Significance
Vocalization is a primary method of communication in many species and relies on coordinated muscle activity. Vocalization and breathing must be synchronized, because calls can be evoked only during expiration. How vocalization and breathing are coordinated is not well understood. Here, we show that newborn mice with impaired development of the nucleus tractus solitarius (NTS) are mute and cannot generate the expiratory pressure needed for vocalization. Furthermore, they do not receive appropriate maternal care. We demonstrate that the NTS contains premotor neurons that directly project to and entrain the activity of spinal (L1) and nucleus ambiguus motor neurons known to control expiratory pressure and laryngeal tension, respectively. We conclude that the NTS is an essential component of the vocal circuit.
Keywords: vocalization, expiration, hindbrain, premotor neurons, Olig3
Abstract
Vocalization in young mice is an innate response to isolation or mechanical stimulation. Neuronal circuits that control vocalization and breathing overlap and rely on motor neurons that innervate laryngeal and expiratory muscles, but the brain center that coordinates these motor neurons has not been identified. Here, we show that the hindbrain nucleus tractus solitarius (NTS) is essential for vocalization in mice. By generating genetically modified newborn mice that specifically lack excitatory NTS neurons, we show that they are both mute and unable to produce the expiratory drive required for vocalization. Furthermore, the muteness of these newborns results in maternal neglect. We also show that neurons of the NTS directly connect to and entrain the activity of spinal (L1) and nucleus ambiguus motor pools located at positions where expiratory and laryngeal motor neurons reside. These motor neurons control expiratory pressure and laryngeal tension, respectively, thereby establishing the essential biomechanical parameters used for vocalization. In summary, our work demonstrates that the NTS is an obligatory component of the neuronal circuitry that transforms breaths into calls.
Vocalization is the primary mechanism used by many vertebrate species for communication (1). Whereas adult mice call during courtship, mating, and territorial disputes, newborn mice use vocalization to communicate with their mothers (2, 3). Newborn mice, when isolated, produce ultrasonic calls (USCs) that elicit search and retrieval behavior by their mothers. Thus, vocalizations of newborn mice represent an innate behavior that is thought to rely on a genetically determined circuit. Such innate vocalizations are reminiscent of nonverbal utterances of humans like laughing, crying, sighing, and moaning.
The central circuits that control vocalization have been widely studied in adult vertebrates, where they overlap in their executive components with respiratory circuits (4). Forebrain pathways that control the frequency and sequence of ultrasounds in mice are not essential for innate vocalization (5, 6); rather, it is the periaqueductal gray in the midbrain that modulates the activity of motor neurons in the hindbrain and spinal cord to implement calls and modulate breathing (7, 8). Calls are shaped through a biomechanical process that involves variations in subglottal air pressure and laryngeal muscle tension (9, 10). Expiration is an important determinant of subglottal air pressure (11), suggesting that expiratory muscle activity and laryngeal tension are highly coordinated during vocalization. However, because expiratory and laryngeal motor neurons are located at markedly different axial levels of the nervous system, in the spinal cord (T11–L1 levels, expiratory) and hindbrain (nucleus ambiguus, laryngeal), how the activities of these motor pools are coordinated is unclear (12, 13). More importantly, the identity and location of functionally important premotor neurons for vocalization are little known.
Using mouse genetics to investigate the neuronal basis of innate vocalization, we identified the nucleus tractus solitarius (NTS) as a crucial vocal nucleus. Newborn mice that lack the NTS are mute (i.e., unable to call), and do not receive adequate maternal care; however, these mice do display other vocalization-associated behaviors, such as mouth opening and the production of clicks. We found that in these mice, muteness is accompanied by a deficit in the ability to generate the expiratory pressure necessary for vocalization. We also show that the NTS directly connects to and activates spinal (L1) and nucleus ambiguus motor neurons at positions where expiratory and laryngeal motor neurons reside (12, 13). Our data suggest that NTS neurons establish the biomechanical parameters essential for call production by controlling the activity of expiratory and laryngeal motor neurons. Thus, we identify the NTS as an obligatory component of the circuit that links breathing and vocalization.
Results
Identification of Hindbrain Neurons Associated with Vocalization.
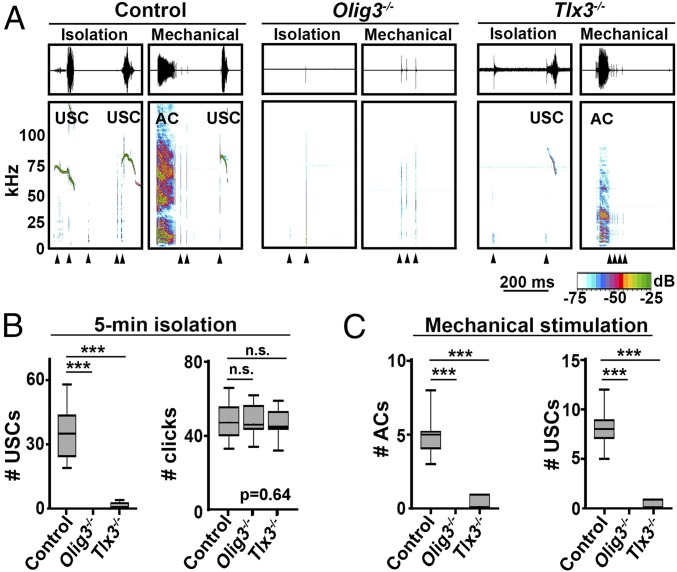
The vocalizations of young mice in the postnatal period provides a model for exploring the neuronal circuitry responsible for this behavior. When isolated, newborn mice produce USCs and short clicks (Fig. 1A; spectrograms illustrate sound frequencies and intensity of calls). Calls are particularly frequent during the first hours of life (Fig. S1). Newborn mice also vocalize with a combination of stereotypic broadband audible calls (ACs), USCs, and short clicks in response to mechanical stimulation (Fig. 1A).
Fig. 1.
Vocal impairment of Olig3 and Tlx3 mutant mice. (A) Representative waveforms (Upper) and spectrograms (Lower) illustrating ACs, USCs, and clicks (arrowheads) produced by control, Olig3−/−, and Tlx3−/− newborn mice in isolation or after mechanical stimulation. Sound intensity (in dB) is color-coded. (B) Quantification of USCs and clicks produced by control (n = 24), Olig3−/− (n = 12), and Tlx3−/− (n = 12) mice during a 5-min isolation period. (C) Quantification of ACs and USCs produced by control (n = 24), Olig3−/− (n = 12), and Tlx3−/− (n = 12) mice after a single tail stimulation. The boxplots in B and C show median (black line), quartiles (boxes), and ranges (whiskers). The numbers and types of calls produced by Olig3+/− and Tlx3+/− heterozygous mice were indistinguishable from those produced by WT animals and are displayed together as controls (Fig. S1B). ***P < 0.0001.
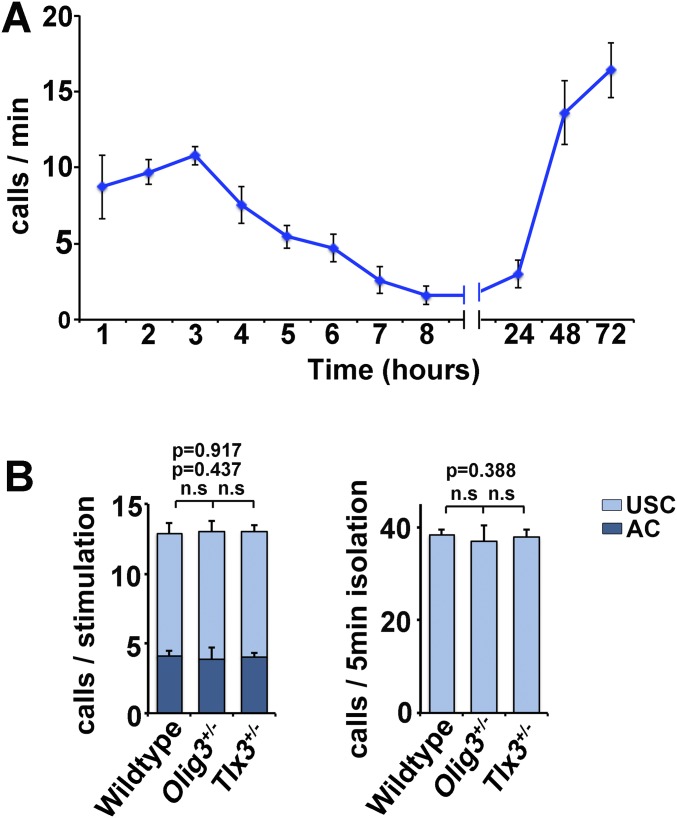
Fig. S1.
USCs produced in newborn mice. (A) Quantification of the number of isolation-evoked USCs in WT (n = 6) newborn mice at different times after birth. (B) Quantifications of ACs and USCs evoked by mechanical stimuli or isolation in WT (n = 8), Olig3+/− (n = 8), and Tlx3+/− (n = 8) heterozygous newborn mice.
Given the central role of hindbrain circuits in breathing and breathing-associated behaviors, we analyzed call production in mutant mouse strains that display deficits in hindbrain development. In doing so, we identified two strains, Oligodendrocyte transcription factor 3 (Olig3) and T-cell leukemia homeobox 3 (Tlx3) mutants, with severe vocalization impairment (Fig. 1A) (14, 15). Whereas Tlx3 mutants produced weak and rare calls, Olig3 mutant newborns produced no calls either after mechanical stimulation or when isolated from the mother (Fig. 1 B and C). Both mutants were able to generate other behaviors associated with vocalization, including mouth openings and click production (Fig. 1B). This indicates that these mice have a deficit in the neuronal circuitry that specifically controls the production of calls.
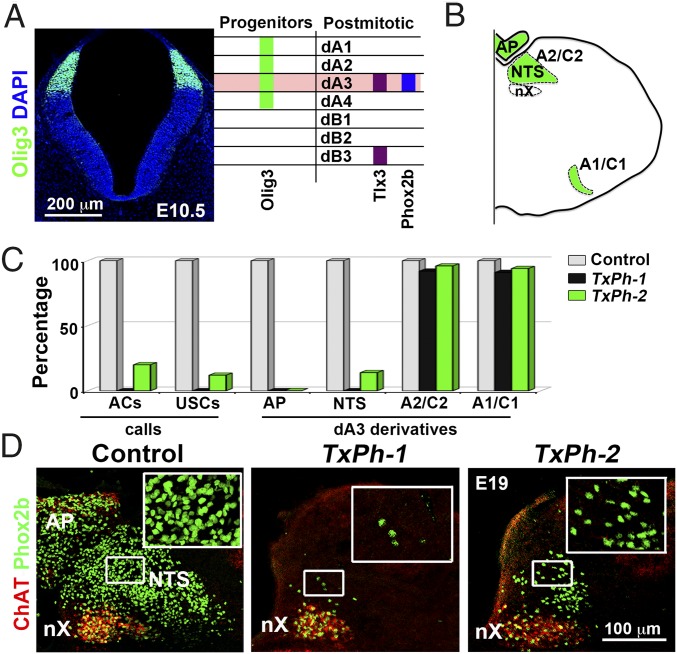
Previous studies have shown that mutations in Olig3 and Tlx3 affect the development of largely nonoverlapping neuronal populations in the mouse hindbrain (14, 15). The single exception is the dA3 neuronal type produced exclusively in rhombomeres 4–7, which is impaired in both mutant strains. This raises the possibility that dA3 neurons are required for call production. The Olig3 transcription factor is expressed in a dorsal hindbrain progenitor domain that generates dA1–dA4 neuronal types; among these, dA3 neurons express the Tlx3 transcription factor (Fig. 2A). dA3 neurons contribute to four distinct hindbrain centers: the NTS, area postrema, and A1/C1 and A2/C2 adrenergic groups (shown schematically in Fig. 2B) (14–17). During development, all dA3 neurons are excitatory (vGluT2+) and coexpress the Paired-like homeobox 2b (Phox2b) (Fig. S2 A–K). Thus, all vGluT2+ NTS neurons express Phox2b at P0, but some down-regulate Phox2b subsequently. Inhibitory neurons associated with the NTS and area postrema are not derived from dA3, but emerge from dB neurons (18). A1/C1 and A2/C2 adrenergic groups express tyrosine hydroxylase, but again some neurons down-regulate Phox2b and vGlut2 in postnatal life (19, 20).
Fig. 2.
NTS neurons are essential for vocalization. (A, Left) Transverse section of rhombomere 7 stained with Olig3 antibodies (green) and DAPI (blue). Olig3 is expressed in dorsal progenitor cells at E10.5. (A, Right) Scheme showing genes expressed in progenitor cells and neurons of the dorsal hindbrain. Olig3+ progenitors generate dA1-4 neurons; dA3 neurons express Tlx3 and Phox2b. (B) Scheme of a transverse hindbrain section depicting dA3 neuronal derivatives (green): area postrema (AP), NTS, A1/C1, and A2/C2 adrenergic groups. (C) Column plot comparing the numbers of ACs, USCs, and dA3-derived neuronal types in control (n = 22), TxPh-1 (n = 14), and TxPh-2 (n = 9) animals (Table S1). (D) Transverse sections of control, TxPh-1, and TxPh-2 mice costained with Phox2b (green) and ChAT (red) to visualize NTS neurons at E19.
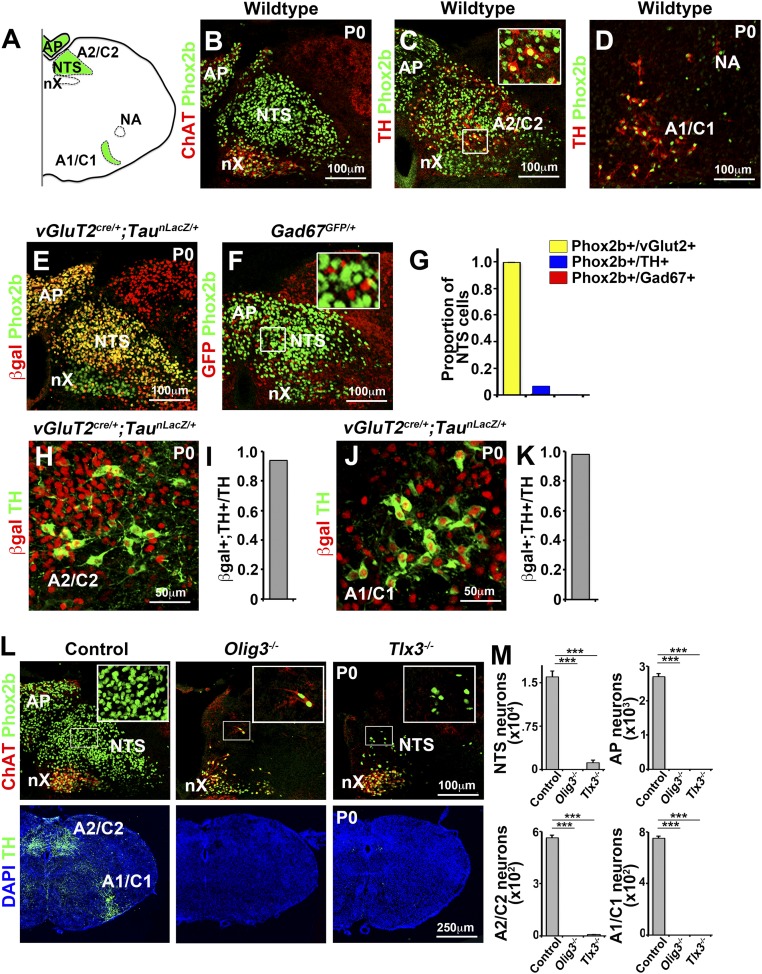
Fig. S2.
dA3 neuronal subtypes in the hindbrain. (A) Scheme of a transverse hindbrain section showing dA3 neuronal derivatives in green: area postrema (AP), NTS, A1/C1, and A2/C2 groups. In addition, vagal (nX) and ambiguus (NA) nuclei that serve as anatomic landmarks but do not derive from dA3 are outlined. (B–L) Histological analysis of dA3 derivatives in hindbrain transverse sections. (B) Neurons of the area postrema and NTS express Phox2b (green) but not acetyl-choline transferase (ChAT; red) that is expressed by ventrally abutting motor neurons of the vagal (nX) motor nucleus. (C and D) The adrenergic groups A2/C2 (C) and A1/C1 (D) coexpress Phox2b (green) and tyrosine hydroxylase (TH, red). (C, Inset) Magnification of the boxed area. (E and F) The neurotransmitter identity of NTS neurons was examined by costaining of Phox2b (green) with (i) antibodies against β-gal (product of the LacZ gene; in E) in mice carrying vGluT2-cre and a cre-dependent nuclear indicator allele (vGlut2cre/+;TaunLacZ-mGFP/+; n = 3), to visualize excitatory neurons, or (ii) with antibodies against GFP (red, false color; in F) in Gad67GFP/+ (n = 3) mice to visualize inhibitory neurons. (F, Inset) Magnification of the boxed area. (G) Proportion of Phox2b+ neurons in the NTS expressing excitatory (β-gal), adrenergic (TH), and inhibitory (GFP) markers in newborn mice. (H–K) The neurotransmitter identity of A2/C2 (H) and A1/C1 (J) neurons was examined by immunohistology and antibodies directed against tyrosine hydroxylase (TH; green) and β-gal (red) in vGlut2cre/+;TaunLacZ-mGFP/+ newborn (n = 3) mice. (I and K) Proportion of TH+ neurons coexpressing β-gal in A2/C2 (I) and A1/C1 (K) adrenergic groups. Note that Phox2b+ NTS and area postrema neurons are excitatory but not inhibitory; the neurons of the A2/C2 and A1/C1 groups are Phox2b+/TH+ and excitatory, and vagal motor neurons are Phox2b+/ChAT+. (L) Transverse hindbrain sections were used for histological analysis of dA3 neuronal derivatives in Olig3−/− and Tlx3−/− mice using antibodies against Phox2b, ChAT, and TH. (M) Quantification of NTS, AP, A1/C1, and A2/C2 neurons in control (n = 6), Olig3−/− (n = 6), and Tlx3−/− (n = 6) newborn mice. ***P < 0.0001.
Changes in the generation of dA3 neuronal derivatives were quantified in Olig3 and Tlx3 mutant mice. Costaining of serial hindbrain sections with antibodies against Phox2b and choline acetyltransferase (ChAT) distinguished residual ChAT−/Phox2b+ NTS neurons from nearby ChAT+/Phox2b+ vagal motor neurons that emerge ventrally and have no history of Olig3 or Tlx3 expression. Tyrosine hydroxylase served as a marker for adrenergic neurons. In Olig3 mutants, all dA3 derivatives (NTS, area postrema, and A1/C1 and A2/C2 neurons) were absent. In Tlx3 mutants, the number of ChAT−/Phox2b+ NTS neurons was reduced by 86%, and the area postrema, A1/C1, and A2/C2 neurons were absent (Fig. S2 L and M). Thus, Phox2b+ NTS neurons are impaired to different degrees in Olig3 and Tlx3 mutant mice. Most strikingly, the degree of vocal impairment was correlated with the reduction of Phox2b+ NTS neurons; Olig3 mutants lost all Phox2b+ NTS neurons and were unable to produce calls, whereas Tlx3 mutants displayed a severe reduction in these neurons and produced weak and infrequent calls. Although we reasoned that the Phox2b+ NTS neurons play an important role in vocalization, we could not exclude the possibility that other neuronal deficits in Olig3 and Tlx3 mutants might contribute to their vocal impairment.
NTS Neurons Are Essential for Vocalization.
To directly assess the function of NTS neurons in vocalization, we set out to specifically eliminate Phox2b+ NTS neurons. Because neuronal progenitors commonly generate different neuronal subtypes in a defined temporal order (21), we performed a lineage trace analysis of dA3 neuronal derivatives. We found that dA3 neurons that produce the NTS and area postrema emerge after embryonic day (E) 10.5, whereas those that form adrenergic A2/C2 and A1/C1 groups arise earlier (Fig. S3 A–C). Therefore, we selectively ablated late-born Phox2b+ neurons by combining the inducible Olig3creERT2 and conditional Phox2bFlox alleles, and induced recombination with tamoxifen treatment at E10.5 (Olig3creERT2/+;Phox2bFlox/Flox mice, hereinafter called TxPh mutants; Fig. S3 D–F) (14, 22). All TxPh mutants displayed major deficits in vocalization, whereas tamoxifen-treated control littermates vocalized normally (Fig. 2C and Fig. S3 G–I). The TxPh mutants were classified into two subgroups according to the degree of vocal impairment: 18 of 31 pups in the TxPh-1 subgroup produced no calls, and 13 of 31 pups in the TxPh-2 subgroup produced only a few weak calls.
Fig. S3.
Selective elimination of late-born dA3 neuronal subtypes. (A) Schematic display illustrating the genetic strategy for birthdating dA3 neuronal derivatives using a tamoxifen-dependent cre recombinase driven by Olig3 and a cre-dependent nLacZ indicator (Olig3creERT2/+;TaunLacZ-mGFP/+). On tamoxifen treatment, a stop cassette upstream of nuclear LacZ (nLacZ) and membrane GFP (mGFP) is removed, allowing visualization of recombined cells by β-gal and GFP expression. (B) Tamoxifen was applied at indicated developmental stages (one application/time point), and neurons were analyzed at E19. (C) Percentage of indicated neuronal types that were β-gal+ when embryos were exposed to tamoxifen at E9.5, E10.5, or E11.5 (n = 3 each). (D) Scheme illustrating the strategy for mutating Phox2b by removing exon 2 (E2) using the Olig3creERT2 allele. (E) Recombination was induced at E10.5, and embryos were analyzed at either E11.5 or E19. (F) Representative transverse sections of the dorsal hindbrain in control (TxControl: Olig3creERT/+;Phox2bFlox/+) and TxPh (Olig3creERT/+;Phox2bFlox/Flox) mutants stained with antibodies against Olig3 (blue), Phox2b (green), and Foxd3 (red) at E11.5. Note that Phox2b+ dA3 neurons were no longer specified in TxPh embryos, but Foxd3+ dA4 neurons were unaffected. (G) Spectrograms (Lower) and waveforms (Upper) illustrating ACs and USCs from control, TxPh-1, and TxPh-2 mice after mechanical stimulation; arrowheads indicate clicks. The sound intensity (in dB) is color-coded. (H) Quantification of ACs and USCs evoked by mechanical stimuli or USCs produced in isolation by control (n = 22), TxPh-1 (n = 14), and TxPh-2 (n = 9) newborn mice (Table S1). (I) Quantification of ACs and USCs evoked by mechanical stimuli or isolation in WT (n = 11) and tamoxifen-treated control mice (Txcontrol; n = 22). (J) Representative transverse hindbrain sections of control, TxPh-1, and TxPh-2 mice stained with antibodies against tyrosine hydroxylase (TH, green) and counterstained with DAPI at E19 to identify the A2/C2 and A1/C1 adrenergic groups. Note that development of adrenergic groups is unaltered in TxPh mice (Table S1). ***P < 0.0001.
We next quantified Phox2b+ NTS neurons, and found that >98% of these neurons were absent in the TxPh-1 mice and 84% were lacking in the TxPh-2 mice (Fig. 2D and Table S1). This finding supports a direct correlation between the depletion of NTS neurons and the inability to emit calls. In contrast, area postrema neurons were completely eliminated in all TxPh mutants, whereas A2/C2 and A1/C1 neurons were mostly spared (Fig. 2C, Fig. S3J, and Table S1). Therefore, TxPh-1 and Olig3 mutant mice are similar; they lack Phox2b+ NTS neurons and are unable to vocalize. Tlx3 and TxPh-2 mutants also are alike, in that they lose most, but not all, Phox2b+ NTS neurons and produce infrequent and weak calls. A2/C2 and A1/C1 neurons are present in TxPh mutant mice and absent in Olig3 and Tlx3 mutant mice, whereas the area postrema is lacking in all mutants. Thus, deficits in adrenergic neurons and the area postrema do not correlate with impaired vocalization. Taken together, these findings reveal that ablation of Phox2b+ NTS neurons causes muteness, pointing to an essential role of NTS neurons in vocalization.
Table S1.
Number of dA3 derivatives (NTS, area postrema, A1/C1, A2/C2 neurons) and calls in individual TxPh-1 and TxPh-2 mutant animals
| Genotype | dA3 neuronal derivatives, n | Calls | |||||
| NTS neurons | AP neurons | A2/C2 neurons | A1/C1 neurons | Mechanical stimulation | Isolation, USCs, n | ||
| ACs, n | USCs, n | ||||||
| WT 1 | 17,144 | 2,947 | 621 | 841 | 3 | 8 | 36 |
| WT 2 | 16,891 | 2,781 | 579 | 785 | 2 | 5 | 40 |
| WT 3 | 15,997 | 2,877 | 584 | 794 | 4 | 10 | 58 |
| WT 4 | 17,581 | 2,864 | 548 | 765 | 3 | 5 | 35 |
| WT 5 | 16,985 | 2,854 | 601 | 763 | 3 | 8 | 37 |
| WT 6 | 17,304 | 2,994 | 586 | 789 | 5 | 8 | 40 |
| WT 7 | ND | ND | ND | ND | 8 | 8 | 41 |
| WT 8 | ND | ND | ND | ND | 6 | 6 | 38 |
| WT 9 | ND | ND | ND | ND | 6 | 12 | 36 |
| WT 10 | ND | ND | ND | ND | 5 | 8 | 38 |
| WT 11 | ND | ND | ND | ND | 5 | 9 | 37 |
| TxCon 1 | 16,988 | 2,674 | 611 | 712 | 7 | 9 | 55 |
| TxCon 2 | 16,995 | 2,596 | 599 | 852 | 6 | 8 | 54 |
| TxCon 3 | 17,288 | 2,892 | 614 | 764 | 8 | 7 | 44 |
| TxCon 4 | 16,788 | 2,955 | 568 | 729 | 4 | 6 | 35 |
| TxCon 5 | 16,459 | 2,711 | 674 | 781 | 3 | 12 | 34 |
| TxCon 6 | 16,598 | 2,859 | 626 | 728 | 3 | 8 | 27 |
| TxCon 7 | ND | ND | ND | ND | 4 | 8 | 4 |
| TxCon 8 | ND | ND | ND | ND | 5 | 7 | 58 |
| TxCon 9 | ND | ND | ND | ND | 5 | 12 | 37 |
| TxCon 10 | ND | ND | ND | ND | 4 | 11 | 22 |
| TxCon 11 | ND | ND | ND | ND | 5 | 10 | 27 |
| TxCon 12 | ND | ND | ND | ND | 4 | 8 | 22 |
| TxCon 13 | ND | ND | ND | ND | 3 | 7 | 24 |
| TxCon 14 | ND | ND | ND | ND | 8 | 9 | 22 |
| TxCon 15 | ND | ND | ND | ND | 4 | 7 | 44 |
| TxCon 16 | ND | ND | ND | ND | 5 | 6 | 96 |
| TxCon 17 | ND | ND | ND | ND | 5 | 6 | 22 |
| TxCon 18 | ND | ND | ND | ND | 4 | 5 | 48 |
| TxCon 19 | ND | ND | ND | ND | 7 | 8 | 71 |
| TxCon 20 | ND | ND | ND | ND | 6 | 12 | 57 |
| TxCon 21 | ND | ND | ND | ND | 5 | 10 | 34 |
| TxCon 22 | ND | ND | ND | ND | 4 | 7 | 23 |
| TxPh-1 #1 | 23 | 0 | 551 | 729 | 0 | 0 | 0 |
| TxPh-1 #2 | 3 | 0 | 517 | 702 | 0 | 0 | 0 |
| TxPh-1 #3 | 29 | 0 | 541 | 704 | 0 | 0 | 0 |
| TxPh-1 #4 | 0 | 0 | 502 | 722 | 0 | 0 | 0 |
| TxPh-1 #5 | 36 | 0 | 488 | 689 | 0 | 0 | 0 |
| TxPh-1 #6 | 7 | 0 | 583 | 703 | 0 | 0 | 0 |
| TxPh-1 #7 | 42 | 0 | 511 | 748 | 0 | 0 | 0 |
| TxPh-1 #8 | 32 | 0 | 557 | 716 | 0 | 0 | 0 |
| TxPh-1 #9 | 14 | 0 | 531 | 678 | 0 | 0 | 0 |
| TxPh-1 #10 | 18 | 0 | 502 | 719 | 0 | 0 | 0 |
| TxPh-1 #11 | 28 | 0 | 562 | 764 | 0 | 0 | 0 |
| TxPh-1 #12 | 36 | 0 | 523 | 750 | 0 | 0 | 0 |
| TxPh-1 #13 | 28 | 0 | 590 | 721 | 0 | 0 | 0 |
| TxPh-1 #14 | 51 | 0 | 589 | 748 | 0 | 0 | 0 |
| TxPh-2 #1 | 1,819 | 0 | 602 | 841 | 1 | 0 | 4 |
| TxPh-2 #2 | 1,545 | 0 | 587 | 785 | 0 | 1 | 2 |
| TxPh-2 #3 | 2,254 | 0 | 599 | 794 | 2 | 2 | 6 |
| TxPh-2 #4 | 1,324 | 0 | 610 | 765 | 1 | 0 | 0 |
| TxPh-2 #5 | 1,245 | 0 | 547 | 769 | 1 | 0 | 2 |
| TxPh-2 #6 | 1,873 | 0 | 541 | 787 | 1 | 1 | 1 |
| TxPh-2 #7 | 1,956 | 0 | 622 | 712 | 2 | 2 | 2 |
| TxPh-2 #8 | 1,138 | 0 | 516 | 758 | 0 | 1 | 1 |
| TxPh-2 #9 | 1,596 | 0 | 561 | 794 | 0 | 1 | 1 |
ND, not determined.
NTS Neurons Regulate Vocal Expiration.
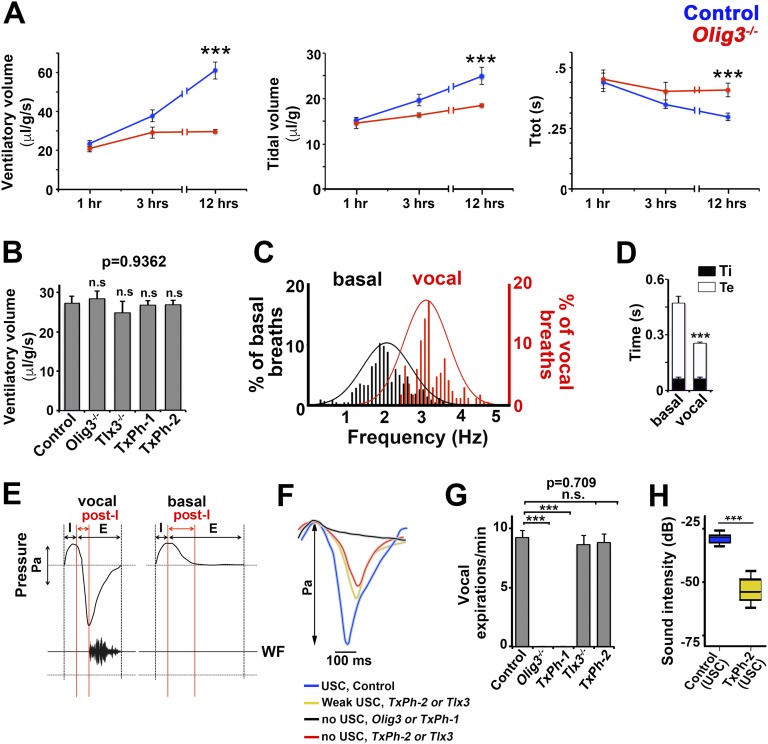
We next tested whether perturbations in the development of the NTS compromise basal breathing in early postnatal life. Basal breathing parameters (ventilatory volume/min; Ttot, which assesses the length of the breathing cycle; and tidal volume, which assesses ventilatory volume per breath) of Olig3 mutants were normal during the first 3 postnatal hours; however, breathing maturation was disturbed thereafter because of insufficient increases in breathing frequency, tidal volume, and ventilation minute volume (Fig. S4A). Olig3 mutants did not survive beyond the first 12 h (14, 17). Likewise, the basal breathing parameters of Tlx3 and TxPh mice were unchanged in the first hours of life (Fig. S4B), but nevertheless, the Tlx3 (15) and TxPh mice also failed to survive. Consequently, all experiments assessing vocalization were restricted to the first 2 postnatal hours, during which basic ventilatory parameters were normal in mutant mice and the production of calls was highest in control animals.
Fig. S4.
Vocal breathing behavior. (A) Quantification of the ventilatory volume, tidal volume, and breathing cycle length (Ttot) in control (blue lines; n = 9) and Olig3−/− (red lines; n = 6) mice at various postnatal time points. (B) Quantification of the average ventilatory volume of control (n = 22), Olig3−/− (n = 10), Tlx3−/− (n = 6), TxPh-1 (n = 6), and TxPh-2 (n = 7) mice during the first 2 h of life. (C) Breathing frequencies during basal and vocal breathing in control newborns (n = 4). (D) Length of expiration (Te) and inspiration (Ti) during basal and vocal breathing in control newborns (n = 4). (E) Plethysmographic recording and waveforms (WF) during a representative vocal and a basal expiration. Inspiratory (I), expiratory (E), and postinspiration phases (post-I, red lines) are indicated. (F) Representative plethysmographic traces of individual postinspiratory phases during fast breathing episodes in control (blue), Olig3−/−, and TxPh-1 mice (indistinguishable, shown together in black), as well as TxPh-2 or Tlx3−/− mice (indistinguishable, displayed together), that were associated with weak or no calls are shown in yellow and red, respectively. (G) Quantification of vocal (active) expirations in control (n = 22), Olig3−/− (n = 10), Tlx3−/− (n = 6), TxPh-1 (n = 6), and TxPh-2 (n = 7) mice at birth. (H) Quantification of USC intensity in control (n = 4; 128 USCs) and TxPh-2 (n = 9; 27 USCs) mice. ***P < 0.0001.
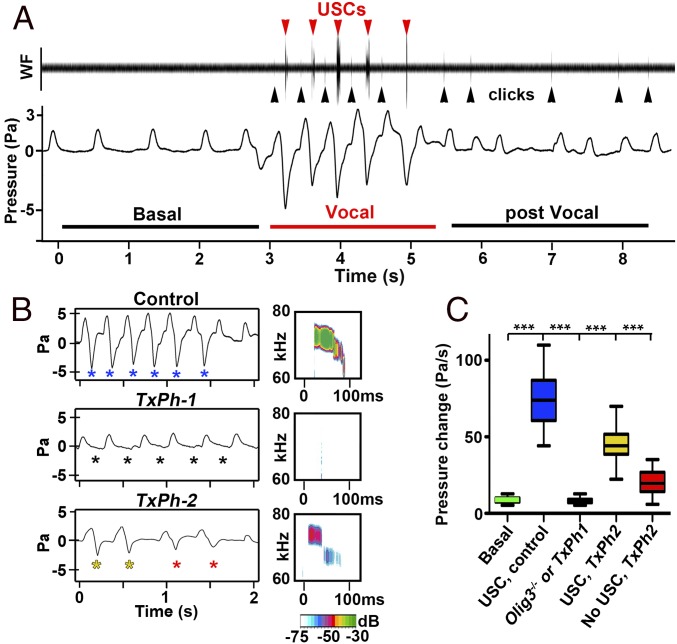
We next simultaneously investigated vocalization and breathing in newborn mice using a plethysmograph equipped with a microphone. In the plethysmograph, newborn control mice produced characteristic isolation-evoked calls—rhythmically repeated bouts of four to six USCs intermingled with clicks, but never with ACs. The bouts of USCs were associated with specific breathing episodes that we call “vocal breathing” (Fig. 3A; 5.9 ± 0.6 vocal breathing episodes were detected during a 5-min isolation period). Vocal breathing was characterized by (i) fast breathing, i.e., an average frequency of 3 Hz due to a reduction in the duration of expirations but not inspirations, which distinguishes it from basal breathing with an average frequency of 2 Hz; (ii) greater expiratory pressure in the postinspiratory phase, detected by the plethysmograph as negative signal, which were not observed during basal breathing; and (iii) production of USCs (Fig. 3A and Fig. S4 C–E). During vocal breathing, the expiratory pressure increased rapidly and peaked immediately before ultrasound emission. We refer to this large postinspiratory pressure change as vocal or active expiration.
Fig. 3.
Vocal breathing behavior in newborn mice. (A) Waveform and plethysmographic traces illustrating slow basal and fast vocal breathing episodes in control mice. Red and black arrowheads point to USCs and clicks, respectively. (B, Left) Plethysmographic recordings of fast breathing episodes in control, TxPh-1, and TxPh-2 mice. Blue asterisks indicate expirations of control mice that produced USCs. Black asterisks indicate expirations of TxPh-1 mice that produce no USCs. Red and yellow asterisks indicate expirations of TxPh-2 mice that produced no calls or weak calls, respectively. (B, Right) spectrograms from control, TxPh-1, and TxPh-2 mice. Note that only clicks were observed in TxPh-1 mice. Sound intensity (in dB) is color-coded. (C) Boxplots of expiratory pressure changes during vocal breathing. Pressure changes observed in basal expirations and vocal expirations of control (n = 9) mice are shown in green and blue, respectively. Pressure changes during fast breathing episodes of Olig3−/− (n = 4) and TxPh-1 (n = 14) mice are shown in black. Pressure changes that were/were not associated with USCs in TxPh-2 mice (n = 7) are shown in yellow and red, respectively. ***P < 0.0001.
We next simultaneously recorded breathing and vocalization in Olig3 and TxPh-1 mutant mice whose vocal behavior was the most severely affected. These animals completely lacked active expirations but had normal incidences of fast breathing episodes (Fig. 3B and Fig. S4 F and G). In Tlx3 and TxPh-2 mutant mice that rarely vocalize, we observed fast breathing episodes associated with blunted active expirations that were characterized by smaller pressure changes (Fig. 3B and Fig. S4 F and G). Interestingly, only a fraction of these expirations were associated with USCs (Fig. 3B; expirations producing calls are indicated by yellow asterisks, and those not producing calls are shown by red asterisks), and those calls were weak (Fig. S4H). We used TxPh-2 mice to estimate the rate of pressure change during expiration in which a USC was produced. This revealed that successful USCs occurred when the pressure change was >35 Pa/s (yellow and blue in Fig. 3C). Lower values usually were not sufficient to produce calls (red in Fig. 3C). We conclude that the NTS is essential for the generation of postinspiratory pressure required for USCs.
Direct Projections of Phox2b+ NTS Neurons to Expiratory and Laryngeal Motor Neurons.
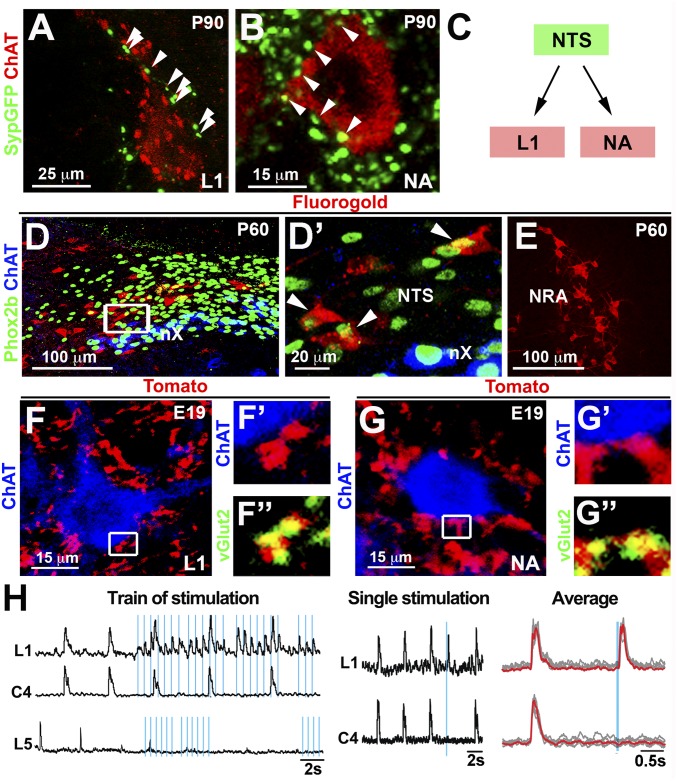
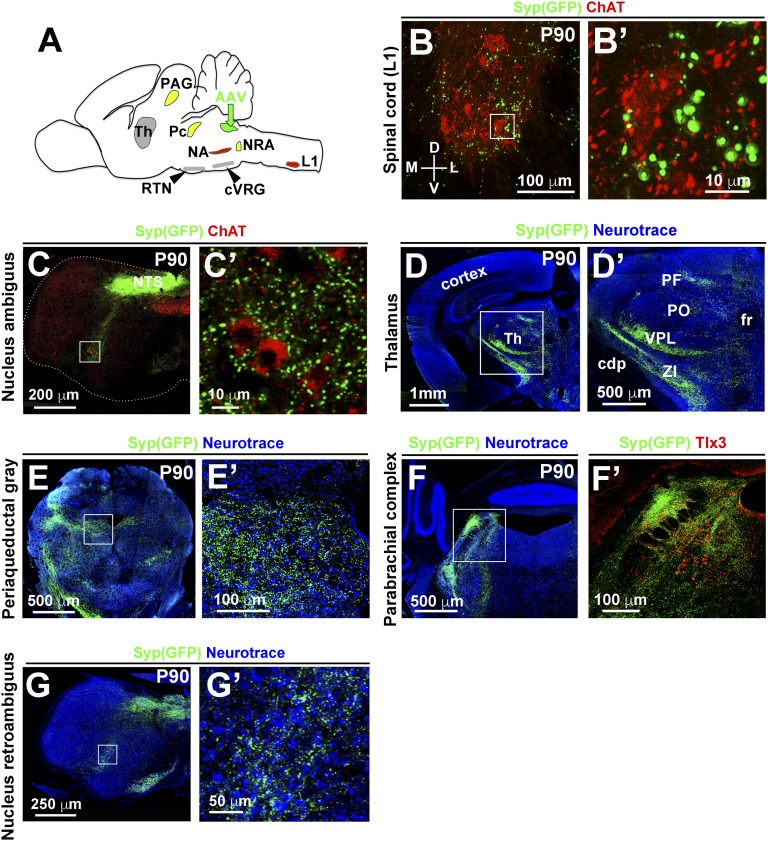
To investigate whether the connectivity of NTS neurons is consistent with its function in vocalization, we stereotactically injected an adeno-associated virus (AAV) encoding a synaptophysin-GFP fusion protein (SypGFP) into the NTS of adult mice and mapped NTS synaptic connections. We observed a dense labeling of GFP+ synapses on motor neurons at lower thoracic (T11–13) and upper lumbar (L1) levels of the spinal cord (Fig. 4 A and C and Fig. S5 A and B). We also observed synaptic contacts with other known targets of the NTS, including motor neurons of the semicompact area and loose formation of the nucleus ambiguus known to innervate the larynx (Fig. 4 B and C and Fig. S5C). The retrotrapezoid nucleus, caudal ventral respiratory group, A1/C1, nucleus retroambiguus, parabrachial complex, periaqueductal gray, and thalamus also were seen to be innervated by the NTS (Fig. S5 D–G). Among these, the ambiguus, retroambiguus, parabrachial, and periaqueductal gray nuclei have been implicated in controlling vocalization in mammals (4, 23).
Fig. 4.
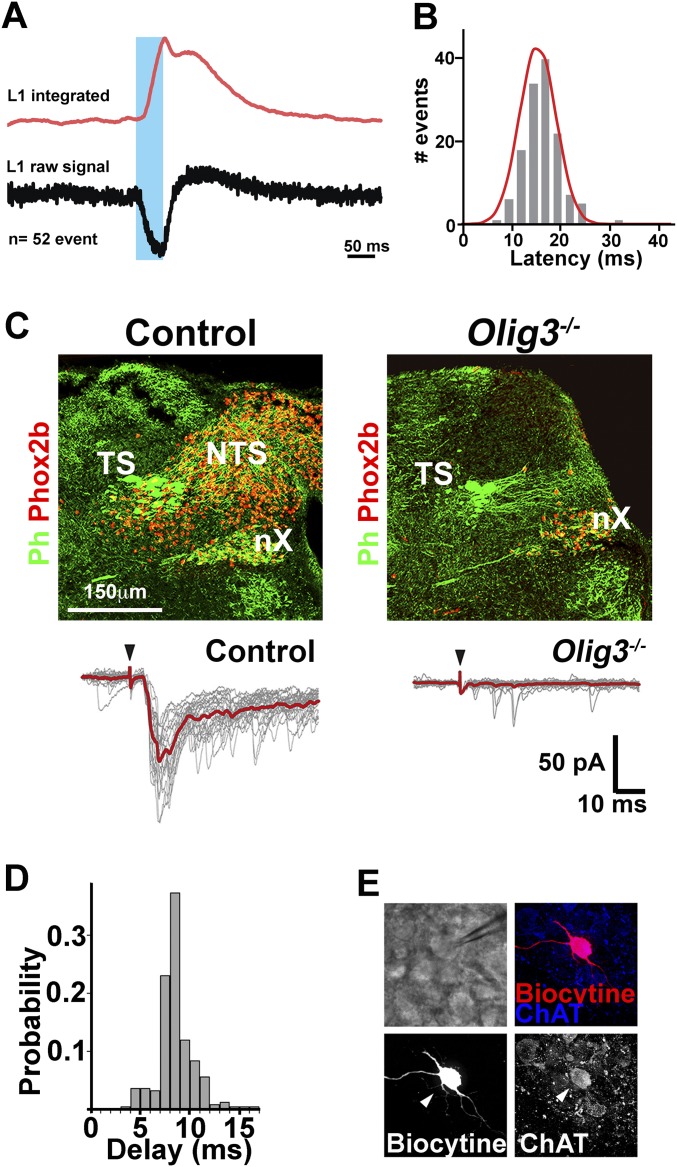
NTS neurons innervate expiratory and laryngeal motor neurons in newborn mice. (A and B) Injection of AAV encoding SypGFP into the NTS (n = 3) at P60 demonstrating the presence of GFP+ synaptic boutons (arrowheads) on L1 spinal (A) and nucleus ambiguus (NA) (B) motor neurons (ChAT+; red; single motor neurons are shown (Fig. S5 B and C). (C) Scheme illustrating that premotor neurons of the NTS connect to laryngeal and expiratory motor neurons. (D and D′) After injection of Fluorogold into the ventral L1 spinal cord, Fluorogold+ (red; arrowheads) neurons were present in the caudal NTS. Phox2b (green) and ChAT (blue) were used to distinguish Phox2b+ NTS neurons from Phox2b+/ChAT+ vagal motor nucleus (nX) on transverse sections. D′ shows a magnification of the boxed area in D. (E) Fluorogold+ cells (red) in the nucleus retroambiguus. (F and G) Intersectional strategy (Olig3creERT2/+;Phox2bFlpo/+;Ai65−/+ mice) to specifically label NTS axons and their synaptic terminals with Tomato fluorescent protein (Fig. S6). ChAT+ (blue) motor neurons at L1 spinal cord levels (F) and in the nucleus ambiguus (G) display numerous Tomato+ (red)/vGluT2+ (green) contacts. F′ and F″ and G′ and G″ show magnifications of the boxed areas in F and G, respectively. Single motor neurons are shown in F and G (overview provided in Fig. S6 C and D). (H, Left) Recordings of L1 (expiratory), C4 (inspiratory), and L5 (locomotion) motor roots in hindbrain-spinal cord preparations (n = 4) after trains of light stimulation (blue lines) on channelrhodopsin+ NTS cells. Light triggered only L1 responses, and not C4 or L5 responses. Note that inspiratory (C4) and expiratory motor roots (L1) are rhythmically active and fire synchronously in such preparations. (H, Middle) An evoked burst of L1 but not C4 motor root activity after a single light stimulation of the NTS. (H, Right) Superimposition of individual (gray) and average (red) traces of L1 and C4 recordings.
Fig. S5.
NTS projections in adult mice. (A) Scheme illustrating injection of AAV-SypGFP (green) into the NTS (n = 3) at P60. Histological analysis was done at P90. Identified NTS targets with a known function in vocalization include L1 spinal and nucleus ambiguus (NA) motor neurons shown in red, as well as periaqueductal gray (PAG), parabrachial complex (Pc), and nucleus retroambiguus (NRA) (yellow). Other known NTS targets displayed in gray were also observed: thalamus (TH), retrotrapezoid nucleus (RTN), and the caudal respiratory group (cVRG). (B and B′) Transverse section of the spinal cord at L1 level of an AAV-SypGFP–infected animal. The sections were stained with antibodies against GFP (green) and ChAT (red). B′ shows a magnification of the boxed area in B. (C–G′) The tile scan modus was used to acquire photomicrographs of caudal hindbrain (C and C′ and G and G′), forebrain (D and D′), midbrain (E and E′) and pons (F and F′), and assembled using ZEN2012 software (0% overlap between tiles). (C and C′) Transverse sections of the hindbrain at the level of the semicompact formation of the nucleus ambiguus. The section was costained with GFP (green) and ChAT (red) antibodies. Note that the ChAT+ motor neurons display numerous GFP+ synaptic boutons. C′ shows a magnification of the boxed area in C. (D and D′) GFP+ projections in the ventral posterolateral (VPL) and zona incerta (ZI) of the thalamus (Th). The fasciculus retroflexus (fr), cerebral peduncle (cpd), and posterior complex of the thalamus (PO) are indicated as landmarks. D′ shows a magnification of the boxed area in D. (E and E′) GFP+ projections in the ventromedial periaqueductal gray (PAG). E′ shows a magnification of the boxed area in E. (F and F′) GFP+ projections in the parabrachial complex (Pc). F′ shows a magnification of the boxed area in F. Tlx3 staining (red) reveals neurons in the parabrachial complex. (G and G′) GFP+ synaptic boutons were observed on neurons in the nucleus retroambiguus (NRA). G′ shows a magnification of the boxed area in G.
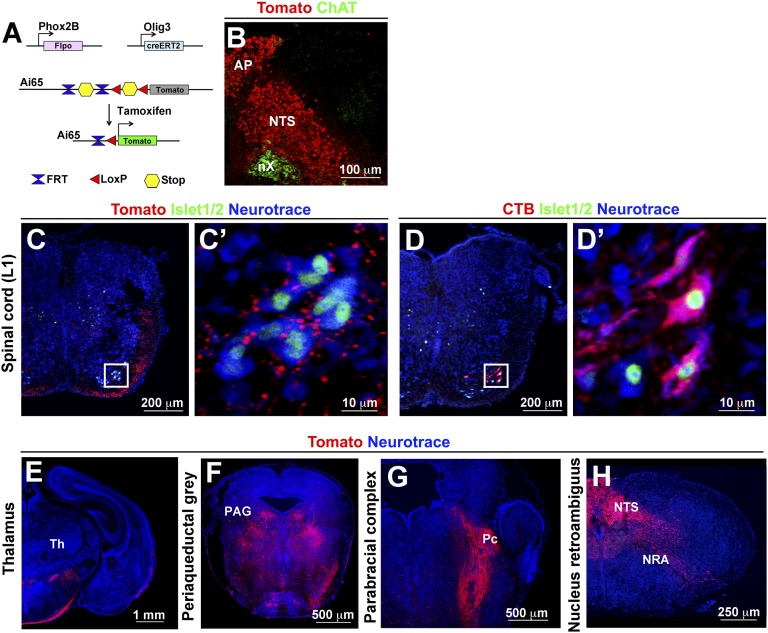
To confirm direct projections of NTS neurons to L1 motor neurons, we injected the retrograde tracer Fluorogold into the ventral L1 spinal cord of adult mice. Fluorogold+ neurons were observed in the caudal part of the NTS (121 ± 7 Fluorogold+ neurons/NTS; n = 3), as well as in the nucleus retroambiguus (Fig. 4 D and E). The majority of Fluorogold+ cells in the NTS were Phox2b+ (76 ± 3.5%; n = 3). In contrast, injections at L3 or L5 levels did not label neurons in the NTS. We next tested whether projections of Phox2b+ NTS neurons to T11–L1 motor neurons exist in newborn mice. To achieve this, we used the Ai65 reporter mice, which conditionally express Tomato fluorescent protein on the removal of two stop cassettes flanked by either FRT or LoxP sites (24). The stop cassettes were removed by cre recombinase under the control of the Olig3 (Olig3creERT2; tamoxifen induction at E10.5) and through expression of FLPo recombinase under the control of Phox2b (Phox2FlpO; Fig. S6 A and B) (25). This demonstrated direct excitatory (vGluT2+) innervation of Phox2b+ NTS neurons to T11–L1 motor neurons (Fig. 4F and Fig. S6C). Injection of fluorescent cholera toxin subunit B (CTB) into the oblique muscles of newborn mice confirmed that motor neurons controlling abdominal expiratory muscles reside at similar positions (Fig. S6D). In addition, excitatory Tomato+ synaptic terminals were observed on motor neurons of the semicompact area and loose formation of the nucleus ambiguus (Fig. 4G). Among the additional NTS targets identified in adults, only projections to the thalamus were not present in newborn mice (Fig. S6 E–H). Thus, NTS projections to brainstem and spinal cord motor neurons are established at birth, whereas projections to the thalamus form postnatally. We conclude that Phox2b+ NTS neurons innervate both spinal motoneurons at T11–L1 levels that control abdominal muscles needed for active expiration and motor neurons of the semicompact area and loose formation of the nucleus ambiguus that innervate the larynx.
Fig. S6.
NTS projections in newborn mice. (A) Scheme illustrating the strategy for selectively labeling NTS neurons, axons, and terminals using intersectional recombination. The mice carry a tamoxifen-dependent CreERT2 driven by Olig3, Flp recombinase (FlpO) driven by Phox2b, and the Tomato reporter allele Ai65 (Olig3creERT2/+;Phox2bFlpo/+;Ai65−/+ mice; n = 4). Cre recombination was induced by tamoxifen at E10.5. Ai65 contains two stop cassettes flanked by LoxP and FRT sites. Tomato is expressed after dual recombination. (B) Immunostaining for Tomato (red) fluorescent protein in NTS and area postrema neurons on a transverse section of the hindbrain. ChAT (green) labeled the vagal motor nucleus (nX). (C and C′) Analysis of Tomato+ (red) NTS projections to L1 motor neurons in Olig3creERT2/+;Phox2bFlpo/+;Ai65−/+ newborn mice (n = 4). Neurotrace (blue) was used as a counterstain, and anti-Islet1/2 antibodies (green) identified motor neurons. C′ shows a magnification of the boxed area in C. (D and D′) Retrograde labeling of L1 motor neurons after CTB injection into the oblique muscles. Shown is a transverse section of the spinal cord at L1 level costained with antibodies against CTB (red) and Islet1/2 (green). Neurotrace (blue) was used as a counterstain. D′ shows a magnification of the boxed area in D. (E–H) The tile scan modus was used to acquire photomicrographs of the forebrain (E), midbrain (F), pons (G), and caudal hindbrain (H), which were assembled using ZEN2012 software (10% overlap between tiles). Tomato+ projections were visualized in transverse sections of Olig3creERT2/+;Phox2bFlpo/+;Ai65−/+ mice at the level of the periaqueductal gray (PAG; in F), parabrachial complex (Pc; in G), and nucleus retroambiguus (NRA; in H), but not the thalamus (E).
Activation of NTS Neurons Suffices to Drive Expiratory and Laryngeal Motor Activity.
We next tested whether activation of Phox2b+ NTS neurons entrains expiratory-like activity by targeting channelrhodopsin expression to these neurons (Olig3creERT2;Ai32 mice; tamoxifen treatment at E10.5). Using hindbrain-spinal cord preparations from these mice, recordings were made from L1 and C4 ventral roots, which contain axonal projections of expiratory and inspiratory motor neurons, respectively, or from L5 ventral roots, which contain projections from locomotor neurons. In accordance with previous studies, L1 and C4 roots were coactive in the absence of light in such preparations (Fig. 4H) (26). Light-dependent activation of NTS neurons selectively recruited activity of L1, but not of C4 or L5 motor roots (Fig. 4H; Fig. S7 A and B quantifies the latency of the L1 response). This result shows that NTS neurons control the activity of L1 motor neurons already at birth. In addition, we electrically stimulated the solitary tract that innervates NTS neurons and patch-clamped neurons of the nucleus ambiguus in transverse slice preparations (27). In control slices, 28 of 28 motor neurons in the semicompact formation of the nucleus ambiguus responded to a stimulus of the solitary tract with a short latency of excitatory postsynaptic inward current, which is indicative of a direct connection between the NTS and the motor pool (Fig. S7 C–E). In contrast, the same stimulus failed to evoke synaptic responses in nucleus ambiguus motor neurons (9 of 9) from Olig3 mutant preparations. Taken together, these findings demonstrate that the NTS contains functional premotor neurons to spinal T11–L1 and nucleus ambiguus motor neurons.
Fig. S7.
NTS activity entrains L1 and nucleus ambiguus motor neurons in newborn mice. (A, Upper) Representative average (red line) of 52 individual integrated traces of L1 motor root activity. The traces are synchronized to the onset of the light pulse used to stimulate the NTS (blue bar, 50 ms). Recordings were performed on hindbrain spinal cord preparations of Olig3creERT2;Ai32+/− mice (tamoxifen treatment at E10.5) at E19. (A, Lower) Representative average of the same electrical event based on the raw data signal. (B) Distribution plot representing the time latency separating the onset of the NTS light stimulation and the start of the triggered L1 motor response. The graph shows the latency measures on the raw data signal of 150 nonconsecutive light stimulations (mean latency, 15.9 ± 3.8 ms). (C, Upper) Representative transverse sections of the hindbrain of control and Olig3−/− mice. Sections were stained with peripherin (Ph, green), a marker of the tractus solitarius (TS), and Phox2b (red) to show Phox2b+/Peripherin+ vagal (nX) and Phox2b+/Peripherin− NTS neurons. (C, Lower) Patch-clamp recordings of semicompact nucleus ambiguus motor neurons after solitary tract stimulation in slices prepared from control (n = 11; 28 neurons) and Olig3−/− (n = 3; 9 neurons) newborn mice. The red traces show averages of 25 individual excitatory postsynaptic currents, and the individual traces are shown in gray. Black arrowheads indicate the onset of stimulation. (D) Delay between solitary tract stimulation and motor neuro depolarization in control mice. (E) The motor neuron identity of the patch-clamped neurons was verified by biocytin filling and ChAT immunostaining.
Lack of Vocalization Impairs Mother–Offspring Interactions.
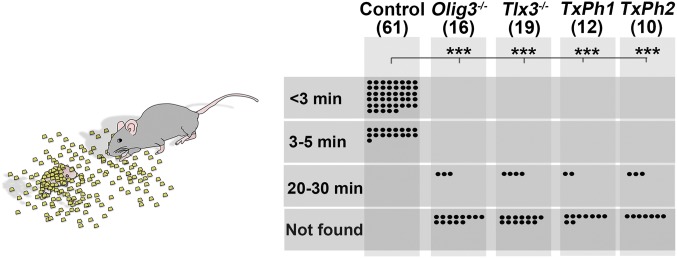
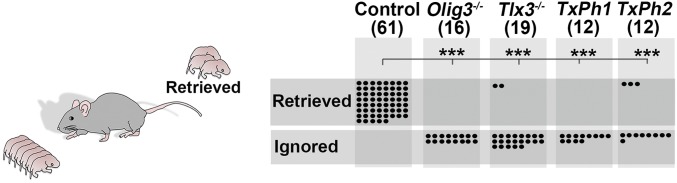
Vocalization of newborn mice elicits search and retrieval behaviors and thus is an important cue for mother–offspring interactions (2). TxPh-1 and Olig3 mutants are mute mice, yielding a model for directly testing the function of vocalization in maternal care. Examination of postpartum behaviors showed that vocally impaired (Olig3, Tlx3, and TxPh mutants) mice were licked after birth like control littermates, indicating acceptance by the mothers, but were frequently found outside of the nest. We assessed two behaviors of mother–offspring interaction, search and retrieval. In the first test, we covered individual pups with bedding material from their cages and observed that mothers found vocalizing pups faster than vocally impaired pups, and that many vocally impaired pups were not found within a 30-min period (Fig. S8). In the second test, we placed mixed litters containing vocalizing and nonvocalizing pups outside of the nest, and allowed the mothers to freely interact with the pups. In all cases, the mothers retrieved vocalizing pups and returned them to the nest. In contrast, Olig3 and TxPh-1 mutants that did not vocalize were not retrieved, whereas TxPh-2 and Tlx3 mutant pups that produced only a few calls were rarely retrieved (Fig. 5). We conclude that USCs in newborn mice are an important cue that elicits maternal care.
Fig. S8.
Vocally impaired pups are not found by their mothers. (Left) Schematic display of searching behavior. Individual pups of indicated genotypes were covered with bedding material, and mothers were allowed to freely search for them. (Right) Quantification of the time needed by the mother to uncover the hidden pups. Pups that were not uncovered by the mother after 30 min were classified as not found. ***P < 0.0001.
Fig. 5.
Vocally impaired pups are neglected by their mothers. (Left) Schematic display of retrieval behavior. Control and mutant newborns were placed together outside of the nest; the mother was allowed to move freely in the cage to retrieve the pups. (Right) Quantification of retrieved/ignored pups with indicated genotypes. Dots represent individual pups. The number of newborns of each genotype is indicated in brackets. ***P < 0.0001.
Discussion
In this study, we show that the NTS is essential for innate vocalization in mice. When an intersectional genetic strategy was used to prevent the development of Phox2b+ NTS neurons, newborn mice were found to be mute, although other vocalization-associated behaviors— mouth openings and click production—were maintained. This mutism has severe consequences for newborn pups, interfering with maternal care. Vocalization relies on coordinated expiratory and laryngeal activity. We show that Phox2b+ NTS neurons form functional connections with L1 and nucleus ambiguus motor pools at positions where expiratory and laryngeal motor neurons reside. When Phox2b+ NTS neurons are absent, pups can breathe but fail to produce the postinspiratory airway pressure required to elicit calls.
The NTS Is a Vocal Nucleus at Birth.
The NTS is one of few sites in the hindbrain containing neurons that have vocalization-correlated activity (28). Our work provides an animal model that selectively lacks Phox2b+ NTS neurons and a direct experimental route to assess the consequences of NTS disruption. As expected, ablation of NTS neurons is incompatible with homeostasis in postnatal life, owing to a loss of chemosensory and viscerosensory reflexes linked to breathing and cardiovascular functions (29). For this reason, we examined mutants in the first hours of life, a period when wild-type (WT) pups vocalize most frequently and when ventilation parameters of WT and mutant pups are similar. Our major finding is that complete ablation of Phox2b+ NTS neurons causes the elimination of both USCs and ACs.
The NTS receives projections from and projects to the periaqueductal gray, a region in the midbrain in which stimulation elicits calls in many species (30). The periaqueductal gray has been discussed as a gating center for vocal behavior but has no direct connections with motor neurons (7), and as such it depends on other premotor neurons to control vocalization. The nucleus retroambiguus, a loose neuronal cluster located posterior to the nucleus ambiguus, has been proposed to serve this role (23). Our work reveals that the NTS, like the nucleus retroambiguus with which it is reciprocally connected, projects to and entrains expiratory and laryngeal motor neurons. Therefore, the expiratory force and the degree of vocal fold contraction, which determine the subglottal pressure critical for call production, appear to be controlled by a parallel and convergent premotor drive from the NTS and the retroambiguus nucleus. The mutations analyzed in this study disrupt Phox2b+ NTS neurons, but not neurons of the nucleus retroambiguus that have no history of Olig3 or of Phox2b expression. Thus, in the absence of the NTS, the nucleus retroambiguus does not suffice for vocalization.
The NTS at the Interface of Breathing and Vocalization Circuits.
Our finding that call production and vocal expirations, but not basal expiration, depend on the integrity of the NTS is accounted for by the fact that basal breathing is accompanied by passive expiration. Basal expirations rely on lung recoil after inspiration, whereas vocal breathing relies on active expiration. Innate vocalization and vocal NTS activity are integrated into the respiratory cycle and produced strictly during postinspiratory phases. The parabrachial complex or the postinspiratory complex might provide postinspiratory drive to the NTS (31–33). Vocalization and breathing rely on viscerosensory feedback; in particular, information on airflow, pressure, and expansion of the lungs as well as laryngeal muscle activity is conveyed to the NTS by pulmonary and upper airway afferents, respectively (34, 35). Such feedback modifies vocal behavior and is required for the breathing phase-dependent Hering–Breuer reflexes (36). Thus, the NTS as a nucleus that integrates viscerosensory information and vocal commands is particularly well suited to coordinate vocalization with respiration. We propose that the NTS is an essential component of the vocal circuit that depends on viscerosensory feedback to modulate vocal and respiratory neurons.
Vocalization is used in distinct behavioral contexts in young and adult mice that also differ in their “readiness” to vocalize. In the monkey, two descending pathways control vocalization, one originating in the motor cortex that is used for learned vocalization and the other from the anterior cingulate cortex gating vocalization that acts via the periaqueductal gray (7). Cortical pathways are dispensable for innate vocalizations, however (5, 6). In contrast, the NTS is essential, thus substantiating and refining the notion that vocalization in newborn mice is supported by a “hard-wired” circuitry in the brainstem. Elements of this circuitry likely are shared among multiple postinspiratory behaviors, such as swallowing, coughing, and sneezing, that rely on glottis closure (31). In addition, innate vocalizations have been related to nonverbal utterances in humans like laughing, crying, sighing, and moaning, which represent postinspiratory behaviors (37, 38).
Communication Behavior.
The USCs of young mice are known to elicit approach and retrieval behavior of their mothers, and even the playback of such calls suffices to trigger an approach (2). We show here that mute newborn mice are not retrieved when placed outside of the nest. It should be noted, however, that deaf mothers are able to provide their pups with maternal care, and thus auditory cues can be replaced by other signals recognized by a deaf mother (39). In the retrieval tests used here, the entire litter of vocalizing and nonvocalizing pups was placed together outside the nest, and the calls of vocalizing littermates provided guidance cues. Thus, the inability of dams to locate nonvocalizing pups is not the sole factor responsible for their neglect. We suggest that not only does innate vocalization serve as a guidance cue during searches, but also that calls are mandatory for pup retrieval, indicating that vocalization represents one parameter by which mice assess the fitness of their offspring.
Speech disorders are frequently observed in patients with various neurodegenerative conditions, autistic spectrum disorder, and in rare cases of neurogenic mutism caused by developmental or acquired nervous system damage (40, 41). The identity of the affected brain area(s) or neuronal cell types often remains undefined, however. Our data indicate that damage to the NTS should be considered as a potential cause of speech pathologies.
Materials and Methods
Animals.
Experimental procedures and animal handling were conducted according to institutional protocols and guidance approved by the Max Delbrueck Center (Berlin), CNRS (Gif sur Yvette), and Columbia University (New York). Details on mouse strains and plethysmographic, audio, and behavioral analyses are provided in SI Materials and Methods.
Histology.
The development of dA3 neuronal derivatives and connectivity of NTS neurons were assessed in 20-μm transverse hindbrain and spinal cord sections from control and mutant mice. Details on antibodies, viruses, and retrograde tracers are provided in SI Materials and Methods.
Electrophysiology.
Patch-clamp and optogenetic studies were performed using 450-μm transverse sections and hindbrain-spinal cord preparations, respectively. The electrophysiological experiments are described in detail in SI Materials and Methods.
SI Materials and Methods
Animals.
All experimental procedures were done in accordance with the guidelines and policies of the Max Delbrueck Center for Molecular Medicine, Berlin, Germany; the Institut des Neurosciences Paris-Saclay, Gif-sur-Yvette, France; and the Institutional Animal Care and Use Committee of Columbia University.
The following mouse strains were used in this study: Ai32 (42), Ai65 (24), GAD67GFP (43), Olig3−/− (44), Olig3creERT2 (14), Phox2bflox (22), Phox2bFlpo (25), vGlut2cre (45), TaumGFP-nLacZ (46), and Tlx3−/− (47). All strains were maintained in a mixed genetic background.
Pregnant dams were treated with tamoxifen as described previously (14). Tamoxifen delays labor in rodents and humans; therefore, offspring from tamoxifen-treated dams were delivered by cesarian section at E19, and the pups were provided with a foster mother.
Audio Recording and Quantification of Vocalizations.
Audio recordings were obtained with an UltraSoundGate condenser microphone capsule CM16 (sensitive to frequencies from 20 Hz to 180 kHz) and Avisoft Recorder software (sampling rate, 250 kHz; format, 16 bit) from Avisoft Bioacoustics. ACs, USCs, and clicks were elicited by applying a gentle pinch on the tails of the pups, and 15-s recordings were made after the mechanical stimulation. USCs and clicks were spontaneously produced when pups were isolated from the litter. Recordings (5-min) were taken with the pups in this isolation state. ACs, USCs, and clicks were quantified using the Automatic Parameter Measurement option from the Avisoft SASLab Pro software (resolution, 488 Hz, 1 ms; threshold, −50 dB). The spectrograms were visually inspected to validate the correct counts from the automatic option.
Histology, Cell Quantification, and Anatomic Connectivity.
Immunofluorescence and tissue processing were performed as described previously (48). The following antibodies were used: chicken anti–β-gal (Abcam), goat anti-ChAT (EMD Millipore), guinea pig anti-Fluorogold (Santa-Cruz Biotechnology), guinea pig anti-Foxd3 (14), rat anti-GFP (Nacalai Tesque), guinea pig anti-Islet1/2 (kindly provided by Thomas Jessell), rabbit anti-Nk1R (Sigma-Aldrich), guinea pig anti-Olig3 (44), rabbit anti-Phox2b (16), rabbit anti-RFP (Abcam), rabbit anti-somatostatin (EMD Millipore), sheep anti-TH (EMD Millipore), guinea pig anti-Tlx3 (14), guinea pig anti-vGluT2 (EMD Millipore), and Cy2-, Cy3-, and Cy5-conjugated secondary antibodies (Jackson ImmunoResearch).
For quantification of the total number of neurons in the NTS, AP, A1/C1, and A2/C2 of control, Olig3−/−, Txph-1, Txph-2, and Tlx3−/− mutant mice, 20-μm transverse sections encompassing the entire NTS were used (10–12 sections/animal). Lineage tracing of dA3 derivatives was done using Olig3creERT2/+;TaumGFP-nLacZ mice recombined at E9.5, E10.5, and E11.5.
Olig3creERT2/+;Phox2bFlpo/+;Ai65−/+ embryos were treated with tamoxifen at E10.5 and analyzed by immunohistochemistry at E19. AAV-2/1 containing a 1:1 ratio of type 1 and type 2 capsid proteins was prepared by transfection of human embryonic kidney (HEK-293) cells with the AAV backbone plasmids pAM-synaptophysin-GFP, AAV1 (pH21), AAV2 (pRV1), and AAV pFdelta6 helper plasmids (49). Virions were harvested, purified (using a 1-mL HiTrap heparin column; Sigma-Aldrich), and concentrated (using Amicon Ultra centrifugal filter devices; EMD Millipore). Then 100 nL of AAV-2/1 virions (109 IU) was injected into the caudomedial part of the NTS at P60 using a Nanoject II (Drummond) and a Kopf stereotaxic frame with the following coordinates (posterior, 7.48 mm; lateral, 0.2 mm; depth, 2.1 mm) relative to bregma.
Histological analysis of the animals was done 4 weeks after injection. To define NTS neurons projecting to the adult ventral spinal cord by retrograde tracing, 20 nL of 2% Fluorogold solution in PBS (Sigma-Aldrich) was injected into the ventral horn of the spinal cord at the L1, L3, and L5 levels at P60. The injection was carried out at a depth of 1.2 mm from the dorsal surface of the spinal cord. Each injection site was tested to ensure that Fluorogold did not spread to the dorsal spinal cord. Histological analysis of the animals was done at 4–5 d after the injections.
To localize laryngeal motor neurons in newborn mice, we stained consecutive transverse sections of the hindbrain with ChAT antibodies to visualize motor neurons and define the distinct compartments of the nucleus ambiguus: pars compacta, semicompact area, and loose formation. The anatomic region containing the transition between the semicompact area and loose formation of the nucleus ambiguus was observed to expand from 550 μm to 900 μm and from 900 μm to 1,200 μm after the end of the facial nucleus in newborn and adult mice, respectively. Laryngeal motor neurons locate to the transition zone between the semicompact area and loose formation (50), and we analyzed neurons from the semicompact area in this study (Fig. 4 and Fig. S5).
To localize spinal expiratory motor neurons, we injected CTB (recombinant; Alexa Fluor 594-conjugated) into the external and internal oblique abdominal muscles. After 4 d of incubation, spinal cords were transversely sectioned and counterstained with Neurotrace and antibodies against CTB and Islet1/2. Expiratory motor neurons were located in the lateral motor column at lumbar 1 level. We used these coordinates to identify expiratory motor neurons in newborn and adult mice (Fig. 4 and Figs. S5 and S6).
Plethysmography, Audio Recordings, and Electrophysiology.
To perform unrestrained whole-body plethysmography, we connected the plethysmograph chamber (20 mL), equipped with a LM335Z temperature sensor (Texas Instruments), to a reference chamber of the same volume. The pressure difference between the two chambers was measured with a differential pressure transducer (Validyne DP 103–14) connected to a sine wave carrier demodulator (Validyne CD15). The spirogram was acquired at a sampling frequency of 1 kHz and was stored on a computer using a LabMaster interface. Calibrations were recorded at the end of each recording session by injecting a defined volume of air (1–5 μL) into the chamber using a Hamilton syringe. Newborn mice were placed individually in the plethysmographic chamber, which was kept hermetically sealed and maintained at 31 °C during the recording session (5 min). To calculate breathing parameters (ventilatory volume/min; Ttot, which assesses the length of the breathing cycle; tidal volume, which assesses ventilatory volume per breath), recordings were analyzed with Elphy software (developed by G. Sadoc at CNRS).
To synchronize the acquisitions of plethysmographic and audio signals, a “send trigger” command is written into the Elphy program. This generates a 10-ms trigger pulse sent to both an analog input of the automated data collection system acquiring the plethysmographic signal and the Avisoft UltraSound Gate system acquiring the audio signal. Although synchronization of the two signals can be obtained within 1-ms accuracy, the clocks of the two systems have a ±50 ppm rating, which can result in deviations of ≥10 ms/min of recording. To take this drift into account, we gently knocked on the plethysmographic chamber with a metal rod before the end of the recording session to create synchronous signals of a pressure change and sound that were used after acquisition to adjust the timing for both signals. Repeated tests demonstrated that this procedure ensured that the latencies between the plethysmographic and the audio signals were <2 ms in a 10-min recording. The relevant Elphy configuration (.gfc) and program (.pg2) files are available at neuro-psi.cnrs.fr/IMG/ElphyConfigVocal/breath7-usb.gfc and neuro-psi.cnrs.fr/IMG/ElphyConfigVocal/breath7-usb.pg2.
Vocal breathing was quantified using simultaneous audio and breathing recordings. To determine the frequencies of vocal and basal breathing, we analyzed the following periods: breathing 10 s before a vocal event (USC production), breathing during the vocal event, and breathing 10 s after the vocal event. Vocal breaths in control mice were defined by the presence of three criteria: fast breathing episodes, active expiration, and USC production. In Olig3 and TxPhx-1 mice, fast breathing episodes were present, but the two other criteria (sound and active expiration) were absent. The incidence of fast breathing episodes was comparable to that observed in control mice. In Tlx3 and TxPhx-2 mice, the number of successful vocal expirations (all three criteria fulfilled) was rare. Fast breathing episodes with blunted deep expiration and without sound production were identifiable (“blunted” vocal breathing). The sum of blunted vocal breathing events plus successful vocal breathing events in such mutants was similar to the vocal breathing episodes in control mice.
Calibration of plethysmographic signals was done by injecting air into the plethysmograph, and the recorded signal was used to estimate pressure changes during breathing. Plethysmographic signals and volume of injected air (2.5–10 μL) were linearly related. To estimate pressures, we applied Boyle’s law: PV = k and P1V1 = P2V2, where P2 is the atmospheric pressure (100 kPa), V2 is the calibration volume (2.5 μL) applied to the chamber, and V1 is the volume of the plethysmographic chamber (20 μL). The calibration volume generated a pressure increase of 12.5 Pa, which evoked a 2,000-mV signal on the plethysmograph transducer. Signals measured during breathing were then used to calculate pressures during expiration.
Patch-clamp recordings were performed on brainstem transverse slices of P0 mouse pups. Progressing in caudal directions, 100-µm-thick sections were generated with a vibratome (Leica VT 1000S) until the caudal end of the facial motor nucleus was reached. At this point, a single 500- to 550-µm-thick slice was cut to set the anterior limit of the subsequent 450-µm-thick slice used for recording. This exposed the semicompact formation of the nucleus ambiguus that contains laryngeal motor neurons (50). Slices were transferred into a recording chamber mounted on a Nikon E-600-FN upright microscope equipped with a DIC illumination system and a CoolSNAP HQ2 CCD camera (Photometrics). The chamber was perfused at 2.5 mL/min with a Carbogen (95% O2/5% CO2) saturated artificial cerebrospinal fluid composed of 120 mM NaCl, 8 mM KCl, 1.26 mM CaCl2, 1.5 mM MgCl2, 21 mM NaHCO3, 0.58 mM Na2HPO4, and 30 mM glucose, pH 7.4. Whole-cell patch-clamp recordings were performed using a 4–6 MΩ pipette electrode pulled from borosilicate glass tubes (Clark GC 150TF) and filled with a solution containing 144 mM K-gluconate, 3 mM MgCl2, 10 mM Hepes, 0.5 mM EGTA, and 1 mg/mL biocytine (Molecular Probes) at pH 7.2. Electrophysiological signals were amplified using an Axopatch 700B amplifier, digitized on a Digidata 1322A and acquired by pClamp10 software (Molecular Devices). A concentric bipolar stimulating electrode (FHC Neural microTargeting Worldwide) was positioned onto the medial tractus solitarius, and single electrical stimulations (50 µs, 0.15 Hz) were delivered using intensities (range 0.2–1.0 mA) approximately double that of the threshold intensity eliciting a response. After the recordings, slices were fixed and immunostained for biocytin and ChAT to visualize the recorded motor neurons. Eleven control slices (n = 28 motor neurons) and three Olig3 mutant slices (n = 9 motor neurons) were recorded.
Optogenetic stimulation was done on E19 hindbrain-spinal cord preparations. The preparation was placed in prone position to expose the dorsal aspect of the hindbrain to enable photostimulation of the NTS by digital holography. Motor nerve recordings from C4, L1, and L5 motor roots were made using suction electrodes as described previously (51). After detection of spontaneous respiratory-like C4 motor bursts, bilateral light pulses (50–70 ms, 1–5 mW/mm2) on the NTS were triggered either manually or with a 4-s latency following onset of a spontaneous C4 burst detected by a threshold device. Raw signals from the motor nerves were amplified (high-gain AC 7P511; Grass Technologies), filtered (0.1–3 kHz), integrated (Neurolog system; Digitimer), digitized, and acquired using pClamp10 software (Molecular Devices).
Mother–Offspring Behavior.
Pregnant female mice were maintained on a 12-h light/dark cycle, housed individually from postcoitus until delivery, and provided with food and water ad libitum. The last cleaning of the cages was done 2 d before the expected delivery.
For the analysis of searching behavior, the mothers were briefly removed from the cage and placed in a separate cage to keep them from seeing the pups. Individual pups were covered with bedding material. Subsequently, mothers were returned to the cage and allowed to freely search for the pups. The test was terminated after the mother uncovered the pups or 30 min after the reintroduction of the mother. Pups that were not uncovered by the mother after 30 min were classified as not found.
For the analysis of retrieval behavior, pregnant females were observed to determine labor onset. Pups were monitored to assess whether they were licked and cleaned by the mother. At 10 min after the end of labor, all pups were removed from the cage, placed on a humid 37 °C plate for 30 min, and then placed back into the cage in the opposite corner of the nest. The mother approached the litter soon after, and the first olfactory exploration of the litter marked the beginning of the behavioral test. Mothers usually licked all pups but chose particular pups for retrieval into the nest. The test was terminated after the mother remained in the nest for 15 min, and all pups left outside were scored as ignored. Mothers did not attack or display other types of aggressive behavior toward ignored pups.
For the analysis of retrieval behavior from tamoxifen-treated animals, pups were isolated by cesarean section at E19. All pups were cleaned with moist, odorless tissue and placed on a humid 37 °C plate for 30 min before being transferred into a cage with a foster mother. Foster mothers of a CD1 genetic background that had delivered by natural labor and had remained with their pups were transferred to the new litter at 30 min before the start of behavioral tests. Foster mothers approached the litter shortly (1–5 min) after the pups were introduced into the cage and began to sniff all individuals of the litter. The subsequent test for retrieval was done as described above. Again, no attacks or aggressive behavior toward ignored pups was observed.
Statistics.
Statistical analyses were performed with GraphPad Prism 3 software. The statistical significance between group means was tested by either the two-tailed t test or one-way ANOVA followed by Bonferroni's post hoc test (for multiple comparisons). Significance was set with the following values: *P ≤ 0.01, **P ≤ 0.001, and ***P ≤ 0.0001.
Acknowledgments
We thank Christo Goridis (IBENS, Paris) and Thomas Müller, Russ Hodge, and Elijah Lowenstein (all Max Delbrueck Center, Berlin) for a critical reading of the manuscript, and Sven Buchert, Petra Stallerow, Claudia Päseler, and Sandra Autran for technical support. Funding for this work was provided by the European Commission (Marie Curie Fellowship 302477, to L.R.H.-M.), Deutsche Forschungsgemeinschaft (SFB 665), Excellenzcluster NeuroCure and Helmholtz Association (to C.B.), Agence Nationale pour la Recherche (10-BLAN1410-02, BSV5-001-02), Fondation pour la Recherche Médicale (DEQ20120323709, to G.F.), and the European Molecular Biology Organization (P.-L.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702893114/-/DCSupplemental.
References
- 1.Konopka G, Roberts TF. Insights into the neural and genetic basis of vocal communication. Cell. 2016;164:1269–1276. doi: 10.1016/j.cell.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehret G. Infant rodent ultrasounds: A gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- 3.Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Curr Opin Neurobiol. 2014;28:115–120. doi: 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 5.Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: The mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerschmidt K, Whelan G, Eichele G, Fischer J. Mice lacking the cerebral cortex develop normal song: Insights into the foundations of vocal learning. Sci Rep. 2015;5:8808. doi: 10.1038/srep08808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jürgens U. The neural control of vocalization in mammals: A review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amador A, Margoliash D. A mechanism for frequency modulation in songbirds shared with humans. J Neurosci. 2013;33:11136–11144. doi: 10.1523/JNEUROSCI.5906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amador A, Perl YS, Mindlin GB, Margoliash D. Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature. 2013;495:59–64. doi: 10.1038/nature11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011;106:2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: Patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiba K, Nakazawa K, Ono K, Umezaki T. Multifunctional laryngeal premotor neurons: Their activities during breathing, coughing, sneezing, and swallowing. J Neurosci. 2007;27:5156–5162. doi: 10.1523/JNEUROSCI.0001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storm R, et al. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136:295–305. doi: 10.1242/dev.027193. [DOI] [PubMed] [Google Scholar]
- 15.Qian Y, et al. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dauger S, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, et al. Control of precerebellar neuron development by Olig3 bHLH transcription factor. J Neurosci. 2008;28:10124–10133. doi: 10.1523/JNEUROSCI.3769-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieber MA, et al. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–4909. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang BJ, et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- 20.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Miranda LR, Müller T, Birchmeier C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Coppola E, et al. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proc Natl Acad Sci USA. 2010;107:2066–2071. doi: 10.1073/pnas.0910213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holstege G, Subramanian HH. Two different motor systems are needed to generate human speech. J Comp Neurol. 2016;524:1558–1577. doi: 10.1002/cne.23898. [DOI] [PubMed] [Google Scholar]
- 24.Madisen L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch MR, d’Autréaux F, Dymecki SM, Brunet JF, Goridis C. A Phox2b:FLPo transgenic mouse line suitable for intersectional genetics. Genesis. 2013;51:506–514. doi: 10.1002/dvg.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurons in vitro: Evidence for re-excitatory processing. Brain Res. 1993;630:125–135. doi: 10.1016/0006-8993(93)90650-c. [DOI] [PubMed] [Google Scholar]
- 28.Lüthe L, Häusler U, Jürgens U. Neuronal activity in the medulla oblongata during vocalization: A single-unit recording study in the squirrel monkey. Behav Brain Res. 2000;116:197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- 29.Blessing WW. The Lower Brainstem and Bodily Homeostasis. Oxford Univ Press; Oxford, UK: 1997. [Google Scholar]
- 30.Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson TM, et al. A novel excitatory network for the control of breathing. Nature. 2016;536:76–80. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis PJ, Zhang SP, Bandler R. Pulmonary and upper airway afferent influences on the motor pattern of vocalization evoked by excitation of the midbrain periaqueductal gray of the cat. Brain Res. 1993;607:61–80. doi: 10.1016/0006-8993(93)91490-j. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa K, et al. Role of pulmonary afferent inputs in vocal on-switch in the cat. Neurosci Res. 1997;29:49–54. doi: 10.1016/s0168-0102(97)00071-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol. 2014;4:287–324. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 37.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheiner E, Hammerschmidt K, Jürgens U, Zwirner P. The influence of hearing impairment on preverbal emotional vocalizations of infants. Folia Phoniatr Logop. 2004;56:27–40. doi: 10.1159/000075326. [DOI] [PubMed] [Google Scholar]
- 39.D’Amato FR, Populin R. Mother-offspring interaction and pup development in genetically deaf mice. Behav Genet. 1987;17:465–475. doi: 10.1007/BF01073113. [DOI] [PubMed] [Google Scholar]
- 40.Newbury DF, Monaco AP. Genetic advances in the study of speech and language disorders. Neuron. 2010;68:309–320. doi: 10.1016/j.neuron.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent RD. The MIT Encyclopedia of Communication Disorders. The MIT Press; Cambridge, MA: 2004. [Google Scholar]
- 42.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 44.Müller T, et al. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005;19:733–743. doi: 10.1101/gad.326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, et al. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernández-Miranda LR, et al. Robo1 regulates semaphorin signaling to guide the migration of cortical interneurons through the ventral forebrain. J Neurosci. 2011;31:6174–6187. doi: 10.1523/JNEUROSCI.5464-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClure C, Cole KL, Wulff P, Klugmann M, Murray AJ. Production and titering of recombinant adeno-associated viral vectors. J Vis Exp. 2011;57:e3348. doi: 10.3791/3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patrickson JW, Smith TE, Zhou SS. Motor neurons of the laryngeal nerves. Anat Rec. 1991;230:551–556. doi: 10.1002/ar.1092300415. [DOI] [PubMed] [Google Scholar]
- 51.Bouvier J, et al. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]