Significance
We describe a physiological role for uncoupling protein 1 (UCP1) in the regulation of reactive oxygen species. Notably, the molecular differences between brown fat mitochondria from wild-type and UCP1 knockout (UCP1-KO) mice extend substantially beyond the deletion of UCP1 itself. Thus, caution must be taken when attributing a brown fat phenotype solely to UCP1 deletion when these animals are used. Given the wide utilization of the UCP1-KO mouse model, these data are of critical importance for the scientific communities studying obesity, thermogenesis and energy metabolism, and mitochondrial biology.
Keywords: brown fat, mitochondria, ROS, UCP1, electron transport chain
Abstract
Brown adipose tissue (BAT) mitochondria exhibit high oxidative capacity and abundant expression of both electron transport chain components and uncoupling protein 1 (UCP1). UCP1 dissipates the mitochondrial proton motive force (Δp) generated by the respiratory chain and increases thermogenesis. Here we find that in mice genetically lacking UCP1, cold-induced activation of metabolism triggers innate immune signaling and markers of cell death in BAT. Moreover, global proteomic analysis reveals that this cascade induced by UCP1 deletion is associated with a dramatic reduction in electron transport chain abundance. UCP1-deficient BAT mitochondria exhibit reduced mitochondrial calcium buffering capacity and are highly sensitive to mitochondrial permeability transition induced by reactive oxygen species (ROS) and calcium overload. This dysfunction depends on ROS production by reverse electron transport through mitochondrial complex I, and can be rescued by inhibition of electron transfer through complex I or pharmacologic depletion of ROS levels. Our findings indicate that the interscapular BAT of Ucp1 knockout mice exhibits mitochondrial disruptions that extend well beyond the deletion of UCP1 itself. This finding should be carefully considered when using this mouse model to examine the role of UCP1 in physiology.
Uncoupling protein 1 (UCP1) plays a role in acute adaptive thermogenesis in interscapular brown adipose tissue (BAT). UCP1 dissipates the mitochondrial protonmotive force (Δp) generated by the electron transport chain (ETC) and is important for thermal homeostasis in rodents and human infants (1, 2). Ucp1 orthologs are not limited to mammals, but are also expressed in ectothermic vertebrates (3) and protoendothermic mammals (4), suggesting that UCP1 may have an important role in biology beyond thermal control. For example, it is becoming increasingly evident that in specific respiratory states, UCP1 can reduce reactive oxygen species (ROS) levels in vitro (4–9). The mitochondrial ETC is a major source of ROS production in the cell, and ROS play important roles in physiology and pathophysiology (10–12). Reverse electron transport (RET) through mitochondrial complex I is a key mechanism by which ROS are generated in vivo (11, 13). Interestingly, RET relies critically on high Δp, whereas dissipation of Δp by UCP1 can lower ROS levels in isolated mitochondria (5–7).
Thermogenic respiration in BAT is triggered by external stimuli that activate adrenergic signaling (14). Most notably, environmental cold induces the capacity for adrenergic-mediated BAT respiration in wild type (WT) animals, but only minimally in UCP1-KO animals (15, 16). It is understood that the respiratory response of BAT under these conditions is indicative of UCP1-mediated respiration; however, the rate of maximal chemically uncoupled oxygen consumption, an UCP1-independent parameter, is also lower in UCP1-KO adipocytes compared with WT (15, 16). Moreover, the basal respiratory rate of UCP1-KO BAT mitochondria is reduced after cold exposure, whereas it is increased in WT BAT mitochondria (7). These data strongly suggest broader functional changes to brown adipocyte mitochondrial function on increased adrenergic tone following Ucp1 deletion.
Here we demonstrate that extensive down-regulation of ETC abundance and concomitant triggering of host defense signaling occurs in BAT of UCP1-KO mice following cold acclimation. Remarkably, UCP1-KO BAT mitochondria are highly sensitive to calcium overload-induced mitochondrial dysfunction, which can be inhibited by reducing ROS levels. These findings suggest a critical physiological role of UCP1 in maintaining a mitochondrial environment that can mitigate ROS-dependent dysfunction in vivo. In addition, these data demonstrate that the absence of UCP1 in BAT results in widespread mitochondrial proteomic alterations that should be considered when using this mouse model to examine the role of UCP1 in physiology.
Results
Aberrant Cristae Morphology and Reduced ETC Abundance in UCP1-KO BAT.
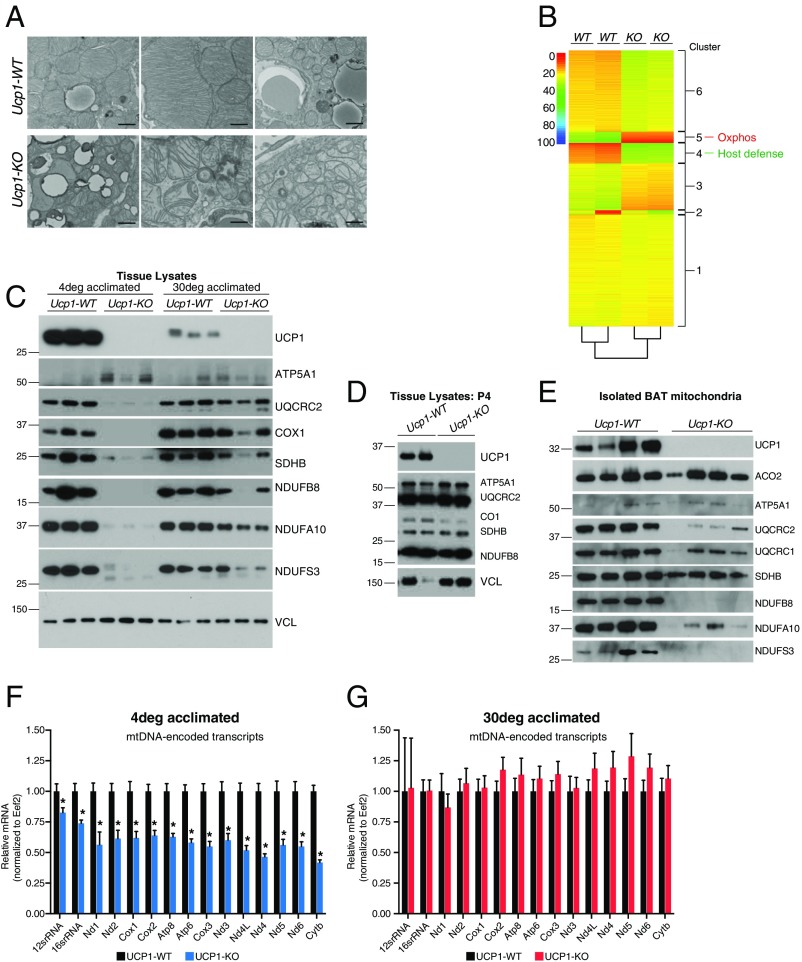
Environmental cold increases state 4 respiration in mitochondria isolated from BAT of WT mice, and substantially reduces it in BAT mitochondria from UCP1-KO mice (7, 17). These previous studies have suggested that ETC expression is impaired after adrenergic stimulation of BAT in the absence of UCP1. To investigate the role of UCP1 on BAT mitochondria on adrenergic stimulation, we gradually acclimated WT and UCP1-KO mice to cold (4 °C) and assessed gross mitochondrial morphology by electron microscopy. Remarkably, this analysis revealed highly disorganized and in some cases absent cristae from UCP1-KO organelles relative to WT organelles (Fig. 1A). This aberrant morphology prompted us to examine the molecular components of BAT mitochondria that might be affected by the absence of UCP1 under conditions of adrenergic stimulation.
Fig. 1.
Mitochondrial morphology and protein expression in BAT of WT and UCP1-KO animals. (A) Electron microscopy images of WT and UCP1-KO BAT following 3 wk cold exposure of mice. (B) Heatmap of BAT proteomics data (from Dataset S1). (C) Western blot of mitochondrial proteins from WT and UCP1-KO BAT after housing adult animals at 4 °C or 30 °C. (D) Western blot of mitochondrial proteins from WT and UCP1-KO BAT from postnatal day 4 pups. (E) Western blot of mitochondrial proteins from WT and UCP1-KO BAT mitochondria. (F) qRT-PCR of mtDNA-encoded transcripts in BAT from WT and UCP1-KO mice housed at 4 °C; n = 5 mice per genotype. (G) qRT-PCR of mtDNA-encoded transcripts in BAT from WT and UCP1-KO mice housed at 30 °C; n = 5 mice per genotype. Data are presented as mean ± SEM. *P < 0.05; ***P < 0.01.
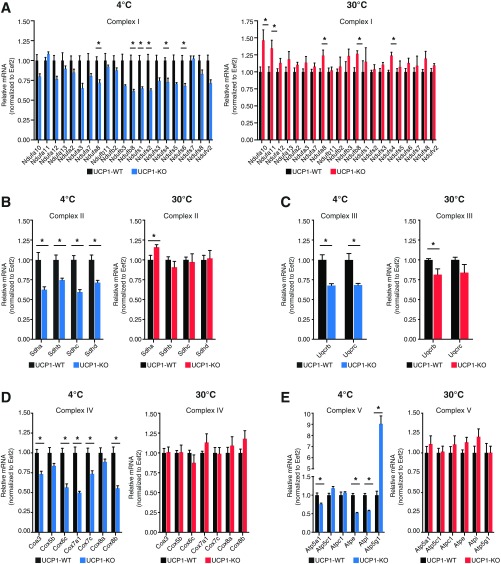
To determine the mitochondrial molecular response in WT and UCP1-KO BAT after cold exposure, we began by measuring the mRNA abundance of nuclear-encoded subunits of the oxidative phosphorylation complexes. A subset of transcripts was significantly reduced in response to environmental cold in a UCP1-dependent manner; however, the extent of reduction was modest (Fig. S1). In contrast, no differences in mRNA abundance in BAT were detected between UCP1-KO and WT mice under thermoneutral (30 °C) housing (Fig. S1). This was a general feature for nuclear-encoded genes of all the oxidative phosphorylation complexes.
Fig. S1.
The role of ROS in mitochondrial calcium buffering capacity and membrane potential in the BAT of BATI4KO mice. (A) qRT-PCR of Complex I gene expression in BAT from WT and UCP1-KO mice housed at 4 °C or 30 °C; n = 5 mice per genotype. (B) qRT-PCR of Complex II gene expression in BAT from WT and UCP1-KO mice housed at 4 °C or 30 °C; n = 5 mice per genotype. (C) qRT-PCR of Complex III gene expression in BAT from WT and UCP1-KO mice housed at 4 °C or 30 °C; n = 5 mice per genotype. (D) qRT-PCR of Complex IV gene expression in BAT from WT and UCP1-KO mice housed at 4 °C or 30 °C; n = 5 mice per genotype. (E) qRT-PCR of ATP Synthase gene expression in BAT from WT and UCP1-KO mice housed at 4 °C or 30 °C; n = 5 mice per genotype. Data are presented as mean ± SEM. *P < 0.05; ***P < 0.01.
The mild diminution of nuclear-encoded oxidative phosphorylation complexes (Fig. S1) prompted us to investigate their abundance at the protein level. To do so, we performed global quantitative proteomics in BAT of cold-acclimated WT and UCP1-KO mice using isobaric tagging (18), which provided quantitation of 6,354 proteins (Dataset S1). Unsupervised k-means clustering of the proteomic data revealed a total of six clusters (Datasets S2–S7), two of which (clusters 4 and 5) were robustly distinct between WT and UCP1-KO BAT (Fig. 1B). The top KEGG pathway of cluster 5 was designated as “oxidative phosphorylation,” consisting primarily of ETC proteins (Dataset S6). The protein abundance in this cluster was substantially reduced in UCP1-KO relative to WT BAT (Fig. 1B and Dataset S1). The percentage reduction (>80%) at the protein level (Dataset S1) was discordant with the relatively mild decrease in corresponding transcript abundance (Fig. S1). Most subunits of complexes I and IV were down-regulated (up to 95%) compared with BAT from WT mice (Dataset S1).
We next examined protein abundance by Western blot analysis in a separate cohort of mice housed at 4 °C or 30 °C. As in the MS analysis, ETC subunits were considerably reduced in UCP1-KO BAT tissue lysates, primarily on 4 °C exposure. Interestingly, these reductions in ETC abundance were not nearly as drastic in adult animals housed at 30 °C (Fig. 1C). Notably, there were no differences in ETC protein expression in young (postnatal day 4) pups (Fig. 1D), suggesting that the impaired ETC expression seen at older ages was primarily a response to environmental cold specifically and not due to aberrant BAT development. We next examined the expression of these proteins after mitochondrial purification. Strikingly, the depletion in ETC proteins was also observed in isolated organelles (Fig. 1E), demonstrating that the decrease in protein expression was not due solely to a reduction in mitochondrial mass. Interestingly, the succinate dehydrogenase complex iron sulfur subunit B of complex II showed minimal differences when we controlled for total mitochondrial abundance (Fig. 1E). Because succinate dehydrogenase is the only ETC complex that is entirely nuclear-encoded, these data suggest a specific effect on ETC complexes containing gene products encoded by mitochondrial DNA (mtDNA) owing to the absence of UCP1.
Environmental Cold Triggers Loss of mtDNA Transcripts in UCP1-KO BAT.
mtDNA is physically associated with the mitochondrial inner membrane in DNA-protein complexes known as nucleoids (19, 20), and a disturbance in this association is known to disrupt mtDNA-encoded gene expression. Thus, given the aberrant cristae morphology of UCP1-KO BAT mitochondria after exposure to 4 °C (Fig. 1A), along with the specific reduction in ETC complexes containing mtDNA-encoded gene products (Fig. 1E), we examined the expression level of mtDNA-encoded transcripts. Strikingly, all mtDNA-encoded transcripts, as well as the two mitochondrial rRNA transcripts, were significantly reduced in BAT of UCP1-KO mice after exposure to environmental cold (Fig. 1F). In contrast, when mice were housed at 30 °C, no change in mRNA abundance between the genotypes was detected (Fig. 1G).
Environmental Cold Triggers Host Defenses in UCP1-KO BAT.
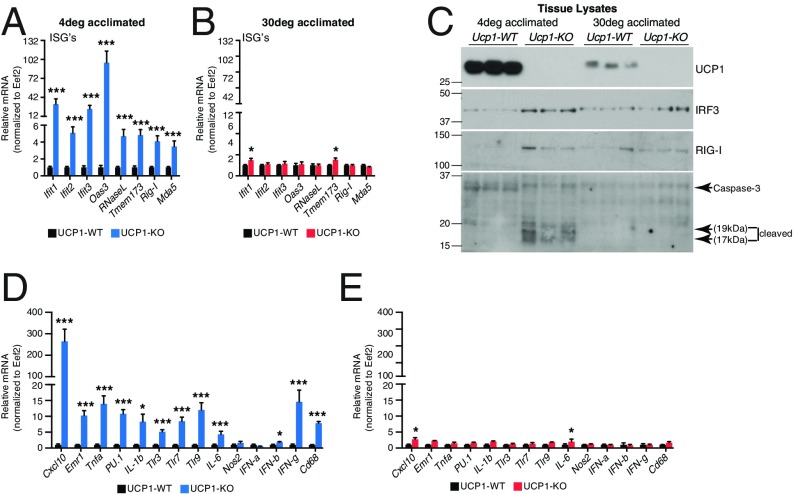
In contrast to the large reduction in ETC subunits, quantitative proteomic profiling of BAT from WT and UCP1-KO mice revealed an additional cluster (cluster 4), highlighting increased levels of proteins involved in host defense (Fig. 1B and Dataset S5). Included in this cluster was an increase in IFN-stimulated genes (ISGs) and antiviral factors encoding DNA and RNA sensors, such as Ifi204, Ifi205, Ifih1, and Ddx58, as well as the transcription factors Stat1, Stat2, and Irf3 (Dataset S1).
Because these factors function to reinforce the innate and adaptive immune response (21), we next examined the control of ISG expression by ambient temperature. ISGs (Ifit1, Ifit2, Ifit3, Oas3, and RNase L) were significantly elevated at the mRNA level in BAT of 4 °C-exposed UCP1-KO mice relative to WT controls (Fig. 2A); however, these changes were largely abolished when mice were housed at 30 °C (Fig. 2B). We found elevated levels of the nucleic acid-sensing factor RIG-I and the antiviral transcription factor IRF3 in UCP1-KO mice compared with WT mice housed at 4 °C, whereas housing at 30 °C abrogated the difference between the genotypes (Fig. 2C and Dataset S1). IRF3 represses thermogenic gene expression (22). Interestingly, the thermogenic transcription factor Prdm16 suppresses ISG expression (23). Our data extend these findings by demonstrating that the capacity for thermogenic respiration itself may regulate ISG abundance. This result highlights the interconnected relationship between the thermogenic and host defense transcriptional machinery and the effectors of thermogenic respiration. Furthermore, in line with the higher levels of ISG expression, cleaved caspase-3 levels were elevated in UCP1-KO BAT after exposure to 4 °C, but not when mice were housed at 30 °C (Fig. 2C). Caspase activation dampens the immune response and triggers mitochondrial-mediated programmed cell death (24).
Fig. 2.
Expression of genes and proteins involved in host defense in BAT of WT and UCP1-KO animals. (A) qRT-PCR of ISG expression in BAT from WT and UCP1-KO mice housed at 4 °C; n = 5 mice per genotype. (B) qRT-PCR of ISG expression in BAT from WT and UCP1-KO mice housed at 30 °C; n = 5 mice per genotype. (C) Western blot of antiviral and cell death proteins in BAT from WT and UCP1-KO animals after exposure to 4 °C or 30 °C. (D) qRT-PCR of inflammatory gene expression in BAT from WT and UCP1-KO mice housed at 4 °C; n = 5 mice per genotype. (E) qRT-PCR of inflammatory gene expression in BAT from WT and UCP1-KO mice housed at 30 °C; n = 5 mice per genotype. Data are presented as mean ± SEM. *P < 0.05; ***P < 0.01.
To explore whether activation of the immune response is regulated by ambient temperature, we examined a panel of inflammatory genes from BAT of WT and UCP1-KO mice housed under cold or thermoneutral conditions by quantitative RT-PCR (qRT-PCR). We detected robust elevation of inflammatory genes, including those associated with innate immunity, IFN-γ signaling, and macrophage infiltration, in the UCP1-KO mice housed at 4 °C (Fig. 2D). Remarkably, housing mice at 30 °C largely abolished this inflammatory response (Fig. 2E). Taken together, the foregoing data demonstrate that environmental cold triggers mitochondrial dysfunction and antiviral signaling in UCP1-deficient BAT. The data suggest that on cold stimulation, absence of Ucp1 results in aberrant BAT mitochondrial function, leading to mitochondrial-mediated triggering of immune activation.
Calcium Overload Induces Mitochondrial Dysfunction in UCP1-KO BAT.
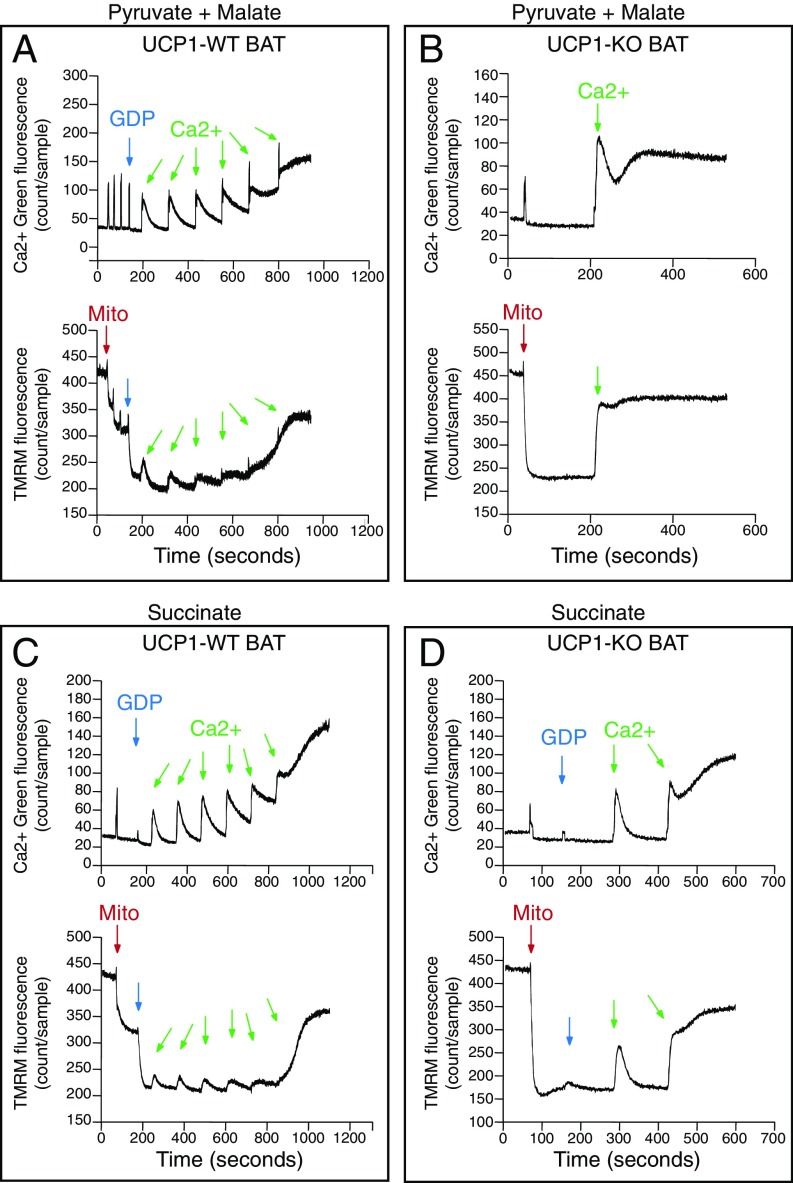
Cold stimulation drives the activation of innate immune pathways linked to cell death. Because mitochondrial permeability transition is a well-established regulator of this process (25), we examined whether UCP1-KO BAT mitochondria are inherently sensitive to permeability transition by challenging organelles with calcium. We simultaneously measured the mitochondrial membrane potential (Δψ) and calcium-buffering capacity in BAT mitochondria isolated from WT and UCP1-KO mice. WT BAT mitochondria respiring on pyruvate and malate exhibited a modest Δψ, indicated by the downward inflection in the tetramethylrhodamine, methyl ester (TMRM) signal on addition of the organelles. This was increased after GDP administration, demonstrating inhibition of UCP1-dependent leak (Fig. 3A, Bottom).
Fig. 3.
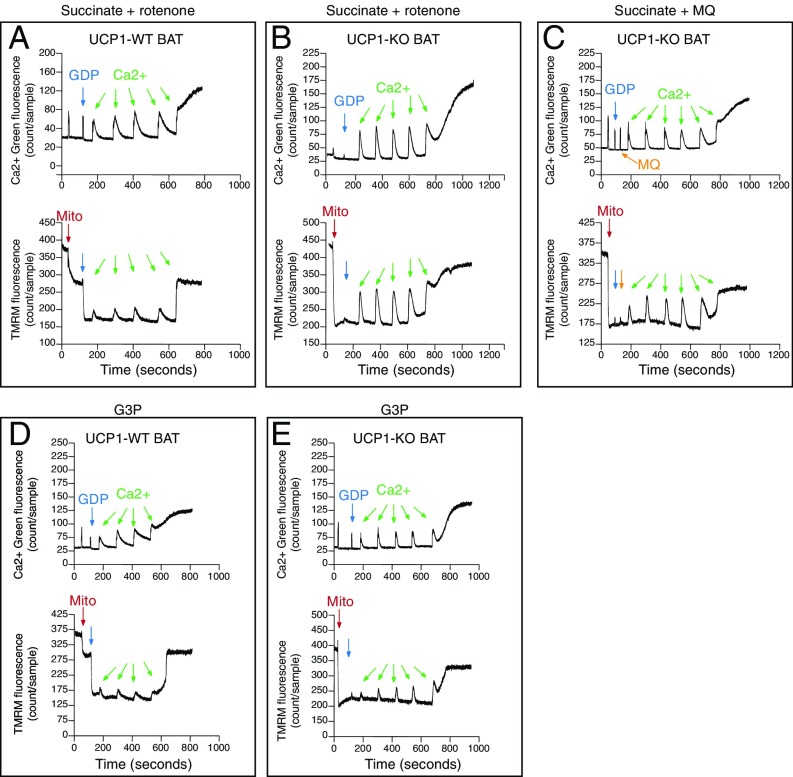
Simultaneous monitoring of mitochondrial calcium buffering capacity and membrane potential. (A) Calcium (Ca2+) and TMRM monitoring of WT BAT mitochondria respiring on pyruvate/malate. (B) Ca2+ and TMRM monitoring of UCP1-KO BAT mitochondria respiring on pyruvate/malate without GDP. (C) Ca2+ and TMRM monitoring of WT BAT mitochondria respiring on succinate. (D) Ca2+ and TMRM monitoring of UCP1-KO BAT mitochondria respiring on succinate. Images shown are representative of at least three independent mitochondrial preparations for each substrate used. Arrows indicate the addition of mitochondria (red), GDP (blue), and calcium (green).
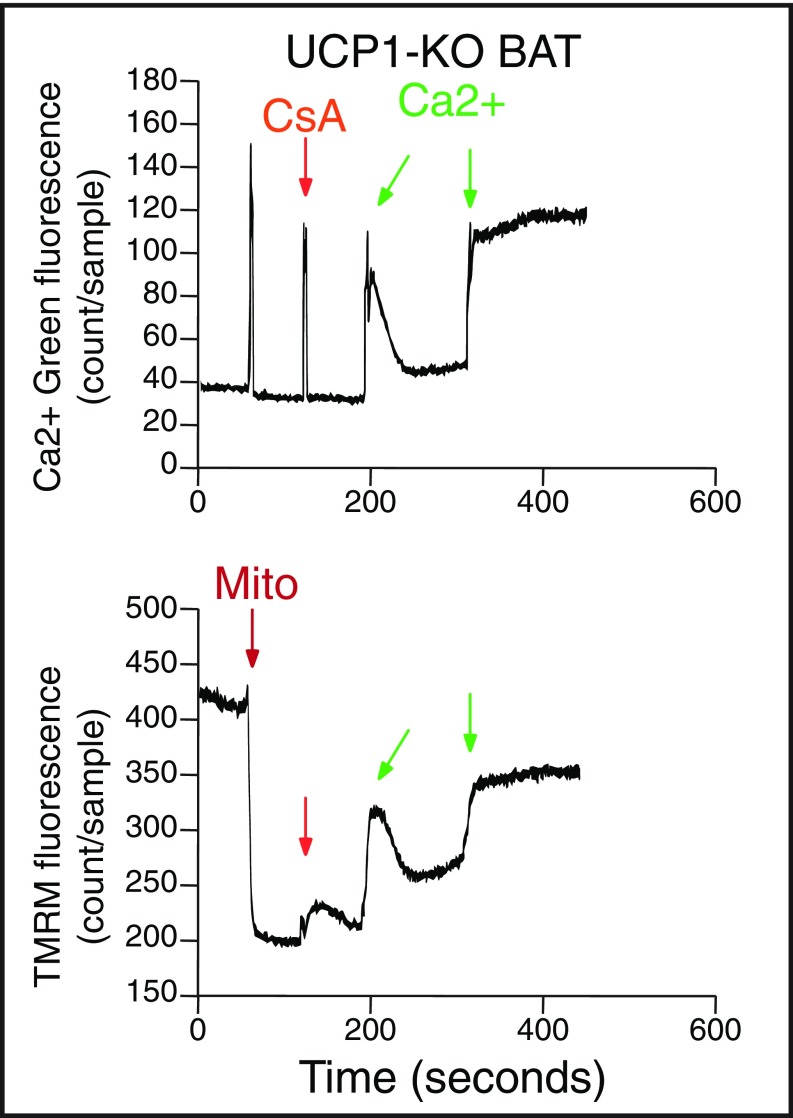
We next treated mitochondria with sequential additions of calcium (26). Each calcium addition could be observed as an increase in Calcium Green fluorescence in the buffer, whereas uptake into the organelles was reflected in a reduction in buffer calcium (Fig. 3A, Top). Mitochondrial membrane depolarization occurred concomitantly with each addition of calcium (60 nmol calcium/mg mitochondrial protein), followed by rapid repolarization (Fig. 3A, Bottom). Here 300 nmol calcium/mg mitochondrial protein could be buffered by WT BAT mitochondria, whereas calcium release occurred thereafter (Fig. 3A, Top). Depolarization of Δψ occurred concomitantly with calcium release. UCP1-KO BAT mitochondria exhibited a similar baseline Δψ as GDP-treated WT mitochondria (Fig. 3B, Bottom); however, the addition of calcium to UCP1-KO organelles resulted in a significant depolarization of Δψ (Fig. 3B, Bottom). Furthermore, a single calcium addition (60 nmol calcium/mg mitochondrial protein) was poorly buffered by UCP1-KO mitochondria and was sufficient to result in concomitant calcium release and depolarization (Fig. 3B). These data indicate that UCP1-KO BAT mitochondria have substantially reduced calcium-buffering capacity compared with WT organelles. Permeability transition can be inhibited by the drug cyclosporin A (27), and we detected a modest protective effect of this compound on UCP1-KO mitochondrial calcium buffering capacity (Fig. S2), which is consistent with previous findings (28). Taken together, these data demonstrate that UCP1-KO BAT mitochondria are sensitized to calcium overload-induced mitochondrial dysfunction, a component of which is partly inhibited by cyclosporin A.
Fig. S2.
The role of cyclosporine A in UCP1-KO BAT mitochondrial calcium buffering capacity. Ca2+ and TMRM monitoring of UCP1-KO BAT mitochondria respiring on pyruvate/malate.
We next examined calcium kinetics and Δψ during succinate-dependent respiration. WT BAT mitochondria respiring on succinate responded similarly as when pyruvate and malate were used to drive respiration through complex I. A total of 300 nmol calcium/mg mitochondrial protein was efficiently buffered by WT BAT mitochondria (Fig. 3C). In contrast, UCP1-deficient BAT mitochondria could buffer only 60 nmol calcium/mg mitochondrial protein (Fig. 3D). Therefore, UCP1 deficiency results in a profound sensitization to calcium-overload induced dysfunction in mitochondria respiring on succinate.
ROS Triggers Calcium Overload-Induced Mitochondrial Dysfunction in UCP1-KO BAT.
Succinate oxidation can drive RET, and so can be used to investigate complex I-dependent ROS (11, 29–31). Complex I is the dominant site of superoxide production when mitochondria respire on succinate (29, 32). Under conditions of high membrane potential, succinate reduces ubiquinone (Q) to ubiquinol (QH2), resulting in a reverse electron flow through complex I (11, 30). Under these circumstances, single electron reduction of oxygen can generate substantial levels of superoxide, and this has been proposed to occur at the FMN site of complex I (32, 33) or, alternatively, the IQ site (30). The complex I Q site inhibitor rotenone inhibits RET-driven superoxide production during succinate oxidation (29, 34–37). Critically, mitochondrial ROS has been demonstrated to potentiate calcium-dependent permeability transition (25, 38). Moreover, succinate has been shown to generate significant amounts of ROS in BAT, which is exacerbated in the absence of UCP1 (7, 8). Thus, we evaluated the contribution of ROS to the observed calcium sensitivity of UCP1-KO BAT mitochondria. Inhibition of RET with rotenone had no effect on the calcium-buffering properties of WT BAT mitochondria (Fig. 4A); however, inhibition of RET rescued the calcium-induced dysfunction of UCP1-KO BAT mitochondria (Fig. 4B, Top). Although each addition of calcium still caused a large transient depolarization and slow repolarization (Fig. 4B, Bottom), the amount of calcium required to drive complete membrane depolarization was completely normalized. Therefore, inhibiting RET through complex I essentially restored the calcium retention time and buffering capacity of UCP1-KO BAT mitochondria to WT levels.
Fig. 4.
The role of ROS in mitochondrial calcium buffering capacity and membrane potential. (A) Ca2+ and TMRM monitoring of WT BAT mitochondria respiring on succinate/rotenone. (B) Ca2+ and TMRM monitoring of UCP1-KO BAT mitochondria respiring on succinate/rotenone. (C) Ca2+ and TMRM monitoring of UCP-KO BAT mitochondria respiring on succinate in the presence of 100 nM MitoQ. (D) Ca2+ and TMRM monitoring of WT BAT mitochondria respiring on G3P. (E) Ca2+ and TMRM monitoring of UCP1-KO BAT mitochondria respiring on G3P. Images shown are representative of at least three independent mitochondrial preparations for each substrate used. Arrows indicate the addition of mitochondria (red), GDP (blue), calcium (green), or MitoQ (yellow).
To further explore whether elevated ROS levels are responsible for the calcium-induced dysfunction, we treated UCP1-KO BAT mitochondria with the mitochondria-targeted antioxidant MitoQ (39) just before the calcium challenge. Like rotenone, MitoQ rescued the calcium buffering capacity of UCP1-KO organelles (Fig. 4C). The large depolarization and slow repolarization of Δψ (Fig. 4C, Bottom) are consistent with abrogated ETC expression. Collectively, these results suggest that UCP1-KO BAT mitochondria are extraordinarily sensitive to ROS-dependent permeability transition. Interestingly, we did not observe large differences between genotypes when G3P was used as a respiratory substrate (Fig. 4 D and E).
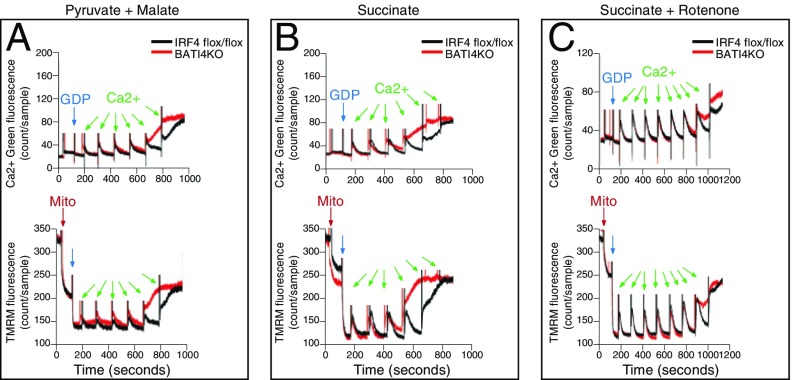
Finally, to examine whether the calcium-induced sensitivity of UCP1-KO BAT mitochondria is a feature of these organelles specifically or a feature of UCP1 deficiency generally, we examined the calcium-buffering capacity and Δψ in mitochondria from BAT-specific IRF4 KO mice (BATI4KO) that exhibit reduced UCP1 levels in BAT (40). Similar to what we observed in UCP1-KO BAT mitochondria, organelles from BATI4KO mice demonstrated increased sensitivity to calcium overload-induced permeability transition when respiring on pyruvate/malate (Fig. 5A) or succinate (Fig. 5B). Although the sensitivity to calcium overload of BAT14KO mitochondria was not as striking as that of UCP1-KO BAT mitochondria, it still could be partially rescued by rotenone treatment (Fig. 5C). Because BAT mitochondria from BATI4KO mice contain lower levels of UCP1 and complex I protein (40), these results suggest that calcium overload-induced mitochondrial dysfunction may be a general phenomenon of BAT mitochondria under conditions with reduced UCP1 expression.
Fig. 5.
The role of ROS in mitochondrial calcium buffering capacity and membrane potential in BAT of BATI4KO mice. (A) Ca2+ and TMRM monitoring of WT and BATI4KO BAT mitochondria respiring on pyruvate/malate. (B) Ca2+ and TMRM monitoring of WT and BATI4KO BAT mitochondria respiring on succinate. (C) Ca2+ and TMRM monitoring of WT and BATI4KO BAT mitochondria respiring on succinate/rotenone.
Discussion
UCP1 is a key feature of thermogenic fat cells, both brown and beige. We have demonstrated here that on cold exposure, interscapular BAT of UCP1-KO mice exhibits global mitochondrial disruptions that extend well beyond the deletion of UCP1 itself. Our data reveal physiological interactions between UCP1 and ROS. The role of UCP1 itself in the regulation of ROS production is incompletely understood. Evidence in support of a robust role for UCP1-mediated uncoupling in the regulation of ROS production in vitro has been provided (6, 7, 41), as have findings suggesting a limited role for UCP1 activity in controlling ROS in vitro (8, 42–44). Importantly, UCP1 appears to play a role in regulating BAT redox tone in vivo (9), and acute adrenergic stimulation in vivo drives ROS production to support UCP1-dependent thermogenesis (10).
Our findings demonstrate that UCP1-deficient BAT mitochondria are poorly equipped to buffer calcium in a ROS-dependent manner. Most importantly, we have shown that the acquired molecular and functional differences between BAT mitochondria from WT and UCP1-KO mice are more widespread than the deletion of UCP1 itself. Considering the striking alterations to the BAT mitochondrial proteome (i.e., substantial reduction of ETC abundance) in UCP1-KO mice, caution must be observed when attributing a BAT phenotype solely to UCP1 deletion in these animals. In addition, reduced ETC expression may be commonly associated with decreased UCP1 levels more generally, which should be kept in mind when studying genetic models with reduced BAT UCP1 expression. Notably, our findings reported here suggest that the reduced capacity of UCP1-KO BAT to activate oxidative metabolism after adrenergic administration (cold or chemical) is due at least in part to reduced expression of the ETC, and not solely to lack of UCP1-mediated uncoupling.
Examination of calcium sensitivity of BAT mitochondria with and without UCP1 adds further evidence supporting the relevance of the mechanisms of ROS production in BAT. Previous studies comparing ROS production between WT and UCP1-KO BAT mitochondria when using G3P as a respiratory substrate have indicated either comparable (8) or enhanced (6, 7) levels. Importantly, G3P-mediated mitochondrial energization can drive ROS production by RET or from mitochondrial G3P dehydrogenase (GPD2) itself (7, 30). Moreover, GPD2 appears to have the capacity to produce ROS in the mitochondrial intermembrane space (30, 41, 45), as opposed to complex I, which produces superoxide in the mitochondrial matrix (12). This compartmentalization of ROS production is a plausible explanation for the sensitivity of UCP1-KO mitochondria to succinate-mediated ROS production, which drives superoxide production principally through complex I (12). Because G3P-mediated ROS production can drive ROS independently of complex 1 (i.e., at GPD2 itself) (7, 30, 41), our data suggest that complex I-mediated ROS production by RET is a major contributor to mitochondrial dysfunction in UCP1-KO BAT. This interpretation is in line with the recognized importance of ROS originating in the mitochondrial matrix supporting permeability transition (46, 47). Interestingly, GPD2 abundance was unaltered in UCP1-KO BAT (Dataset S1), suggesting that in the absence of UCP1, G3P-mediated electron flux to coenzyme Q is maintained. Previous investigations have noted quantitatively different effects of G3P-driven ROS production in BAT mitochondria between WT and UCP1-KO animals (6–8). Considering our findings, these discrepancies may be predictive of differential mitochondrial adaptation in different UCP1-KO mice colonies to mitigate ROS sensitivity owing to a genetic absence of UCP1. Such differences might be expected to arise on congenic (i.e., C57BL/6J and 129/SvImJ) backgrounds, which are particularly sensitive to the ablation of UCP1 (48) and thus may be prone to selection against enhanced ROS production, depending on breeding strategy. More generally, the functional effects that arise from distinct ROS sites in BAT on thermogenesis is an interesting avenue for future research.
The data presented herein indicate that mice genetically lacking UCP1 exhibit a plethora of acquired features that extend substantially beyond the deletion of UCP1 itself. These defects, such as the striking reduction of mitochondrial ETC components, should be considered when using this mouse model to study UCP1 function. Nonetheless, this model may have utility for examining the general features of mitochondrial dysfunction; for example, the molecular processes regulating the discordance between ETC protein and mRNA abundance in cold-exposed UCP1-KO animals may be an appropriate model for studying the fundamental mechanisms of mitochondrial proteostasis.
Materials and Methods
Animals.
Mice were housed at 23 °C under a 12-h light/dark cycle with free access to food and water. All experiments used matched littermates. UCP1-KO mice and littermate UCP1-WT controls were generated by breeding heterozygous male and female (B6.129-Ucp1tm1Kz/J) mice as described previously (49). All animal experiments were performed in accordance with procedures approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Mitochondrial Purification.
BAT mitochondria were isolated as reported previously (17).
Measurement of Mitochondrial Ca2+ Uptake Capacity and Membrane Potential.
The measurement of these parameters was performed simultaneously on a multichannel dye fluorimeter (C&L Instruments). The mitochondrial membrane potential (ΔΨ) was estimated by measuring changes in the fluorescence intensity of TMRM (60 nM; Molecular Probes) at excitation and emission wavelengths of 543 and 590 nm, respectively. Mitochondrial Ca2+ fluxes were measured as changes in extramitochondrial [Ca2+], which were followed by monitoring the fluorescence intensity of Calcium Green-5N (125 nM; Molecular Probes) at excitation and emission wavelengths of 482 and 535 nm, respectively. Mitochondria were challenged with single or multiple Ca2+ additions. Mitochondrial calcium retention capacity was determined as the amount of Ca2+ sequestered by mitochondria without incurring depolarization. Fluorimeter-based data were analyzed using Origin 8.0 (OriginLab).
Succinate + MitoQ.
Mitochondria were added to buffer containing succinate (5 mM), followed by the addition of MitoQ (100 nM) and then GDP (1 mM). The mitochondria were challenged with calcium (15 µM) every 2 min until depolarization occurred.
Brown Fat Mitochondrial Calcium Uptake and Membrane Potential.
Mitochondrial calcium uptake and membrane potential were monitored using a fluorescent spectrophotometer (C&L Instruments). Brown fat mitochondria were added to buffer (62.5 mM KCl, 5 mM Hepes pH 7.4, 0.1% BSA, 6 µM EDTA, 2 mM KH2PO4, 60 nM Calcium Green, and 1 µM TMRM). Mitochondria were respired off pyruvate and malate (5 mM each), succinate (5 mM) with or without rotenone (1 µM), or glycerol phosphate disodium salt hydrate (containing 50% β-isomer and 50% α-isomer) (5 mM) with or without rotenone. Next, GDP (1 mM) was added, and mitochondria were challenged with calcium (60 nmol calcium/mg mitochondrial protein) every 2 min until depolarization occurred.
Statistical Analysis.
Data are presented as mean ± SEM. The unpaired two-tailed Student’s t test and two-way ANOVA were used to determine statistical differences, with P < 0.05 considered to indicate statistical significance. Additional materials and methods can be found in SI Materials and Methods.
SI Materials and Methods
Gene Expression Analysis (qRT-PCR).
Total RNA was extracted from frozen tissue using TRIzol (Invitrogen), purified with Qiagen RNeasy Mini spin columns, and reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resultant cDNA was analyzed by qRT-PCR. In brief, 20 ng of cDNA and 150 nmol of each primer were mixed with SYBR GreenER qPCR SuperMix (Applied Biosystems). Reactions were performed in a 384-well format using an ABI PRISM 7900HT Real-Time PCR System (Applied Biosystems). Relative mRNA levels were calculated using the comparative CT method and normalized to Eef2 mRNA. A complete list of primers and sequences is provided in Dataset S8.
Western Blot Analysis.
Samples were prepared in Lysis Buffer (50 mM Tris pH 7.4, 500 mM NaCl, 1% Nonidet P-40, 20% glycerol, 5 mM EDTA, and 1 mM PMSF, supplemented with a mixture of Roche protease inhibitors). The homogenates were centrifuged at 16,000 × g for 10 min at 4 °C, and the supernatants were used for subsequent analyses. Protein concentration was determined using the bicinchoninic acid assay (Pierce). The quantity of protein lysate to use for each antibody was determined empirically. Protein lysates were denatured in Laemmli Buffer (60 mM Tris pH 6.8, 2% SDS, 10% glycerol, 0.05% bromophenol blue, and 0.7 M β-mercaptoethanol), resolved by 4–12% NuPAGE Bis-Tris SDS/PAGE (Invitrogen), and transferred to a PVDF membrane. Primary antibodies were diluted in TBS containing 0.05% Tween (TBS-T), 5% BSA, and 0.02% NaN3. Membranes were incubated overnight with primary antibodies at 4 °C. For secondary antibody incubation, anti-rabbit or anti-mouse HRP (Promega) was diluted in TBS-T containing 5% milk. Results were visualized with enhanced chemiluminescence Western blotting substrates (Pierce).
Antibodies.
The following commercially available antibodies were used for Western blotting: NDUFA10 (Santa Cruz Biotechnology; sc-376357), NDUFS3 (Abcam; ab110246), total OXPHOS (Abcam; ab110413), UCP1 (Abcam; ab10983), ACO2 (Abcam; ab110321), VDAC (Abcam; ab34726), VCL (Cell Signaling Technology; 13901), UQCRC1 (Abcam; ab110252), COX5B (Abcam; ab110263), TFAM (Abcam; ab131607), IRF3 (Cell Signaling Technology; 4302), RIG-I (Cell Signaling Technology; 3743), Cleaved Caspase-3 (Cell Signaling Technology; 9661), and p62/SQSTM1 (Abcam; ab56416).
Mass Spectrometry: Protein Extraction and Proteolytic Digestion.
Mitochondria enriched fractions were lysed with a dounce homogenizer with SDS lysis buffer [2% (wt/vol) SDS, 250 mM NaCl, EDTA-free protease inhibitor mixture (Promega), and 50 mM Hepes, pH 8.5] at a 1:1 ratio. Fractions were reduced with 5 mM DTT for 30 min at 57 °C and then cooled for 15 min at room temperature, after which cysteine residues were alkylated with 14 mM iodoacetamide in the dark. Protein content was extracted by methanol-chloroform precipitation followed by two washes in cold acetone. Protein pellets were air-dried and dissolved with 500 μL of 8 M urea containing 50 mM Hepes, pH 8.5. Protein concentrations were measured by the bicinchoninic acid assay (Thermo Fisher Scientific) before protease digestion. Mitochondrial proteins were diluted to 4 M urea and digested with LysC (Wako) at a 1:200 enzyme:protein ratio overnight. Mitochondrial proteins were further diluted with 25 mM Hepes, pH 8.5 to a 1 M urea concentration, and then trypsin (Promega) was added to a final 1:250 enzyme:protein ratio for 6 h at 37 °C. Digests were acidified with 250 μL of 20% formic acid (FA) to a pH ∼2 and concentrated using C18 SPE on Sep-Pak cartridges (50 mg; Waters).
Tandem Mass Tagging and Labeling.
Isobaric labeling of the enriched peptides was performed using 8-plex tandem mass tag (TMT) reagents (Thermo Fisher Scientific). TMT reagents (1 mg) were dissolved in 50 μL of dry acetonitrile (I), and 10 μL was added to 100 μg of peptides dissolved in 100 μL of 200 mM Hepes, pH 8.5. After 1 h at room temperature, the reaction was quenched by adding 4 μL of 5% hydroxylamine. TMT-labeled peptides were combined, acidified with 20 μL of FA, and diluted to a final acetonitrile concentration of ∼5% before C18 SPE on Sep-Pak cartridges (50 mg).
MS Data Processing and Spectra Assignment.
A compilation of in-house software was used to convert mass spectrometric data (RAW file) to the mzXML format, as well as to correct monoisotopic m/z measurements and erroneous peptide charge state assignments. Assignment of MS/MS spectra was performed using the Sequest algorithm by searching the data against a protein sequence database including all entries in the Mouse Uniprot database (download date, June 2014) containing known contaminants, such as human keratins and its reverse decoy components (50). Sequest searches were performed using a 10-ppm precursor ion tolerance and requiring each peptide’s N/C termini to have trypsin protease specificity, while allowing up to three missed cleavages. TMT tags on peptide N termini/lysine residues (+229.162932 Da) and carbamidomethylation of cysteine residues (+57.02146 Da) were set as static modifications, and methionine oxidation (+15.99492 Da) was set as a variable modification. An MS2 spectra assignment false discovery rate (FDR) of <1% was achieved by applying the target-decoy database search strategy (50). Filtering was performed using an in-house linear discrimination analysis algorithm to create a single combined filter parameter from the following peptide ion and MS2 spectra metrics: Sequest parameters XCorr and ΔCn, peptide ion mass accuracy and charge state, peptide length, and missed cleavages. Linear discrimination scores were used to assign a probability for being assigned correctly to each MS2 spectrum, and these probabilities were used to filter the dataset to a 1% protein-level FDR (Huttlin et al., 2010).
Determination of TMT Reporter Ion Intensities and Quantitative Data Analysis.
For quantification, a 0.003-m/z window centered on the theoretical m/z value of each of the eight reporter ions and the most intense signal intensity from the theoretical m/z value were recorded. Reporter ion intensities were further denormalized based on their ion accumulation time for each MS3 spectrum, and adjusted based on the overlap of isotopic envelopes of all reporter ions in accordance with the manufacturer’s specifications. Total signal-to-noise values for all peptides were summed for each TMT channel, and all values were adjusted to account for variance in sample handling. For each peptide, a total minimum signal-to-noise value of 200 was required (18).
Supplementary Material
Acknowledgments
We thank the members of the B.M.S. laboratory for helpful discussions. This work was supported by a Canadian Institutes of Health Research postdoctoral fellowship (to L.K.), a Human Frontier Science Program postdoctoral fellowship (to E.T.C.), the Wellcome Trust (Investigator Award 110159/Z/15/Z, to M.P.M.), the National Institutes of Health (Grants R01 DK102170 and R01 DK085171 to E.D.R., and R01 DK31405 to B.M.S.), and the JPB Foundation (B.M.S.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 7744.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705406114/-/DCSupplemental.
References
- 1.Enerbäck S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 2.Lidell ME, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 3.Jastroch M, Wuertz S, Kloas W, Klingenspor M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol Genomics. 2005;22:150–156. doi: 10.1152/physiolgenomics.00070.2005. [DOI] [PubMed] [Google Scholar]
- 4.Oelkrug R, Goetze N, Meyer CW, Jastroch M. Antioxidant properties of UCP1 are evolutionarily conserved in mammals and buffer mitochondrial reactive oxygen species. Free Radic Biol Med. 2014;77:210–216. doi: 10.1016/j.freeradbiomed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Clarke KJ, Porter RK. Uncoupling protein 1 dependent reactive oxygen species production by thymus mitochondria. Int J Biochem Cell Biol. 2013;45:81–89. doi: 10.1016/j.biocel.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Dlasková A, Clarke KJ, Porter RK. The role of UCP 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta. 2010;1797:1470–1476. doi: 10.1016/j.bbabio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Oelkrug R, Kutschke M, Meyer CW, Heldmaier G, Jastroch M. Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. J Biol Chem. 2010;285:21961–21968. doi: 10.1074/jbc.M110.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabalina IG, et al. ROS production in brown adipose tissue mitochondria: The question of UCP1-dependence. Biochim Biophys Acta. 2014;1837:2017–2030. doi: 10.1016/j.bbabio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Stier A, et al. Mitochondrial uncoupling prevents cold-induced oxidative stress: A case study using UCP1 knockout mice. J Exp Biol. 2014;217:624–630. doi: 10.1242/jeb.092700. [DOI] [PubMed] [Google Scholar]
- 10.Chouchani ET, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chouchani ET, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialò F, et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016;23:725–734. doi: 10.1016/j.cmet.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–E357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 16.Matthias A, et al. Thermogenic responses in brown fat cells are fully UCP1-dependent: UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 17.Kazak L, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAlister GC, et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem. 2014;86:7150–7158. doi: 10.1021/ac502040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, et al. The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. J Cell Biol. 2007;176:141–146. doi: 10.1083/jcb.200609158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Bogenhagen DF. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J Biol Chem. 2006;281:25791–25802. doi: 10.1074/jbc.M604501200. [DOI] [PubMed] [Google Scholar]
- 21.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumari M, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest. 2016;126:2839–2854. doi: 10.1172/JCI86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kissig M, et al. PRDM16 represses the type I interferon response in adipocytes to promote mitochondrial and thermogenic programing. EMBO J. 2017;36:1528–1542. doi: 10.15252/embj.201695588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 28.Crichton PG, Parker N, Vidal-Puig AJ, Brand MD. Not all mitochondrial carrier proteins support permeability transition pore formation: No involvement of uncoupling protein 1. Biosci Rep. 2009;30:187–192. doi: 10.1042/BSR20090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 30.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Meis L, Ketzer LA, Camacho-Pereira J, Galina A. Brown adipose tissue mitochondria: Modulation by GDP and fatty acids depends on the respiratory substrates. Biosci Rep. 2012;32:53–59. doi: 10.1042/BSR20100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chouchani ET, et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: A single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem. 2004;90:405–421. doi: 10.1111/j.1471-4159.2004.02489.x. [DOI] [PubMed] [Google Scholar]
- 35.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 36.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 38.Carraro M, Bernardi P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium. 2016;60:102–107. doi: 10.1016/j.ceca.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelso GF, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 40.Kong X, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic Biol Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 42.Mailloux RJ, Adjeitey CN, Xuan JY, Harper ME. Crucial yet divergent roles of mitochondrial redox state in skeletal muscle vs. brown adipose tissue energetics. FASEB J. 2012;26:363–375. doi: 10.1096/fj.11-189639. [DOI] [PubMed] [Google Scholar]
- 43.Schönfeld P, Wojtczak L. Brown adipose tissue mitochondria oxidizing fatty acids generate high levels of reactive oxygen species irrespective of the uncoupling protein-1 activity state. Biochim Biophys Acta. 2012;1817:410–418. doi: 10.1016/j.bbabio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Shabalina IG, et al. UCP1 and defense against oxidative stress: 4-hydroxy-2-nonenal effects on brown fat mitochondria are uncoupling protein 1-independent. J Biol Chem. 2006;281:13882–13893. doi: 10.1074/jbc.M601387200. [DOI] [PubMed] [Google Scholar]
- 45.Orr AL, Quinlan CL, Perevoshchikova IV, Brand MD. A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase. J Biol Chem. 2012;287:42921–42935. doi: 10.1074/jbc.M112.397828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madungwe NB, Zilberstein NF, Feng Y, Bopassa JC. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. Am J Cardiovasc Dis. 2016;6:93–108. [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem. 2001;276:12460–12465. doi: 10.1074/jbc.M100466200. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, et al. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.