Abstract
Discoveries about the cultures and cultural capacities of the great apes have played a leading role in the recognition emerging in recent decades that cultural inheritance can be a significant factor in the lives not only of humans but also of nonhuman animals. This prominence derives in part from these primates being those with whom we share the most recent common ancestry, thus offering clues to the origins of our own thoroughgoing reliance on cumulative cultural achievements. In addition, the intense research focus on these species has spawned an unprecedented diversity of complementary methodological approaches, the results of which suggest that cultural phenomena pervade the lives of these apes, with potentially major implications for their broader evolutionary biology. Here I review what this extremely broad array of observational and experimental methodologies has taught us about the cultural lives of chimpanzees, gorillas, and orangutans and consider the ways in which this knowledge extends our wider understanding of primate biology and the processes of adaptation and evolution that shape it. I address these issues first by evaluating the extent to which the results of cultural inheritance echo a suite of core principles that underlie organic Darwinian evolution but also extend them in new ways and then by assessing the principal causal interactions between the primary, genetically based organic processes of evolution and the secondary system of cultural inheritance that is based on social learning from others.
Keywords: social learning, culture, evolutionary biology, chimpanzee, orangutan
Recent decades have revealed social learning (learning from others) to be pervasive across the animal kingdom, with important implications for evolutionary biology at large (1) and the subject of the Sackler Colloquium published here (2). This article focuses on great apes: chimpanzee (Pan troglodytes), gorilla (Gorilla gorilla), and orangutan (Pongo pygmaeus). Other primates are dealt with elsewhere in the issue (3, 4). Despite an early report (5), we still know little about cultural phenomena in chimpanzees’ rarer sister species, the bonobo (Pan paniscus) so bonobos are omitted here. I also make only limited reference to human culture, although we are technically also great apes. Human culture is extensively treated in other papers in this issue.
I first survey the nature and scope of social learning and associated aspects of cultural transmission in great apes, concluding that the depth and diversity of observational and experimental evidence for cultural phenomena are unparalleled among nonhuman species. The evidence thus accumulated suggests that culture permeates the lives of the great apes in the breadth of behavioral repertoires affected and also in their time-depth. These properties may be evolutionarily significant. The authors of a comprehensive recent review of cetacean culture concluded that “Culture … is a major part of what the whales are” (ref. 6, p. 7; and see ref. 7). Such a statement is obviously true for our own species (8–11); here I examine the justifications for thinking the phrase also has validity for great apes.
Following a sister review ranging much more widely across both vertebrates and invertebrates (1), I take eight core principles of evolution illuminated by Darwin (12) and assess the extent to which they apply to cultural phenomena in the great apes (henceforth simply “apes”), as they do in humans (13). I then explore ways in which cultural inheritance goes yet further beyond these principles, creating new evolutionary phenomena. Finally I address interactions between the primary manifestations of organic evolution based on genetic inheritance and the “second inheritance system” (14) based on social learning. In a now long-standing body of literature for humans, this interaction has been called “gene–culture coevolution” (15); the logic of such coevolution (10, 16) may apply to other cultural animals (1, 7).
Diverse and Convergent Evidence for the Scope of Great Ape Culture
Geographic Variation in Traditions in the Wild.
In 1986 Goodall began to chart differences in behavior patterns among chimpanzee study sites across Africa (17), proposing these differences as cultural variants when no genetic or environmental explanation was apparent (later called the “method of exclusion”). The approach became more comprehensive with time (18, 19), eventually benefitting from a systematic collaboration between multiple long-term research groups (20, 21). Similar collaborative analyses were soon achieved by orangutan field researchers (22) and more recently by a gorilla consortium (23). These analyses converged in reporting multiple cultural variants in all three genera: 39 in Pan; 24 in Pongo, and 23 in Gorilla. The variants spanned apes’ behavioral repertoires, including a great variety of tool use, food processing, and social behavior, as discussed further below. Further variants have continued to be reported intermittently for Pan (24) and Pongo, in the latter case leading to a revised tally of 26–35 variants, depending upon the criteria applied (25).
These surveys are vulnerable to false positives (it can be difficult to be sure that all alternatives to social learning have been excluded) and also to false negatives (cultural adaptations to local environmental properties may be inappropriately excluded) (26). However, these pioneering efforts provided essential platforms for more refined approaches, some incorporating both genetic and environmental variables into analyses (27). Other advances yielded confirmatory evidence for culture through (i) more focused microhabitat analyses for specific behaviors such as ant-dipping (28, 29); (ii) comparisons between neighboring communities sharing genes and habitat properties (30); and (iii) social learning experiments, as for nut-cracking (31, 32).
The broad geographic surveys thus provide an initially imperfect but progressively refined overall picture of ape cultural repertoires. The approach has been systematically applied to spider monkeys (Ateles, reporting 23 cultural variants) (33) but not yet, to my knowledge, to other animals. Evidence exists for multiple cultural variants in other species such as killer whales, which display very different hunting repertoires (e.g., for fish versus seals), song repertoires, and migratory patterns (6), but systematic tabulations have yet to facilitate direct cross-species comparisons.
Intergroup Variation in Traditions in Captive Communities.
A parallel approach has compared neighboring communities in captive contexts, with the advantage that genetic and environmental explanations for group differences can be dismissed more cleanly. For example in the Chimfunshi chimpanzee sanctuary in Zambia, a bizarre habit of inserting a blade of grass into one ear and leaving it there spread in one group but not in others (34). Moreover a distinctive “hand-clasp” form of grooming was absent in this and one other group but was customary in others, in which it additionally took different forms (35). Similar group contrasts were found in the means by which hard-shelled Strychnos fruits were opened (36). At the Yerkes Center in the United States a further contrast in hand-clasp grooming emerged and spread in one group over several years but remained absent in another group (37). These results reinforce those derived from the studies in the wild outlined above.
Quantitative Evidence for Vertical Mother-to-Offspring Transmission.
A study of the ontogeny of using stem-tools for termite-fishing found that juvenile female chimpanzees spent significantly more time attending to their mother’s fishing than did their male peers (38). Consistent with the skills being learned by observation, the young females tended to master the technique a whole year ahead of the males, with a significant tendency to match even the length of probe their mother typically inserted into the mound (38).
Researchers studying orangutans have called the focused visual attention of juveniles “peering” (Fig.1) (39). Building on studies documenting correlations between maternal and juvenile foraging profiles (40, 41), a suite of predictions were confirmed that were consistent with peering functioning to facilitate learning key survival skills (39). In foraging and nest-building contexts, in which peering is most frequent, it was found that (i) the frequency of peering in foraging contexts was predicted by the quantified complexity of processing operations and also by the skill’s rarity; (ii) peering was followed by a higher rate of exploration of the item concerned, as was also confirmed specifically for the use of sticks in foraging (seen only at one of the two sites studied); (iii) peering rose along with the learning of new skills and diminished as competence was achieved; (iv) peering at nest-building was followed by a rise in nest-building over the next hour; (iv) developmentally, peering tracked the peak time of learning to make nests; and (v) by about age 5 y, peering directed at a juvenile’s mother tipped below 50% and was directed more toward others, from whom there was still something to learn (39). Such observations offer a compelling case that juvenile apes’ close peering facilitates the learning of major life skills.
Fig. 1.
Peering (39) by a juvenile orangutan as her mother extracts termites from dead wood. Image courtesy of Christiaan Conradie and Caroline Schuppli.
Quantitative Evidence for Horizontal Transmission.
There is both intracommunity and intercommunity evidence for horizontal transmission in the wild. An example of the former was tracked by network-based diffusion analysis, confirming that a novel chimpanzee behavior, using moss as a water-sponge, spread from the alpha male along lines of social affiliation, providing quantitative circumstantial evidence for transmission (42). Examples of intercommunity transmission include (i) a significant acceleration in habituation to human observers in a chimpanzee community being newly habituated after two females immigrated from a well-habituated community (43); and (ii) the spread of ant-fishing to a new community after the immigration of a proficient individual from a neighboring community where fishing was habitual (44).
Quantitative and Qualitative Evidence for Investment in Transmission.
Videos of termite-fishing have documented skilled chimpanzee mothers donating tools to less competent juveniles (Fig. S1), thus suffering a diminished duration and rate of termite-fishing while the recipient enjoyed improved fishing (45). The authors propose these observations meet commonly accepted criteria for a functional (as opposed to intentional) concept of “teaching.” They also document mothers orally splitting their tool lengthwise neatly to make two functional tools or bringing multiple tools and suggest these behaviors partially buffer mothers from the costs of youngsters’ demands. Alternatively it might be argued that these actions are essentially unnecessary and thus represent the more compelling evidence that the behavior has costs and therefore counts as teaching, even if the teaching is not as active as teaching by scorpion provision by meerkats (46) or beaching to catch seals by killer whales (6). However, the pattern of costs and benefits suggests that this support has positive fitness benefits for the young, and parallel reports concerning the use of tools for nut-cracking have also been described (47).
Fig. S1.
Investment in offspring competence. (Upper) A mother donates her tool to a begging offspring. (Lower) The offspring has begun to termite-fish. Following the tool transfer, the termite-gathering efficiency of the tool donor is depressed, as illustrated by the mother’s selecting a replacement tool in the second frame. Still frames reused with permission from ref. 45 and thanks to Stephanie Musgrave, Crickette Sanz, Dave Morgan, and Goualougo Triangle Ape Project.
An earlier report described more active involvement in curbing youngsters’ exploration of potentially dangerous food-types. Haraiwa-Hasegawa reported that when an infant, PN, reached to touch some fig leaves, “her mother, FT, took PN’s hand and moved it away from the leaves. As PN continued … FT took the leaves from PN’s hand, plucked all the leaves within her arm’s reach and dropped them to the ground” (ref. 48, p. 280). At least one other mother behaved similarly and “prohibited … infants only from feeding on the individual trees that they themselves never fed on.”
Dyadic Experimental Studies of Social Learning.
Experimental reports of social learning by naive individuals from proficient models multiplied for more than a century in all great ape genera and have been tabulated and enumerated in successive reviews (49: n = 19 studies; 50: n = 33 further studies; 51: n = 25 studies comparing two or more ape species). The more recent experiments often adopt a highly informative two-action approach in which participants see one of two models, each trained to tackle a problem such as opening a foraging box in a different way. Ideally a third, no-model control group is included. This method has demonstrated social learning in captive chimpanzees (52), gorillas (53), and orangutans (54) that implies the copying of the action (imitation) or movements of the manipulanda (emulation) that were seen, rather than the simpler process of mere enhancement of the manipulanda (55). These approaches have further dissected the particular social learning processes at work, a topic beyond the scope of this review (see refs. 56–59 for in-depth treatment). More relevant to the present discussion and reviewed further below are extensions of these approaches to track the successive cultural transmissions necessary to sustain traditions.
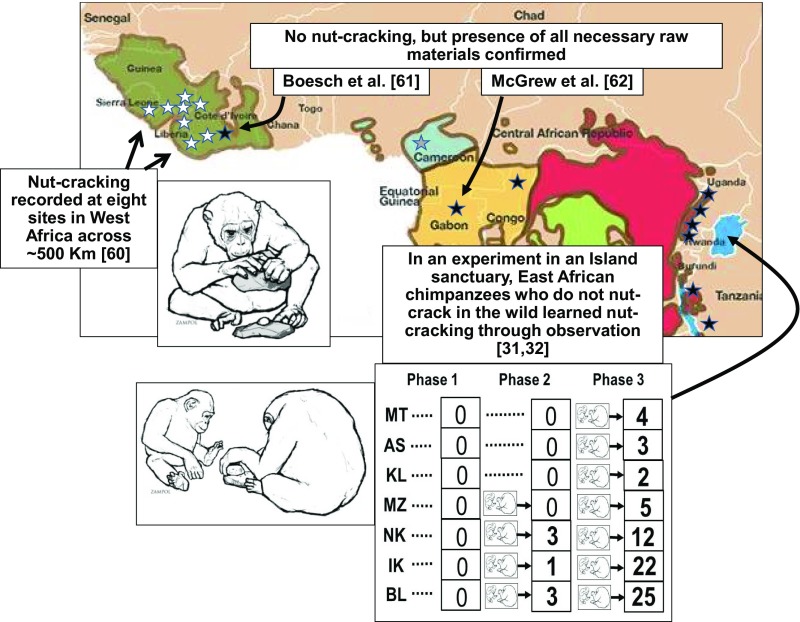
Nevertheless these dyadic experiments can importantly complement results from the field. For example, nut-cracking with natural hammer materials, found naturally only in West Africa (Fig. 2), is shown not to be explicable as an instinct in the West that is simply absent in the East, because East African chimpanzees exposed to proficient models became proficient, whereas controls in a no-model control condition did not, showing that the behavior is (socially) learned (Fig. 2) (31, 32).
Fig. 2.
Convergent evidence for a culture of nut-cracking in chimpanzees. Evidence for nut-cracking is seen at multiple sites in West Africa (20, 21, 60) (white stars) but is absent at others (black stars). The gray star indicates an early report in Cameroon, which was not subsequently confirmed. Independent studies confirmed availability of raw materials at two such sites (61, 62). Experiments showed East African chimpanzees (two-letter ID codes) did not initially nut-crack (Phase 1), but when half of the population was exposed to a proficient model, they began to do so (Phase 2), and all did so once exposed (Phase 3) (31, 32).
Cultural Diffusion Experiments.
Experiments focused on the broader phenomenon of cultural diffusion typically begin with models displaying different solutions to a task and then track the potential spread of the solutions in others who witness them. Alternative variants provide important complementary information (63, 64). For example, the “transmission chain” design pairs a first model (A) with a naive individual (B); then when B achieves some competence criterion, B becomes a model for a further individual (B for C, C for D, and so on). Achieving such configurations peacefully with apes requires sensitive experimental maneuvering, but transmission has been demonstrated along chains of up to five participants in chimpanzees (65) and orangutans (66), as well as in children (65). These experiments provide important models of transmission across cultural “generations,” implying a potential for cultural transmission across what would naturally be decades of ape life.
By contrast, “open diffusion” designs mimic transmission in the wild in which whole groups are exposed to alternative models; watching and copying is “open” to any individual in the group. This design has been applied in chimpanzees and children in several experiments (67–69), including two experiments in which tool-use behavioral variants were transmitted across three groups with significant fidelity (69). These results are important for interpreting putative cultures in the wild, such as the nut-cracking distributed across several hundred kilometers of West Africa, which would have required repeated intercommunity transmission.
How Pervasive Is the Role of Cultural Inheritance in the Lives of Great Apes?
Social Learning Shapes a Broad Repertoire of Cultural Variants.
We can first examine how pervasive the role of cultural inheritance is in the lives of great apes by surveying the range of behaviors described in the cross-site comparisons summarized above (20–23, 27).
The chimpanzee lists of 1999–2001 include 30 different kinds of tool use ascribed to culture, all suggesting functional and adaptive payoffs beneficial to the performer’s biological fitness (14, 21, 24). Others have been reported since then, including sticks bitten and thus sometimes made sharp and used to stab or evict bush baby prey at Fongoli in Senegal (70, 71); a kit of stout tools used to make tunnels; and fine stems used to fish down these tunnels and extract termites from nests deep underground (72). A majority of such tools are used in food extraction, but others are used in hygienic actions such as wiping blood or semen off fur, in protective comfort roles such as creating leaf-cushions on wet ground, and in local courtship gambits such as bending small shrubs on the ground (20, 21). Other diverse items include forms of food processing without tools, ways of dispatching ecto-parasites located during grooming, and grooming customs such as the hand-clasp that shows variant forms even in neighboring communities (73).
The orangutan list of 2003 (22) also includes a dozen different forms of tool use, several used for food extraction, such as holding a small stick in the mouth to extract seeds from Neesia fruits or using “leaf-gloves” to handle spiky fruit. Hygiene/comfort examples include using a leaf napkin to wipe off sticky latex. The list as a whole is diverse, incorporating forms of arboreal locomotion, vocal sounds (some modified using leaves), variant nest constructions such as adding sun covers, and whether slow lorises are eaten (irrespective of lorises’ availability).
The recent gorilla list of putative cultural variants (23) also displays diversity that includes making bridges across water, rubbing fruit to clean it or remove spines, incorporating tree-slapping into displays, using teeth as a “fifth limb” in climbing, forms of bodily contact while traveling together, and forms of social play.
The Extent of Vertical Intergenerational Transmission.
A detailed study of the foraging behavior of young wild orangutans before and after weaning concluded that their “diets were essentially identical to their mothers’ even though not all mothers had the same diet” … “immatures selectively observed their mothers during extractive foraging, which increased goal-directed practice but not general manipulation of similar objects, suggesting observational forms of learning of complex skills” (ref. 40, p. 62). This conclusion was reinforced by a later study focused on peering (39), referred to earlier. At 2–4 y of age, infants foraged with their mother more than 90% of the time; 94% of their feeding time occurred when the mother was also feeding; and 96% of their feeding was on the same items as the mother’s (39). The extent of cofeeding is clearly massive in preweaning years and is likely to engender the vertical social transmission of dietary profiles.
These years of mother–offspring association and cofeeding are typical of all the great apes and appear to lay down dietary preferences that change relatively little after weaning. Although the social learning implied may be as simple as enhancement of a food type by the mother’s feeding on it, such effects are likely to be profoundly important, because large diet-sets need to be mastered and selected from the even more vast options a tropical forest offers. This mastery includes avoiding the numerous plant parts that are toxic, selecting relatively nutritious options, and avoiding relatively poor ones. Chimpanzees may eat more than 300 different food types (species × parts) in a year (74); in the Lopé Park of Gabon, for example, fruit alone is taken from 114 different plants (75) selected from among many hundreds of potential food types available. The diet of gorillas may be similarly diverse; gorillas in the Alfi Mountains of Cameroon eat more than 200 different food types, including fruits, seeds, leaves, stems, pith, flowers, bark, roots, and invertebrates (75). For the orangutans of Tanjung Putting in Borneo, the figure is again more than 300 different food types (76). However, the dietary profiles of different populations may vary greatly, as suggested by earlier chimpanzee studies (77) and confirmed more recently in neighboring orangutan populations separated by a large river, which displayed 60% difference in diet, contrasting with intrapopulation homogeneity (78). Years of close apprenticeship to a mother who daily displays her knowledge of such a large but selective diet-set likely provide an important means of achieving an adaptive response to this challenging complexity.
Time Depth of Cultural Transmission.
Long-term field sites have shown that techniques such as termite-fishing have continued across several generations during the half-century of research now achieved. However, this time-frame pales in comparison with the discoveries of real archaeological excavation, which in the Tai Forest of Ivory Coast reached a depth corresponding to 4,300 y, where remains of nut-cracking were identified (illustrated in figure 1 of ref. 1) beneath those currently generated on the surface by chimpanzees (79). Of course, this behavior may be very much older. Once such a beneficial technology becomes customary, it may continue in perpetuity, pending major ecological perturbation. This example suggests that ape cultural inheritance spans not only the breadth of behavioral repertoires outlined earlier but also a potentially significant time depth comparable to that familiar in organic evolution via genetic inheritance.
Does Ape Culture Instantiate a New Form of Evolution?
Ape culture may have pervasive effects in shaping the behavioral repertoires of successive generations in the ways reviewed above, but do these effects imply an extension of biology in the sense of instantiating a new form of evolution based not on genetic but on social inheritance? This development is what Dawkins proposed in his concept of culturally replicated “memes” as analogies of genes, creating a new form of evolution in the case of human culture (80). The idea of aspects of culture such as language evolving through variation, (cultural) inheritance, and selection goes back to Darwin’s own writings (81) and was highlighted as the tenth and latest of the major evolutionary transitions proposed by Maynard-Smith and Szathmary (82). Mesoudi et al. (13) tackled the issue in finding abundant evidence for counterparts in human culture of eight major principles Darwin set out in the Origin (12): variation, selection, inheritance, adaptation, accumulation of modifications, geographic variation, convergence, and changes of function. How does ape culture compare?
In addressing this question we must be clear about the phenomena for which we are querying a potential “evolution.” If a chimpanzee invents a better hammer for nut-cracking (perhaps using a stone rather than wood), this innovation may enhance that individual’s biological fitness, so that its genes are better represented in future generations, i.e., natural selection shapes biological evolution. However, if others copy use of the new tool, the fitness (reproductive success) of that cultural entity—stone-tool use—will be enhanced through its spread, and to that extent there is cultural evolution of this behavior. It is this second phenomenon that we are addressing here. Effects on an individual culture-bearer’s biological, inclusive fitness are a different matter to which we return in a later section. We can now consider the eight evolutionary principles noted above.
Variation, Selection, and Inheritance.
The three principles of variation, selection, and inheritance can together be regarded as the core trinity of Darwinian evolution. Their joint working is an evolutionary algorithm that has been suggested to have the power to explain a multitude of phenomena beyond the living systems to which Darwin applied it (83, 84).
As we have seen above, there is plentiful evidence in the great apes for the feature of inheritance through social learning that provides sufficient fidelity to sustain traditions. There is also cultural variation, in part because, compared with gene replication, social learning is prone to imperfect copying. In the arrays of cultural variants among great apes discussed earlier in this article, there are plenty of behaviors that are displayed by many but not all individuals in a community (these behaviors are classed as “habitual” rather than “customary”).
By contrast, as yet there seems to be little direct recording of cultural evolutionary change through competition and selection within this variation. This absence of evidence of evolutionary change is perhaps unsurprising. During the human Stone Age, even when sophisticated, bilaterally symmetric Acheulian blades showed an advance over earlier crude Oldowan tools, they changed relatively little over a million years (85). If, as is plausible, such stability characterizes chimpanzee nut-cracking and other cultural variants of apes, then we will see little evidence of cultural selection in human lifetimes. Of course, organic evolutionary change is itself often slow compared with scientific lifetimes, although instructive exceptions have often followed human-caused environmental perturbations that create new selection pressures. The classic example is selection favoring dark morphs of peppered moths, better camouflaged against the sooty surfaces of the industrial revolution, and then flipping to favor light-colored morphs as the world became cleaner again.
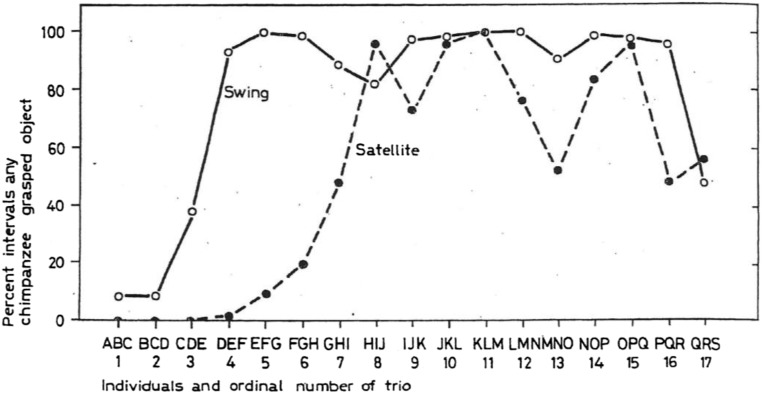
Accordingly I have suggested that similar contexts of anthropogenic change may be fruitful for investigating cultural evolution in animals (1). Scientific experiments may offer a convenient instance. For example, in a pioneering cultural diffusion study, three juvenile chimpanzees were confronted with and avoided two novel objects (86). One youngster was then replaced with a naive one, and this replacement was repeated, so after every third such cycle the triplet contained different individuals. Nevertheless, approaches to the objects increased steadily and in later generations of triplets became customary (Fig. S2). Accordingly, here there was variation in boldness inheritance insofar as naive youngsters learned from bolder ones that the objects could be safely approached and explored, and competitive selection favored small, progressive steps in boldness. Playing with the objects thus could evolve as the norm in the later generations composed of different youngsters than the original shy ones. In a counterpart from the wild, two individuals from a human-habituated community of wild chimpanzees immigrated into a neighboring community that scientists were beginning to habituate, and at that point habituation accelerated significantly (43).
Fig. S2.
Three young chimpanzees, A, B, and C, were exposed to two initially alarming objects, a swing and a satellite (moving ball). Every 2 mo, one of the three chimpanzees was replaced by a naive one, as indicated in the sequence BCD, CDE, and so on. Adaptive bolder approaches were socially inherited and over time came to dominate, resulting in a culture of common contact with the objects (86).
In these examples an initially common variant (caution) was replaced competitively by another (boldness) which was adaptively superior (fear was unnecessary in these contexts). Likewise, in all the cultural diffusion experiments with apes cited earlier, improved foraging techniques spread across test groups to replace the less competitive behavioral state characterized by their absence. Here, again, there was variation (although in this case the variation was engineered by the experimenter), inheritance via social learning, and competitive selection favoring the cultural spread of the new foraging technique.
I suggest experiments such as the one reported in ref. 86 may allow us to explore the capacity of apes to exemplify, even if in a limited way, the operation of the Darwinian-trinity algorithm in a cultural context. Presumably, at some previous time, all the cases of clearly beneficial cultural variants in the wild, such as nut-cracking and other tool use, did not exist; so, where they are customary, their common use is likely to have arisen through the operation of this algorithm.
Adaptation.
The growth of boldness in the studies discussed above (43, 86) indicates culturally evolved instances of adaptation, although the adaptive payoffs were likely only mild. In the wild there is evidence that a more crucial level of adaptiveness has been delivered. Chimpanzees in Bossou, Guinea, were shown to be reliant on two forms of technology, in particular nut-cracking and pestle-pounding (a means of extracting nutritious pulp from the apex of palm trees) during the dry season when fruit became scarce (87); these cultural variants allow these apes to inhabit otherwise inadequate habitats. How often culturally inherited technology is this critical remains difficult to judge at present, but many forms of tool use allow chimpanzees and orangutans to gain otherwise unavailable foodstuffs.
Such adaptations concern the local physical environment. Other adaptations may be societal. In a community of chimpanzees that customarily practices hand-clasp grooming, it may be adaptive to learn this behavior from those already using it; and where a particular courtship gambit such as leaf-clipping has become common, it will likely be beneficial to adopt this behavior as an action already recognized by one’s potential mating partner.
Accumulation of Modifications.
Human cultural modifications accumulate in an elaborate fashion that has no match in other animals and display the most striking analogies with the richness of the evolved forms of the living world (9, 10, 13, 16, 83, 84). Many authors assert that we are the only species to exhibit cumulative culture (8, 9, 49, 56), but I suggest this view may be premature. For example, chimpanzees in Goualougo use a stout stick to make a deep tunnel to reach subterranean termite nests and then use long stems to fish down the tunnels, first creating a distinct brush tip effective for fishing by stripping the stem ends through their teeth (72). They do so in a context in which the effective procedure is highly opaque, so it is difficult to see how the technique could have developed other than by a series of cumulative steps beginning with the more transparent context of fishing near the surface. Boesch (47) describes several other candidates for cumulative cultural evolution in chimpanzees. Direct evidence for the origins of such routines is lost in the past, but their complexity suggests elementary forms of cumulative culture, comparable perhaps to the achingly slow forms that characterized the early hominin Stone Age.
Geographic Variation.
Because the Darwinian algorithm operates in different regions, organic characteristics differentiate, and speciation may occur. Parallel effects occur in human cultural evolution (13, 88). As we have seen, great apes show evidence of different traditions at geographically separated locations, and there is evidence from all of the great ape genera that differences in putative cultural profiles are correlated with the geographic separation of communities (23, 24). As humans or other apes disperse over greater distances, one would expect both genetic and cultural similarities to diminish, and indeed Langergraber et al. (89) showed that cultural variation in chimpanzees is also correlated with genetic variation (but this correlation does not mean genes explain the behavioral differences; see the supplementary information in ref. 79 for further discussion of this study). Kamilar and Atkinson (90) demonstrated a nested structure in four samples of human cultural repertoires in North America and New Guinea, which would occur if, as people disperse, traits are sequentially added in or lost. Consistent with earlier cladistic analyses of the branching pattern of chimpanzee profiles (91), chimpanzees were found to display this pattern of nestedness across African sites. However, orangutans did not, in agreement with an earlier detailed orangutan study (27) and possibly reflecting a greater preponderance of vertical, mother-to-offspring transmission than horizontal transmission between communities.
Convergent Evolution.
The Darwinian algorithm delivers some similar organic evolutionary outcomes in different places, despite different foundations. Cultural convergences of this kind appear to occur at different layers of relatedness among apes. An example within the same species is hand-clasp grooming, which has emerged and spread in some chimpanzee communities in the wild, but not in others (20, 21, 73), as well as in an African sanctuary (35) and in groups in the United States (37). This hand-clasp grooming is not simply an individual invention because within-group spread of the behavior has been documented, indicating social transmission. Other convergences span different ape genera, such as use of fly swats and leaf napkins by both chimpanzees and orangutans; again, these behaviors are neither species instincts nor individually learned, because they are habitual at some locations but are absent in the same species at other locations. Finally there are convergences between apes and other primates, e.g., the use of stones as hammers to break open hard-cased food by distantly related long-tailed macaques (92) and capuchins (3, 93).
Change of Function.
Change of function is perhaps the category for which we are most limited by lack of historical records. In humans, historical records suggest that, just as morphology can evolve to serve a new function (arms becoming wings, for example), cultural elements may evolve new functions different from their original one (10). A candidate in great apes is that in chimpanzees leaf-clipping (noisily shredding leaves with the teeth) is reported as a courtship bid in some communities but is used for other functions, such as play, in others (47); such variations suggest that some of these alternatives may have evolved from each other or from a common ancestral function. However, it is possible that the lack of evidence in this category is explained by the lack of evidence concerning limited cultural cumulation, noted above.
Culture Extends Biology into New Realms of Evolution
The above discussion focuses on how cultural evolution may match the template for genetically based Darwinian evolution, but cultural transmission by social learning also extends the scope of biological systems by incorporating additional dimensions of inheritance and evolution. Some of these dimensions have long been recognized in the literature concerning human cultural evolution, including the fact that, in addition to intergenerational transmission shared with genetic inheritance, cultural transmission can be horizontal (within or between groups, and extending to nonkin) or oblique (with learning from nonrelatives in the prior generation) (15). Above I have reviewed some of the evidence for learning from parents, typically the mother, in apes (38–41, 45). Horizontal and oblique transmissions are commonly demonstrated in diffusion experiments (63–69) as well as in observational studies in the wild (42–44) and in sanctuaries (34). Such transmission makes cultural learning a powerful adaptive process, as does the fact that, because it hinges on neural rather than genetic changes, it can act very much faster; some important things can be learned observationally in a matter of minutes (94), although the acquisition of complex skills may require a more extended observational apprenticeship (32, 39).
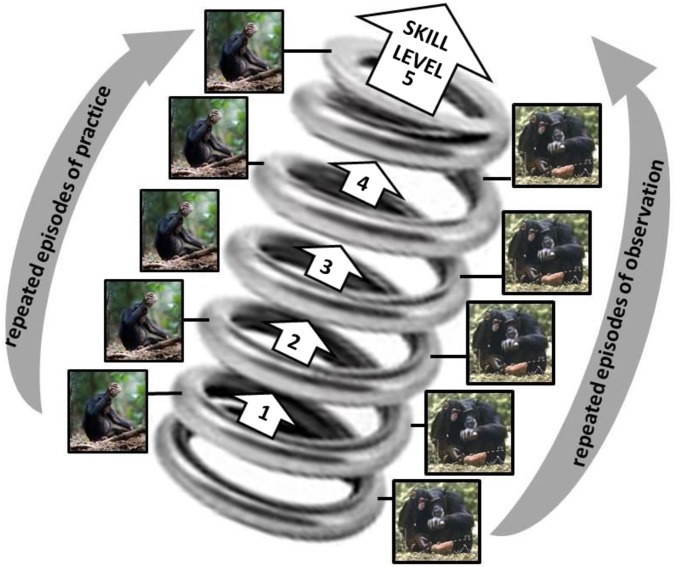
Additionally, although adaptive information is inherited genetically in a package at conception (even if activation is later adaptively contingent on environmental inputs), the adaptive information that feeds into social learning can be temporally distributed in at least two major ways. First, cultural transmission can be Lamarckian-like, with the adaptive features acquired through one individual’s lifetime passed on to those who learn from them. Second, a learner can build up complex skills, such as some forms of ape tool use, progressively by repeated cycling through a process of observe, practice, observe again, and practice again. This pattern can be thought of as a spiral or helical process of learning in which cycles of observation and practice allow the learner to assimilate more in later observations than was possible in the earlier, more naive stages (Fig. 3) (32, 39).
Fig. 3.
Helical curriculum model of skill development (after ref. 32). Over repeated cycles of observation-of-expert and practice, the social learner is able to assimilate more information from the expert and gradually improve his/her skill level. See the text for more explanation.
Social learning also may be selective in the assimilation of information, variously referred to as “directed social learning” (95), “biased transmission” (15), or “social-learning strategies” (96), which can in principle shape adaptation and consequent evolutionary change, with no clear counterparts in the gene-based processes.
Evidence in great apes has been adduced for a number of the potential learning rules these analyses highlight (97). Evidence for a copy-the-majority rule, suggested by the apparent conformity of chimpanzees in diffusion experiments (68), came in further experiments showing that both children and chimpanzees would copy the choices of three other conspecifics rather than those of a single individual repeating the same act three times (98). Orangutans did not show this selectivity (98), possibly reflecting their less community-based social life. Evidence for recognizing and copying more successful or productive options came from further experiments with chimpanzees (99). It has been suggested that preferentially copying individuals of high rank could serve this function also, and two studies have shown chimpanzees preferring to copy a high-ranked rather than a lower-ranked individual (100, 101). Finally, a tendency to learn from kin is shown by the studies of peering reviewed earlier, which showed extensive learning from the mother during apes’ extended preweaning period (39, 41). After weaning, this behavior widened to include peering at the activities of others, a plausibly adaptive shift from initially learning basic information from parents and then later targeting others to learn more specific skills, a trend identified in both observational (102) and experimental (103, 104) studies of human children. However, we still have only limited understanding of when and why an ape opts to learn socially (or does not): For example, what determines when immigrants will either conform to local norms (30) or instead transmit their habitual skills to others (44)?
Interactions Between Genetic and Cultural Modes of Inheritance and Evolution
At the broadest level, culture extends biology insofar as some culturally transmitted behaviors are evolutionarily consequential, i.e., they have implications for practitioners’ survival, reproduction, and ultimate inclusive fitness (as opposed to the reproductive success of the cultural items themselves, discussed earlier). Some cultural variants that appear relatively frivolous, such as staring at one’s reflection in water in gorillas (23) or applying an autoerotic tool in orangutans (22), may have less evolutionary significance, but varied forms of tool use by orangutans and chimpanzees appear to be highly functional in gaining access to rich resources such as insect prey, nut kernels, and honey. Indeed, some of these behaviors appear to be vital for chimpanzees to exploit niches that would otherwise exclude them (87). Other culturally transmitted behaviors play functional roles in grooming, social interactions, and sexual courtship.
Another sense in which culturally transmitted behaviors may have been evolutionary important concerns their effects on organic evolution. Cetacean researchers have proposed that cultural differentiation among whales has led to genetic differences (7, 105). For example, killer whales display eco-types that specialize in hunting alternative prey such as seals or fish using very different techniques, and different clans exhibit other behavioral differences in their songs and migratory/resident patterns, despite often being sympatric (6, 7, 105, 106). Such effects are suggested to have driven other morphological and genetic differentiation, ultimately leading to incipient speciation, because it becomes difficult for a member of one culture to enter another and successfully manage the different foraging and courtship requirements of that culture. This causal pathway would be an instance of “behavioral drive” (107–109), in which plasticity in behavior allows a species to exploit or create a new niche, in this case a culturally dependent one (e.g., hunting fish versus seal, a cultural drive). This niche in turn may create selection pressures acting on organic evolution, with effects such as the evolution of more robust jaws in the seal-hunters (6). Parallel hypotheses have been developed in the case of birdsong dialects driving speciation (110, 111).
Dramatically different specialisms such as seen in killer whales are not apparent among great apes, although the extent to which similar processes are at work, e.g., in contrasts between nut-cracking communities of chimpanzees and the nearest neighbors that do not crack, would repay attention. However, one principal effect of complex culture on organic evolution in apes has been proposed concerning encephalization and the cognitive sophistication it can provide: the cultural intelligence hypothesis.
The Cultural Intelligence Hypothesis
In accord with a previously advanced social (or Machiavellian) intelligence hypothesis, relative brain size in different primate species was found to be predicted by the typical size of their social group and the concomitant demands on social cognition (59, 112). Great apes do not fit this pattern, showing high relative and absolute brain sizes although gorillas and orangutans do not live in large communities. However, because all appear to display relatively complex cultures, the cultural intelligence hypothesis suggests that this complexity has selected for encephalization, either in a culture-first or an entwined culture–gene–brain coevolution scenario (112–115). One side of this proposition may be glossed as “culture makes you smart,” as is self-evident in the human case (9), insofar as present-day humans are smarter than those a century earlier by virtue of the cumulative cultural achievements from which they benefit. On a more modest scale, the same is proposed for the cultural endowment of great apes. The converse side of the proposal is that there is selection on the socio-cognitive capacities necessary to assimilate and store all the potential cultural repertoire available. In turn, it has been suggested that there will be correlated selection on technical and general intelligence, so as to benefit from the cultural input, as in intelligent tool-use, for example (114). One recently offered test of such ideas showed that when tested on the level playing field of zoo contexts, Sumatran orangutans scored higher on general intelligence than their Bornean cousins, as predicted by the more elaborate cultural repertoires of the Sumatran populations in the wild; moreover Sumatrans have brains that are 2–10% larger (116).
Summary and Conclusions
Research, particularly in the last two decades or so, has shown that a second inheritance system of social learning is widespread among animals, extending to all main classes of vertebrate and also to insects (1, 2). Apes merit a special focus, insofar as they have been subjected to an unmatched diversity and volume of observational and experimental studies by multiple research teams, whose work has revealed what appear to be the richest nonhuman cultural repertoires identified to date (although some cetaceans, e.g., killer whales, may show greater cultural differentiation). This article has attempted to indicate the scope of ape culture research and the key points of its discoveries, particularly with respect to the theme of the present issue: how these cultural phenomena may extend biology and its core evolutionary theory in particular. I have argued that the evidence supports the conclusion that the nature of social learning and its consequences in cultural transmission create new forms of evolution. These new forms echo well the established core principles of organic evolution but also go beyond them in a number of fundamental ways, such as horizontal transmission and inheritance of acquired characteristics, thereby extending the scope of evolutionary processes we must now entertain. Moreover the primary genetically based forms of evolution shaped and are also shaped by the consequences of this second inheritance system in complex ways we are only now starting to uncover.
Acknowledgments
I thank Sarah Davis, Bill McGrew, Stephanie Musgrave, Crickette Sanz, and Stuart Watson for comments on early versions of this paper.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “The Extension of Biology Through Culture,” held November 16–17, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Extension_of_Biology_Through_Culture.
This article is a PNAS Direct Submission. K.N.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620733114/-/DCSupplemental.
References
- 1.Whiten A. 2017 A second inheritance system: The extension of biology through culture. Interface Focus, in press. [Google Scholar]
- 2.Whiten A, Ayala FJ, Feldman MW, Laland KN. The extension of biology through culture. Proc Natl Acad Sci USA. 2017;114:7775–7781. doi: 10.1073/pnas.1707630114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fragaszy DM, et al. Synchronized practice helps bearded capuchin monkeys learn to extend attention while learning a tradition. Proc Natl Acad Sci USA. 2017;114:7798–7805. doi: 10.1073/pnas.1621071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry SE, Barrett BJ, Godoy I. Older, sociable capuchins (Cebus capucinus) invent more social behaviors, but younger monkeys innovate more in other contexts. Proc Natl Acad Sci USA. 2017;114:7806–7813. doi: 10.1073/pnas.1620739114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohmann G, Fruth B. Culture in Bonobos? Between species and within species variation in behavior. Curr Anthropol. 2003;44:563–571. [Google Scholar]
- 6.Whitehead H, Rendell L. The Cultural Lives of Whales and Dolphins. Univ of Chicago Press; Chicago: 2015. [Google Scholar]
- 7.Whitehead H. Gene–culture coevolution in whales and dolphins. Proc Natl Acad Sci USA. 2017;114:7814–7821. doi: 10.1073/pnas.1620736114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagel M. Wired For Culture: The Natural History of Human Communication. Allen Lang; London: 2012. [Google Scholar]
- 9.Henrich J. The Secret of Our Success: How Culture Is Driving Human Evolution, Domesticating Our Species, and Making Us Smarter. Princeton Univ Press; Princeton, NJ: 2015. [Google Scholar]
- 10.Creanza N, Kolodny O, Feldman MW. Cultural evolutionary theory: How culture evolves and why it matters. Proc Natl Acad Sci USA. 2017;114:7782–7789. doi: 10.1073/pnas.1620732114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legare CH. Cumulative cultural learning: Development and diversity. Proc Natl Acad Sci USA. 2017;114:7877–7883. doi: 10.1073/pnas.1620743114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwin CD. On the Origin in Species by Natural Selection. Murray; London: 1859. [Google Scholar]
- 13.Mesoudi A, Whiten A, Laland KN. Perspective: Is human cultural evolution Darwinian? Evidence reviewed from the perspective of the Origin of Species. Evolution. 2004;58:1–11. doi: 10.1111/j.0014-3820.2004.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 14.Whiten A. The second inheritance system of chimpanzees and humans. Nature. 2005;437:52–55. doi: 10.1038/nature04023. [DOI] [PubMed] [Google Scholar]
- 15.Boyd R, Richerson P. Culture and the Evolutionary Process. Univ of Chicago Press; Chicago: 1985. [Google Scholar]
- 16.Mesoudi A. Pursuing Darwin’s curious parallel: Prospects for science of cultural evolution. Proc Natl Acad Sci USA. 2017;114:7853–7860. doi: 10.1073/pnas.1620741114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Harvard Univ Press; Boston: 1986. [Google Scholar]
- 18.McGrew WC. Chimpanzee Material Culture: Implications for Human Evolution. Cambridge Univ Press; Cambridge, UK: 1992. [Google Scholar]
- 19.Boesch C, Tomasello M. Chimpanzee and human cultures. Curr Anthropol. 1998;39:591–614. [Google Scholar]
- 20.Whiten A, et al. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 21.Whiten A, et al. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]
- 22.van Schaik CP, et al. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 23.Robbins MM, et al. Behavioural variation in gorillas: Evidence of potential cultural traits. PLoS One. 2016;11:e0160483. doi: 10.1371/journal.pone.0160483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiten A. Social learning, traditions and culture. In: Mitani J, Call J, Kappeler P, Palombit R, Silk J, editors. The Evolution of Primate Societies. Univ of Chicago Press; Chicago: 2012. pp. 681–699. [Google Scholar]
- 25.van Schaik CP, et al. Orangutan cultures re-visited. In: Wich SA, Atmoko SSU, Setia TM, van Schaik CP, editors. Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Oxford Univ Press; Oxford, UK: 2009. pp. 299–309. [Google Scholar]
- 26.Laland KN, Janik VM. The animal cultures debate. Trends Ecol Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Krützen M, Willems EP, van Schaik CP. Culture and geographic variation in orangutan behavior. Curr Biol. 2011;21:1808–1812. doi: 10.1016/j.cub.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Schöning C, Humle T, Möbius Y, McGrew WC. The nature of culture: Technological variation in chimpanzee predation on army ants revisited. J Hum Evol. 2008;55:48–59. doi: 10.1016/j.jhevol.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Möbius Y, Boesch C, Koops K, Matsuzawa T, Humle T. Cultural differences in army ant predation by West African chimpanzees? A comparative study of microecological variables. Anim Behav. 2008;76:37–45. [Google Scholar]
- 30.Luncz LV, Boesch C. Tradition over trend: Neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. Am J Primatol. 2014;76:649–657. doi: 10.1002/ajp.22259. [DOI] [PubMed] [Google Scholar]
- 31.Marshall-Pescini S, Whiten A. Social learning of nut-cracking behavior in East African sanctuary-living chimpanzees (Pan troglodytes schweinfurthii) J Comp Psychol. 2008;122:186–194. doi: 10.1037/0735-7036.122.2.186. [DOI] [PubMed] [Google Scholar]
- 32.Whiten A. Experimental studies illuminate the cultural transmission of percussive technologies in Homo and Pan. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140359. doi: 10.1098/rstb.2014.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santorelli CJ, et al. Traditions in spider monkeys are biased towards the social domain. PLoS One. 2011;6:e16863. doi: 10.1371/journal.pone.0016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Leeuwen EJC, Cronin KA, Haun DB. A group-specific arbitrary tradition in chimpanzees (Pan troglodytes) Anim Cogn. 2014;17:1421–1425. doi: 10.1007/s10071-014-0766-8. [DOI] [PubMed] [Google Scholar]
- 35.van Leeuwen EJC, Cronin KA, Haun DB, Mundry R, Bodamer MD. Neighbouring chimpanzee communities show different preferences in social grooming behaviour. Proc Biol Sci. 2012;279:4362–4367. doi: 10.1098/rspb.2012.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlings B, Davila-Ross M, Boysen ST. Semi-wild chimpanzees open hard-shelled fruits differently across communities. Anim Cogn. 2014;17:891–899. doi: 10.1007/s10071-013-0722-z. [DOI] [PubMed] [Google Scholar]
- 37.Bonnie KE, de Waal FBM. Affiliation promotes the transmission of a social custom: Handclasp grooming among captive chimpanzees. Primates. 2006;47:27–34. doi: 10.1007/s10329-005-0141-0. [DOI] [PubMed] [Google Scholar]
- 38.Lonsdorf EV, Eberly LE, Pusey AE. Sex differences in learning in chimpanzees. Nature. 2004;428:715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- 39.Schuppli C, et al. Observational learning and socially induced practice of routine skills in immature orangutans. Anim Behav. 2016;119:87–98. [Google Scholar]
- 40.Jaeggi AV, et al. Social learning of diet and foraging skills by wild immature Bornean orangutans: Implications for culture. Am J Primatol. 2010;72:62–71. doi: 10.1002/ajp.20752. [DOI] [PubMed] [Google Scholar]
- 41.Jaeggi AV, van Noordwijk MA, van Schaik CP. Begging for information: Mother-offspring food sharing among wild Bornean orangutans. Am J Primatol. 2008;70:533–541. doi: 10.1002/ajp.20525. [DOI] [PubMed] [Google Scholar]
- 42.Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 2014;12:e1001960. doi: 10.1371/journal.pbio.1001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuni L, Mundry R, Terkel J, Zuberbühler K, Hobaiter C. Socially learned habituation to human observers in wild chimpanzees. Anim Cogn. 2014;17:997–1005. doi: 10.1007/s10071-014-0731-6. [DOI] [PubMed] [Google Scholar]
- 44.O’Malley RC, Wallauer W, Murray CM, Goodall J. The appearance and spread of ant fishing in the Kasekela chimpanzees of Gombe: A possible case of intercommunity cultural transmission. Curr Anthropol. 2012;53:650–663. doi: 10.1086/666943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musgrave S, Morgan D, Lonsdorf E, Mundry R, Sanz C. Tool transfers are a form of teaching among chimpanzees. Sci Rep. 2016;6:34783. doi: 10.1038/srep34783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton A, McAuliffe K. Teaching in wild meerkats. Science. 2006;313:227–229. doi: 10.1126/science.1128727. [DOI] [PubMed] [Google Scholar]
- 47.Boesch C. Wild Cultures: A Comparison Between Chimpanzee and Human Cultures. Cambridge Univ Press; Cambridge, UK: 2012. [Google Scholar]
- 48.Haraiwa-Hasegawa M. A note on the ontogeny of feeding. In: Nishida T, editor. The Chimpanzees of the Mahale Mountains: Sexual and Life History Strategies. Univ of Tokyo Press; Tokyo: 1990. pp. 277–283. [Google Scholar]
- 49.Tomasello M, Call J, editors. Primate Cognition. Oxford Univ Press; Oxford, UK: 1997. [Google Scholar]
- 50.Whiten A, Horner V, Litchfield CA, Marshall-Pescini S. How do apes ape? Learn Behav. 2004;32:36–52. doi: 10.3758/bf03196005. [DOI] [PubMed] [Google Scholar]
- 51.Galef BG, Whiten A. The comparative psychology of social learning. In: Call J, Burghardt G, Pepperberg I, Snowdon C, Zentall T, editors. APA Handbook of Comparative Psychology. APA; Washington: 2017. [Google Scholar]
- 52.Whiten A, Custance DM, Gomez J-C, Teixidor P, Bard KA. Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes) J Comp Psychol. 1996;110:3–14. doi: 10.1037/0735-7036.110.1.3. [DOI] [PubMed] [Google Scholar]
- 53.Stoinski TS, Wrate JL, Ure N, Whiten A. Imitative learning by captive western lowland gorillas (Gorilla gorilla gorilla) in a simulated food-processing task. J Comp Psychol. 2001;115:272–281. doi: 10.1037/0735-7036.115.3.272. [DOI] [PubMed] [Google Scholar]
- 54.Stoinski TS, Whiten A. Social learning by orangutans (Pongo abelii and Pongo pygmaeus) in a simulated food-processing task. J Comp Psychol. 2003;117:272–282. doi: 10.1037/0735-7036.117.3.272. [DOI] [PubMed] [Google Scholar]
- 55.Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philos Trans R Soc Lond B Biol Sci. 2009;364:2417–2428. doi: 10.1098/rstb.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tennie C, Call J, Tomasello M. Ratchetting up the ratchet: On the evolution of cumulative culture. Phil Tran R Soc B. 2009;36:2405-2415. doi: 10.1098/rstb.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subiaul F. What’s special about human imitation? A comparison with enculturated apes. Behav Sci (Basel) 2016;6:13. doi: 10.3390/bs6030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whiten A. Social learning and culture in child and chimpanzee. Annu Rev Psychol. 2017;68:129–154. doi: 10.1146/annurev-psych-010416-044108. [DOI] [PubMed] [Google Scholar]
- 59.Whiten A, van de Waal E. Social learning, culture and the ‘socio-cultural brain’ of human and non-human primates. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho S, McGrew W. The origins of the Oldowan: Why chimpanzees are still good models for technological evolution in Africa. In: Domínguez-Rodrigo M, editor. Stone Tools and Fossil Bones. Cambridge Univ Press; Cambridge, UK: 2010. pp. 201–221. [Google Scholar]
- 61.Boesch C, Marchesi P, Marchesi N, Fruth B, Joulian F. Is nutcracking in wild chimpanzees a cultural behaviour? J Hum Evol. 1994;26:325–338. [Google Scholar]
- 62.McGrew WC, Ham RM, White LJT, Tutin CEG, Fernandez M. Why don’t chimpanzees in Gabon crack nuts? Int J Primatol. 1997;18:353–374. [Google Scholar]
- 63.Whiten A, Mesoudi A. Review. Establishing an experimental science of culture: Animal social diffusion experiments. Philos Trans R Soc Lond B Biol Sci. 2008;363:3477–3488. doi: 10.1098/rstb.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whiten A, Caldwell CA, Mesoudi A. Cultural diffusion in humans and other animals. Curr Op Psychol. 2016;8:15–21. doi: 10.1016/j.copsyc.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Horner V, Whiten A, Flynn E, de Waal FBM. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. Proc Natl Acad Sci USA. 2006;103:13878–13883. doi: 10.1073/pnas.0606015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dindo M, Stoinski T, Whiten A. Observational learning in orangutan cultural transmission chains. Biol Lett. 2011;7:181–183. doi: 10.1098/rsbl.2010.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whiten A, Horner V, de Waal FBM. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- 68.Bonnie KE, Horner V, Whiten A, de Waal FBM. Spread of arbitrary conventions among chimpanzees: A controlled experiment. Proc Biol Sci. 2007;274:367–372. doi: 10.1098/rspb.2006.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whiten A, et al. Transmission of multiple traditions within and between chimpanzee groups. Curr Biol. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Pruetz JD, Bertolani P. Savanna chimpanzees, Pan troglodytes verus, hunt with tools. Curr Biol. 2007;17:412–417. doi: 10.1016/j.cub.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 71.Pruetz JD, et al. New evidence on the tool-assisted hunting exhibited by chimpanzees (Pan troglodytes verus) in a savannah habitat at Fongoli, Sénégal. R Soc Open Sci. 2015;2:140507. doi: 10.1098/rsos.140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanz C, Call J, Morgan D. Design complexity in termite-fishing tools of chimpanzees (Pan troglodytes) Biol Lett. 2009;5:293–296. doi: 10.1098/rsbl.2008.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura M, Uehara S. Proximate factors of different types of grooming hand-clasp in Mahale chimpanzees: Implications for chimpanzee social customs. Curr Anthropol. 2004;45:108–114. [Google Scholar]
- 74.Inskipp T. Chimpanzee (Pan troglodytes) In: Caldecott J, Miles L, editors. World Atlas of Great Apes and Their Conservation. Univ of California Press; Berkeley, CA: 2005. pp. 53–81. [Google Scholar]
- 75.Ferriss S. Western gorilla (Gorilla gorilla) In: Caldecott J, Miles L, editors. World Atlas of Great Apes and Their Conservation. Univ of California Press; Berkeley, CA: 2005. pp. 105–127. [Google Scholar]
- 76.McConkey K. Bornean orangutan (Pongo pygmaeus) In: Caldecott J, Miles L, editors. World Atlas of Great Apes and Their Conservation. Univ pof California Press; Berkeley, CA: 2005. pp. 161–183. [Google Scholar]
- 77.Nishida T, Wrangham RW, Goodall J, Uehara S. Local differences in plant feeding habits of chimpanzees between the Mahale Mountains and Gombe National Park, Tanzania. J Hum Evol. 1983;12:467–480. [Google Scholar]
- 78.Bastian ML, Zweifel N, Vogel ER, Wich SA, van Schaik CP. Diet traditions in wild orangutans. Am J Phys Anthropol. 2010;143:175–187. doi: 10.1002/ajpa.21304. [DOI] [PubMed] [Google Scholar]
- 79.Mercader J, et al. 4,300-year-old chimpanzee sites and the origins of percussive stone technology. Proc Natl Acad Sci USA. 2007;104:3043–3048. doi: 10.1073/pnas.0607909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dawkins R. The Selfish Gene. Oxford Univ Press; Oxford. UK: 1976. [Google Scholar]
- 81.Darwin C. The Descent of Man and Selection in Relation to Sex. Murray; London: 1871. [Google Scholar]
- 82.Maynard-Smith J, Szathmary E. The Major Transitions in Evolution. Freeman; Oxford, UK: 1995. [Google Scholar]
- 83.Dennett D. Darwin’s Dangerous Idea. Penguin; London: 1995. [Google Scholar]
- 84.Ridley M. The Evolution of Everything. Fourth Estate; London: 2015. [Google Scholar]
- 85.Stout D. Stone toolmaking and the evolution of human culture and cognition. Philos Trans R Soc Lond B Biol Sci. 2011;366:1050–1059. doi: 10.1098/rstb.2010.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menzel EW, Jr, Davenport RK, Rogers CM. Protocultural aspects of chimpanzees’ responsiveness to novel objects. Folia Primatol (Basel) 1972;17:161–170. doi: 10.1159/000155425. [DOI] [PubMed] [Google Scholar]
- 87.Yamakoshi G. Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: Possible implications for ecological importance of tool use. Am J Phys Anthropol. 1998;106:283–295. doi: 10.1002/(SICI)1096-8644(199807)106:3<283::AID-AJPA2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 88.Gray RD, Watts J. Cultural macroevolution matters. Proc Natl Acad Sci USA. 2017;114:7846–7852. doi: 10.1073/pnas.1620746114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Langergraber KE, et al. Genetic and ‘cultural’ similarity in wild chimpanzees. Proc Biol Sci. 2011;278:408–416. doi: 10.1098/rspb.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamilar JM, Atkinson QD. Cultural assemblages show nested structure in humans and chimpanzees but not orangutans. Proc Natl Acad Sci USA. 2014;111:111–115. doi: 10.1073/pnas.1313318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lycett SJ, Collard M, McGrew WC. Cladistic analyses of behavioural variation in wild Pan troglodytes: Exploring the chimpanzee culture hypothesis. J Hum Evol. 2009;57:337–349. doi: 10.1016/j.jhevol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Gumert MD, Malaivijitnond S. Marine prey processed with stone tools by Burmese long-tailed macaques (Macaca fascicularis aurea) in intertidal habitats. Am J Phys Anthropol. 2012;149:447–457. doi: 10.1002/ajpa.22143. [DOI] [PubMed] [Google Scholar]
- 93.Fragaszy D, Izar P, Visalberghi E, Ottoni EB, de Oliveira MG. Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. Am J Primatol. 2004;64:359–366. doi: 10.1002/ajp.20085. [DOI] [PubMed] [Google Scholar]
- 94.Whiten A. Imitation of the sequential structure of actions by chimpanzees (Pan troglodytes) J Comp Psychol. 1998;112:270–281. doi: 10.1037/0735-7036.112.3.270. [DOI] [PubMed] [Google Scholar]
- 95.Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Anim Behav. 1995;50:1441–1450. [Google Scholar]
- 96.Laland KN. Social learning strategies. Learn Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- 97.Price EE, Wood LA, Whiten A. 2016 Adaptive cultural transmission biases in children and nonhuman primates. Infant Behav Dev, 10.1016/j.infbeh.2016.11.003. [Google Scholar]
- 98.Haun DB, Rekers Y, Tomasello M. Majority-biased transmission in chimpanzees and human children, but not orangutans. Curr Biol. 2012;22:727–731. doi: 10.1016/j.cub.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Vale GL, Flynn EG, Lambeth SP, Schapiro SJ, Kendal RL. Public information use in chimpanzees (Pan troglodytes) and children (Homo sapiens) J Comp Psychol. 2014;128:215–223. doi: 10.1037/a0034420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Horner V, Proctor D, Bonnie KE, Whiten A, de Waal FBM. Prestige affects cultural learning in chimpanzees. PLoS One. 2010;5:e10625. doi: 10.1371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kendal R, et al. Chimpanzees copy dominant and knowledgeable individuals: Implications for cultural diversity. Evol Hum Behav. 2015;36:65–72. doi: 10.1016/j.evolhumbehav.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henrich J, Broesch J. On the nature of cultural transmission networks: Evidence from Fijian villages for adaptive learning biases. Philos Trans R Soc Lond B Biol Sci. 2011;366:1139–1148. doi: 10.1098/rstb.2010.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris PL, Corriveau KH. Young children’s selective trust in informants. Philos Trans R Soc Lond B Biol Sci. 2011;366:1179–1187. doi: 10.1098/rstb.2010.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lucas AJ, et al. The development of selective copying: Children’s learning from an expert versus their mother. Child Dev. 2016 doi: 10.1111/cdev.12711. [DOI] [PubMed] [Google Scholar]
- 105.Carroll EL, et al. Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci Rep. 2015;5:16182. doi: 10.1038/srep16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foote AD, et al. Genome-culture coevolution promotes rapid divergence of killer whale ecotypes. Nat Commun. 2016;7:11693. doi: 10.1038/ncomms11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wyles JS, Kunkel JG, Wilson AC. Birds, behavior, and anatomical evolution. Proc Natl Acad Sci USA. 1983;80:4394–4397. doi: 10.1073/pnas.80.14.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson AC. The molecular basis of evolution. Sci Am. 1985;253:164–173. doi: 10.1038/scientificamerican1085-164. [DOI] [PubMed] [Google Scholar]
- 109.Bateson PPG. The active role of behavior in evolution. Biol Philos. 2004;19:283–298. [Google Scholar]
- 110.Grant BR, Grant PR. Simulating secondary contact in allopatric speciation: An empirical test of premating isolation. Biol J Linn Soc Lond. 2002;76:545–556. [Google Scholar]
- 111.Grant PR, Grant BR. Adaptive radiation of Darwin’s finches. Am Sci. 2002;90:130–139. [Google Scholar]
- 112.Whiten A, van Schaik CP. The evolution of animal ‘cultures’ and social intelligence. Philos Trans R Soc Lond B Biol Sci. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Schaik CP, Burkart JM. Social learning and evolution: The cultural intelligence hypothesis. Philos Trans R Soc Lond B Biol Sci. 2011;366:1008–1016. doi: 10.1098/rstb.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burkart JM, Schubiger MN, van Schaik CP. 2016 The evolution of general intelligence. Behav Brain Sci1–65. [Google Scholar]
- 115.Street SE, Navarette AF, Reader SM, Land KN. Coevolution of cultural intelligence, extended life history, sociality, and brain size in primates. Proc Natl Acad Sci USA. 2017;114:7908–7914. doi: 10.1073/pnas.1620734114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Forss SIF, Willems E, Call J, van Schaik CP. Cognitive differences between orang-utan species: A test of the cultural intelligence hypothesis. Sci Rep. 2016;6:30516. doi: 10.1038/srep30516. [DOI] [PMC free article] [PubMed] [Google Scholar]