 (E–W-oriented rows) recorded at maturity in Exp.1 (see details in Table S1). The direction of inclination of each plant in the row is represented by the ladder of bars to the side of the pictures. (B) Plant inclination increases with stand density; recorded at the floral-bud stage (49 DAE) in Exp. 1. (C) Dynamics of stem inclination in a high density stand (14 plants per m2) from 27 to 49 DAE in Exp. 1. In B and C, data are means ± SEM. See details in Table S1.
Table S1.
Detail of the plant material, use of plots or pots, plot size, sowing date, stand density, experimental design, treatments, measurements and statistical analysis that were used in the experiments
| Exp. | Description | Plant material; stand density | Experimental design | Sowing date | Treatments | Measurements | Statistical analysis |
| 1 | Effects of stand density on the pattern of stem inclination. | Paraíso 20 (Nidera Semillas); 5.1, 10, and 14 plants per m2 | Randomized design with 3 replicates, each replicate 6 rows × 6 m per row and 0.70 m between rows (plot size 21 m2). This experiment was done once. | November 4, 2003 | Stand density | Number and direction of inclination of 45 plants per replicate at 49 DAE (Fig. 1B) and from 27 to 49 DAE (Fig. 1C). | To test the null hypothesis that the alternate inclination in neighboring plants was random, we used a permutation test (28). ANOVA with one factor, stand density. The Tukey test (41) was used to determine the significance of differences between treatment means. |
| 2 | Effects of row orientation on the pattern of stem inclination. | Paraíso 20; 14 plants per m2 | Randomized design with 3 replicates, each replicate 6 rows × 6 m per row and 0.70 m between rows (plot size 21 m2). This experiment was done once. | January 10, 2004 | Row orientation:N–SE–W | Direction of inclination of 35 plants per replicate at 45 DAE (floral bud stage). | ANOVAs with one factor, stand density. The Fisher test (41) was used to determine the significance of differences between treatment means. |
| 3 a–e | Time-lapse photography | Paraíso 20; 20 plants per m2 | Four cameras were set at 1 m above the ground to capture images of apex position and leaf area shade of plants (5 plants per camera) cultivated in a row. The experiment was repeated five times. | January 17, 2006 (14 plants, Exp. 3a); February 1, 2006 (11 plants, Exp. 3b); March 7, 2006 (18 plants, Exp. 3c); April 10, 2006 (17 plants, Exp. 3d); May 12 (18 plants, Exp. 3e) | Evolution of apex position (six data points for each plant taken at 9 AM., 11 AM, 1 PM, 3 PM, 5 PM, and 6:30 PM of each day). Daily time integral of leaf area of each plant shaded by neighbors. Measurements were taken from 8 to 18 DAE. | Pearson’s χ2 test (n = 78 plants, pooled data from the 5 experiments) (42) was used to test whether the directions of shading and of stem bending were independent. | |
| 4 | Manipulation of quality of vertically incident light using filters. | Paraíso 20; 5.1 plants per m2 | Randomized design with 10 plants per light quality treatment. Plants cultivated in 6-m rows spaced at 0.70 m between rows. | February 15, 2005 | Light quality:Neutral filter;low blue filter (blue light absorbed);Simulated leaf shading (low PAR, low R/FR, and low blue) | Angle of stem inclination and number of inclined plants at 8 d from the start (19 DAE) of light quality manipulation treatments. | ANOVAs with one factor, light quality. The Fisher test (41) was used to determine the significance of differences between treatment means. |
| 5 | Manipulation of quality of horizontally incident light with distance from FR or mock (i.e., control treatment) sources. | Paraíso 20 | Randomized design with two factors (R/FR ratio and hybrids) and 3 replicates per combination of R/FR ratio and hybrid, with 6 plants per replicate. Plants n pots. | March 13, 2006 | R/FR ratio:<0.1 0.8 1.1 | Angle of stem inclination at 11 d after the start (17 DAE) of light quality manipulation treatments. For relative spectral photon fluence rates of the FR source, see ref. 40. | ANOVAs with one factor, R/FR ratio. The Fisher test (41) was used to determine the significance of differences between treatment means. |
| 6 a and b | Oil yield in response to stand density and stem inclination (forcibly verticalized or allowed to incline naturally). | Paraíso 20; 10 and 14 plants per m2 | Randomized design with two factors (stand density and stem position) and 3 replicates per combination of density and stem position. Each replicate had 6 rows x 6 m per row and 0.70m between rows (plot size 21m2). The experiment was repeated twice. | December 20, 2004 (Exp. 6a) and November 17, 2005 (Exp. 6b) | Stand density;Stem position:Inclined (natural);vertical (forced) | Oil yield and components (i.e., flower number, grain number, grain weight and grain oil concentration), and biomass at anthesis (Exp. 6 a and b);Biomass at physiological maturity and harvest index, both corrected by oil-synthesis costs (Exp. 6a). | Linear-mixed model (41). Stand density and stand inclination (fixed factors), year x treatment (random factors) for oil yield, its components, and biomass at anthesis (Exp. 6 a and b). ANOVAs with two factors (stand density and stand inclination) for biomass at maturity and harvest index corrected by oil-synthesis costs (Exp. 6a). The Fisher test (41) was used to determine the significance of differences between treatment means. |
| 7 a and b | Intraspecific variability of stem inclination in response to stand density. | MG50(Dow Agro Sciences), DK 4050 (Monsanto) ACA885 (Asociación de Cooperativas Argentinas); 5.1, 10, and 14 plants per m2 | Randomized design with two factors (stand density and hybrids) and 3 replicates per combination of two factors. Each replicate had 4 rows × 5 m per row and 0.70m between rows (plot size 14 m2). The experiment was repeated twice. | December 20, 2004 (Exp. 7a) and November 23, 2005 (Exp. 7b) | Hybrids stand density | Angle and direction of inclination of 34 plants per replicate at 20 DAE. | ANOVA with two factors (hybrid and density). The Fisher test (41) was used to determine the significance of differences between treatment means. |
| 8 | Intraspecific variability of stem inclination in response to R/FR ratio of horizontally incident light. | MG50 (Dow AgroSciences), DK 4050 (Monsanto) ACA885 (Asociación de Cooperativas Argentinas) | Randomized design with two factors (R/FR ratio and hybrids) and 3 replicates per combination of R/FR ratio and hybrid, each replicate 6 plants. Plants in pots. | March 13, 2006 | Hybrids;R/FR ratio <0.1 0.8 1.1 | Angle of stem inclination at 11 d after the start (17 DAE) of light quality manipulation treatments. For the relative spectral photon fluence rates of the FR source, see ref. 40. | ANOVA with two factors (hybrid and F/FR ratio). The Fisher test (41) was used to determine the significance of differences between treatment means. |
In high-density stands, incipient inclination commenced 27 d after emergence (DAE), progressed gradually, became fixed at 41 DAE, and persisted through maturity (Fig. 1C). At maturity, a similar (P > 0.60) proportion of plants inclined toward the N or the S of the row. The frequency of alternately inclined plants was significantly (P < 0.0001) higher than expected from permutation analysis (28), demonstrating that this process is not a random one.
Dynamic Pattern of Alternate Inclination of Neighboring Plants.
To capture the rapid development of the alternate arrangement of high-density stands, we used time-lapse photography. Pioneer plants 10, 13, and 18 in Fig. 2A initiated waves of alternate inclination. With time, these waves started to converge. When the waves dictated contrasting directions of inclination at the convergence point, the alternate pattern broke down (e.g., plants 12 and 13 in Fig. 2A). The number of pattern failures increased significantly (P < 0.05) with plant density (0 ± 0% and 13 ± 2% at 10 and 14 plants per m2, respectively; Exp. 1).
Fig. 2.
Propagation of plant inclination in high density sunflower stands conforms to self-organization. (A) Observed progression of inclination of plants grown at 20 plants per m2 (high density) with E–W-oriented rows from 8 to 22 DAE in Exp. 3c (see details in Table S1). (B and C) Progression of stem inclination obtained with cellular automata simulations of plants sown at 10 plants per m2 (B) and at 20 plants per m2 (C). States: S, inclined toward the S; N, inclined toward the N; X, upright. Gray areas indicate the waves of inclination, and dark gray areas indicate convergence of waves of inclination. The example series indicated by row numbers in these panels are subsets of 18 observed plants (A) or of 100 simulations (B and C).
Dynamic Pattern of Inclination Conforms to Self-Organization.
Cellular automata (i.e., mathematical systems constructed from many identical components, each simple, but together capable of complex behavior) are particularly suitable to test for self-organization (18, 20). To investigate whether the dynamic pattern of shoot inclination conforms to self-organization, we compared the key features of the actual phenomenon with simulations where the state of a plant is driven by its own state and the state of its two adjacent neighbors in the row. Using simple transition rules, we were able to simulate the progression of the waves, the final alternate pattern, and the apparent failure of this pattern at the intersection of the waves (Fig. 2B). In field experiments, the number of ”early incliners” increased with plant density (e.g., inclined plants at 27 DAE: 17 ± 9% and 28 ± 7% at 10 and 14 plants per m2, respectively; P < 0.06; Fig. 1B). Therefore, in our cellular automaton simulations, we altered the proportion of initially inclined plants (q) as a function of crop population density. This alteration was enough to capture the density-dependent differences in the rate of plant inclination and of the failures in the pattern of alternate inclination (Fig. 2 B and C).
Inclination Correlates with Neighbor Shading.
We also used the time-lapse photographs to quantify the degree of shading from neighbors in the row experienced by the leaves of specified plants that were oriented to the N or to the S of the E–W-oriented rows. The daily integral indicated that some seedlings experienced more shade from the N and others from the S (as exemplified in Fig. 3A). The plants inclined toward the N if they received shade from the S and vice versa (plants 10 and 14, respectively; Fig. 3A). Analysis of the pooled data from Exps. 3a–e showed (Table S2) the following: (i) that there was a global significant association between plant condition, described as upright, bent to the N, or bent to the S, and shading status, described as no shading, shading from the N, or shading from the S (P < 0.0001); (ii) that irrespective of the directions of shading and bending, bending was significantly associated with shading (P < 0.0001); and (iii) that among plants that were both shaded and bent, there was a significant association of bending toward the N with shading from the S and vice versa (P < 0.0001). In addition, apex position correlated (P < 0.001; Fig. S1) with the integral of daily shade of plants in Exp. 3c, including those shown in Fig. 3A.
Fig. 3.
Sunflower plants incline in response to shading by neighbors and to gradients to R/FR ratio. (A) Dynamics of apex position and daily integral of foliage shading of six representative plants selected from a row of 18 plants grown at 20 plants per m2 between 8 and 16 DAE. Data are from Exp. 3c (see details in Table S1). Open circles indicate apex positions at the six times of measurement (9 and 11 AM and, 1, 3, 5 and 6:30 PM) during the daylight period of each day. The dotted horizontal line shows the position of the vertical plane passing through the row axis, and the letters N and S indicate positions to the N and the S of the E–W row. Vertical arrows close to abscissae show the onset of inclination (apex deviation from vertical plane passing through row axis ≥ 2 cm). See global analyses in Table S2 and Fig. S1. (B and C) Inclination responds to the gradient of R/FR ratio. In B, plants of 11 DAE were placed under horizontal filters that simulated leaf shade or lowered blue irradiance impinging on leaves of one of the two sides of the plant during 8 d. Controls were placed under a clear filter. Data are from Exp. 4 (see details in Table S1). (C) Responses of plants placed at different distances from a source of FR to lower the R/FR ratio reaching the plant from that side, the source was lit from 11 to 16 DAE. Data are from Exp. 5 (see details in Table S1). Spectral composition of the light produced by the laterally placed FR source is shown in ref. 40. (D) Inclined plants generate a horizontal gradient of R/FR ratio. The light sensor (∢) was placed next to an inclined plant at the height of the apex of a neighbor and facing either toward (right) or against (left) the direction of inclination. Pooled data for plants inclined to the N- and S-inclined plants from Exp. 3c (see details in Table S1). In B, numbers above the bars indicate the proportion of inclined plants. In B–D, data are means and SE of 10 (B), 6 (C), or 10 (D) replicate plants. Different letters above the bars indicate significant differences at P < 0.01.
Table S2.
Observed frequencies of plants shaded from either the N or S directions or that remained unshaded, that bent to either the N or the S or remained upright
| Direction of stem bending | Direction of shading | |||
| N | S | Unshaded | Total | |
| N | 0 | 27 | 3 | 30 |
| S | 24 | 0 | 2 | 26 |
| Upright | 0 | 3 | 19 | 22 |
| Total | 24 | 30 | 24 | 78 |
Pooled data from Exps. 3 a–e. The global hypothesis of independence between shading (Un, N, or S) and bending status (Up, N, or S) is rejected (P < 0.0001, 4 degrees of freedom, χ2 test). The partial hypothesis of independence between shading (Un, N + S) and bending (Up, N + S) is rejected (P < 0.0001, 1 degree of freedom, χ2 test). The partial hypothesis of independence between the directions of shading (N or S), and the directions toward which plants bent (N or S) among plants that were both shaded and bent is rejected (P < 0.0001, 1 degree of freedom, χ2 test). Un, unshaded; Up, upright.
Fig. S1.
Plot of the relationship between apex position (which deviates from the row due to stem bending) on day 18 and the degree of shading on day 16. Day 18 was selected because, at that point, many of the plants were in the process of bending and day 16 was selected to investigate whether the direction of shading predicts the direction of bending. Note that a negative apex position indicates bending toward the S and a positive apex position bending toward the N. A negative integral of daily shade indicates shading coming from the S and a positive value shading from the N. Data correspond to 13 plants (including those shown in Fig. 3A) that were followed in detail. The relationship is significant at P < 0.001.
Plant Inclination Responds to Gradients of R/FR Ratio.
We used a filter to simulate leaf shade (low irradiance, low blue, and low R/FR ratio), a filter to reduce blue light [because blue-light gradients induce classical phototropic responses (29)], and a control filter that did not affect the spectral distribution of the light. Control plants showed no significant inclination. Plants treated unilaterally with simulated leaf shade inclined their shoot in the direction opposite to the simulated neighbor signal, whereas unilaterally lowering blue light was not effective (Fig. 3B). In additional experiments, we grew plants in pots placed on the N side of artificial sources of FR (i.e., the sources never shaded the plants from the sun), thus lowering the R/FR ratio of the light incident on the plants from their S side. Plants bent significantly against FR (i.e., toward high R/FR ratios) (Fig. 3C), indicating that the R/FR ratio is the primary cue.
Plant Inclination Generates a Gradient of R/FR.
Because plants incline toward the side of high R/FR, we investigated whether once a plant responds, it generates a gradient for the next plant of the row that would help to propagate the wave. To do so, we placed the light sensor horizontally and at a right angle to the vertical plane passing through the row axis, with the sensor window on and parallel to the vertical plane, next to an inclined plant (at a distance corresponding to a neighbor in high-density stands), at the height of its apex and facing either toward or against the direction of plant inclination. The R/FR ratio was lower when the light sensor faced the direction of plant inclination (Fig. 3 D, Right) than when it faced the opposite direction (Fig. 3 D, Left). The R/FR ratio for plants inclined either to the N or to the S of E–W-oriented rows showed no significant differences (P > 0.05), so in Fig. 3D, data for both categories were pooled.
Alternate Arrangement of Plants in Dense Stands Increases Crop Yield.
To quantify the impact of the alternate stand arrangement on crop yield, we compared stands in which plants inclined spontaneously and stands in which plants were forced to remain vertical using wire frames. The wiring treatment constrained stem bending, but not the ability of individual leaves to grow in any direction (Fig. 4B). In both 10 and 14 plants-per-m2 stands, oil yield was substantially greater in the alternate arrangement than in the vertical control (Fig. 4B). Yield is the end result of components that develop during the life cycle of the crop and can be expressed as a product. In the case of oil-seed crops, yield equals flower number × grain set (grain/flower ratio) × grain weight × grain oil concentration. Crops with spontaneous alternate inclination produced a similar number of flowers, more grains, and heavier grains than the controls (Tables S3 and S4). This result highlights how the early established pattern of inclination (Fig. 1C) has consequences for grain set and filling, processes that occur late (i.e., after anthesis) in the crop season. To compare between crop total biomass at anthesis and at physiological maturity for crops allowed to incline naturally and those constrained to the vertical, we corrected observed biomass at physiological maturity to account for the oil-synthesis costs of the oil-rich grain (30, 31). This conversion allowed for direct comparison of the increases in crop biomass values between developmental stages and across treatments of differing oil yield (Tables S3 and S4). The greater oil-synthesis cost-corrected biomass at physiological maturity in crops with spontaneous inclination (Tables S5 and S6), and a similar biomass at anthesis between both arrangements (Tables S3 and S4) indicates that stem inclination increased biomass production during seed filling.
Fig. 4.
A light-mediated, self-organized stand structure increases oil yield in sunflower crops. (A) Spontaneous pattern of stem inclination compared with a stand in which wire frames were used to maintain plant stems in the vertical position. (B) Stands with spontaneous pattern of stem inclination out-yielded their counterparts in which inclination was prevented at 10 and 14 plants per m2. Data are from Exps. 6 a and b (see details in Table S1). Vertical arrows in B indicated supporting wires. Data are means and SEM of pooled data from the two experiments. Different letters above bars indicate significant differences at P < 0.05.
Table S3.
Oil yield and its components and biomass at anthesis in crops allowed to incline or forced to remain vertical
| Plant inclination | Plant density, plants.m−2 | Oil yield, g⋅m−2 | Florets, florets.m−2 | Grains, grains.m−2 | Grain weight, mg⋅grain−1 | Grain oil concentration, % | Biomass at anthesis, g⋅m−2 |
| Yes | 10 plants | 155 (c) | 12,512 (a) | 10,388 (a) | 29.6 (b) | 51.6 (a) | 771.21 (a) |
| No | 105.3 (d) | 11,175 (a) | 8,557 (b) | 23.8 (c) | 51.2 (a) | 818.18 (a) | |
| Yes | 14 plants | 246.9 (a) | 16,258 (a) | 13,332 (a) | 36.3 (a) | 51.2 (a) | 1,260.93 (a) |
| No | 208.6 (b) | 16,044 (a) | 13,158 (a) | 31.5 (ab) | 51.4 (a) | 1,128.87 (a) |
Biomass at anthesis (pooled data for 2 y, Exps. 6 a and b) and number of flowers per plant were determined in four plants per replicate at anthesis [stage R5.5 (39)]. Yield and its components were determined at maturity in six plants from each replicate plots Different letters in brackets after each value in a column indicate significant (P < 0.05) differences.
Table S4.
Significance of treatment effects on oil yield and its components and on biomass at anthesis in crops allowed to incline or forced to remain vertical
| Factor | P | |||||
| Oil yield | Floret number | Grain number | Grain weight | Grain oil concentration | Biomass at anthesis | |
| Density | 0.04 | 0.40 | 0.45 | 0.14 | 0.84 | 0.65 |
| Inclination | 0.0001 | 0.09 | 0.005 | 0.0015 | 0.65 | 0.60 |
| Dens. × Inc. | 0.14 | 0.38 | 0.06 | 0.60 | 0.52 | 0.33 |
Biomass at anthesis (pooled data for 2 y, Exps. 6 a and b) and number of flowers per plant were determined in four plants per replicate at anthesis [stage R5.5 (39)]. Summary results of restricted maximum likelihood analysis for Table S3. In this analysis, fixed factors were stand density and inclination. Year was taken as a random effect. Dens., density; Inc., inclination. P < 0.05 indicates significant differences.
Table S5.
Biomass at physiological maturity and harvest index means of Exp. 6a
| Plant inclination | Plant density, plants.m−2 | Biomass at physiological maturity corrected for oil-synthesis cost, g⋅m−2 | HI corrected for oil-synthesis cost |
| Yes | 10 plants | 1,642 (b) | 0.41 (a) |
| No | 1,299 (a) | 0.43 (ab) | |
| Yes | 14 plants | 2,141 (d) | 0.50 (c) |
| No | 1,898 (c) | 0.47 (bc) |
Biomass at physiological maturity and harvest index (both corrected for oil-synthesis costs) were determined in six plants per replicate and corrected for energy expended in oil synthesis using the published production values (30) and method described in ref. 31. Different letters in brackets after each value in a column indicate significant (P < 0.05) differences.
Table S6.
Significance of treatment effects on biomass at physiological maturity and on harvest index, means of Exp. 6a
| Factor | P | |
| Biomass at physiological maturity corrected for oil-synthesis cost, g⋅m−2 | HI corrected for oil-synthesis cost | |
| Density | 0.0001 | 0.005 |
| Inclination | 0.0009 | 0.83 |
| Dens. x Inc. | 0.40 | 0.23 |
Table summarizes the factorial ANOVA with two factors (inclination and stand density). Dens., density; HI, harvest index; Inc., inclination. P < 0.05 indicates significant differences.
Stem Inclination in Response to Stand Density and R/FR Ratio Is Genotype-Dependent.
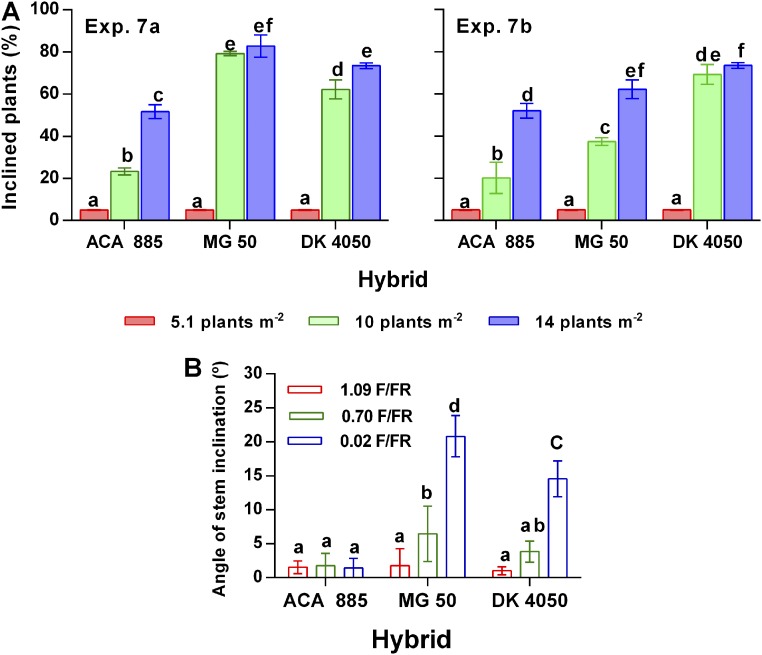
We compared stem inclination responses of three hybrids to either stand density or manipulation of light (i.e., natural light vs. different R/FR ratios of laterally incident light impinging on the plants). The response of stem inclination to stand density differed among hybrids (Tables S7 and S8). The genotype-dependent inclination in response to stand density (Fig. S2A) correlated with the genotype-dependent response to the R/FR ratio of laterally incident light (Table S9 and Fig. S2B). Paraiso 20 responded to both plant density (i.e., the sum of plants inclined to the N and to the S shown in Fig. 1B) and R/FR ratio of laterally incident light (Fig. 3C) approximately as DK4050 (Fig. S2).
Table S7.
F values and significance levels from an ANOVA for the effects of hybrid, population density, and for the hybrid × density interaction for stem inclination Exp. 7a
| Factors | F |
| Stem bending | |
| Hybrid | 92.8*** (13.03) |
| Density | 561.69*** (78.87) |
| Hybrid × density interaction | 28.83*** (8.10) |
Hybrids were MG50, DK 4050, and ACA885. Population density was 5, 10, and 14 plants per m2. The values between parentheses next to each F value show the proportion of nonerror variance that is explained by each factor and by their interaction. ***P < 0.001.
Table S8.
F values and significance levels obtained in ANOVA by the main effects of hybrid and plant density factors, and the hybrid × density interaction for stem inclination responses to density in in Exp. 7b
| Factors | F |
| Stem bending | |
| Hybrid | 26.41*** (9) |
| Density | 252.05*** (85) |
| Hybrid × density interaction | 9.42*** (6.4) |
Plant density was 5, 10, and 14 plants per m2. The proportion of nonerror variance that is explained by each factor and by their interaction is shown in parentheses next to each F value. ***P < 0.001.
Fig. S2.
Genetic variability in stem inclination responses. (A) Stem inclination at 21 DAE in plants of three sunflower hybrids grown at three stand densities in two experiments (Exp. 7 a and b). We measured the number of inclined plants in total of 34 plants per replicate at the flower bud stage, when the process had stabilized. (B) Stem inclination in response to different R/FR ratios of the horizontally incident light on the plants of three sunflower hybrids at 17 DAE in Exp. 8. Data are means and SEM of six plants. Letters above bars indicate significant differences between means at P < 0.05.
Table S9.
F values and significance levels obtained in ANOVA by the main effects of hybrid and plant density factors, and the hybrid × density interaction for stem inclination responses to density in Exp. 8
| Factors | F |
| Angle of stem inclination | |
| Hybrid | 32.60*** (25) |
| R/FR ratio | 60.53*** (48) |
| Hybrid × R/FR ratio | 16.86*** (26) |
Population density was 5, 10, and 14 plants per m2. The proportion of nonerror variance that is explained by each factor and by their interaction is shown in parentheses next to each F value. ***P < 0.001.
Discussion
The experimental and modeling evidence presented here supports the self-organization of high-density sunflower plant stands, triggered by an initial process of random inclination and followed by a positive feedback mediated by light signals that are perceived by phytochrome. This process results in the alternate orientation of plant shoots that improves oil yield per unit area. This finding is completely different from other plant self-organized population structures (21–25), where the occurrence of changes in demography is a stringent condition for emergence of self-organized patterns (26). The propagation of a sensory signal among plants is central to the system presented here, which therefore compares better to the communication networks leading to self-organized structures in social insects (27) than to previously described self-organized plant population structures.
A detailed analysis of the kinetics of the process complemented by manipulative experiments revealed the steps that cause the alternate orientation of sunflower shoots in dense stands. We found that in sunflower crops grown at densities higher that those used commercially, a few pioneer plants become shaded by neighbors and incline their shoot toward the least shaded side of the row (Figs. 2A and 3A) in response to the gradient in R/FR ratio (Fig. 3B). Shoot inclination of the pioneer plant generates a gradient of the R/FR ratio (lower toward the inclined plant; Fig. 3D, right-hand bar), thus influencing the quality of the light perceived by the next neighbor plant in the row. This neighbor bends in response to the R/FR gradient caused by the pioneer plant and, in turn, generates a R/FR gradient in the opposite direction for the next neighbor in the row, thus propagating the wave. Experimental blue-light gradients failed to elicit bending, indicating that this response is not a classical phototropic response (29). Blue-light signals were not effective per se, but might reinforce the response to R/FR as demonstrated in other systems (32, 33).
In crops like sunflower, where breeding has favored strong apical dominance (34, 35), responses to neighbors mediated by branching are precluded, and stem-driven foliage position in response to R/FR is particularly relevant. Leaf-growth responses to horizontal gradients of R/FR can relax competition for radiation by avoidance of shading between matching leaves of neighbors in maize stands (36) and in Arabidopsis plants grown with kin neighbors (37), and this behavior increases the seed yield of the population (37). At high population densities, self-organized sunflower stands produced 19–47% more oil than their counterparts where plants were forced to remain vertical (Fig. 4B). Furthermore, self-organized stands produced more biomass between anthesis and physiological maturity, but not before anthesis (Tables S3–S6). This result highlights how the early established pattern of inclination (Fig. 1C) has consequences for processes that occur late in the crop season, including crop growth, grain set and filling, and, ultimately, oil yield. Our findings have implications for breeding and agronomy. Stem inclination is a trait that meets two criteria essential for breeding (38): It relates to yield in the field, and there is coherent genetic variability in the response to stand density and light quality (Fig. S2 A and B). Furthermore, imaging technologies could be developed for high-throughput screening of stem inclination (Fig. 1A). Agronomically, changes in row spacing and rectangularity could be used to enhance the expression of this trait.
Materials and Methods
Plant Material, Experimental Design, and Statistical Analyses.
We used plants of Helianthus annuus. Details of experimental design, treatments, hybrids, and statistical analyses used in the experiments are described in Table S1.
Field and Pot Experiments.
Experiments were carried out in the field of Facultad de Agronomía, Universidad de Buenos Aires (34° 36' S, 58° 26' W). Plants were grown in silty clay (Vertic Argiudol) soils except for Exp. 5 and 8 (Table S1), in which plants were grown in 4-L pots filled with top soil and sand [1:1 (vol/vol)]. Field crops were oversown and thinned to reach the target density at the two-leaf stage. Phenological development was monitored regularly by using the scale in ref. 39. Plants were irrigated and fertilized to avoid shortage of nutrients; weeds, diseases, and insects were controlled chemically as required.
Plant Inclination.
To define plant status (either inclined or vertical), we considered that a plant was inclined when the apical bud was at a distance of ≥2 cm from the vertical line arising from the base of the plant. Alternatively, the angle of stem inclination relative to the vertical was calculated by using trigonometry in combination with measurements of apex height and distance between the base of the stem and the intercept, on the soil plane, of the normal to the apex.
Time-Lapse Photography.
Four webcams (Genius) were set 1 m above the ground to capture images of 18 plants at 20 plants per m2. Records started at emergence (expanded cotyledons) and were completed when the inclination pattern stabilized. Images were 8.4 × 11.4 cm (1:4 scale). To quantify the dynamics of stem inclination and shading between neighboring plants, images taken at five moments, at intervals of 2 h during the photoperiod, were used. Stem inclination was assessed from the change in apex position between two successive images. Shading was estimated as the proportion of leaf area shaded by neighbors integrated throughout the photoperiod. The crop population density used in Exp. 3 was higher than in Exps. 1 and 6 (Table S1) to advance the completion of stem inclination while the plants were still young. Large (older) plants could leave the radius of capture of the webcams, and a reduced number of expanded leaves in young plants (only two) facilitated a more accurate quantification of the proportion of shaded leaves.
Plant Effects on R/FR Ratio.
We used a Skye SKR 100/SKR110 (Skye Instruments) with the probe at a right angle to the plane passing through the row axis and the sensing window of the remote probe placed vertically, facing toward each side of the row.
Selective Shade Filters.
At 11 DAE, 0.10- by 0.10-m horizontal filters were placed 4 cm above the uppermost leaves to generate three light regimes: control (neutral filter), simulated leaf shade (low irradiance, low blue, low R:FR), and low blue (blue light was absorbed). The neutral filter was constructed by using 2.4-mm transparent acrylic sheet, the simulated leaf shade filter using 2.4-mm blue acrylic sheet (Paolini 2031, La Casa del Acetato) covered with red acetate film (La Casa del Acetato). The low blue filter was made with transparent acrylic sheet covered with orange acetate film (La Casa del Acetato). Light under the filters was characterized by using a FieldSpec spectro-radiometer (Analytical Spectral Devices, Inc.) during a sunny midday (see spectra in Fig. S3).
Fig. S3.
Spectral photon distribution spectra of light under the different filters used in Exp. 4 (sunlight is shown as a reference). Clear filter (2.4 mm-thick transparent acrylic sheet, ≥ 85% transmittance between 400 and 800); low blue light filter (2.4 mm-thick transparent acrylic sheet covered with orange acetate film, 15% transmittance between 400 and 500 nm and 85% at longer wavelengths); and simulated leaf shade filter (2.4 mm-thick blue acrylic sheet Paolini 2031, covered with red acetate film, 23% transmittance between 400 and 700 nm, 19% between 600 and 700 nm and 75% between 700 and 800 nm).
Supplementary FR.
Plants with the first two true leaves fully expanded were arrayed in E–W rows at either 10 or 90 cm to the N of FR or mock (control) sources. The spectral distribution of the FR source was as in ref. 40.
Oil Yield.
Seed oil concentration was measured with RMN (Oxford 4000, Oxford Analytical Instruments) in 10-g subsamples.
Cellular Automata.
We used cellular automata to test the occurrence of self-organization in our experimental system (18, 20). Cellular automata consists of an array of cells that can change their state following transition rules, applied recursively over time, which are a function of both the cell’s own state and the state of its adjacent neighbors (20). A cell (plant) in our model could be in one of three states: S, inclined toward the S; N, inclined toward the N; or X, upright. The time step was set to 2 d, and rules to drive transitions between states were: (i) at time = 10 DAE, all plants were in state X; (ii) at time = 12 DAE, a proportion (q) of plants shifts randomly to stage N or S; (iii) at times ≥ 12 DAE, every 2 d the state of X plants changes depending on the state of its two neighbors in the row as follows: (i) to N if both neighbors are S, or if one neighbor is S and the other is X; (ii) to S, if both neighbors are N, or if one neighbor is N and the other X; (iii) to N or S randomly if one neighbor is S and the other N; (iv) X remains unchanged if both neighbors are X; and (v) N and S plants remain unchanged. The initial conditions were set at q = 0.10 for 10 plants per m2 (Fig. 2B) and q = 0.15 for 20 plants per m2 (Fig. 2C).
Acknowledgments
We are grateful to Abelardo de la Vega for his support in quantifying stem bending in his high crop density experimental plots in which the stem bending phenomenon was first detected. We thank the support staff of the Instituto de Investigaciones Fisiológicas y Ecológicas Vinculadas a la Agronomia (IFEVA) for help in the design and assembly of the time-lapse photography system. This work was supported by grants from the Academia Nacional de Agronomía y Veterinaria; Universidad de Buenos Aires Grant G048; and Agencia Nacional de Promoción Científica y Técnica (Argentina) Grant PICTO 8/13159 (to A.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618990114/-/DCSupplemental.
References
- 1.Bruinsma J. Expert Meeting on How to Feed the World in 2050. Food and Agriculture Organization of the United Nations; Rome: 2009. The resource outlook to 2050. [Google Scholar]
- 2.Connor DJ, Mínguez MI. Evolution not revolution of farming systems will best feed and green the world. Glob Food Secur. 2012;1:106–113. [Google Scholar]
- 3.Fischer RA. Farming systems of Australia, exploiting the synergy between genetic improvement and agronomy. In: Sadras VO, Calderini DF, editors. Crop Physiology, Applications for Genetic Improvement and Agronomy. Academic; New York: 2009. pp. 23–54. [Google Scholar]
- 4.Sinclair TR, Rufty TW. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob Food Secur. 2012;1:94–98. [Google Scholar]
- 5.Fischer RA, Byerlee D, Edmeades G. Crop Yields and Global Food Security. Will Yield Increase Continue to Feed the World? Australian Centre for International Agricultural Research; Canberra, Australia: 2014. [Google Scholar]
- 6.López Pereira M, et al. 2004 Responses of sunflower to stand structure and crop population density, effects on leaf area and yield. Proceedings of the 16th International Sunflower Conference, Fargo, ND, 29 August-2 September. Available at isasunflower.org/publications/isc-symposia/single-view/article/16th-intern-sunflower-conference-fargo-north-dakota-usa-aug-29-sept-2-2004-vol1.html.
- 7.Vega CC, Andrade FH, Sadras VO. Reproductive partitioning and seed set efficiency in soybean, sunflower and maize. Field Crops Res. 2001;72:163–175. [Google Scholar]
- 8.Villalobos FJ, et al. Planting density effects on dry matter partitioning and productivity of sunflower hybrids. Field Crops Res. 1994;36:1–11. [Google Scholar]
- 9.Sarlangue T, et al. Why do maize hybrids responds differently to variations in plant density? Agron J. 2007;99:984–991. [Google Scholar]
- 10.Jennings PR, Dejesus J. Studies on competition in rice. I. Competition in mixtures of varieties. Evolution. 1968;22:119–124. doi: 10.1111/j.1558-5646.1968.tb03455.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamblin J, Donald CM. The relationships between plant form, competitive ability and grain yield in a barley cross. Euphytica. 1974;23:535–542. [Google Scholar]
- 12.Donald CM. Competitive plants, communal plants, and yield in wheat crops. In: Evans LT, Peacock WJ, editors. Wheat Science—Today and Tomorrow. Cambridge Univ Press; Cambridge, UK: 1981. pp. 223–247. [Google Scholar]
- 13.Denison RF. Darwinian Agriculture: How Understanding Evolution Can Improve Agriculture. Princeton Univ Press; Princeton: 2012. [Google Scholar]
- 14.Casal JJ. Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol. 2013;64:403–427. doi: 10.1146/annurev-arplant-050312-120221. [DOI] [PubMed] [Google Scholar]
- 15.Gommers CM, Visser EJ, St Onge KR, Voesenek LA, Pierik R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013;18:65–71. doi: 10.1016/j.tplants.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Libenson S, Rodríguez V, López Pereira M, Sánchez RA, Casal JJ. Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Sci. 2002;42:1180–1185. [Google Scholar]
- 17.Ugarte CC, Trupkin SA, Ghiglione H, Slafer G, Casal JJ. Low red/far-red ratios delay spike and stem growth in wheat. J Exp Bot. 2010;61:3151–3162. doi: 10.1093/jxb/erq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfram S. Cellular automata as models of complexity. Nature. 1984;311:419–424. [Google Scholar]
- 19.Heylighen F. 2001. The science of self-organization and adaptivity. The Encyclopedia of Life Support Systems (EOLSS, Paris), Vol. 5, pp 253–280.
- 20.Bauchau V. Emergence and reductionism: from the game of life to science of life. In: Feltz B, Crommelinck M, Goujon P, editors. Self-Organization and Emergence in Life Sciences. Springer; Dordrecht: 2006. pp. 29–40. [Google Scholar]
- 21.Ganeshaiah KN, Shaanker RU. Frequency distribution of seed number per fruit in plants: A consequence of the self-organizing process? Curr Sci. 1992;62:359–365. [Google Scholar]
- 22.Kauffman S. At Home in the Universe: The Search for the Laws of Self-Organization and Complexity. Oxford Univ Press; Oxford: 1996. [Google Scholar]
- 23.Lejeune O, Tlidi M, Couteron P. Localized vegetation patches: A self-organized response to resource scarcity. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66:010901. doi: 10.1103/PhysRevE.66.010901. [DOI] [PubMed] [Google Scholar]
- 24.Sheffer E, et al. Why do plants in resource deprived environments form rings? Ecol Complex. 2007;4:192–200. [Google Scholar]
- 25.Couteron P, et al. Plant clonal morphologies and spatial patterns as self-organized responses to resource limited environments. Philos Trans A Math Phys Eng Sci A. 2014;372:140102. doi: 10.1098/rsta.2014.0102. [DOI] [PubMed] [Google Scholar]
- 26.Rietkerk M, van de Koppel J. Regular pattern formation in real ecosystems. Trends Ecol Evol. 2008;23:169–175. doi: 10.1016/j.tree.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Seely T. When is self-organization used in biological systems? Bio Bull. 2002;202:314–318. doi: 10.2307/1543484. [DOI] [PubMed] [Google Scholar]
- 28.Good PI. 2005. Permutation, Parametric, and Bootstrap Tests of Hypotheses, Springer Series in Statistics (Springer, New York), 3rd Ed.
- 29.Liscum E, et al. Phototropism: Growing towards an understanding of plant movement. Plant Cell. 2014;26:38–55. doi: 10.1105/tpc.113.119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penning de Vries FWT, Van Laar HH, Chardon MC. Bioenergetics of growth of seed, fruits and storage organs. In: Smith WH, Banta SJ, editors. Potential Productivity of Yield Crops Under Different Environment. IRRI; Los Baños, Las Filipinas: 1983. pp. 37–59. [Google Scholar]
- 31.Hall AJ, Connor DJ, Whitfield DM. Contribution of pre-anthesis assimilates to grain-filling in irrigated and water-stressed sunflower crops. I. Estimates using labeled carbon. Field Crops Res. 1989;20:95–112. [Google Scholar]
- 32.Goyal A, et al. Shade promotes phototropism through phytochrome B-controlled auxin production. Curr Biol. 2016;26:3280–3287. doi: 10.1016/j.cub.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 33.de Wit M, et al. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr Biol. 2016;26:3320–3326. doi: 10.1016/j.cub.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Seiler GJ. 1997. Anatomy and morphology of sunflower. Sunflower Technology and Production, Agronomy Monograph 35, eds Seiler GJ, Miller JF, Charlet LD, Meyer DW (American Society of Agronomy, Madison, WI) pp 67–111.
- 35.Rieseberg L, Seiler G. Molecular evidence and the origin and development of the domesticated sunflower (Helianthus annuus, Asteraceae) Econ Bot. 1990;44:79–91. [Google Scholar]
- 36.Maddonni GA, Otegui ME, Andrieu B, Chelle M, Casal JJ. Maize leaves turn away from neighbors. Plant Physiol. 2002;130:1181–1189. doi: 10.1104/pp.009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crepy MA, Casal JJ. Photoreceptor-mediated kin recognition in plants. New Phytol. 2015;205:329–338. doi: 10.1111/nph.13040. [DOI] [PubMed] [Google Scholar]
- 38.Sadras VO, Richards RA. Improvement of crop yield in dry environments: benchmarks, levels of organisation and the role of nitrogen. J Exp Bot. 2014;65:1981–1995. doi: 10.1093/jxb/eru061. [DOI] [PubMed] [Google Scholar]
- 39.Schneiter A, Miller JF. Description of sunflower growth stages. Crop Sci. 1981;21:901–903. [Google Scholar]
- 40.Casal JJ. Novel effects of phytochrome status on reproductive shoot growth in Triticum aestivum L. New Phytol. 1993;123:45–51. [Google Scholar]
- 41.Di Rienzo JA, et al. 2013 InfoStat (Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba, Argentina). www.infostat.com.ar/
- 42.Agresti A. An Introduction to Categorical Data Analysis. Vol 135 Wiley; New York: 1996. [Google Scholar]