Abstract
Social learning is important to the life history of many animals, helping individuals to acquire new adaptive behavior. However despite long-running debate, it remains an open question whether a reliance on social learning can also lead to mismatched or maladaptive behavior. In a previous study, we experimentally induced traditions for opening a bidirectional door puzzle box in replicate subpopulations of the great tit Parus major. Individuals were conformist social learners, resulting in stable cultural behaviors. Here, we vary the rewards gained by these techniques to ask to what extent established behaviors are flexible to changing conditions. When subpopulations with established foraging traditions for one technique were subjected to a reduced foraging payoff, 49% of birds switched their behavior to a higher-payoff foraging technique after only 14 days, with younger individuals showing a faster rate of change. We elucidated the decision-making process for each individual, using a mechanistic learning model to demonstrate that, perhaps surprisingly, this population-level change was achieved without significant asocial exploration and without any evidence for payoff-biased copying. Rather, by combining conformist social learning with payoff-sensitive individual reinforcement (updating of experience), individuals and populations could both acquire adaptive behavior and track environmental change.
Keywords: social learning, animal culture, conformity, Parus major
Social learning, the acquisition of behavior by observation of, or interaction with, other individuals, is common to many animal species. It provides a relatively cheap way of acquiring valuable information and shields naive individuals from the risks of engaging in trial and error learning. A range of studies have further highlighted the crucial role of social learning in promoting cultural behavior and shared traditions (1–4) and suggested that the cultural inheritance of information across generations may be an important component of the behavioral ecology of some animals (1, 5, 6). However, social learning may also be disadvantageous, if copied information is outdated or mismatched to the observing individual. How individuals balance costs and benefits of social learning has therefore been the focus of much recent research aiming to understand how natural selection has shaped learning (7).
The use of social learning “strategies” is one possible route by which animals can combine and filter different kinds of information to optimize learning outcomes (8–10). Here, individuals use social cues, often from multiple conspecifics, to bias learning in favor of better quality information. These include preferences to copy kin or more prestigious or older individuals, as well as conformist and payoff-biased social learning (11, 12). Such learning strategies also have implications for the spread and persistence of information in populations and for cultural evolution more broadly (13, 14). For example, conformist transmission, here defined as the disproportionate tendency to copy the most common behavioral variant (15, 16), may evolve as a means of providing naive individuals with a quick way of ascertaining locally adaptive information. However, it may also have the outcome of maintaining group differences in behavior, with within-group traditions resilient to invasion by alternative variants.
Empirical evidence for conformity in nonhuman animals is currently limited, but hints at a wide taxonomic occurrence, with proposed cases in fish (17), birds (18), and primates (4, 19). Furthermore, theoretical modeling has suggested that conformist transmission should evolve under a wide range of conditions and be particularly favored when environments are spatially heterogeneous (15, 20). Yet if individuals are exclusively conformist, then any new environmental change may result in a mismatch with the majority behavior, leading to a perpetuation of suboptimal or maladaptive traditions over time (21) and exaggerating the disadvantages of social information use. Evolutionary modeling has gone as far as to suggest that in socially learning animals, coupling of conformist learning with environmental change could lead to population collapse (22).
This apparent paradox of nonadaptive culture has thus been the subject of much debate (23–28), with two individual-level strategies proposed as a potential means of evading this evolutionary trap. First, individuals could switch from socially learned behavior to engaging in asocial learning (individual innovation) when the rewards gained for performing the established tradition is smaller than previously (7, 12, 27, 29). Second, individuals could combine conformist tendencies with payoff-biased social learning, using a “behavioral toolbox” of social learning strategies when choosing what behavior to adopt, thus integrating information about both the frequency of behavior and the relative rewards gained by demonstrators (8, 30). Such a strategy has been observed in laboratory experiments in humans (8). However, empirical tests for the occurrence or emergence of suboptimal or maladaptive traditions in animals have been limited (31, 32).
In a previous study, we demonstrated an influence of conformist transmission on the social learning of novel foraging techniques in wild great tits (Parus major) (18). There, we trained two demonstrators in each of five subpopulations to one of two equal alternative solutions to a novel task, where food could be gained from a puzzle box by pushing a bidirectional door to the right (technique A) or the left (technique B). Using automated tracking of individuals and their choices, we then mapped the spread and establishment of these seeded behaviors through each subpopulation. Results showed a sigmoidal relationship between a naive individual’s probability of adopting one of the two techniques and its frequency in their foraging group, with birds disproportionately likely to learn the majority technique. Across each subpopulation, the most common behavior therefore became increasingly prevalent over time (18). These strong preferences at the subpopulation for one technique persisted over the two generations measured, suggesting that technique choice had become established as a stable cultural behavior.
Here, we vary the rewards gained by these techniques to investigate whether a reliance on conformist social learning will result in mismatched behavior after conditions change. First, in four subpopulations, two with established traditions for technique A (“left”) and two for technique B (“right”), we stocked all puzzle boxes with a less preferred food reward over 2 days, so that solving using either technique was rewarded with a lower payoff. This “equal low payoffs” condition was designed to test whether individuals would explore alternative foraging behaviors when confronted with a lower payoff from their previously learned behavior. Second, and immediately following this condition, all puzzle boxes were stocked with unequal rewards for a period of 14 days. Solving using the established tradition was thus rewarded with this same less preferred food, but solving with the uncommon behavioral variant now resulted in a high payoff. Whereas most individuals had no experience of the uncommon solution, a small minority of individuals already preferred it and so provided available social information for the new difference between the two techniques. Finally, all visits and behaviors at the puzzle boxes were monitored using automated tracking. By quantifying the decision-making process for each individual’s visit to the puzzle box we then examined (i) whether individuals switched from conformist to payoff-biased copying when observing others receiving variable rewards, (ii) whether individuals flexibly adjusted their behavior in response to learning about and gaining variable rewards, and (iii) whether this resulted in a population-level shift in behavior.
Results
Condition 1: Equal High Payoffs.
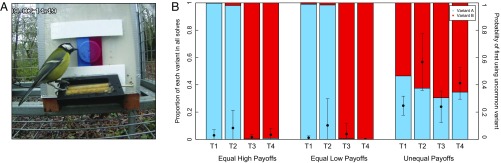
A tradition for pushing a bidirectional door either to the right (variant A, T1–T2) or to the left (variant B, T3–T4) was experimentally induced in four subpopulations of great tits [see Aplin et al. (18) for detailed methods] (Fig. 1A). Either solving technique was rewarded with the same highly preferred food, a live mealworm. In all subpopulations, the large majority (87–98%) of solutions used the technique that was introduced by the trained demonstrator at the beginning of each experiment, with the population-level bias to this technique becoming increasingly entrenched over time (18) (Fig. 1B).
Fig. 1.
(A) A puzzle box where visiting individuals can slide the door open from either the blue/left side (variant A) or the red/right side (variant B) to access a reward in a concealed feeder behind the door. The individual pictured is solving using variant A. Solving using either option can give the same (equal condition) or different (unequal condition) rewards. Puzzle boxes record identity, contact duration, and solution choice and reset after each visit. (B) Proportion of variant A or B used in each replicate (T1–T4) in three sequential conditions after variant A (T1–T2) or variant B (T3–T4) was initially introduced by a trained demonstrator: (i) equally high payoffs for each solving option, proportion for last 5 days shown; (ii) equally low payoffs for each solving option (2 days); and (iii) unequal payoffs, with the established tradition leading to a lower reward (14 days). Solid circles and error bars show mean and 95% CI of the probability of individuals’ first solve in each condition being the uncommon variant.
Condition 2: Equal Low Payoffs.
For a 2-day period following condition 1, three puzzle boxes were distributed in each subpopulation that rewarded both variants A and B with a less preferred food, sunflower seed (Fig. S1) (18). Compared with condition 1, there was no change in the overall proportion of each solution performed in any subpopulation (Welch two-sample test: T1, t = 0.82, P = 0.42; T2, t = −0.16, P = 0.87; T3, t = −0.57, P = 0.57; T4, t = 1.42, P = 0.16) (Fig. 1B). Whereas these patterns may have differed over a longer time frame, these results suggest that, at least over 2 days [average number of solves per individual across replicates = 22(14–30)] birds did not change their sampling behavior in response to experiencing or observing lower rewards.
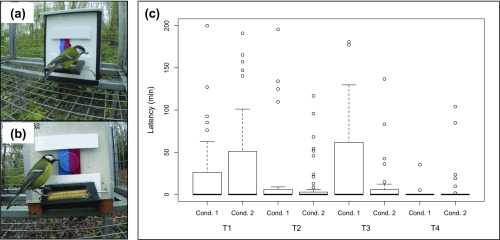
Fig. S1.
(A) Photo of puzzle box in condition 1. (B) Photo of puzzle box in condition 2, showing door modification. (C) The latency for individuals to solve the puzzle box after contacting the openly accessible peanut-granule feeder does not consistently differ between the last day of condition 1 and the first day of condition 2 across four replicates (treatments 1–4). LMM: z = −1.05, P = 0.29. The openly accessible feeder was positioned ∼1 m from the puzzle box and contained peanut granules (a less preferred food source). Similarly, there was also no consistent difference between conditions 1 and 2 in whether individuals contacted the puzzle box before or after the peanut-granule feeder (GLMM, z = −0.97, P = 0.33). This suggests that individuals did not exhibit a neophobic response toward the modified puzzle box in condition 2.
Condition 3: Unequal Payoffs.
Immediately following condition 2, puzzle boxes with unequal rewards were installed at all sites/subpopulations over a period of 14 days. Here, solving using the established tradition was rewarded with this same less preferred food of sunflower seeds, whereas solving with the uncommon variant was rewarded with live mealworms. Overall, there were significantly more solutions of the alternative variant performance in condition 3 than in the previous conditions (Welch two-sample test, first vs. third condition: T1, t = −5.28, P < 0.001; T2, t = −3.87, P < 0.001; T3, t = −3.75, P < 0.001; T4, t = −5.86, P < 0.001) (Fig. 1B). In the last part of condition 1, an average of 8(2–16)% of individuals either showed no preference or preferred the alternative variant. For these individuals, their preference did not change in condition 3. By contrast, 49(33–71)% of other individuals switched to change their variant preference by the end of the experimental period (proportion of all solves for each individual over last 2 days).
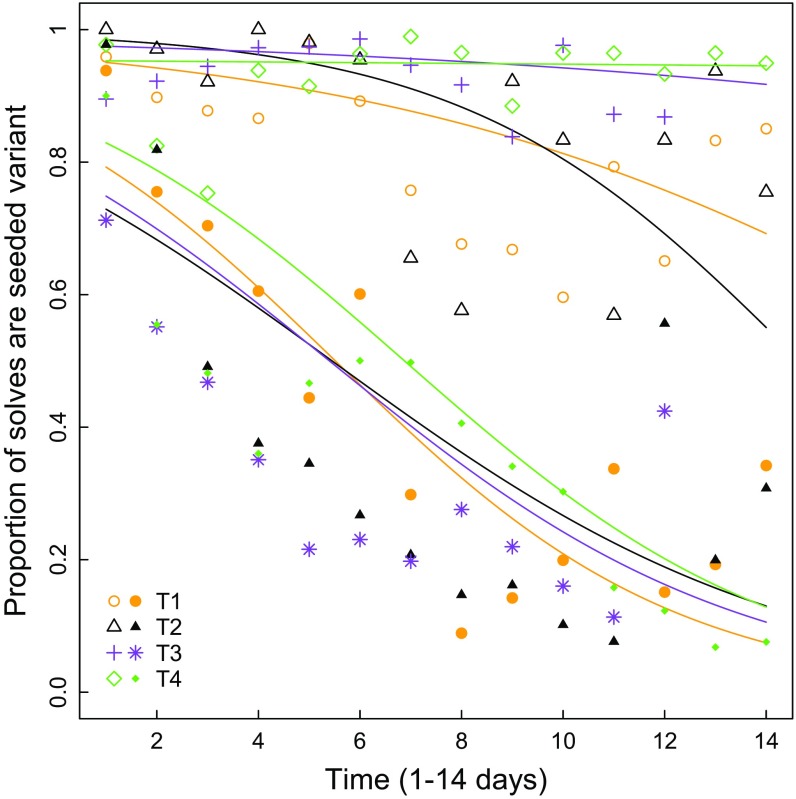
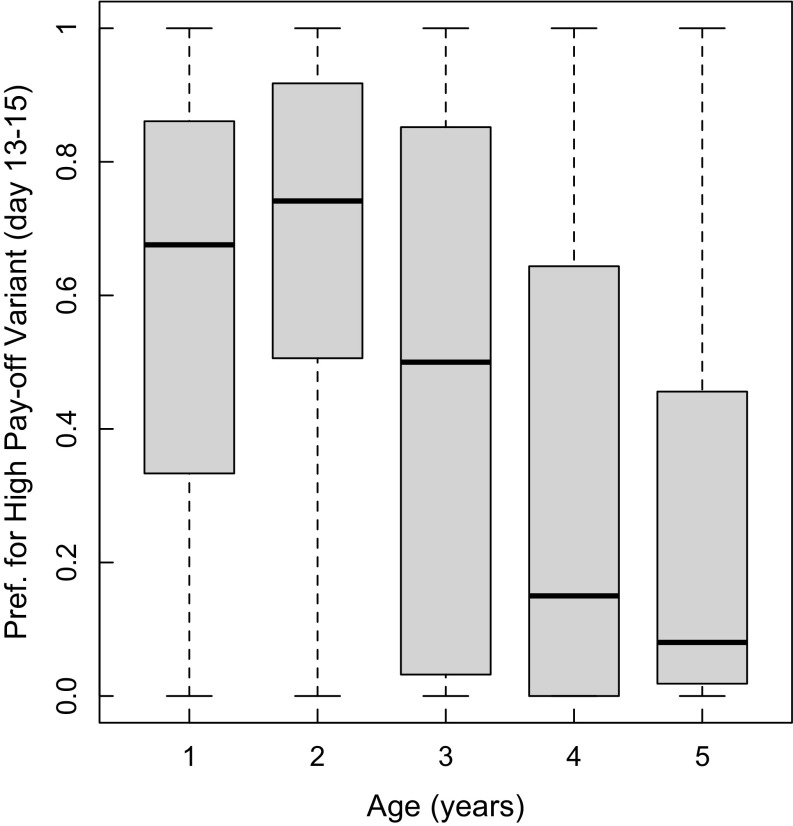
Similarly to Aplin et al. (18), we analyzed the change in individual and population preferences over time. First, as the data were clearly bimodal (Fig. S2), a longitudinal clustering algorithm was used to group individuals into two behavioral trajectories, with 48% (0.33–0.67%) of individuals falling into cluster 1. Clusters were then analyzed separately, using a generalized estimating equation (GEE) model where the dependent variable was the proportion of solves using the established technique on each of 15 days and the explanatory variables were day, individuals weighted by their total number of solves, and subpopulation (Fig. 2). There was strong evidence in cluster 1 that the preference for the established tradition decreased over time (pooled replicate data; coefficient SEM = −0.27 0.02, P < 0.001). Cluster 2 showed a significant but much lower decreasing preference for the established tradition (pooled replicate data; coefficient SEM = −0.13 0.02, P < 0.001) (Fig. 2). This bimodality in the rate of change over time was related to age, with younger individuals more likely to fall into cluster 1 [general linear mixed model (GLMM): z140 = −2.94, P = 0.003]. There was no relationship between cluster membership and any other measured variables (sex GLMM, z116 = 0.26, P = 0.79; prior preference strength GLMM, z116 = −0.89, P = 0.37; number of solves in condition 1 GLMM, z116 = −0.89, P = 0.37). Finally, and in support of this result, there was a negative correlation between age and likelihood of preferring the high-payoff variant in the last 2 days of the experimental period [linear mixed model (LMM): −0.07 0.02, t = −3.83, P < 0.001] (Fig. S3).
Fig. S2.
The distribution of individual preferences for the high-payoff solution technique across the four replicates (T1–T4) on the last 2 days of condition 3. The distribution is clearly not unimodal, and a test for deviance from unimodality on this (R package dip-test) gives a clear affirmative result for the combined data: Hartigans’ dip test (HDT) = 0.07, P < 0.001. When analyzed separately, three of four replicates show a significant or near-significant signal of bimodality (T1, HDT = 0.059, P = 0.057; T2, HDT = 0.089, P = 0.20; T3, HDT = 0.103, P = 0.002; T4, HDT = 0.185, P < 0.001).
Fig. 2.
The proportion of solutions using the seeded technique decreased over time in each replicate, with individuals moving toward preferring the previously uncommon technique. Each replicate is shown in a different color/shape, and solid and open symbols represents the two distinct clusters of individuals identified in the longitudinal clustering algorithm (solid symbols, cluster 1; open symbols, cluster 2). Lines show the generalized estimating equation model fit for each cluster/replicate.
Fig. S3.
Older individuals were less likely to prefer the high-payoff variant at the end of the experimental period. Box and whisker plots show age (in years) by preference for solving using the high-payoff technique for all individuals, summed over the last 2 days of condition 3 (unequal payoffs).
Analysis of Learning Mechanisms.
We next investigated the relative contribution of different learning mechanisms to the observed change in behavior during condition 3. To address these questions statistically, we used a sequential learning model of a form previously used to study the interaction of social and asocial learning (8, 33). This framework allows for individual choices to be modeled as products of time-varying interactions of different modes of learning. Specifically, each solution decision was modeled as a binary outcome in which the probability of an individual choosing either option was a combination of both social cues and accumulated experience. Social cues included the frequencies of each behavior and the relative value of demonstrated rewards and were calculated from activity immediately before a given solve at a given puzzle box (18). Code sufficient to repeat our results is available as an R package, wythamewa, that contains the data, models, and simulation code, as well as code for reproducing each figure to follow (Figs. 3–5).
Fig. 3.
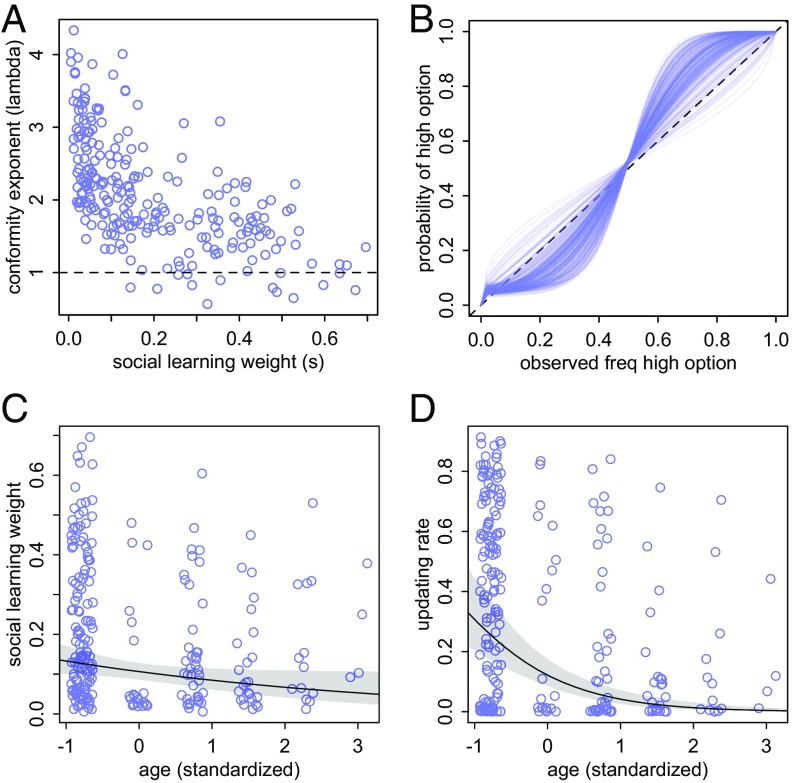
Individual parameter estimates for the mechanistic learning model. Each circle represents the posterior mean for an individual bird. (A) Strength of conformity () is negatively correlated with reliance on social learning (), but most individuals show some conformist bias (exponent above 1). (B) Implied social learning influence functions (expression 5). The diagonal line represents unbiased social learning. S-shaped curves are conformist individuals. The weak influence of payoff bias shifts these curves upward in the lower left corner. (C) Reliance on social cues tends to decline with age, explained mainly by the presence of large values of in the youngest individuals. Individuals with low values are present at all ages. (D) Updating rate tends to decline with age. The youngest individuals can be highly responsive to individual experience, whereas the oldest individuals change their attraction scores more slowly.
Fig. 5.
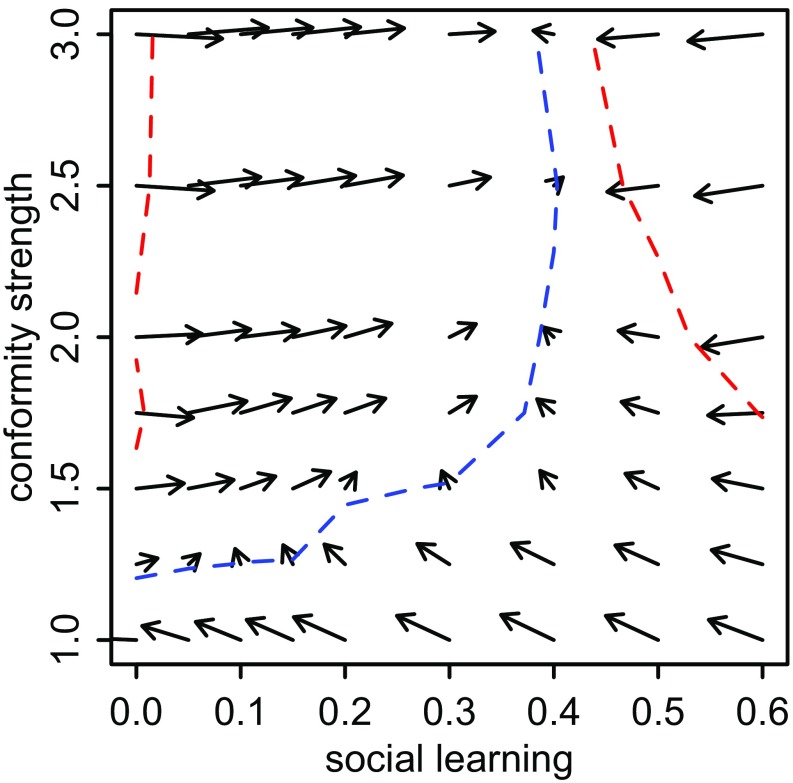
Selection gradient on social learning weight and conformity, represented as a vector field for social learning weight (horizontal axis) and conformity (vertical axis). Selection increases conformity below the outer red contours. Selection increases social learning above and to the left of the central blue contour. For these parameter values, selection does not favor much social learning, unless social learning is also conformist.
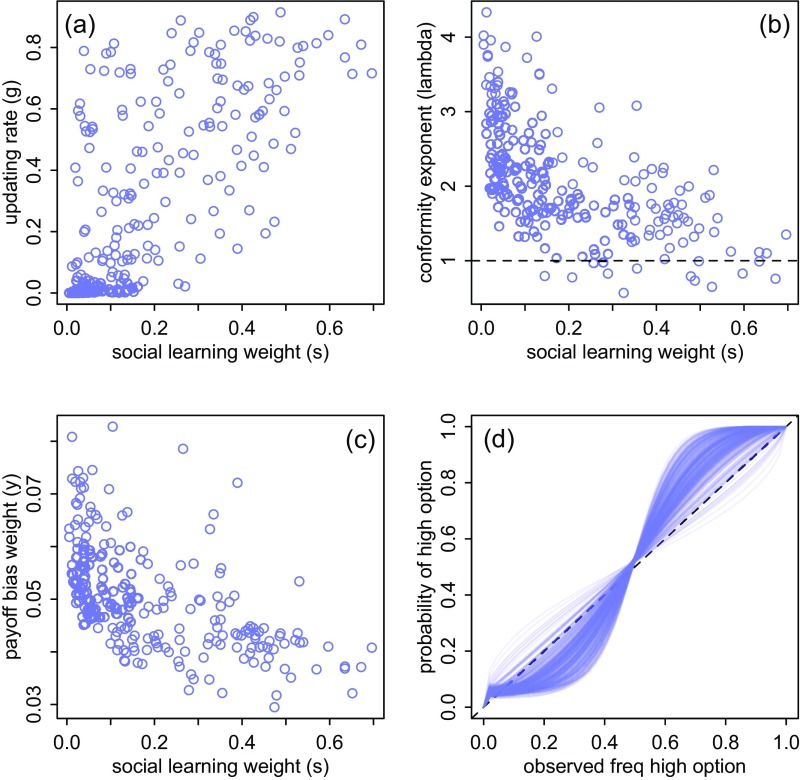
Four model parameters were considered: , , , and , representing, respectively, the influence of social cues on choice, the updating rate (how quickly an individual’s attraction to an option responds to newly experienced payoffs), an individual’s conformist exponent, and its payoff social learning bias (population averages presented in Table S1). There was little evidence for a payoff social learning bias in individuals’ behavior, with no individuals weighting this parameter higher than 0.1 (Fig. S2). All other parameters were more important, yet there was considerable individual variation in each (Table S1 and Fig. S4). Solving individuals ranged in their behavior from little use of social information to placing more than half of their decision-making weight on social cues (). Similarly, the updating rate () ranged from nearly zero to over 0.8. Notably, these two parameters were correlated across individuals, indicating that birds that tended to learn socially also updated their personal information more quickly.
Table S1.
Summary of population means for the mechanistic learning model
| Model parameters | Mean | SD | L89% | U89% | n_eff | Rhat |
| Logit | −2.12 | 0.16 | −2.38 | −1.89 | 767 | 1.00 |
| Logit | −1.96 | 0.28 | −2.39 | −1.53 | 503 | 1.00 |
| 0.17 | 0.27 | −0.25 | 0.60 | 1550 | 1.00 | |
| Logit | −3.40 | 0.52 | −4.26 | −2.59 | 1349 | 1.00 |
| 0.24 | 0.15 | −0.49 | 0.00 | 803 | 1.00 | |
| −1.15 | 0.27 | −1.60 | −0.73 | 650 | 1.00 | |
| 0.09 | 0.23 | −0.25 | 0.48 | 1443 | 1.00 | |
| 0.02 | 0.48 | −0.72 | 0.79 | 3000 | 1.00 | |
| 1.77 | 0.17 | 1.50 | 2.03 | 962 | 1.01 | |
| 3.55 | 0.27 | 3.13 | 3.97 | 812 | 1.01 | |
| 0.79 | 0.39 | 0.05 | 1.31 | 504 | 1.00 | |
| 0.53 | 0.53 | 0.00 | 1.16 | 958 | 1.00 |
Shown are posterior means; SD; lower (L) and upper (U) 89% intervals; and convergence diagnostics for the average learning parameters, age effects, and variance components. See p. 58 of ref. 1 for an explanation of the 89% intervals.
Fig. S4.
Individual parameter estimates for the mechanistic learning model. Each circle represents the posterior mean for an individual bird. (A) Reliance on social learning (s) is correlated with the weight of new experience (g), across individuals. (B) Strength of conformity () is negatively correlated with reliance on social learning (s). (C) The influence payoff bias (y) is small overall. (D) Implied social learning influence functions (main text, expression 5). The diagonal dashed line represents unbiased social learning. S-shaped curves are conformist individuals. The weak influence of payoff bias shifts these curves upward in the lower right corner. B and D are replicated in Fig. 3 A and D.
In contrast to payoff social learning biases, the large majority of individuals had a conformity exponent () above 1, indicating at least mild conformity in their use of social cues (above dotted line, Fig. 3A). However, there was a strong negative correlation with overall reliance on social learning (), such that birds who put lower weights on social learning were more strongly conformist (Fig. 3A). It is easier to understand the impact of these individual differences by translating the estimates for each individual into an implied social learning function, as defined by expression 5. Fig. 3B shows these implied functions, plotted for the posterior mean of each individual. Here, an s-shaped curve corresponds to conformist learning. Whereas a few birds appear slightly anticonformist, it must be noted that Fig. 3B shows posterior means, with considerable uncertainty about the exact function of any one individual. Inspecting individual functions with full uncertainty envelopes confirms that there is no strong evidence for anticonformity in this population, only for a minority of individuals whose behavior is consistent with both weak anticonformity and weak conformity.
There was a consistent negative impact of age on both weight given to social cues () and updating rate (). In contrast, there were no consistent or strong effects of age on conformity () or on payoff bias (). These relationships are shown in Fig. 3 C and D and suggest that older individuals were much less influenced by social cues and also changed their behavior more slowly in response to changes in personal experience. This is in agreement with the descriptive results: Older individuals switched to the high-payoff variant more slowly. Younger individuals, in contrast, adapted more quickly; these “adaptor” individuals showed the strongest use of social information and simultaneously tended to be less conformist, yet also tended to update their own attraction scores more quickly in response to new personal experience.
Modeling the Link Between Individual Behavior and Population-Level Patterns.
The results in the previous section suggest a combination of conformist social learning and payoff-sensitive individual reinforcement (updating) in the population, with individual variation in all measures. Surprisingly, given our initial hypotheses, we found no evidence of individual exploration or payoff-biased social learning of sufficient strength to explain the patterns of behavior change. Therefore, does the conformity present in the population slow the rate of switching? Or does it instead help both individuals and the population show adaptive responses to environmental change?
To test this, we used the same learning model as for data analysis and used it as a forward evolutionary simulation. We simulated groups of individuals learning together, with parameters for the weight of social cues, strength of conformity, and updating rate. We first used these simulations to validate our data analysis code and then explored the population dynamics arising from different parameter settings. Finally, we computed selection gradients on the parameters.
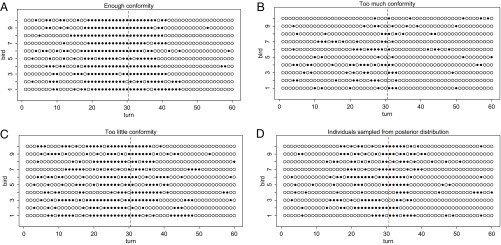
First, we consider three examples to show the role of conformist social learning (Fig. 4). Each plot shows the time series of a simulated group of 10 learners, where payoffs switch for the variants at turn 1 and again at turn 30, with groups starting by preferring the low variant. Only conformist strength is varied, with other parameters held at , , and (representative of the posterior distribution in the fitted model). When conformity is turned off (), individuals take a long time to stabilize on the high-payoff variant and are slow to switch back when the environment changes again (Fig. 4A). Similarly, when conformity is set very high (), adaptive learning is very slow, and most individuals fail to establish on the high-payoff variant at all before the environment switches (Fig. 4B). However, when conformity is set to a moderately high value (), all individuals stabilize on the high-payoff variant before turn 30 and quickly switch back after the variants switch again (Fig. 4C).
Fig. 4.
Simulations of the population consequences of mixes of conformist social learning and individual reinforcement. In each plot, each row is an individual agent and each column is a time period. Open and solid circles represent alternative behavior. Before the vertical dashed line at turn 30, solid is adaptive. After turn 30, open is adaptive. All groups of learners initialized with nonadaptive attraction scores, , , and . (A) , no conformity. (B) , high conformity. (C) , intermediate conformity. (D) Ten birds sampled from posterior distribution of the fitted model.
Second, we ran a simulation using parameter values for 10 birds sampled from the posterior distribution deduced from the experimental data (Fig. 4D). The simulation shown here is representative, and if anything, the birds showed less conformity than is optimal in this setting. Nevertheless, the amount of conformity that is present, combined with payoff-sensitive updating, allows individuals to track changes in behavioral variants.
Finally, selection gradients for social learning weight () and conformity strength () were used to determine whether selection favors larger or smaller values of each parameter, conditional on the value of the other. We calculated the selection gradients by conducting 20,000 simulations at each of 63 combinations of and (for a total of 1,260,000 simulations) to compute the selection differential of a mutant (Fig. 5). In each simulation, we considered the difference in total payoffs between a mutant individual with parameters or and an average common-type individual with parameters and . This difference defines a numerical estimate of the selection gradient for an invader. The parameters and were again fixed at and . We display the results in Fig. 5 as a vector field. Selection adjusts combinations on and in the directions indicated by the arrows, with longer arrows indicating stronger selection. The red dashed contour is the combinations of and at which selection on conformity is neutral. Selection increases conformity below this contour. The blue dashed contour is the combinations where selection on is neutral. Selection increases above this contour. Therefore, selection favors more conformity for most of the gradient space, becoming disadvantageous only at high weights of social learning. Social learning weight increases above and to the left of the blue contour. Here, social learning weight cannot increase from zero unless social learning is slightly conformist (above the central blue contour); however, once conformity is above a threshold value, higher social learning weight is favored only up to a point. These processes combine to produce evolutionary dynamics that favor conformity combined with an intermediate weighting of social learning (Fig. 5).
In summary, the birds in the experiment obviously did not evolve in the experiment, and we do not expect them to be precisely adapted to it. Nevertheless, these simulations help us to understand why conformist social learning, in combination with payoff-sensitive individual reinforcement, facilitated the ability of individuals and groups to track environmental change.
Discussion
Our experiment reveals that socially learned foraging traditions in great tits are flexible in response to environmental change. Indeed when subpopulations with strongly established foraging traditions for a single behavioral variant were subjected to a change in foraging payoffs, after just 14 days 49% of birds switched their behavior to prefer an alternative higher-payoff variant, with almost all individuals sampling this option. By modeling the decision-making process for each individual we show that, perhaps surprisingly, this population-level flexibility was achieved without significant asocial sampling and despite an ongoing bias for conformity at the individual level. Instead, switching depended on two factors. First, there was an interaction between social information and personal experience, with individuals that experienced the higher-payoff behavior having a strong preference for that variant in future solves. Second, there was extensive individual variation, with those individuals that relied more on social information showing a weaker conformist bias. These factors allowed some individuals to switch once fortuitously exposed to, and experiencing, the high-payoff variant. These individuals then provided the correct social information for others, leading to a positive feedback loop and eventual population-level turnover.
There has been extensive speculation about whether a reliance on social learning can lead to mismatched or out-of-date behavior (23, 25). Conformity has been thought to potentially exacerbate this process, as conformist individuals rely on an indirect cue of information quality (the proportion of individuals exhibiting a behavior) rather than assessing the value of the information itself (21, 22). However, there has been a paucity of empirical evidence in nonhuman animals. In the only prior study, guppies (Poceilia reticulata) were trained on a longer, suboptimal route to reach a feeding station. This route preference transmitted and persisted over several days before eroding toward a faster route (31). It was assumed that this erosion was associated with asocial learning, but this was not tested. The learning mechanisms that individuals may be using to optimally exploit variable environments were more explicitly tested in Rendell et al. (7), where a computer tournament was used to compete different sets of learning strategies. Winning strategies invested in social learning over asocial learning learned most when individuals experienced a reduced payoff and relied on recently acquired information. Most interestingly, strategies did not benefit in variable environments either by using conformist learning or by preferentially copying high payoffs.
Our experiment supports the findings from Rendell et al. (7) in two main ways. First, we found no evidence that individuals used asocial sampling to change established behavior. This is contrary to previous theory, which suggested that individuals should use “critical social learning,” switching to individual innovation under reduced payoffs (27, 34). In Rendell et al. (7), indiscriminate social learning was adaptive because other agents were “rational”; that is, they reliably demonstrated the highest-payoff behavior in their repertoire. Again, our results reflect this finding; individuals exhibited a clear payoff bias in their personal experience, preferring to use the high-payoff technique once experiencing both possible options. Second, we found no evidence that individuals were incorporating information about the differences in payoffs achieved by different observed demonstrators. Thus, our results, along with those of ref. 7, suggest that the evolution of adaptive social learning strategies like “copy the high payoff” may not be necessary for individuals and populations to cope with temporally variable environments.
Our results differ in one major respect from those of ref. 7; individuals exhibited a conformist bias in their social information use. This agrees with our previous experiment conducted under stable payoff conditions, where individuals were also conformist in their social learning (18). This conformity did not prevent the population from tracking environmental change. Rather, our simulations demonstrated that the combination of conformist social learning with the payoff-sensitive individual reinforcement we observed allowed naive individuals to learn adaptive behavior, but then actually promoted their ability to learn new information and switch behavior once conditions changed. However, our simulations found that evolutionary dynamics favored conformity only under intermediate social learning. This reflects the model in Kandler and Laland (35), which also suggested that conformity bias should be associated with a weaker influence for social learning (20, 21).
Interestingly, we also found considerable individual variation in these parameters, with individuals that used the most social information also generally being less conformist. The role that this this between-individual variation plays in mediating population-level outcomes merits more investigation. Additionally, future research should investigate whether these underlying individual differences in learning behavior are consistent across contexts and whether they relate to other correlates of behavior, for example differences in personality or in developmental conditions (36, 37).
Our simulations of model parameters therefore suggest that a mix of conformist social learning and individual reinforcement is sufficient to result in population-level switches in groups of free-mixing individuals. However, it is interesting to consider how social structure might have additionally influenced this process in the wild population. Great tits show a fission–fusion social structure, with extensive mixing and remixing of small foraging flocks (38, 39) and with social information moving between individuals via these foraging associations (18, 40). It seems likely that this social system might have acted to increase the rate at which the population could flexibly adjust. That is, if individuals occur in small groups that frequently fission, then if there is any behavioral heterogeneity within the population, then even as conformist learners, they will likely have some opportunity to acquire this alternative information. In species with highly modular networks, by contrast, social structure could instead act to slow the rate at which individuals and populations could flexibly adjust their socially learned behavior, with individuals repeatedly exposed to the same mix of potential demonstrators when copying.
In addition to social structure, population demography could also influence the speed at which populations can flexibly adjust socially learned behavior in a variable environment. Whereas we found no sex differences in learning, unlike in refs. 41 and 42, younger individuals in our population tended to show a faster move away from the established low-payoff technique than older individuals and had a higher probability of preferring the high-payoff technique by the end of the experiment. All individuals had equal opportunity in condition 1 to learn and practice the established behavior, and this result was unrelated to previous experience. Rather, it appears that younger birds were generally more likely than older individuals to use social information and, once having experienced the high-payoff technique, were also more able to flexibly adjust their behavior. As younger individuals are often also more likely to disperse, such flexibility in behavior could be advantageous when moving between new habitats (41). More broadly, future work should model the effects of population demography and social network structure on the ability of socially learning populations to track environmental change. Indeed, both population demography and social structure could also potentially be manipulated, and their effect experimentally tested.
In conclusion, we show that socially learned traditions in wild populations of great tits will track environmental change. We further find that populations can track payoffs while individuals remain conformist social learners and use simulations to elucidate the mechanisms by which this counterintuitive outcome occurs. Indeed, our results suggest that conformist social learning actually helps the population adapt to and retain high-payoff behavior, provided it is not too strong. This adds further weight to arguments that social learning will be adaptive in a wide range of environments and contexts (7, 12). It is intriguing to consider what circumstances might therefore promote the perpetuation of maladaptive traditions. One possibility is that some kinds of socially learned information might be more vulnerable to this, for example, where matching group patterns is more important than the absolute adaptive value of a behavior or where the adaptive value of a behavior is obtuse or delayed. Future work should continue to investigate how general these findings are to other species, including humans, and explore their possible implications for the adaptive significance of animal culture.
Materials and Methods
Study System.
This study was conducted in a population of great tits (P. major) at Wytham Woods, near Oxford (51° 46’ N, 01° 20’ W; 385 ha). This population has been the subject of a long-term study; all resident great tits were caught as chicks or adults and fitted with a British Trust for Ornithology metal leg ring and a plastic leg ring encasing a passive integrated transponder (PIT) tag (IB Technology). In addition to this main marking scheme, regular mist netting targeted individuals immigrating into the population and was also used to age and sex birds (by plumage). Immigrants could be classed only as first year or older on plumage; however, as most individuals disperse as relatively young individuals, in all analyses they were assigned as their youngest possible age based on first capture date. From autumn to winter, birds form loose flocks of unrelated individuals (38, 39) with groups aggregating to exploit patchy food sources. In spring and summer, great tits prefer insect prey, but switch to a seed-based diet in winter when insects are less available [e.g., beech mast, Fagus sylvatica (40)]. All experiments mimicked this diet, using live mealworms, unhusked sunflower seeds, and peanut granules; previous work has established that mealworms are a highly preferred food type, and sunflower seed is preferred to peanut granules (18). All work was conducted with relevant ethics approval from the University of Oxford, and by license holders from the British Trust for Ornithology and the Natural England (Natural England license numbers 20123075, 20131205, 20145171).
Experimental Apparatus.
The social learning task consisted of a plastic box containing a feeder that was accessed by sliding a bidirectional door either to left or to right. The left side of this door was colored blue and the right side red, and it had a raised front section to allow an easier grip. A perch in front in the door functioned as a radio-frequency identification (RFID) antenna registering the identity, visit duration, and action of each visiting individual; these were recorded and controlled by a printed circuit board (Stickman Technology) inside the box. One second after a bird was recorded as departed from the antenna, the door reset back to the middle. When installed in the woodland, each puzzle box was surrounded by a 1 × 1-m cage with a 5 × 5-cm mesh to prevent access by larger species, and a freely accessible bird feeder providing peanut granules was provided at 1 m distance.
In experimental condition 1, the puzzle-box feeder contained live mealworms. However, for experimental conditions 2 and 3, the puzzle box was modified to provide two different rewards, depending on the solving technique. This modification involved widening the door by ∼1.5 cm; however, no other changes were made to the puzzle-box interface.
Experimental Design.
A social learning and foraging experiment was conducted in four relatively isolated subpopulations across the woodland, in 4-wk periods between December 2013 and January 2014 (treatment 1 and treatments 3 and 4) and in a 4-wk period in January 2013 for treatment 2. First, two males were caught from each subpopulation and trained in captivity to solve a novel puzzle box: In two subpopulations (T1 and T2), they were trained to solve using variant A (solving pushing right from the blue side), whereas in T3 and T4 they were trained to solve using variant B (solving pushing left from the red side) (Fig. 1A). All birds were then released to act as the initial demonstrators for this behavior, and three such puzzle boxes were installed 250 m apart in each subpopulation. These then continuously operated from dawn on Monday to dusk on Friday for a total of 20 days (18). In all areas the solving behavior spread rapidly, with 68–83% (n = 37–96 per subpopulation) of resident individuals solving either variant at least once. Puzzle boxes were used frequently, with 7,945–12,411 rewarded visits per subpopulation; for more detail see ref. 18.
In January and February 2014, these four replicates were then exposed to a modified puzzle box providing changed rewards. In condition 2 (2 days), this modified puzzle box provided sunflower seeds as a reward for solving using either technique. This was followed by condition 3 (14 days): Here the behavioral variant introduced in the initial experiment was rewarded with sunflower seed, whereas the alternative technique was rewarded with live mealworms. In three replicates, these conditions occurred immediately following the initial experiment. In T2, the experiment occurred 1 y later; however, the population was given 15 days of exposure to the puzzle box immediately before condition 2, with 73% of resident individuals solving the task (either variant) in this period. The results from this replicate were similar to those from the three other replicates.
Statistical Analysis.
To analyze the change over time in individual- and population-level preferences in condition 3, we used a GEE model where the dependent variable was the proportion of solves using the established tradition and explanatory variables were day, replicate, and individuals, weighted by their total number of solves per day. As the data distribution was bimodal, we first divided the data into two clusters, using a longitudinal clustering algorithm that fitted the data for the relative proportion of events that were variant A for individuals over cumulative 2-h time periods. This method was implemented using the R package kml3d.
Learning mechanisms underlying individual changes in behavior were analyzed using a sequential learning model that modeled individual choices as products of time-varying interactions of different modes of learning. The foundation of this framework is the experience-weighted attraction learning model (43), but with additional terms that allow behavior to be guided by social cues. Specifically, we assume that the probability of observing a choice at time by individual is given by
| [1] |
where is the influence of social cues on choice, the probability of choice according only to accumulative individual attraction, and the probability of according only to social cues. The individual attractions are modeled as ordinary experience-weighted attractions with a simple reinforcement model, such that the attraction score for an option at time and individual is given by
| [2] |
where is the importance of newly experienced payoff . Therefore, when there is no influence of past experience. Here we estimate both for each individual and the unobservable payoff to each option . Attraction scores at time imply choice probability by means of a softmax choice rule:
| [3] |
In fitting the model, we set the initial attraction scores at time for each individual to the empirical preferences from the first condition. This accounts for the fact that most individuals begin the second condition with strong preferences for the formerly high option.
Social cues at time can influence choice by changing the probability . In the simplest example, conformist learning is modeled as
| [4] |
where nkt is the frequency of choice among social cues at time and is individual ’s conformist exponent. When this exponent is 1, social learning is unbiased by frequency and behavior is sampled merely in proportion to its occurrence among cues. When, however, the exponent exceeds 1, social learning is conformist. In this study, we consider also payoff-biased social learning, which favors the highest-payoff choice among choices observed at time . Specifically, we construct a convex combination of conformist and payoff-bias terms
| [5] |
where is individual ’s reliance on payoff bias. When there is no variation in social cues, we assume that , which means that payoff bias is active only when observed payoffs vary.
We allow learning strategy to vary at the individual level, estimating , , , and for each individual in the sample. In each case, we construct each parameter such that its log-odds are a linear combination of an individual random effect and an age-specific offset. For example, the submodel for is
| [6] |
where is the standardized age of individual and is a vector of individual random effects, one for each parameter , , , and . The conformity exponent is given a log rather than a logit link.
Model fitting was performed using Hamiltonian Monte Carlo, as implemented in version 2.12 of Stan (44), to draw samples from the posterior distribution. We assessed convergence by inspection of the trace plots, Gelman–Rubin , and an estimate of the effective number of samples. Finally, model priors were defined to be weakly informative and conservative, so that estimated effects and correlations were shrunk slightly toward zero. Specifically, the averages for , , , and were assigned Normal(0,1) priors on the latent scale. The standard deviations of each random effect were assigned Exponential(2) priors, also on the latent scale. For the correlation matrix of random effects, we used the LKJ family of distributions of matrices and assigned , which shrinks correlations away from extreme values near or and toward zero. For the unobserved payoff advantage of the high-payoff option, we assigned a Cauchy(0,1) prior, which is essentially uninformative. Code sufficient to repeat our results is available as an R package, wythamewa, that contains the data, models, and simulation code.
Acknowledgments
We thank Keith McMahon, Stephen Lang, and other members of the Edward Gray Institute for help with various aspects of fieldwork and data collection and Damien Farine for discussions leading to the formation of the project and for developing the software for the puzzle boxes. This research was supported by a grant from the Biotechnology and Biosciences Research Council (BB/L006081/1) (to B.C.S.). L.M.A. was supported by a junior research fellowship at St. John’s College, University of Oxford.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, "The Extension of Biology Through Culture," held November 16–17, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Extension_of_Biology_Through_Culture.
This article is a PNAS Direct Submission. K.N.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621067114/-/DCSupplemental.
References
- 1.Muller CA, Cant MA. Imitation and traditions in wild banded mongooses. Curr Biol. 2010;20:1171–1175. doi: 10.1016/j.cub.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 2.Whiten A, et al. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 3.van Schaik CP, et al. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 4.van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- 5.Slagsvold T, Wiebe KL. Learning the ecological niche. Proc Biol Sci. 2007;274:19–23. doi: 10.1098/rspb.2006.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slagsvold T, Wiebe KL. Social learning in birds and its role in shaping a foraging niche. Philos Trans R Soc Lond B Biol Sci. 2011;366:969–977. doi: 10.1098/rstb.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendell L, et al. Why copy others? Insights from the social learning strategies tournament. Science. 2010;328:208–213. doi: 10.1126/science.1184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElreath R, et al. Beyond existence and aiming outside the laboratory: Estimating frequency-dependent and pay-off-biased social learning strategies. Philos Trans R Soc Lond B Biol Sci. 2008;363:3515–3528. doi: 10.1098/rstb.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd R, Richerson P. Culture and the Evolutionary Process. Univ of Chicago Press; Chicago: 1985. [Google Scholar]
- 10.Henrich J, McElreath R. The evolution of cultural evolution. Evol Anthropol. 2003;12:123–135. [Google Scholar]
- 11.Laland K. Social learning strategies. Learn Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- 12.Rendell L, et al. Cognitive culture: Theoretical and empirical insights into social learning strategies. Trends Cogn Sci. 2011;15:68–76. doi: 10.1016/j.tics.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Cantor M, Whitehead H. The interplay between social networks and culture: Theoretically and among whales and dolphins. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120340. doi: 10.1098/rstb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rendell L, et al. How copying affects the amount, evenness and persistence of cultural knowledge: Insights from the social learning strategies tournament. Philos Trans R Soc Lond B Biol Sci. 2011;366:1118–1128. doi: 10.1098/rstb.2010.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan TJH, Laland K. The biological bases of conformity. Front Neurosci. 2012;6:1–7. doi: 10.3389/fnins.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aplin LM, et al. Counting conformity: Evaluating the units of information in frequency-dependent social learning. Anim Behav. 2015;110:e5–e8. [Google Scholar]
- 17.Pike TW, Laland K. Conformist learning in nine-spined stickleback’s foraging decisions. Biol Lett. 2010;6:466–468. doi: 10.1098/rsbl.2009.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aplin LM, et al. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature. 2015;518:539–541. doi: 10.1038/nature13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiten A, Horner V, de waal FB. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- 20.Nakahashi W, Wakano JY, Henrich J. Adaptive social learning strategies in temporally and spatially varying environments : How temporal vs. spatial variation, number of cultural traits, and costs of learning influence the evolution of conformist-biased transmission, payoff-biased transmission, and individual learning. Hum Nat. 2012;23:386–418. doi: 10.1007/s12110-012-9151-y. [DOI] [PubMed] [Google Scholar]
- 21.Henrich J, Boyd R. The evolution of conformist transmission and the emergence of between-group differences. Evol Hum Behav. 1998;19:215–241. [Google Scholar]
- 22.Whitehead H, Richerson PJ. The evolution of conformist social learning can cause population collapse in realistically variable environments. Evol Hum Behav. 2009;30:261–273. [Google Scholar]
- 23.Galef BG. Why behaviour patterns that animals learn socially are locally adaptive. Anim Behav. 1995;49:1325–1334. [Google Scholar]
- 24.Galef BG. A new model system for studying behavioural traditions in animals. Anim Behav. 1995;50:705–717. [Google Scholar]
- 25.Laland K. Is social learning always locally adaptive? Anim Behav. 1996;52:637–640. [Google Scholar]
- 26.Franz M, Matthews LJ. Social enhancement can create adaptive, arbitrary and maladaptive cultural traditions. Proc Biol Sci. 2010;277:3363–3372. doi: 10.1098/rspb.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enquist M, Eriksson K, Ghirlanda S. Critical social learning: A solution to Rogers’s paradox of nonadaptive culture. Am Anthropol. 2007;109:727–734. [Google Scholar]
- 28.Giraldeau LA, Valone TJ, Templeton JJ. Potential disadvantages of using socially acquired information. Philos Trans R Soc Lond B Biol Sci. 2002;357:1559–1566. doi: 10.1098/rstb.2002.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendal RL, Coolen I, van Bergen Y, Laland KN. Trade-offs in the adaptive use of social and asocial learning. Adv Stud Behav. 2005;35:333–379. [Google Scholar]
- 30.Kendal J, Giraldeau LA, Laland K. The evolution of social learning rules: Payoff-biased and frequency-dependent biased transmission. J Theor Biol. 2009;260:210–219. doi: 10.1016/j.jtbi.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Laland K, Williams K. Social transmission of maladaptive information in the guppy. Behav Ecol. 1998;9:495–499. [Google Scholar]
- 32.Bates L, Chappell J. Inhibition of optimal behaviour by social transmission in the guppy depends on shoaling. Behav Ecol. 2002;13:827–831. [Google Scholar]
- 33.McElreath R, et al. Applying evolutionary models to the laboratory study of social learning. Evol Hum Behav. 2005;26:483–508. [Google Scholar]
- 34.Rendell L, Fogarty L, Laland K. Rogers’ paradox recast and resolved: Population structure and the evolution of social learning strategies. Evolution. 2010;64:534–548. doi: 10.1111/j.1558-5646.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 35.Kandler A, Laland K. Tradeoffs between the strength of conformity and number of conformists in variable environments. J Theor Biol. 2013;332:191–202. doi: 10.1016/j.jtbi.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Mesoudi A, Chang L, Dall SR, Thornton A. The evolution of individual and cultural variation in social learning. Trends Ecol Evol. 2016;31:215–225. doi: 10.1016/j.tree.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Farine DR, Spencer KA, Boogert NJ. Early-life stress triggers juvenile zebra finches to switch social learning strategies. Curr Biol. 2015;25:2184–2188. doi: 10.1016/j.cub.2015.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farine DR, et al. The role of social and ecological processes in structuring animal populations: A case study from automated tracking of wild birds. R Soc Open Sci. 2015;2:150057. doi: 10.1098/rsos.150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aplin LM, et al. Consistent individual differences in the social phenotypes of wild great tits, Parus major. Anim Behav. 2015;108:117–127. doi: 10.1016/j.anbehav.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. Social networks predict patch discovery in a wild population of songbirds. Proc Biol Sci. 2012;279:4199–4205. doi: 10.1098/rspb.2012.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aplin LM, Sheldon B, Morand-Ferron J. Milk-bottles revisited: Social learning and individual variation in the blue tit (Cyanistes caeruleus) Anim Behav. 2013;85:1225–1232. [Google Scholar]
- 42.Brodin A, Urhan AU. Sex differences in learning ability in a common songbird, the great tit - females are better observational learners than males. Behav Ecol Sociobiol. 2015;69:237–241. [Google Scholar]
- 43.Camerer C, Ho TH. Experience-weighted attraction learning in normal form games. Econometrica. 1999;67:827–874. [Google Scholar]
- 44.Stan Development Team 2016 Stan Modeling Language Users Guide and Reference Manual, Version 2.12.0. Available at mc-stan.org/users/documentation/. Accessed June 19, 2017.