Abstract
An important extension to our understanding of evolutionary processes has been the discovery of the roles that individual and social learning play in creating recurring phenotypes on which selection can act. Cultural change occurs chiefly through invention of new behavioral variants combined with social transmission of the novel behaviors to new practitioners. Therefore, understanding what makes some individuals more likely to innovate and/or transmit new behaviors is critical for creating realistic models of culture change. The difficulty in identifying what behaviors qualify as new in wild animal populations has inhibited researchers from understanding the characteristics of behavioral innovations and innovators. Here, we present the findings of a long-term, systematic study of innovation (10 y, 10 groups, and 234 individuals) in wild capuchin monkeys (Cebus capucinus) in Lomas Barbudal, Costa Rica. Our methodology explicitly seeks novel behaviors, requiring their absence during the first 5 y of the study to qualify as novel in the second 5 y of the study. Only about 20% of 187 innovations identified were retained in innovators’ individual behavioral repertoires, and 22% were subsequently seen in other group members. Older, more social monkeys were more likely to invent new forms of social interaction, whereas younger monkeys were more likely to innovate in other behavioral domains (foraging, investigative, and self-directed behaviors). Sex and rank had little effect on innovative tendencies. Relative to apes, capuchins devote more of their innovations repertoire to investigative behaviors and social bonding behaviors and less to foraging and comfort behaviors.
Keywords: innovation, Cebus capucinus, cultural evolution, phenotypic plasticity, learning
Behavioral innovation has long been a topic of interest for researchers dedicated to studying the evolution of culture, because it is a driver of cultural change (1, 2). The types of behavioral traditions that are of greatest interest to evolutionary modelers are those starting with an innovation that then spreads via social learning. Understanding the characteristics of (i) behavioral innovations (which are roughly analogous to genetic mutations) and (ii) the individuals who invent these behaviors is critical to understanding cultural evolution and its relationship to genetic evolution. Innovation is also of interest to evolutionary biologists who study the role that learning plays in macroevolution, because it is a type of phenotypic plasticity that can affect the direction of natural selection (3). Ability to innovate can enhance reproductive success (for example, by enabling individuals to exploit new resources) (4–6). Innovation can generate the Baldwin effect, in which learned traits create recurring phenotypes that select for morphological adaptations, eventually leading to speciation (3, 7). Innovation is also of interest as a correlate of intelligence more generally, and the ability to solve novel cognitive problems presented by experimenters can be positively associated with mating success [e.g., bowerbirds (8)] and parenting success [e.g., great tits (9)] in wild populations.
Despite the obvious theoretical importance of innovation as a key element in cultural evolution, there are relatively few studies of this topic, particularly in wild populations, because of methodological difficulties in stimulating innovation experimentally or detecting innovations in observational studies (10). This paucity of information is partly because of the difficulty in creating operational definitions of innovation that can produce meaningful datasets for comparative analysis. Here, we loosely adopt the definitions by Reader and Laland (11) of innovation (the process) as “a process that results in new or modified learned behaviour and that introduces novel behavioural variants into a population’s repertoire” and innovation (the product) as “a new or modified learned behavior not previously found in the population” (ref. 11, p.14). Following the definitions of Ramsey et al. (12) and van Schaik et al. (13), we emphasize that innovations are not part of the innate repertoire and do not arise predictably in all population members at certain points in the life history; also, they do not predictably emerge in all population members in response to particular social or ecological conditions. The definition by Ramsey et al. (12) and van Schaik et al. (13) differs from the definition by Reader and Laland (11), because it focuses on the individual rather than the population [i.e., Ramsey et al. (12) argue that multiple individuals within the same population could independently create the same behavior]. We take a compromise position, counting a behavior as an innovation if this is the first time that the behavior has been seen in a particular social group during the putative innovator’s lifetime. Operational definitions of innovation and invention differ greatly across fields. Some define innovations as inventions that have subsequently spread throughout the population via social learning (14). We use the definition most commonly and currently used in the animal behavior literature, in which transmission of a new behavior is not a necessary part of our definition of innovation. We assume because of the extreme xenophobia exhibited by white-faced capuchins (15) that widespread social transmission of innovations is inhibited by insufficient exposure to members of other social groups.
The thorniest challenges in the rapidly growing field of animal innovation research arise in devising methods for quantifying innovation rates and determining the characteristics of innovators. Most research on animal innovation thus far has involved either (i) analysis of anecdotes drawn from published literature on topics other than innovation (16–18) or (ii) experiments in either the field or the laboratory, in which experimenters present individuals with a novel problem to solve (8, 9, 19–22). The most prominent body of innovation research using observational data has focused on orangutans (Pongo) using developmental data from rehabilitants (23) or “geographic contrasts methods” akin to those used to diagnose probable social traditions (24, 25) to infer innovation rates by evaluating the patterning of within- and between-group behavioral variation in short-term studies of both captive and field populations (12, 13, 26). Few field studies of innovation are longitudinal or look at the properties of individuals that make them more, or less, likely to innovate within their lifetime.
There are advantages and disadvantages to all of these methods, and none of them seemed entirely suitable for our goals, which are to (i) document the kinds of behaviors present in the innovation repertoire in wild white-faced capuchins, (ii) investigate differences in rates of innovation across behavioral domains, (iii) determine what features of individuals (rank, sex, age, and sociality) predict propensity to innovate, and (iv) determine whether new behaviors become established components of individual or group behavioral repertoires. Our approach differs from previous approaches to documentation of behavioral innovation, in that it was implemented into the core data collection protocol over a 10-y period, for which there was dense behavioral sampling of multiple social groups in the same ecological context. This approach provides the advantage of enabling researchers to detect probable innovations in any behavioral domain and have sufficient time depth to know who the probable innovators are (details are in Methods).
There are several biologically relevant behavioral contexts, or domains, that are difficult to study experimentally. Studies have largely looked at the diffusion of novel foraging tasks for good reasons. Experimentally seeded innovations or problem-solving tasks provide controlled contexts, where both the latency of individuals innovating a solution and the social diffusion of innovations may be studied. Innovating solutions to novel tasks is also of obvious adaptive value. However, many innovations that are ecologically or socially relevant are difficult to study experimentally. Some animals have innovative social interaction, i.e., “social games,” which may serve as bond testing rituals and can be socially transmitted (27). Some wild animals display repetitive self-directed “quirks,” perhaps to self-soothe, akin to the proposed function of some stereotyped coping behaviors in captive and wild animals (28). Other behavioral innovations have no apparent immediate biological or ecological function.
Systematic observational study of innovations across a wide range of behavioral domains permits us to explore whether individual propensity to innovate is generalized or whether individuals will be differentially prone to innovate in different behavioral domains according to their ecology and life history. For example, it has been suggested that “necessity is the mother of invention” and hence, that individuals who are young, low-ranking, and/or socially peripheral will be more prone to inventing new foraging strategies (29); this hypothesis has yielded mixed results in past literature reviews (6). In general, there are few strong theoretical expectations about how age, sex, rank, and sociality affect innovation rates; we need natural history and observational studies to help guide theory.
Capuchin monkeys (genera Cebus and Sapajus) are expected to have unusually high innovation rates for myriad reasons. Comparative studies have shown that brain size covaries with innovation frequency in primates and birds (30, 31), and capuchins are notable for their large relative brain sizes (32). White-faced capuchins (Cebus capucinus) are also omnivorous extractive foragers (33, 34), an ecological trait positively related to innovation in callitrichids (35), and profit greatly from experimenting with new foods and feeding techniques. Capuchins have radiated over a large, ecologically diverse geographic area (34, 36), which suggests that they are capable of adapting their behavior to novel situations. They frequently interact with and investigate other species as predators, prey, and feeding competitors (37, 38). It has been suggested by Sol et al. (39) that innovative tendencies in birds have coevolved with life history in generalist species, with slow-developing, large-brained species prioritizing future over current reproduction and being more likely to innovate and produce plastic responses to changing conditions. If this model holds true in other taxa, capuchins should be excellent candidates for high innovation rate given their life histories and ecological niche. White-faced capuchins live in multimale, multifemale social groups that are highly xenophobic, offering little opportunity to learn from members of other groups, except by migrating (40). Capuchins are notable for the high frequency of coalition formation and the creation of quirky social conventions that seem to serve as means of testing the social bonds that are likely to be important for enhancing fitness (27, 38, 40). The presence of social traditions in the foraging and social domains (41) implies that capuchins innovate as well.
Results
Innovations were classified in four categories or “behavioral domains”: foraging, self-directed, social, and investigative. The foraging category included 17 behaviors related to drinking water or processing food, 4 of which were independently invented in other groups and only 2 of which persisted in the innovator’s repertoire. One of these was a form of tool use: the use of leaves to wrap and scrub the urticating hairs off of Automeris caterpillars. Another was the use of the tail tip as a sponge to access water from tree holes too deep to reach with a hand or foot (Movie S1). The latter was invented only once during the period of 2007–2011 by a female who did this regularly but did not transmit it to other group members. This same tail-dipping behavior was independently invented in the buffer period (2002–2006) by monkeys in three other groups and spread to multiple individuals in one of those groups. (The buffer period was a time period during which we did not score innovations, but we used this time period to confirm whether behaviors seen in the subsequent 5-y period were truly new to that group. Details are in Methods.) It was not possible to reliably score food choice innovations and assign them to particular innovators, but the foraging category would have been much larger had we been able to include them.
The self-directed category included nine behaviors related to enhancing comfort, dental hygiene, self-soothing, and self-stimulation. Capuchins are prone to inventing “personal quirks,” especially involving clutching or poking some part of their own body for prolonged periods of time. These habits may persist for years and might be transmitted to other group members. There were many individuals in the 2007–2011 dataset that were still practicing postural quirks that they invented during the buffer period (2002–2006), and those are not evident in SI Appendix, Table S1. The “body part hold” category lumps together many different types of postural quirks; if we split this category more finely, there would be far more innovations in this domain.
The social category includes 47 forms of social interaction that are not part of the standard species repertoire. Eight of these were independently invented in multiple groups, and most of these inventions involved incorporation of behavioral elements from the foraging repertoire into the exploration or use of the interaction partner’s body (e.g., explorations of the partner’s orifices or mouthing parts of the partner). Some behaviors (e.g., dental examinations, eye poking, hand sniffing, sucking of body parts, toy game, and hair game) have been invented in multiple groups over the years and have become well-established traditions in some groups; hence, many of these behaviors scored as innovations for this 2007–2011 time period were invented before 2007 in other groups and still in practice (and hence, not counted as innovations) during this time period. Both past work and the patterning of results in SI Appendix, Table S1 suggest that those social rituals that involve some discomfort or risk (e.g., having an appendage bitten or damaging an eye) are more prone to remain in individual repertoires and become established in group repertoires. In addition to the aforementioned behaviors that have been proposed as bond-testing behaviors (41), the social category of innovations includes social play, social displays, and some creative ways for females to regulate infant behavior.
The investigative category included creative manipulations of other species (e.g., porcupines, howler monkeys, and turtles), human artifacts, leaves, sand, sticks, water, rocks, and other inanimate objects as well as innovative ways of locomoting through the forest. Most of these behaviors had no obvious immediate purpose and gave the impression that the innovator was engaged in recreational creativity, exploring the affordances of the materials. To give a few examples, in “cow pie seesaw” (Movie S2), the young monkey flips over a dried piece of cattle dung the length of her body, so that the flat side is on top and the rounded side contacts the ground; then, she stands on top, rocking back and forth. In “mango hitting game,” a young monkey routinely finds mangos about one-half the size of her body on the ground and applies hard, two-handed strikes to them for 4–5 min at a stretch, throwing her body weight into the assault on the mango, with no apparent interest in eating it (SI Appendix). Less than 15% of investigative behaviors were seen more than once in individual repertoires, and only 18% were subsequently observed being practiced by the innovator’s groupmates.
The final dataset, described in detail in SI Appendix, Table S1, included 187 innovations, 127 of which were unique behavior types. Of the 187 innovations, 149 (80%) of them we never again saw performed by the innovator, and only 41 (22%) were seen performed by other monkeys in the same group (i.e., had the potential to have become a tradition during the observation period). Of the 127 unique innovations seen, 54 (42.5%) were in the investigative domain, 47 (37.0%) related to social behavior, 9 (7.1%) were self-directed behaviors, and 17 (13.4%) were foraging behaviors. At least one innovation, based on our conservative criteria, was scored in 117 of 234 individuals included in this dataset. Descriptions of all innovations are included in SI Appendix, and SI Appendix, Table S1 reports the distribution of these behavioral variants across social groups and individuals.
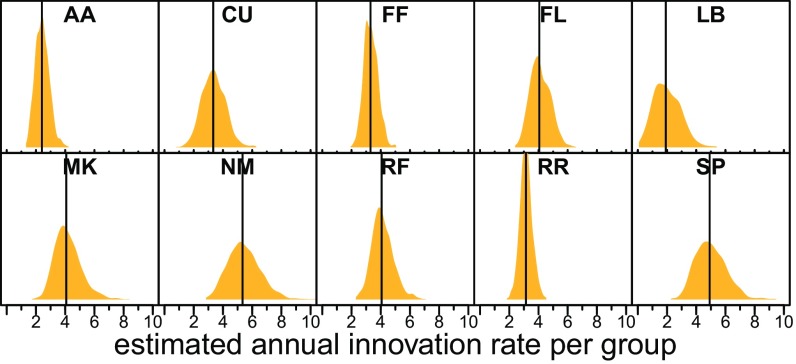
Innovations were rare, being observed, on average, less than once per individual per year in any particular domain. These annual rates may appear low, but our aim is to predict the properties of an individual that make him/her more prone to innovate in particular behavioral domains. If one ignores the properties of individuals or behavioral domains, annual group-wide innovation rates (Fig. 1), which have been the focus of many field studies of innovation, are much higher; however, we can learn much about individual innovators by taking this more detailed approach.
Fig. 1.
Posterior predictions of annual innovation rate (number of innovations per year) for each group. Two-letter names of the social groups are at the top of each panel; vertical lines indicate PMs. n = 44 group-years.
Our global model (referred to as mASRMG in Table 1) received overwhelming support compared with other models [having a Widely Applicable Information Criterion (WAIC) weight of 1.00] and suggests that sociality and age are the most important predictors of innovation (Table 1). From posterior median (PM) estimates and 89% credible intervals (89% CIs), our model suggests that marginal rates of innovation per individual per year (innovation rates) are highest in the social domain (PM = 0.122; 89% CI = 0.064–0.195) followed by the investigative domain (PM = 0.085; 89% CI = 0.047–0.147), foraging domain (PM = 0.028; 89% CI = 0.014–0.052), and self-directed domain (PM = 0.018; 89% CI = 0.008–0.037). Much of the variance in probability of innovating can be explained by individual identity (σid of αp = 1.01) and social group membership (σ group of αp = 1.56). Importantly, differences between behavioral domains account for more variation in rates of innovation (Table 2) than between-individual or -group differences for all other varying effect parameters with the exception of βmalep (SI Appendix, Table S2). Because interpreting the coefficients of these models can be nonintuitive and because they interact multiplicatively to estimate innovation rates in each domain, we instead refer the reader to plots of model predictions (Figs. 2 and 3 and SI Appendix, Figs. S1–S4) for all estimated effects.
Table 1.
WAIC estimates for all evaluated innovation models
| Model | WAIC | dWAIC | wWAIC | SE |
| mASRMG | 1,442.02 | 0 | 1 | 81.97 |
| mA | 1,478.34 | 36.32 | 0 | 84.38 |
| mS | 1,503.03 | 61.01 | 0 | 84.49 |
| m | 1,510.11 | 68.09 | 0 | 84.22 |
| mRG | 1,526.1 | 84.08 | 0 | 86.46 |
| mM | 1,570.25 | 128.23 | 0 | 88.39 |
Capital letters in model names correspond to predictors included: age (A), sociality (S), rank (R), sex (M), and group size (G). dWAIC, difference in WAIC scores from the highest ranked model; wWAIC, WAIC weight.
Table 2.
Posterior mean estimates of varying effects of behavioral domain
| Parameter | Behavioral domain | |||
| Foraging | Investigative | Self-directed | Social | |
| p | −0.12 | −0.45 | 0.01 | 0.06 |
| l | −0.98 | 0.07 | −1.38 | 0.49 |
| agep | 2.37 | 3.96 | 2.97 | −1.64 |
| agel | 0.03 | −0.52 | −0.04 | 0.24 |
| socialityp | −0.03 | −0.31 | 0.23 | −0.13 |
| socialityl | −0.32 | −0.09 | −0.52 | 0.51 |
| rank.highp | 0.14 | −0.46 | −0.02 | 0.00 |
| rank.highl | 0.05 | −0.32 | 0.15 | −0.01 |
| rank.lowp | −0.37 | −0.42 | −0.44 | 0.19 |
| rank.lowl | −0.21 | 0.03 | −0.21 | 0.13 |
| malep | 0.12 | −0.57 | 0.33 | −0.09 |
| malel | −0.09 | 0.08 | −0.26 | 0.20 |
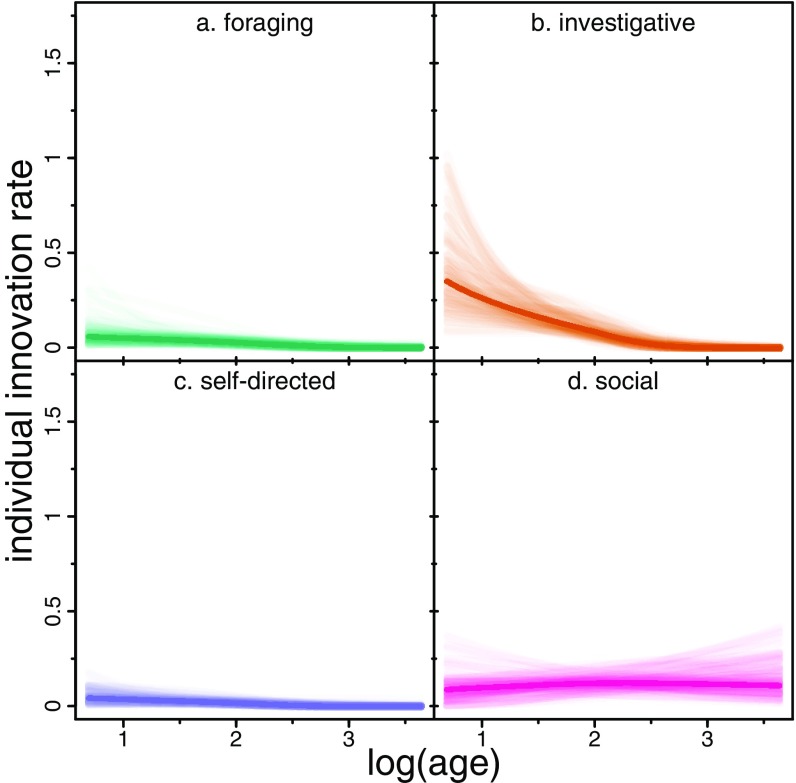
Fig. 2.
Joint model predictions for the effect of age on the number of innovations per individual per year in the domains of (A) foraging, (B) investigative, (C) self-directed and (D) social behaviors. Dark lines are at the PMs; lighter lines are 100 randomly sampled posterior predictions. n = 3,132 individual-years.
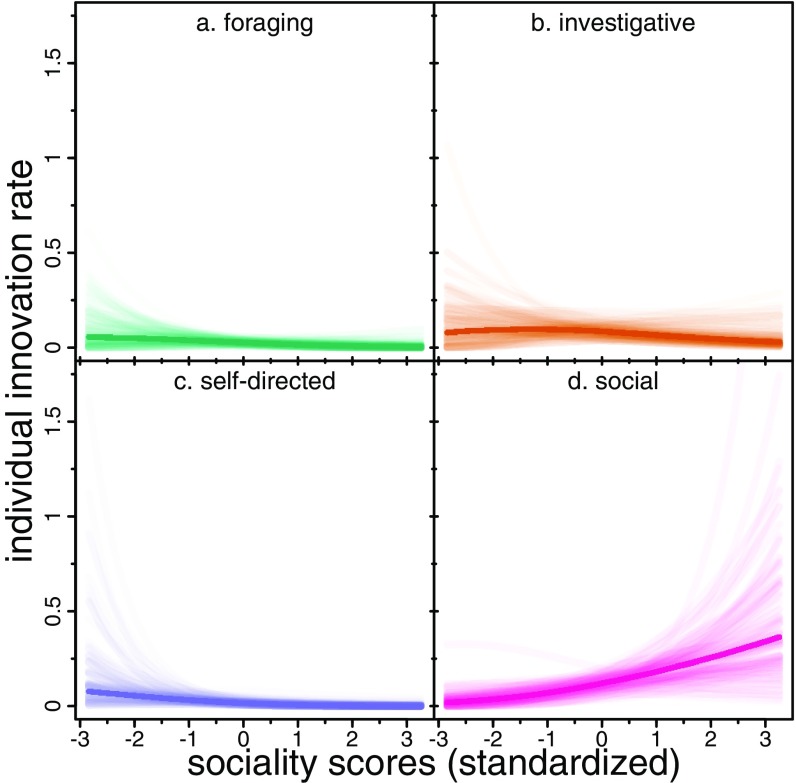
Fig. 3.
Joint model predictions for the effect of sociality on the number of innovations per individual per year. Dark lines are at the PMs; lighter lines are 100 randomly sampled posterior predictions. n = 3,132 individual-years.
For our model predictions, we display the posterior predictions for each individual’s annual innovation rate in each behavioral domain. The y axes may represent (i) the estimated number of innovations per individual monkey per year or the joint probability, (1 − ) × (Figs. 2 and 3 and SI Appendix, Figs. S3A and S4A). In some cases, looking at the individual components of a zero-inflated Poisson (ZIP) model can be informative, and therefore, we also present (ii) the probability of innovating per year, 1 − , (SI Appendix, Figs. S1 A–D, S2 A–D, S3B, and S4B) and (iii) the number of innovations per year estimated to be observed conditional on being an innovator, (SI Appendix, Figs. S1 E–H, S2 E–H, S3, and S4C).
Age.
Age differentially predicts innovativeness across behavioral contexts. Younger individuals innovate at higher rates in the investigative, foraging, and self-directed domains, although the effect size is quite small for self-directed and foraging behaviors (Fig. 2 A–C). Older individuals are slightly more innovative in the social domain (Fig. 2D and Table 2). This effect was more heavily driven by the probability of being an innovator (SI Appendix, Fig. S1 A–D and Table S2) than the number of innovations conditional on being an innovator. Younger innovators seem slightly more likely to produce more innovations, conditional on being an innovator, but this effect is small (SI Appendix, Fig. S1 E–H) and near zero in most domains.
Sociality.
Sociality also differentially predicts innovation across behavioral domains. More social individuals showed higher rates of innovation in the social (Fig. 3D) domain. Less social individuals had higher innovation rates in the foraging (Fig. 3A), investigative (Fig. 3B), and self-directed (Fig. 3C) domains, although these effects are weak and less certain. More social individuals produced a greater number of innovations per year (conditional on being innovators) in the social domain (SI Appendix, Fig. S2H), whereas less social individuals showed a greater number of innovations in the self-directed domain (SI Appendix, Fig. S2G), and there was little effect of sociality on foraging (SI Appendix, Fig. S2E) and investigative (SI Appendix, Fig. S2F) behaviors.
Sex.
Males (PM = 0.034; 89% CI = 0.014–0.074) have slightly higher innovation rates than females (PM = 0.024; 89% CI = 0.011–0.045), ignoring between-domain variation. Males were slightly more likely to be innovators (SI Appendix, Fig. S3B). Within the social and investigative domains, males showed slightly higher innovation rates both overall and conditional on being innovators, but there were no discernable effects of sex in the foraging and self-directed domains (SI Appendix, Fig. S3 A and C). Where these small differences exist, they are uncertain and potentially of no biological significance.
Rank.
Ignoring domain-specific effects, we found that innovation rate is highest in middle-ranking individuals (PM = 0.036; 89% CI = 0.017–0.070) followed by high-ranking individuals (PM = 0.026; 89% CI = 0.011–0.058) and lowest in low-ranking individuals (PM = 0.021; 89% CI = 0.009–0.48). Rank did not have consistent or strong effects on overall innovation rates within domains, although there was a slight tendency for midranking individuals to show higher innovation rates in the social and investigative domains (SI Appendix, Fig. S4A) relative to low- or high-ranking individuals; this pattern held for the rates of innovation conditional on being an innovator (SI Appendix, Fig. S4C). However, within each domain, low-rankers had a slightly higher probability of becoming innovators than mid- or high-ranked individuals (SI Appendix, Fig. S4B). Most of these rank-related domain-specific effects are relatively small and uncertain, and therefore, we hesitate to make strong claims about them.
Discussion
Utility of This Method for Identifying Innovations.
On the surface, it would seem that white-faced capuchins have the largest repertoire of innovations of any primate species studied thus far, but we encourage caution in comparing the sizes of different species’ innovation repertoires or their individual innovation rates because of differences in methods between studies. The method that we used here differs from previous approaches in the following ways.
-
i)
Researchers were vigilant for innovations and recorded them throughout the study period, likely resulting in fewer overlooked innovations than in other long-term studies. It also enabled rigorous recording of innovations across a wider range of behavioral domains than would have been the case had we focused our data collection on a specialized research topic not specifically related to innovation in multiple behavioral domains. Despite efforts to record all possible types of innovations, we failed to record food choice innovations with sufficient rigor to include them in this analysis.
-
ii)
This study generated a high density of behavioral observations compared with most primate field projects because of the year-round presence of a large number of well-trained data collectors. This higher sampling density suggests that, relative to other studies, more true innovations would have been observed and also, perhaps, that fewer false positives would have been generated (i.e., fewer rare species-typical behaviors labeled as innovations simply because they had not been observed in other practitioners because of low sampling density).
-
iii)
The large number of groups monitored in essentially identical ecological circumstances with overlapping home ranges offered better opportunity than most studies for identifying innovations via comparison of presence vs. absence in groups exhibiting similar ecologies. With the exception of five of the innovations concerned with exploration of human artifacts, there is no reason to think that monkeys had differential opportunities to discover particular innovations. Having more groups in the sample might make it easier to find one that lacked some rare behavior, although the long-term nature of the study combined with the dense behavioral sampling probably mitigate this tendency for the large number of groups to produce false positives of innovation caused by presence/absence contrasts.
-
iv)
The long-term nature of the study and the use of a 5-y buffer period before the observation period reduce the chance that behaviors will be falsely termed innovations when they are actually low-frequency behaviors already in the repertoire. If our study had been the length of a typical dissertation project (i.e., 1 y) and if we had assumed that behaviors were new to the practitioners if it was the first time that we had seen them performed in those groups, then we would have had 52% more innovations in our sample than we obtained by using the buffer period method and requiring each recorded innovation to be the first sighting by that individual in its group(s) of residence.
-
v)
Our method is more conservative than the definition used by Ramsey et al. (12), which defines innovations as being new to individual repertoires but not necessarily new to group repertoires. We recognize the possibility of having independent inventions within a single social group, and we suspect that many true innovations in our sample have been discarded because of the suspicion that they may be the products of social learning. On the whole, we think that our method provides a more accurate technique for diagnosing innovations than alternative observational methods. However, overlooking independent inventions within the same social group is one way in which we likely underestimate innovation rates.
-
vi)
Our approach looks at the properties that affect individual propensities to innovate and longitudinally tracks the rate of innovation over 5 y. Previous studies have looked at group-level differences or short windows of time. Our hierarchical statistical approach accounts for unequal sampling effort among individuals, estimates between- and within-individual variation, and avoids the potential problem of falsely making inferences about individual innovation rate from potentially spurious group-level effects.
Rates and Types of Innovation in Capuchin Monkeys.
Evidence that individuals, on average, (i) innovate in any one of these four domains less than once per year, (ii) retain only about 20% of these innovations in their repertoires, (iii) transmit no more than 22% of their innovations to other group members, and (iv) generally produce innovations with no obvious utility may give an artificially low impression of the biological importance that innovation has for behavioral repertoires overall. Most groups have a current repertoire of bond-testing signals that have been steadily practiced by a subset of the group for many years, and these behaviors are not, for the most part, scored as innovations in this dataset, because they first appeared in the buffer period or even earlier. Food choice innovations (not reported here because of the interobserver reliability challenge of being able to correctly identify plants the first time that they are eaten) tend to be adopted quickly and remain in repertoires for long periods of time, such as is the case for chimpanzees (42) and various species of monkeys (29). Particularly useful food processing or drinking techniques, once invented, are likely to persist in repertoires for many years [or even centuries (43)]. Tail-dipping to access water in deep tree holes seems to have been independently invented in four groups at Lomas, originating during the 2002–2006 period in three of these groups (and therefore, not counting as an innovation according to our definition) but persisting during the 2007–2011 period in two of those three groups. Although many of the innovative behaviors recorded during this 5-y period seem to be aimless creativity with no obvious utilitarian goal in mind (aside from the foraging behaviors), it is important to remember that innovations, like mutations, may not be particularly beneficial on their own but may become exapted (44) and acquire a benefit when paired with a particular ecological or social context (even if the initial pairing is accidental). For example, a potentially risky behavior, such as sticking a finger deep into the eye socket of a partner, might yield benefits if it is incorporated into a dyadic ritual that tests the quality of an important social bond (27, 45). Additionally, many of the capuchin innovations in this dataset might inform the developing monkey about the affordances of objects or how his/her body relates to the environment, providing useful feedback, even if there is no practical value to permanently incorporating the new behavior into the behavioral repertoire.
How the Capuchin Innovation Repertoire Compares with Those of Chimpanzees and Orangutans.
The profound methodological differences between studies of these species preclude precise quantitative comparisons of innovation rates in different behavioral domains, but we can at least make some crude qualitative comparisons between capuchins and the other two primate species for which innovations have been systematically cataloged in the wild. The Mahale chimpanzee researchers (42) present data on 26 novel behaviors (after excluding food choice to make their results more comparable with the other datasets) retrospectively extracted from their 43-y study of two chimpanzee communities. Although studying innovation was not an explicit part of their core data collection protocol, many researchers at Mahale described behavioral variation and novel behaviors in detail. They defined innovation as any behavior not seen in the first 15 y of research. Innovation in orangutans has been explicitly studied using the geographic contrasts method in short-term studies by van Schaik et al. (26) at multiple sites, producing a sample of 44 putative innovations. Comparison of the distribution of behaviors across domains in their datasets with the composition of the repertoire in the dataset of 127 unique innovations from Lomas Barbudal suggests that capuchins, relative to these ape species, devote a higher proportion of their creative energy to investigation of their environment and devising new social behaviors and a lower proportion to comfort-related behaviors and foraging. Orangutans are particularly prone to devising new variants on nest-building techniques, and even their novel acoustic behaviors seem to emerge primarily in a nest-building context. Both ape species are more innovative with regard to bodily comfort and hygiene than capuchins. Capuchins rarely seem to prioritize comfort: they do not build nests, and they readily endure stings, bites, urticating hairs, and spiny vegetation in the process of acquiring food. Although nearly 4% of unique innovations may serve to mitigate physical discomfort, this percentage is low compared with both chimpanzees (15%) and orangutans (36%). Capuchins devoted about 42% of their innovation repertoire to the investigative domain compared with chimpanzees (31%) and orangutans (14%). Orangutans are curiously neophobic relative to both chimpanzees and capuchins, and the exploration of the environment that they engage in as young individuals is primarily focused on the mother’s activities rather than independent discovery (13), whereas capuchins engage in much object play and independent exploration of the affordances of the environment at an early age. The preponderance of investigative sorts of innovations in orangutans seems to be devoted to solving the problem of how to safely engage in arboreal locomotion as a large-bodied animal (26, 46)—a problem that is less difficult for a small-bodied animal with a prehensile tail. Within the category of social innovations, capuchins were more prone to inventing behaviors related to social bonding, whereas chimpanzees placed more emphasis on aggressive displays. This emphasis is consistent with the importance of alliances and hence, bond tests in capuchins: males need allies for parallel dispersal and to acquire and maintain breeding positions, and females depend on allies to defend food resources and infants from potentially infanticidal males (40).
What Factors Affect Propensity to Innovate in Other Species?
In this study, younger animals were more innovative in all behavioral domains except for social interaction, in which the tendency was reversed. This result is consistent with capuchins’ slow life history, generalist ecological niche, and complex social relationships. During a long juvenile phase, individuals have much to gain by experimenting with both new foods and more efficient ways of acquiring resources. As they age, capuchins increasingly have reason to form and test the quality of those social relationships that will prove critical for acquiring and protecting access to the food and social resources needed for enhancing reproductive success (40), and therefore, it makes sense that older animals are more prone to innovation in the social domain. Studies of other species provide mixed results regarding the effect of age on innovative tendencies. A review of the published primate literature suggests that adults innovate more than immatures (16), and this pattern has been corroborated by experimental studies of innovation in callitrichids (22) and meerkats (20), in which young animals were less likely than adults to successfully solve a novel extractive foraging task, possibly because of insufficient development of dexterity. Chimpanzees are a notable exception to this general pattern; high rates of innovation, particularly of the social and investigative sort, are observed in immature chimpanzees (16, 29). Among human children, older children are better than younger children at solving novel problems (47). It is worth noting that, in this study, we were probably measuring something more akin to creativity and exploration rather than skill at solving a task, and it is possible that the innovations created by older capuchins could be argued to be more sophisticated in some way.
The other variable that had a strong effect on capuchins’ propensity to innovate was sociality: more social capuchins were more prone to inventing novel social interaction types, and more social monkeys were also slightly more likely to have their social innovations picked up by other group members (although we cannot currently say whether this is because of social learning) (SI Appendix, Fig. S5B). These results can probably be explained simply by the fact that more social individuals have more opportunities to experiment with novel forms of social interaction. The existing literature on innovation does not have much to say about the effects of sociality per se, aside from predicting that technological innovations will be more common in peripheral animals who are less distracted by social life (29). However, the literature does address dominance rank as a predictive variable, and subordinates are often less socially central than dominants. Because there was not a strong rank effect in this dataset, we remain hesitant about making claims regarding rank mediating the relationship between sociality and innovative tendencies.
Although sex and rank (16, 20, 29) are often predictive of innovative tendencies in other species, neither variable had much explanatory power in the capuchin dataset. Capuchin males were slightly more likely to innovate in all domains, which is consistent with observations from other primates (16) and meerkats (20) but not guppies (48); however, these effects were so small and uncertain in the capuchin dataset that they are not likely to have any biological significance.
With a better understanding of innovation, the next logical questions to address are two questions linking this research to the social learning literature. What properties of innovations and what properties of innovators make the innovations most likely to be socially transmitted to other group members? Adequate answers to these questions are beyond the scope of this paper, but preliminary analyses suggest that age and group size of the innovator might be important drivers (SI Appendix, SI Text and Fig. S5).
Methods
Study Site and Subjects.
The study was performed at Lomas Barbudal Biological Reserve in Guanacaste, Costa Rica, a tropical dry forest described in the work by Frankie et al. (49). This population of C. capucinus has been the subject of long-term study by Perry et al. (50) since 1990, starting with a single social group. The number of regularly monitored groups has since expanded by both fission of research groups and habituation of new groups to include 10 groups by the end of 2011.
One of the methodological problems in documenting innovations is the difficulty of knowing whether a particular behavior has ever been performed or observed by a particular individual. In a short-term study, when the researchers have little experience with the animals, many rare behaviors will be falsely scored as innovations simply because the researcher has not seen them previously. Ironically, such sampling biases may result in the misimpression that innovation rates are higher in short-term studies than long-term studies. Such problems are mitigated in this study by (i) using data from a long-term study; (ii) generating a high density of observations by using a large staff to collect year-round behavioral data, in which new behaviors were explicitly recorded; (iii) explicitly training observers to watch for and record new behaviors; and (iv) having a single observer with 26 y of experience collecting data on this population (S.E.P.) make the final determinations about which behaviors are truly new for each group of monkeys, with input from two additional long-term researchers (B.J.B. and I.G.).
Beginning in January of 2002, all research staff were directed to make freeform comments about any behaviors that did not neatly fit into the standard ethogram of species-specific behavior and explicitly mark comment lines as comment innovation when they thought they were seeing a behavior that they had never seen before in that group or a behavior that they thought was a unique behavioral tradition. Naturally, many behaviors seem new to relatively inexperienced observers, and therefore, not all of the behaviors initially coded as innovations were true innovations. Also, some behaviors not coded as innovations in the comments section were, in fact, true innovations. S.E.P., who personally collected 13,770 h of data on this population from 1990 to 2016, read through all data to determine whether behavioral sequences were likely to be true innovations (i.e., behaviors seen for the first time in that particular social group).
This research was performed in compliance with the laws of Costa Rica, and the protocol was approved by the University of California, Los Angeles Animal Care Committee (ARC 1996-122 and 2005-084 plus various renewals).
Buffer Period.
We used a 5-y chunk of observational data (35,196 h collected between January 1, 2007 and December 31, 2011) to look for innovations. We used the 5 preceding years of data (∼37,514 h of observation collected during 2002–2006) as a “buffer period” (i.e., a period in which we could search for prior instances of behaviors that appeared to be innovations within the targeted 2007–2011 time period). If we had not left a large buffer period, we would have falsely concluded that far more behaviors within the time period of interest were innovations. SI Appendix, Table S1 reports, for each innovation, the number of groups in which it occurred, its persistence in the innovator’s repertoire, and whether it spread to other group members.
Data were collected by a team of 50 highly trained observers (∼12 per year), each of whom underwent a training period of approximately 3 mo of dawn to dusk instruction and interobserver reliability testing before contributing data to the database. Interobserver reliability tests for monkey identifications and coding were repeated monthly throughout their tenures at the field site. Obviously, we could not train observers to reliably code innovations (behaviors that had not yet happened) in the same ways. We could, however, ensure that the observers recognized and reliably recorded basic motor movements, gestures, and 205 behaviors considered to be part of the species-typical behavioral repertoire. This repertoire was based on >6,000 h of observation invested in studying these monkeys during 1990–2001 before explicit recording of innovations began, thereby enabling better detection of the idiosyncratic behaviors.
Although we gave instructions to data collection teams to record any type of innovation in any behavioral domain, we decided not to include innovations regarding (i) choices of food or medicinal plants and (ii) vocal behavior in this analysis. Although we witnessed many instances of apparent innovation regarding use of novel foods and medicines, we lacked sufficient confidence in observers’ ability to accurately identify rarely used plants and insects or accurately identify rarely produced vocalizations.
A behavioral observation was scored as an innovation if it met the following criteria: (i) the behavior was absent in some of the groups that we regularly monitor, (ii) it was the first time that this behavior had been seen in this group during 2007–2011, and (iii) this behavior had not been seen in this group during the buffer period time period preceding 2007 during the lifetime of the putative innovator. In cases of group fission or migration, we required that the behavior not have been seen previously in the other groups of which the potential innovator had been a member. There was one class of behaviors on which an additional criterion was imposed: postural habits, such as clutching of one’s own body parts or sniffing one’s own hand (SI Appendix).
The number of innovations was also counted via two less conservative methods for methodological comparison. In one version, we eliminated the buffer period criterion, calling the first observation of a behavior in a particular group an innovation. This method yielded 263 innovations compared with 187 produced with the more conservative method described above. In an even less stringent version that yielded 282 innovations, we termed a behavior an innovation if it had not been seen in that group in the past year.
Another challenge in defining innovation is the “grain” problem (i.e., determining the descriptive breadth of behavioral categories or in other words, the extent to which to lump vs. split behaviors) (51). The grain problem is insoluble; the best that we can do is to use our intuitions about what the animals themselves seem to consider novel and observations of how these behaviors cluster in the repertoires of groups and individuals. Are novel actions used as part of a task already in the behavioral repertoire novelties? In our view, they usually were. Are the same actions applied to different objects? Unless the objects and contexts were quite radically different, we opted to lump these together (i.e., we did not designate them as innovations). This issue was most prominent in our decision-making when evaluating clutching of different body parts (a self-directed behavior), sucking of different body parts (a social behavior), and the “toy game,” which involves passing an object from mouth to mouth. In all of these cases, we chose to lump rather than split behaviors. In the case of object play (e.g., with sand, water, or rocks), different actions used by different individuals in interacting with these same substances were scored as different behaviors. Descriptions of the complete list of behaviors that were included in our analysis are in SI Appendix.
Innovations were classified in four categories or behavioral domains, the content of which is described in greater detail in Results: foraging, self-directed behavior, social behaviors, and investigative behaviors (exploration of the environment).
Data Structure.
We created a different row for every individual monkey/year/behavioral domain combination, and a value was scored for the number of innovations observed for that combination; this number was the output variable. We measured four main predictor variables to determine what predicts the number of innovations per individual per year: age, sex, sociality, and rank. (i) Age. To calculate age, we subtracted the birth year of an individual from the year of observation and added one. Individuals born in the same year as the year of observation were excluded from the dataset. Age was log-transformed and centered for analysis. (ii) Sex. We coded sex as a dummy variable (one for males and zero for females). (iii) Sociality. Sociality was calculated using data from group scans, which were taken opportunistically for all group members, at intervals no closer together than 10 min. For each individual per calendar year, we calculated the proportion of group scans in which the individual was in proximity (i.e., within ∼400 cm) to at least one other group member other than their dependent offspring (i.e., for females, their offspring that are less than 1 y of age). Individuals with less than 20 scans per year were excluded from analysis. Sociality scores were standardized, so that a sociality score of one is 1 SD above the centered mean of zero. (iv) Rank. For individuals older than 3 y old, rank was calculated using the EloRating package in R (52). We used outcomes from dyadic interactions involving avoids, cowers, flees, and supplants to determine dominance. We used default Elo parameters, with initial Elo scores set to 1,000, and the constant k set to 100. Rank was estimated using average Elo scores calculated for each individual per calendar year. Young juveniles less than 3 y old were given Elo Scores of zero to assure a low rank within their social groups. Members of a group were divided up into tertiles, with the corresponding levels receiving rank categories of high, middle, and low. High and low ranks were estimated as dummy variables, with middle rank serving as the intercept-only reference category.
Statistical Methods.
Our outcome variable, number of innovations, is a count variable with many zero values. Each monkey was observed over multiple years. Membership in a particular group may have affected propensities to innovate. Therefore, we analyzed these data using a series of hierarchical ZIP models. ZIP models are mixture models that use two probability distributions. One component assumes a Bernoulli distribution and estimates : the probability of observing a zero. The other component assumes a Poisson distribution and estimates : the estimated mean of a Poisson distribution. ZIP models permit a mixture of causal factors to be evaluated and help better predict outcomes when there is a large number of zeros because of both the rarity of an event and false negatives. The joint likelihood of observing an innovation can be calculated by multiplying the likelihoods of the Bernoulli and Poisson outcomes and converting them to the real number scale using their corresponding link functions. We graphically present joint posterior predictions here (Figs. 2 and 3) along with model predictions of and in SI Appendix (SI Appendix, Figs. S1–S4).
We looked at four predictors in this analysis: (i) age, (ii) sex, (iii) sociality, and (iv) rank. We also estimated unique offsets for each individual, because they differed in observation time or exposure. We analyzed six models, four of which corresponded to one of the single aforementioned predictors. The other two included a global model that looked at all four predictors and an intercepts-only model. In each model, we used varying intercepts for each individual (n = 234), social group (n = 10), and behavioral domain (n = 4). Varying slopes for domains and groups were estimated for all four predictors, and varying slopes for individuals were estimated for sociality and age. Group size was used as a covariate to control for the numerical likelihood that, in smaller groups, observed behaviors might be more likely to be scored as innovations because of our definition of uniqueness and that a greater number of innovations is more likely in larger groups. In another analysis, we used experimental year as a varying effect to see if there were any biases in data collection between field assistant cohorts that would change our inference. There were none, and therefore, we excluded these parameters from our final analyses to simplify the presentation of results.
Offsets and Exposure.
Because observations of innovations were collected not only in focal follows but also, ad libitum, and because individuals varied in their likelihood of being observed because of data collection protocols or their visibility in the group, they also differed in exposure. To account for differences in exposure, we calculated an annual offset for each individual in each calendar year. Offsets for each individual () were estimated using
where is the number of instantaneous group scans calculated per calendar year for each individual , and is the number of point samples collected at 2.5-min intervals during focal follows in a calendar year. These offsets were included alongside linear predictors in each model.
Models were fit using the map2stan function in the R package rethinking (53). Models were fit using Hamilton Markov Chain Monte Carlo in r-STAN (v 2.14) (54) in R v. 3.3.2 (55). Models were compared with widely applicable information criteria (WAIC) using the compare function in rethinking. The corresponding code and data used for each model and graph production can be found through a link in SI Appendix.
To estimate group-level difference in annual innovation rate, we summed the number of innovations observed within each group and across all individuals and behavioral domains. Exposure rates for individuals within groups were summed within years. Counts of annual innovations per group were then fit using a hierarchical Poisson model fit using r-STAN that accounted for exposure rates using metrics previously described and varying intercepts for each group, thus providing an estimate of annual innovation rate per year per group.
Supplementary Material
Acknowledgments
The following field assistants contributed a year or more of data to the Lomas Barbudal Monkey Project dataset: L. Beaudrot, M. Bergstrom, R. Berl, A. Bjorkman, L. Blankenship, T. Borcuch, J. Broesch, J. Butler, F. Campos, C. Carlson, S. Caro, M. Corrales, N. Donati, C. Dillis, G. Dower, R. Dower, K. Feilen, K. Fisher, A. Fuentes J., M. Fuentes A., C. Gault, H. Gilkenson, I. Gottlieb, L. Hack, S. Herbert, C. Hirsch, C. Holman, S. Hyde, L. Johnson, S. Lee, S. Leinwand, T. Lord, K. Kajokaite, M. Kay, E. Kennedy, D. Kerhoas-Essens, E. Johnson, S. Kessler, S. MacCarter, J. Manson, W. Meno, C. Mitchell, Y. Namba, A. Neyer, C. O’Connell, J. C. Ordoñez, J., N. Parker, B. Pav, R. Popa, K. Potter, K. Ratliff, H. Ruffler, S. Sanford, M. Saul, I. Schamberg, C. Schmitt, A. Scott, J. Verge, A. Walker-Bolton, E. Wikberg, and E. Williams. We thank H. Gilkenson, W. Lammers, C. Dillis, M. Corrales, and R. Popa for helping to manage the field site. E. Wikberg and K. Kajokaite contributed a year or more of effort to organizing the dataset. D. Cohen created the MySQL database. R. Hong assisted in the organization and coding of the innovation data. This paper has benefitted from helpful discussions with D. Caillaud, M. Crofoot, M. Grote, K. Kajokaite, R. McElreath, J. Manson, K. Perry, B. Scelza, and M. J. West-Eberhard. We thank Marcus W. Feldman, Andrew Whiten, Kevin N. Laland, and Francisco J. Ayala for the opportunity to participate in this Sackler Symposium. We thank the Costa Rican park service (Sistema Nacional de Áreas de Conservación and Área de Conservación Tempisque), Hacienda Pelon de la Bajura, Hacienda Brin D’Amor, and the residents of San Ramon de Bagaces for permission to work on their land. This project is based on work supported by the funding provided to S.E.P. by the Max Planck Institute for Evolutionary Anthropology, National Science Foundation (NSF) Grants SBR-0613226 and BCS-0848360, two grants from the Leakey Foundation, two grants from the National Geographic Society, and multiple Committee on Research grants from the University of California, Los Angeles (UCLA). B.J.B. was supported by NSF Graduate Research Fellowship (GRF) Grant 1650042 and two Achievement Rewards for College Scientists Foundation, Northern California Chapter fellowships. I.G. was supported by the International Society for Human Ethology, a Ford Foundation fellowship, an NSF GRF, and multiple grants from UCLA. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or other funding agencies.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “The Extension of Biology Through Culture,” held November 16–17, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Extension_of_Biology_Through_Culture.
This article is a PNAS Direct Submission. A.W. is a guest editor invited by the Editorial Board.
Data deposition: The R-code and data used in the statistical analysis reported in this paper are available at https://github.com/bjbarrett/cebusinnovation2017.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620739114/-/DCSupplemental.
References
- 1.Imanishi K. Man. Mainichi-Shinbunsha; Tokyo: 1952. [Google Scholar]
- 2.Kummer H. Primate Societies: Group Techniques of Ecological Adaptation. AHM Publ Corp; Arlington Heights, IL: 1971. [Google Scholar]
- 3.West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford Univ Press; Oxford: 2003. [Google Scholar]
- 4.Giraldeau L-A, Caraco Y, Valone T. Social foraging: Individual learning and cultural transmission of innovations. Behav Ecol. 1994;5:35–43. [Google Scholar]
- 5.Sol D. Behavioural flexibility: A neglected issue in the ecological and evolutionary literature? In: Reader SM, Laland KN, editors. Animal Innovation. Oxford Univ Press; Oxford: 2003. pp. 62–82. [Google Scholar]
- 6.Reader SM, Morand-Ferron J, Flynn E. Animal and human innovation: Novel problems and novel solutions. Philos Trans R Soc Lond B Biol Sci. 2016;371:371. doi: 10.1098/rstb.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyles JS, Kundel JG, Wilson AC. Birds, behavior, and anatomical evolution. Proc Natl Acad Sci USA. 1983;80:4394–4397. doi: 10.1073/pnas.80.14.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keagy J, Savard J-F, Borgia G. Male satin bowerbird problem-solving ability predicts mating success. Anim Behav. 2009;78:809–817. [Google Scholar]
- 9.Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B. Problem-solving performance is correlated with reproductive success in a wild bird population. Anim Behav. 2013;85:19–26. [Google Scholar]
- 10.Whiten A, Ayala FJ, Feldman MW, Laland KN. The extension of biology through culture. Proc Natl Acad Sci USA. 2017;114:7775–7781. doi: 10.1073/pnas.1707630114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reader SM, Laland KN. Animal innovation: An introduction. In: Reader SM, Laland KN, editors. Animal Innovation. Oxford Univ Press; Oxford: 2003. pp. 3–35. [Google Scholar]
- 12.Ramsey G, Bastian ML, van Schaik C. Animal innovation defined and operationalized. Behav Brain Sci. 2007;30:393–407. doi: 10.1017/S0140525X07002373. [DOI] [PubMed] [Google Scholar]
- 13.van Schaik CP, et al. The reluctant innovator: Orangutans and the phylogeny of creativity. Philos Trans R Soc Lond B Biol Sci. 2016;371:371. doi: 10.1098/rstb.2015.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogarty L, Creanza N, Feldman MW. Cultural evolutionary perspectives on creativity and human innovation. Trends Ecol Evol. 2015;30:736–754. doi: 10.1016/j.tree.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Perry S. Intergroup encounters in wild white-faced capuchins, Cebus capucinus. Int J Primatol. 1996;17:309–330. [Google Scholar]
- 16.Reader SM, Laland KN. Primate innovation: Sex, age, and social rank differences. Int J Primatol. 2001;22:787–805. [Google Scholar]
- 17.Lefebrvre L, et al. Feeding innovations and forebrain size in Australasian birds. Behaviour. 1998;135:1077–1097. [Google Scholar]
- 18.Lefebrvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim Behav. 1997;53:549–560. [Google Scholar]
- 19.Boogert NJ, Reader SM, Hoppitt W, Laland KN. The origin and spread of innovations in starlings. Anim Behav. 2008;75:1509–1518. [Google Scholar]
- 20.Thornton A, Samson J. Innovative problem solving in wild meerkats. Anim Behav. 2012;83:1459–1468. [Google Scholar]
- 21.Aplin LM, et al. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature. 2015;518:538–541. doi: 10.1038/nature13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendal RL, Coe RL, Laland KN. Age differences in neophilia, exploration, and innovation in family groups of callitrichid monkeys. Am J Primatol. 2005;66:167–188. doi: 10.1002/ajp.20136. [DOI] [PubMed] [Google Scholar]
- 23.Russon AE. Innovation and creativity in forest-living rehabilitant orangutans. In: Reader SM, Laland KN, editors. Animal Innovation. Oxford Univ Press; Oxford: 2003. pp. 279–306. [Google Scholar]
- 24.Whiten A, et al. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 25.van Schaik CP, et al. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 26.van Schaik CP, van Noordwijk MA, Wich SA. Innovation in wild Bornean orangutans (Pongo pygmaeus wurmbii) Behaviour. 2006;143:839–876. [Google Scholar]
- 27.Perry S, et al. Social conventions in wild white-faced capuchin monkeys: Evidence for traditions in a neotropical primate. Curr Anthropol. 2003;44:241–268. [Google Scholar]
- 28.Koolhaas JM, et al. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Kummer H, Goodall J. Conditions of innovative behaviour in primates. Philos Trans R Soc Lond B Biol Sci. 1985;308:203–214. [Google Scholar]
- 30.Overington S, Morand-Ferron J, Boogert NJ, Lefebvre L. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim Behav. 2009;78:1001–1010. [Google Scholar]
- 31.Navarrete AF, Reader SM, Street SE, Whalen A, Laland KN. The coevolution of innovation and technical intelligence in primates. Philos Trans R Soc Lond B Biol Sci. 2016;371:371. doi: 10.1098/rstb.2015.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephan H, Bauchot R, Andy OJ. Data on size of the brain and various brain parts in insectivores and primates. In: Noback CR, Montagna W, editors. The Primate Brain. Appleton-Century-Crofts; New York: 1970. pp. 289–297. [Google Scholar]
- 33.Perry S, Ordoñez Jiménez JC. In: The Effects of Food Size, Rarity, and Processing Complexity on White-Faced Capuchins’ Visual Attention to Foraging Conspecifics. Hohmann G, Robbins M, Boesch C, editors. Cambridge Univ Press; Cambridge, UK: 2006. pp. 203–234. [Google Scholar]
- 34.Fragaszy DM, Visalberghi E, Fedigan LM. The Complete Capuchin: The Biology of the Genus Cebus. Cambridge Univ Press; Cambridge, UK: 2004. [Google Scholar]
- 35.Day RL, Coe RL, Kendal JR, Laland KN. Neophilia, innovation and social learning: A study of intergeneric differences in callitrichid monkeys. Anim Behav. 2003;65:559–571. [Google Scholar]
- 36.Lynch Alfaro JW, et al. Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. J Biogeogr. 2012;39:272–288. [Google Scholar]
- 37.Rose L, et al. Interspecific interactions between white-faced capuchins (Cebus capucinus) and other species: Preliminary data from three Costa Rican sites. Int J Primatol. 2003;24:759–796. [Google Scholar]
- 38.Perry S, Manson JH. Manipulative Monkeys: The Capuchins of Lomas Barbudal. Harvard Univ Press; Cambridge, MA: 2008. [Google Scholar]
- 39.Sol D, Sayol F, Ducatez S, Lefebvre L. The life-history basis of behavioural innovations. Philos Trans R Soc Lond B Biol Sci. 2016;371:371. doi: 10.1098/rstb.2015.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry S. The behavior of wild white-faced capuchins: Demography, life history, social relationships, and communication. Adv Study Behav. 2012;44:135–181. [Google Scholar]
- 41.Perry S. Social traditions and social learning in capuchin monkeys (Cebus) Philos Trans R Soc Lond B Biol Sci. 2011;366:988–996. doi: 10.1098/rstb.2010.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida T, Matsusaka T, McGrew WC. Emergence, propagation or disappearance of novel behavioral patterns in the habituated chimpanzees of Mahale: A review. Primates. 2009;50:23–36. doi: 10.1007/s10329-008-0109-y. [DOI] [PubMed] [Google Scholar]
- 43.Haslam M, et al. Pre-Columbian monkey tools. Curr Biol. 2016;26:R521–R522. doi: 10.1016/j.cub.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 44.Gould SJ, Vrba ES. Exaptation - a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- 45.Zahavi A. The testing of a bond. Anim Behav. 1977;25:246–247. [Google Scholar]
- 46.Russon AE, Kuncoro P, Ferisa A. Tools for the trees: Orangutan arboreal tool use and creativity. In: Kaufman AB, Kaufman JC, editors. Animal Creativity and Innovation. Elsevier; San Diego: 2015. pp. 419–455. [Google Scholar]
- 47.Beck SR, Williams C, Cutting N, Apperly IA, Chappell J. Individual differences in children’s innovative problem-solving are not predicted by divergent thinking or executive functions. Philos Trans R Soc Lond B Biol Sci. 2016;371:371. doi: 10.1098/rstb.2015.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laland KN, van Bergen Y. Experimental studies of innovation in the guppy. In: Reader SM, Laland KN, editors. Animal Innovation. Oxford Univ Press; New York: 2003. pp. 155–173. [Google Scholar]
- 49.Frankie GW, Vinston SB, Newstrom LE, Barthell JF. Nest site and habitat preferences of Centris bees in the Costa Rican dry forest. Biotropica. 1988;20:301–310. [Google Scholar]
- 50.Perry S, Godoy I, Lammers W. The Lomas Barbudal Monkey Project: Two decades of research on Cebus capucinus. In: Kappeler P, Watts D, editors. Long-Term Field Studies of Primates. Springer; New York: 2012. pp. 141–165. [Google Scholar]
- 51.Russon A, Andrews K, Huss B. Innovation and the grain problem. Behav Brain Sci. 2007;30:423–433. [Google Scholar]
- 52.Neumann C, Kulik L. 2014 EloRating: Animal Dominance Hierarchies by Elo-Rating. R Package, Version 0.43. Available at https://cran.r-project.org/package=EloRating. Accessed January 5, 2017.
- 53.McElreath R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan. Chapman and Hall/CRC; New York: 2016. [Google Scholar]
- 54.Stan Development Team 2016. RStan: The R Interface to Stan. R package version 2.14.1. (R Foundation for Statistical Computing, Vienna)
- 55.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.