Significance
Heterosis, the phenotypic superiority of a hybrid over its parents, has been extensively exploited in agriculture to improve biomass and yield. Despite its great agricultural importance, the genetic components underlying heterosis remain largely unclear. Here, we characterize the genomic architecture of heterosis in Arabidopsis that have not undergone domestication and identify hundreds of genetic loci that collectively contribute to biomass heterosis using genome-wide association studies. The functional investigation of candidate genes and transcriptomic analysis in representative hybrids suggest that the accumulation of superior genes involved in basic biological processes and the repression of stimulus-responsive genes in hybrids contribute to biomass heterosis in Arabidopsis, thus providing a comprehensive understanding of the genetic bases of heterosis in natural populations of plant species.
Keywords: biomass heterosis, GWAS, natural variation, Arabidopsis
Abstract
Heterosis is most frequently manifested by the substantially increased vigorous growth of hybrids compared with their parents. Investigating genomic variations in natural populations is essential to understand the initial molecular mechanisms underlying heterosis in plants. Here, we characterized the genomic architecture associated with biomass heterosis in 200 Arabidopsis hybrids. The genome-wide heterozygosity of hybrids makes a limited contribution to biomass heterosis, and no locus shows an obvious overdominance effect in hybrids. However, the accumulation of significant genetic loci identified in genome-wide association studies (GWAS) in hybrids strongly correlates with better-parent heterosis (BPH). Candidate genes for biomass BPH fall into diverse biological functions, including cellular, metabolic, and developmental processes and stimulus-responsive pathways. Important heterosis candidates include WUSCHEL, ARGOS, and some genes that encode key factors involved in cell cycle regulation. Interestingly, transcriptomic analyses in representative Arabidopsis hybrid combinations reveal that heterosis candidate genes are functionally enriched in stimulus-responsive pathways, including responses to biotic and abiotic stimuli and immune responses. In addition, stimulus-responsive genes are repressed to low-parent levels in hybrids with high BPH, whereas middle-parent expression patterns are exhibited in hybrids with no BPH. Our study reveals a genomic architecture for understanding the molecular mechanisms of biomass heterosis in Arabidopsis, in which the accumulation of the superior alleles of genes involved in metabolic and cellular processes improve the development and growth of hybrids, whereas the overall repressed expression of stimulus-responsive genes prioritizes growth over responding to environmental stimuli in hybrids under normal conditions.
Heterosis, also known as hybrid vigor, refers to the biological phenomenon that the hybrid progeny exhibits superior performance over its parents in many traits, such as biomass, growth rate, yield, and fitness (1‒4). Three quantitative genetic models, namely dominance (5), overdominance (6, 7), and epistasis (8), are largely conceptual and do not explain the molecular mechanism of heterosis. Although omics studies have described genome-wide changes in gene expression (9‒13), small RNAs (14), DNA methylation (15), and histone modifications (12, 13) between hybrids and parents in plants, the underlying genetic mechanism remains elusive. With the increased availability of genome sequences and the revolution in computational methods, genome-wide association studies (GWAS) has been developed into a powerful tool to explore the genetic loci and candidate genes responsible for traits in plants (16‒20). Using this approach, numerous superior alleles contributing to yield-related heterosis have been identified in rice (21). The mapping of heterosis quantitative trait loci (QTL) for yield in rice hybrids has recently been reported (22). These studies have greatly improved the current understanding of the genetic bases of heterosis in plants. However, genetic loci associated with heterosis may not be completely preserved in rice, as natural genetic diversity decreases constantly during rice domestication (23). By contrast, exploration in native plant species that have not undergone domestication may help uncover the full spectrum of the genetic basis of heterosis.
Arabidopsis, an undomesticated plant species, serves as an excellent model to study the genetic mechanism of heterosis, owing to its short life cycle, extensive naturally occurring genetic variation, and sufficient amount of heterosis (24‒27). To date, few genetic loci associated with heterosis have been identified through QTL mapping in Arabidopsis (28‒30). Remarkably, a recent GWAS performed in Arabidopsis hybrids, generated by intercrossing 30 accessions, discovered significant loci and candidate genes for hybrid performance in flowering time and rosette traits (31), which demonstrated the feasibility of GWAS to dissect the genetic architecture of heterosis in Arabidopsis. However, in this case, limited genetic loci can be detected because sequence divergence among 30 accessions represented only a small proportion of the natural genetic variation in Arabidopsis.
Here, we generated 200 Arabidopsis hybrids by crossing Columbia-0 (Col-0) with other natural accessions collected worldwide and phenotyped these hybrids together with their parents for biomass-related traits during early development. We conducted GWAS for biomass heterosis and discovered 750 associated single-nucleotide polymorphisms (SNPs) that showed collective contributions. We identified 779 candidate heterosis genes, some of which encoded key regulators of the cell cycle and plant growth and development. By performing a transcriptomic analysis in representative hybrids, we found that stimulus-responsive genes were highly overrepresented among candidates and exhibited an overall repression in hybrids with high biomass heterosis. Taken together, these data provide comprehensive insights into the genetic bases of biomass heterosis in Arabidopsis.
Results
Collective Contribution of Different Growth Traits to Biomass Heterosis in Arabidopsis.
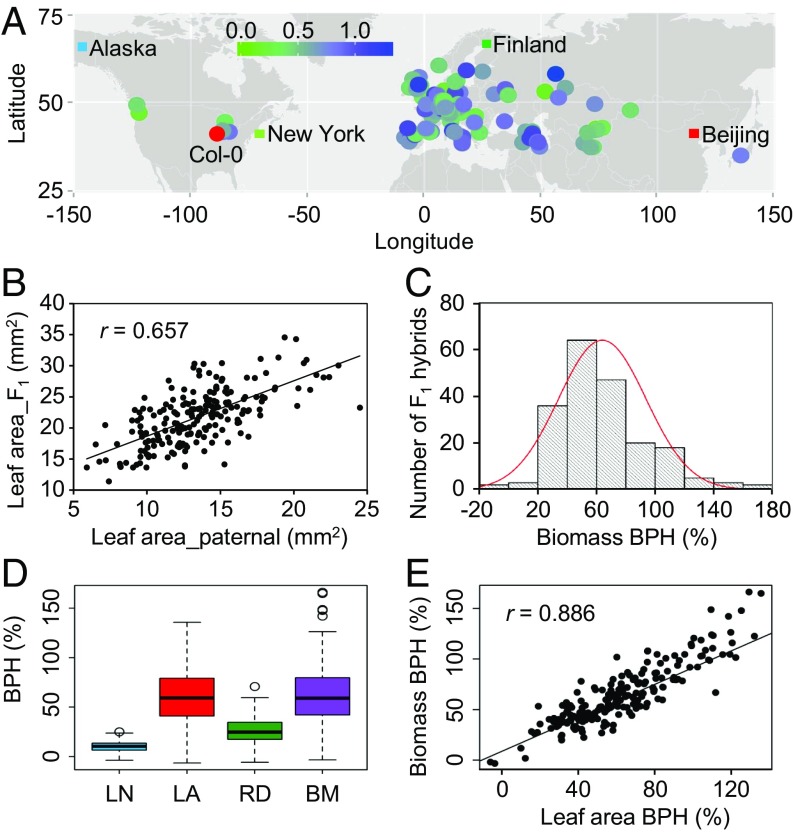
Previous studies have suggested that the degree of heterosis observed in hybrids is proportional to the parental genetic distance (3). In this scenario, if multiple A. thaliana accessions are crossed with a common maternal accession, then the biomass heterosis in these hybrids should be positively correlated with the parental genetic distance, and the differences in biomass heterosis among hybrids should result from the sequence divergence of paternal accessions. This correlation may provide clues to revealing the genetic basis of biomass heterosis. Considering this hypothesis, we generated 200 intraspecific hybrids by crossing 200 A. thaliana accessions with one common maternal accession, Col-0 (Dataset S1). The collected 201 accessions originated from Europe, Asia, and North America and displayed a broad range of geographic diversity (Fig. 1A). The hybrids and their parents were phenotyped for four traits, with one trait corresponding to shoot biomass (represented as shoot fresh weight) and three traits that potentially contributed to shoot biomass (leaf number, leaf area, and rosette diameter), at 14 d after sowing (DAS) to avoid the impact of flowering on biomass heterosis (SI Appendix, Fig. S1). We observed strong positive correlations among biomass, leaf area, and rosette diameter in hybrids (correlation coefficient r = 0.769–0.847; SI Appendix, Table S1), indicating that both leaf area and rosette diameter were the main contributors to hybrid biomass. Whereas the leaf number also partially contributed to biomass in hybrids (r = 0.435), no correlation was detected between the leaf number and leaf area or rosette diameter (r = 0.051 and r = 0.06, respectively) (SI Appendix, Table S1). These data suggested that in hybrids, the genetic components associated with leaf area or rosette diameter are distinct from those associated with leaf number. Furthermore, compared between hybrids and paternal accessions for each trait, significantly positive correlations were detected (Fig. 1B and SI Appendix, Fig. S2), suggesting that the genetic variations among paternal accessions are associated with differences in growth vigor in hybrids.
Fig. 1.
Heterosis in 200 Arabidopsis hybrids. (A) Geographic distribution of 201 A. thaliana accessions used in this study. Each dot indicates the original isolation location for an accession. The colors represent the levels of biomass heterosis, except for red, which represents the common maternal accession Col-0. (B) High correlation of leaf area between paternal accessions and F1 hybrids. (C) BPH for biomass in 200 Arabidopsis hybrids at 14 DAS. (D) Comparison among heterosis for different traits in 200 Arabidopsis hybrids. LN, LA, RD, and BM represent leaf number, leaf area, rosette diameter, and biomass, respectively. (E) BPH for biomass strongly and positively correlates with BPH for leaf area in 200 Arabidopsis hybrids.
Better-parent heterosis (BPH) and middle-parent heterosis (MPH) describe the degree of phenotypic difference between a hybrid and its better parent and between a hybrid and the mean of two parents, respectively. We used BPH and MPH to evaluate the heterosis of the 200 hybrids and found that heterosis widely occurred during early Arabidopsis development. All 200 hybrids showed positive MPH, and no hybrid was inferior to its better parent for all four traits (SI Appendix, Fig. S3). Most hybrids (98%, 196) showed significant positive BPH for all four traits, although with large variation (Fig. 1C and SI Appendix, Fig. S3). Further analyses focused primarily on BPH. We found that both biomass and leaf area heterosis were higher than leaf number or rosette diameter heterosis (Fig. 1D). Furthermore, the BPH for biomass, leaf area, and rosette diameter were highly correlated with each other (r = 0.859–0.886; Fig. 1E and SI Appendix, Table S2). Interestingly, although leaf number was not correlated with leaf area or rosette diameter in hybrids, the BPH for leaf number was significantly correlated with that for leaf area or rosette diameter (r = 0.48 and r = 0.494, respectively) and that for biomass (r = 0.598) (SI Appendix, Table S2). These results indicated a potential common genetic mechanism underlying the heterosis of different traits and the collective contribution of different traits to biomass heterosis in Arabidopsis.
Genome-Wide Heterozygosity of Hybrids Makes a Limited Contribution to Biomass Heterosis.
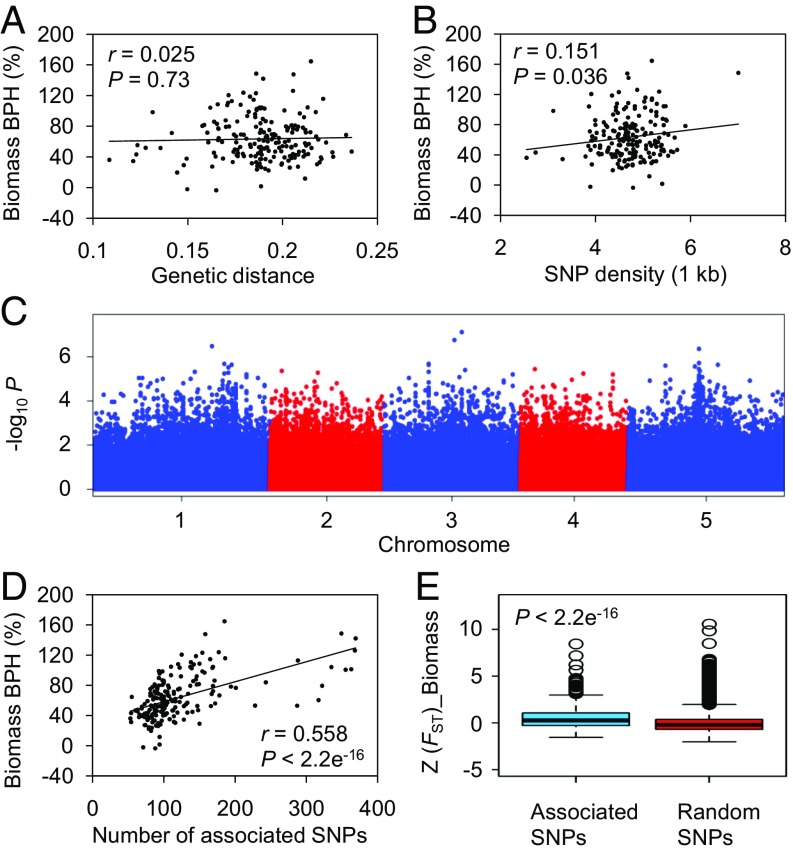
According to the observation that leaf area contributed most significantly to biomass heterosis during early Arabidopsis development (Fig. 1E), further analyses focused on heterosis for these two traits. We observed no obvious correlation between the extent of biomass heterosis and the geographic distance of paternal accessions, and many hybrids of the paternal accessions from nearby locations showed significantly different levels of heterosis (Fig. 1A). To further determine whether the genetic distance was correlated with the heterosis of hybrid populations in this study, a total of 722,000 SNPs with high reliability for 191 of the 200 paternal accessions obtained from the 1001 Genomes Project were used for the pairwise analysis of the genetic distance between Col-0 and each of the 191 paternal accessions using PLINK's identity by descent (IBD) analysis. We found that although the paternal accessions used in this study had extensive genetic diversity relative to Col-0, no correlation between parental genetic distance and heterosis for biomass or leaf area in hybrids was detected (Fig. 2A and SI Appendix, Fig. S4A). Thus, these results suggested that the degree of genetic distance between parents does not necessarily contribute to heterosis in intraspecific hybrids of Arabidopsis and that the natural genetic variation associated with microenvironmental divergence may contribute to biomass heterosis in Arabidopsis.
Fig. 2.
Characterization of genomic features associated with biomass heterosis in Arabidopsis. (A) No correlation is detected between BPH for biomass and parental genetic distance. Parental genetic distance was calculated between Col-0 and the paternal accessions using PLINK's IBD analysis. (B) A weak but significant positive correlation is detected between BPH for biomass and genome heterozygosity in Arabidopsis hybrids. The genome heterozygosity of hybrids was represented by SNP density per kilobase between two parents. (C) Manhattan plot of GWAS for biomass heterosis. Negative log10-transformed P values from a genome-wide scan were plotted against positions on each of the five Arabidopsis chromosomes. (D) BPH for biomass strongly and positively correlates with the number of 750 associated SNPs accumulated in paternal accessions. Associated SNPs were identified using a Benjamini–Hochberg test with a threshold of 0.2. (E) Z (FST) of 10-kb genomic regions surrounding 750 associated SNPs between the high-BPH group and the low-BPH group for biomass is significantly higher than control (random SNPs).
A prerequisite for heterosis manifestation is the genetic heterozygosity resulting from divergence between parental lines. Accordingly, we further investigated the effect of hybrid genome heterozygosity on heterosis. We used SNP density (SNPs per kilobase) to represent hybrid genome heterozygosity. Notably, a weak but significant positive correlation was observed between the genome-wide heterozygosity of hybrids and heterosis for biomass and leaf area (Fig. 2B and SI Appendix, Fig. S4B). These data indicated that not all heterozygous sites in hybrids derived from parental genomic divergence are involved in biomass heterosis, and heterosis should be contributed by the heterozygosity at specific genetic loci in hybrids.
Accumulation of Associated GWAS Loci Correlates with Biomass Heterosis in Arabidopsis.
To identify loci or genes with the potential contribution to Arabidopsis biomass heterosis, we conducted GWAS on heterosis for biomass and leaf area using an algorithm for multivariate linear mixed models in Genome-Wide Efficient Mixed-Model Association (GEMMA) software (32). No clear signals resembling a peak were observed in the resulting Manhattan plot (Fig. 2C), indicating that Arabidopsis biomass heterosis results from many alleles with additive effects. Using a modest significance threshold [false discovery rate (FDR) < 0.2], 750 associated SNPs were identified. Remarkably, heterosis strongly and positively correlated with the accumulation of associated SNPs in the paternal accessions (Fig. 2D and SI Appendix, Fig. S5A), but not with accumulated SNPs that were randomly selected from our SNP library (SI Appendix, Fig. S5 B and C). Therefore, the combination of heterozygous loci containing these 750 associated SNPs in hybrids may contribute to biomass heterosis in Arabidopsis.
As linkage disequilibrium (LD) decays within ∼10 kb in Arabidopsis (33), we focused our analyses on 10-kb genomic regions around these 750 associated SNPs. For convenience of analysis, we divided the paternal accessions into two groups: the high-BPH group consisted of the top third of accessions ranked according to BPH for biomass or leaf area, and the low-BPH group consisted of the bottom third of these ranked accessions (SI Appendix, Fig. S6A). The 10-kb genomic regions around these 750 SNPs exhibited extensive sequence variation between the high-BPH and low-BPH groups, which was reflected by the higher population-differentiation statistic (FST) than control (Wilcoxon’s rank–sum test, P < 2.2 × 10−16) (Fig. 2E and SI Appendix, Fig. S6B). This extensive sequence variation may provide the basis for the collective contribution of heterozygous loci containing 750 associated SNPs to biomass heterosis in Arabidopsis.
Identification and Functional Characterization of Candidate Genes for Biomass Heterosis in Arabidopsis.
Based on the Arabidopsis genome annotation in TAIR, a total of 779 protein-coding genes within the 10-kb genomic regions surrounding 750 heterosis-associated SNPs were identified and thought to be candidate genes for biomass BPH in Arabidopsis (Dataset S2). The biological function of these candidates was characterized based on GO (gene ontology) annotations. Of the 779 genes, 474 genes had functional annotations for biological process categories in the Arabidopsis GO database. These genes were divided into diverse functional categories, including cellular processes, metabolic processes, response to stimulus, biological regulation, and developmental processes (SI Appendix, Fig. S7), indicating the collective contribution of these biological pathways to biomass heterosis in Arabidopsis.
Further detailed inspection revealed that several genes encoding key regulators of cell cycle processes, including CELL DIVISION CYCLE 20.1 (CDC20.1), CELL DIVISION CYCLE 20.2 (CDC20.2), CYCLIN D1;1 (CYCD1;1), CYCLIN D2;1 (CYCD2;1), CYCLIN P2;1 (CYCP2;1), E2F TRANSCRIPTION FACTOR 1 (E2F1), DP-E2F-LIKE PROTEIN 3 (DEL3), HOBBIT (HBT) and KIP-RELATED PROTEIN 2 (KRP2), were among the candidate heterosis genes. Notably, WUSCHEL (WUS), a gene required to control the stem cell pool in the shoot apical meristem (SAM), and AUXIN-REGULATED GENE INVOLVED IN ORGAN SIZE (ARGOS) were also in the candidate list. Other important candidate genes involved in plant growth and development included INDOLE-3-ACETIC ACID INDUCIBLE 28 (IAA28), a negative regulator of auxin signaling, and GA REQUIRING 3 (GA3), which plays an essential role in the gibberellin biosynthetic pathway (Dataset S2). The natural divergence of these genes may occur during the evolution of Arabidopsis, and the accumulation of their superior alleles could be important contributors to the growth vigor in hybrids.
Candidate Genes for High BPH Are Enriched in Stimulus-Responsive Pathways.
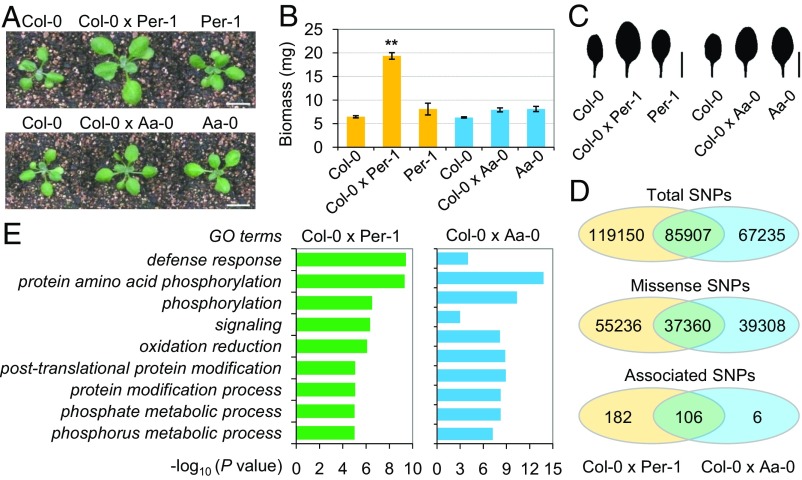
We further characterized heterosis candidates from a gene expression aspect. Because leaf area heterosis and biomass heterosis were highly correlated, the candidate genes underlying biomass heterosis should be expressed in the leaves. Accordingly, we narrowed the candidate genes for biomass heterosis by investigating the expression of these genes in representative Arabidopsis hybrid combinations. The leaves of two hybrid combinations, Col-0 × Per-1 and Col-0 × Aa-0, were selected for whole-genome expression profile analyses. Col-0 × Per-1 exhibited one of the highest levels for biomass BPH (139.4%) and leaf area BPH (129.4%), whereas Col-0 × Aa-0 showed one of the lowest levels for biomass BPH (−2.4%) and leaf area BPH (−7.3%) (Fig. 3 A–C). Comparison of the genotypes between Col-0 and Per-1 or Aa-0 showed that Col-0 × Per-1 contained more total and missense SNPs and accumulated more heterosis-associated SNPs than did Col-0 × Aa-0 (Fig. 3D). Interestingly, genes with multiple missense SNPs were significantly enriched in defense response pathways, which were much more evident in Col-0 × Per-1 (Fig. 3E). Of the 779 genes adjacent to heterosis-associated SNPs, 453 genes were detectably expressed in the leaves of at least one genotype in Col-0 × Per-1 and were considered candidate genes for biomass heterosis in this hybrid. Further GO analysis showed that these candidate genes were significantly enriched in response to stimulus pathways (153 genes), including immune responses, responses to both biotic and abiotic stimuli, and responses to stress (defense responses) (Fig. 4A). These results suggested that the natural genetic variation in stimulus-responsive genes might be associated with biomass heterosis in Arabidopsis.
Fig. 3.
The phenotypes and genotypes of representative hybrids with the highest BPH or the lowest BPH. (A) Growth vigor of two representative Arabidopsis hybrids, Col-0 × Per-1 and Col-0 × Aa-0, at 14 DAS. (Bar, 1 cm.) (B) Col-0 × Per-1 exhibits the highest BPH for biomass, whereas Col-0 × Aa-0 shows no significant BPH for biomass. The data are presented as the means ± SD; n > 30. **P < 0.01 between the hybrids and parents (Student’s t test). (C) Silhouette images showing differences in the size of the first leaf of plants in A. (Bar, 0.5 cm.) (D) Col-0 × Per-1 accumulates more total, missense, and GWAS-associated SNPs than Col-0 × Aa-0. (E) Genes with multiple missense SNPs (>10) are significantly enriched in defense pathways, which were more evident in Col-0 × Per-1.
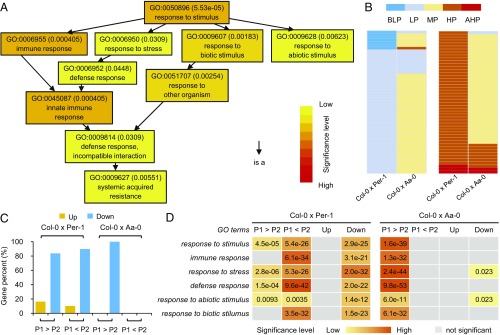
Fig. 4.
Distinct expression patterns of stimulus-responsive genes between Col-0 × Per-1 and Col-0 × Aa-0. (A) GO analysis of 453 candidate genes for biomass heterosis, which were significantly enriched in response to stimuli. (B) Candidate genes exhibiting low-parent expression (LP) or below–low-parent expression (BLP) and high-parent expression (HP) or above–high-parent expression (AHP) in Col-0 × Per-1 largely show middle-parent expression (MP) in Col-0 × Aa-0. (C) Genome-wide comparison of expression patterns between Col-0 × Per-1 and Col-0 × Aa-0. P1 in Col-0 × Per-1 and Col-0 × Aa-0 represents Col-0; P2 in Col-0 × Per-1 and Col-0 × Aa-0 represents Per-1 and Aa-0, respectively. Up, up-regulation in hybrid; Down, down-regulation in hybrid. (D) GO analysis of genes with expression variation in Col-0 × Per-1 and Col-0 × Aa-0.
Overall Repression of Stimulus-Responsive Genes in Hybrids with High Biomass Heterosis.
To gain more in-depth insight into how candidate genes contribute to Arabidopsis biomass heterosis, we compared the expression patterns of these 453 candidate genes between Col-0 × Per-1 and Col-0 × Aa-0. Notably, most candidate genes showing high- or low-parent expression in Col-0 × Per-1 were expressed at the middle-parent level in Col-0 × Aa-0 (Fig. 4B and SI Appendix, Fig. S8). This result further demonstrated that candidate genes exhibited distinct expression patterns between hybrids with different degrees of biomass heterosis. Most importantly, candidate genes displaying low-parent or below–low-parent expression in Col-0 × Per-1 were overrepresented in response to stimuli (P = 4.6 × 10−4). However, candidate genes expressed at high-parent or above–high-parent levels in Col-0 × Per-1 did not exhibit GO enrichment. These data indicated a role for stimulus-responsive genes in the establishment of biomass heterosis in Arabidopsis. This conclusion was further substantiated by a comparative analysis of variations in the expression patterns of stimulus-responsive genes at the genome-wide level between Col-0 × Per-1 and Col-0 × Aa-0 hybrids. In the Col-0 × Per-1 combination, regardless of the direction of expression divergence between parents, gene expression was predominantly down-regulated in the hybrid plants. By contrast, in the Col-0 × Aa-0 combination, only genes in one direction of divergence between parents showed down-regulation in the hybrid (Fig. 4C and SI Appendix, Fig. S9). Further GO enrichment analysis between parents revealed that genes showing higher expression in Col-0 or in Per-1 were significantly enriched in stimulus-responsive pathways and that these response genes were down-regulated in the Col-0 × Per-1 hybrid (Fig. 4D). Nevertheless, only genes with higher expression levels in Col-0 than in Aa-0 were significantly enriched in stimulus responses, and these response genes were only partially down-regulated in the Col-0 × Aa-0 hybrid. Notably, in both combinations, genes showing up-regulation in hybrids were not enriched in stimulus responses (Fig. 4D). Transcriptomic analysis of another hybrid Col-0 × Ak-1 with high biomass BPH revealed similar stimulus-responsive gene expression patterns (SI Appendix, Fig. S10). These data suggested that the bidirectional divergent expression of stimulus-responsive genes between parents and their overall down-regulation in hybrids contribute to better-parent biomass heterosis in Arabidopsis.
Discussion
In this study, we performed GWAS for biomass heterosis established during early development in Arabidopsis and identified 750 associated SNPs showing collective contribution. A total of 779 candidate genes around the 10-kb genomic regions of heterosis-associated SNPs were identified and implicated in diverse biological functions. One of the most important candidate genes was WUS, which is expressed in the stem-cell–organizing center of meristems and functions in controlling the size of the stem cell population (34). It will be intriguing to experimentally investigate whether this gene contributes to biomass heterosis by inducing larger SAM in hybrids. Interestingly, some candidate genes encoded key regulators of the cell cycle. CYCD1;1, CYCD2;1, CYCP2;1, E2F1, DEL3, and KRP2 are critical factors for the regulation of the G1-to-S transition in the cell cycle (35, 36). In addition, HBT is a core component of the anaphase-promoting complex (APC), an E3 ubiquitin ligase that is activated by CDC20 during the G2-to-M transition (35, 36). Because the cell cycle plays a critical role in regulating organ growth and size (37, 38), and greater leaf growth in the Arabidopsis hybrid was tightly associated with increased cell number (39), which may reflect the acceleration of the cell cycle, the cell cycle-related candidate genes identified in this study may make important contributions to biomass heterosis in Arabidopsis. Furthermore, some phytohormone-responsive genes involved in the regulation of cell cycle processes were identified as biomass heterosis gene candidates. For example, ARGOS, an auxin-inducible gene, controls Arabidopsis leaf size by accelerating the cell cycle (40). The expression of ARGOS was activated in Arabidopsis hybrids (39), and the overexpression of the maize ortholog of ARGOS, ZmARGOS1, affected the hybrid yield (41).
Through a transcriptomic analysis, heterosis candidates for a representative hybrid with high BPH were narrowed to 453 genes, which displayed significant enrichment in response to biotic and abiotic stimuli and immune processes. Remarkably, the stimulus-responsive genes showed distinct expression patterns between Col-0 × Per-1 with the highest heterosis and Col-0 × Aa-0 with the lowest heterosis and exhibited overall repression in Col-0 × Per-1. Miller et al. suggested the effect of stress response genes on growth heterosis in Arabidopsis by comparative transcriptomic analysis in hybrids (10). Our results provided evidence from both genetic and transcriptional aspects for the contribution of stimulus-responsive genes to biomass heterosis in Arabidopsis. Notably, stimulus-responsive genes, particularly defense response genes, are highly divergent in sequence among natural Arabidopsis accessions. Thus, although the candidate genes for biomass heterosis are enriched in stimulus-responsive pathways, a specific set of genes involved in these pathways may vary depending on hybrid combinations, which should be identified by additional analyses of the mRNA expression in different organs and at different developmental stages.
Recent studies have uncovered the tradeoff between stress responses and growth in Arabidopsis (42‒45). Therefore, Arabidopsis biomass heterosis may result from the divergence of stimulus-responsive genes between parents and the overall repression of these genes in hybrids (Fig. 5). This mechanism should be independent of plant species, organs, or developmental stages. Potential evidence is that BPH for both leaf number and leaf area is positively correlated and contributes to biomass BPH, whereas the two traits themselves are not correlated in hybrids (SI Appendix, Tables S1 and S2). The regulatory mechanisms for the repression of stimulus-responsive genes may involve the parental divergence of genetic components, such as missense variation in repressors for stimulus-responsive pathways. Epigenetic components, including small RNAs, may also be involved. Indeed, several key genes involved in small-RNA biogenesis and functional processes, including ARGONAUTE 1 (AGO1), DICER-LIKE 4 (DCL4), and HUA ENHANCER 1 (HEN1), were among the candidate genes identified in this study (Dataset S2). Notably, a previous study has demonstrated an important role for HEN1 in biomass heterosis in Arabidopsis (15).
Fig. 5.
A model for biomass heterosis in Arabidopsis. The expression levels of one group of stimulus-responsive genes (R1) are lower in one parent (P1) than in another parent (P2), associated with stronger growth in P1 than in P2 (represented by G1). The expression levels of another group of stimulus-responsive genes (R2) are higher in P1 than in P2, associated with weaker growth in P1 than in P2 (represented by G2). The growth and response are balanced in both parents. The differential expression levels of two groups of response genes between parents may result from divergent negative regulators, such as transcriptional repressors, and both groups of response genes (R1 and R2) showed low-parent expression due to the trans-action of negative regulators in the hybrid. As a result, balanced growth-response is disrupted, and the hybrid shows stronger growth (G1 and G2) than both parents.
These findings differed from those of a recent GWAS in Arabidopsis hybrids that were generated after intercrossing 30 accessions, which reported multiple heterosis-associated loci and candidate genes for flowering time and rosette traits (31). It is likely that the Arabidopsis hybrid populations used for GWAS were derived from different experimental designs and were different in scale. It is also likely that there are different mechanisms underlying different heterotic traits. The heterosis candidate genes identified in this study were also different from those reported in rice (22). Rice is a domesticated crop subjected to extensive human selection. The genetic loci identified in that study should reflect improvements of yield-related traits in hybrids instead of a general mechanism for biomass or growth heterosis throughout plant species, which could be identified in undomesticated natural populations.
In conclusion, our study demonstrated the efficiency of combining GWAS and transcriptome data to dissect the comprehensive genomic architecture of heterosis. The combinational contribution of accumulated superior alleles of the genes involved in basic biological processes and the repressed expression of stimulus-responsive genes should enable more vigorous growth of hybrids relative to their parents.
Materials and Methods
A total of 201 A. thaliana accessions obtained from the Arabidopsis Biological Resource Center were used in this study (Dataset S1). Accession Col-0 was used as the common maternal line and was crossed with the 200 other accessions through hand-pollination. Details of plant materials and growth conditions, phenotyping, and population genetic analyses are described in SI Appendix, SI Materials and Methods.
For GWAS, 722,000 SNPs were used. GWAS was conducted between the strongly and positively correlated biomass BPH and leaf area BPH using an algorithm for multivariate linear mixed models in the GEMMA software (32) (www.xzlab.org/software.html). To correct for multiple testing, a Benjamini–Hochberg test was performed. Thresholds of 0.2 were applied to identify modestly significant SNPs associated with biomass heterosis.
The details and procedures of RNA-seq and data analysis and quantitative RT-PCR are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFD0100801), the National Natural Science Foundation of China (31330048, 31621001), Peking-Tsinghua Center for Life Sciences (to X.W.D), State Key Laboratory of Protein and Plant Gene Research, and in part by the Postdoctoral Fellowship of Peking–Tsinghua Center for Life Sciences.
Footnotes
The authors declare no conflict of interest.
Data deposition: All original data sets have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession nos. GSE85759 and GSE100595.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705423114/-/DCSupplemental.
References
- 1.Chen ZJ. Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet. 2013;14:471–482. doi: 10.1038/nrg3503. [DOI] [PubMed] [Google Scholar]
- 2.Schnable PS, Springer NM. Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol. 2013;64:71–88. doi: 10.1146/annurev-arplant-042110-103827. [DOI] [PubMed] [Google Scholar]
- 3.Chen ZJ. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010;15:57–71. doi: 10.1016/j.tplants.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippman ZB, Zamir D. Heterosis: Revisiting the magic. Trends Genet. 2007;23:60–66. doi: 10.1016/j.tig.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J, Li J, Yuan L, Tanksley SD. Dominance is the major genetic basis of heterosis in rice as revealed by QTL analysis using molecular markers. Genetics. 1995;140:745–754. doi: 10.1093/genetics/140.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, et al. Dominance, overdominance and epistasis condition the heterosis in two heterotic rice hybrids. Genetics. 2008;180:1725–1742. doi: 10.1534/genetics.108.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li ZK, et al. Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. I. Biomass and grain yield. Genetics. 2001;158:1737–1753. doi: 10.1093/genetics/158.4.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu SB, et al. Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA. 1997;94:9226–9231. doi: 10.1073/pnas.94.17.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto R, Taylor JM, Shirasawa S, Peacock WJ, Dennis ES. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA. 2012;109:7109–7114. doi: 10.1073/pnas.1204464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M, Song Q, Shi X, Juenger TE, Chen ZJ. Natural variation in timing of stress-responsive gene expression predicts heterosis in intraspecific hybrids of Arabidopsis. Nat Commun. 2015;6:7453. doi: 10.1038/ncomms8453. [DOI] [PubMed] [Google Scholar]
- 11.Groszmann M, et al. Hormone-regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc Natl Acad Sci USA. 2015;112:E6397–E6406. doi: 10.1073/pnas.1519926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He G, et al. Global epigenetic and transcriptional trends among two rice subspecies and their reciprocal hybrids. Plant Cell. 2010;22:17–33. doi: 10.1105/tpc.109.072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He G, et al. Conservation and divergence of transcriptomic and epigenomic variation in maize hybrids. Genome Biol. 2013;14:R57. doi: 10.1186/gb-2013-14-6-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groszmann M, et al. Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc Natl Acad Sci USA. 2011;108:2617–2622. doi: 10.1073/pnas.1019217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen H, et al. Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell. 2012;24:875–892. doi: 10.1105/tpc.111.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijon M, Satbhai SB, Tsuchimatsu T, Busch W. Genome-wide association study using cellular traits identifies a new regulator of root development in Arabidopsis. Nat Genet. 2014;46:77–81. doi: 10.1038/ng.2824. [DOI] [PubMed] [Google Scholar]
- 17.Atwell S, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet. 2012;44:32–39. doi: 10.1038/ng.1018. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42:961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 20.Kump KL, et al. Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat Genet. 2011;43:163–168. doi: 10.1038/ng.747. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, et al. Genomic analysis of hybrid rice varieties reveals numerous superior alleles that contribute to heterosis. Nat Commun. 2015;6:6258. doi: 10.1038/ncomms7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, et al. Genomic architecture of heterosis for yield traits in rice. Nature. 2016;537:629–633. doi: 10.1038/nature19760. [DOI] [PubMed] [Google Scholar]
- 23.Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Koornneef M, Alonso-Blanco C, Vreugdenhil D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol. 2004;55:141–172. doi: 10.1146/annurev.arplant.55.031903.141605. [DOI] [PubMed] [Google Scholar]
- 25.Weigel D. Natural variation in Arabidopsis: From molecular genetics to ecological genomics. Plant Physiol. 2012;158:2–22. doi: 10.1104/pp.111.189845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genomes Consortium 1,135 Genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell. 2016;166:481–491. doi: 10.1016/j.cell.2016.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barth S, Busimi AK, Friedrich Utz H, Melchinger AE. Heterosis for biomass yield and related traits in five hybrids of Arabidopsis thaliana L. Heynh. Heredity. 2003;91:36–42. doi: 10.1038/sj.hdy.6800276. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell-Olds T. Interval mapping of viability loci causing heterosis in Arabidopsis. Genetics. 1995;140:1105–1109. doi: 10.1093/genetics/140.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kusterer B, et al. Heterosis for biomass-related traits in Arabidopsis investigated by quantitative trait loci analysis of the triple testcross design with recombinant inbred lines. Genetics. 2007;177:1839–1850. doi: 10.1534/genetics.107.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer RC, et al. QTL analysis of early stage heterosis for biomass in Arabidopsis. Theor Appl Genet. 2010;120:227–237. doi: 10.1007/s00122-009-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seymour DK, et al. Genetic architecture of nonadditive inheritance in Arabidopsis thaliana hybrids. Proc Natl Acad Sci USA. 2016;113:E7317–E7326. doi: 10.1073/pnas.1615268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Stephens M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods. 2014;11:407–409. doi: 10.1038/nmeth.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, et al. Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat Genet. 2007;39:1151–1155. doi: 10.1038/ng2115. [DOI] [PubMed] [Google Scholar]
- 34.Schoof H, et al. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 35.Dewitte W, Murray JA. The plant cell cycle. Annu Rev Plant Biol. 2003;54:235–264. doi: 10.1146/annurev.arplant.54.031902.134836. [DOI] [PubMed] [Google Scholar]
- 36.Inze D, De Veylder L. Cell cycle regulation in plant development. Annu Rev Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez N, Vanhaeren H, Inzé D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012;17:332–340. doi: 10.1016/j.tplants.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Polyn S, Willems A, De Veylder L. Cell cycle entry, maintenance, and exit during plant development. Curr Opin Plant Biol. 2015;23:1–7. doi: 10.1016/j.pbi.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Groszmann M, et al. Intraspecific Arabidopsis hybrids show different patterns of heterosis despite the close relatedness of the parental genomes. Plant Physiol. 2014;166:265–280. doi: 10.1104/pp.114.243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, Xie Q, Chua NH. The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell. 2003;15:1951–1961. doi: 10.1105/tpc.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo M, et al. Maize ARGOS1 (ZAR1) transgenic alleles increase hybrid maize yield. J Exp Bot. 2014;65:249–260. doi: 10.1093/jxb/ert370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todesco M, et al. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature. 2010;465:632–636. doi: 10.1038/nature09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozano-Duran R, et al. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife. 2013;2:e00983. doi: 10.7554/eLife.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan M, et al. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. Plant Cell. 2014;26:828–841. doi: 10.1105/tpc.113.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malinovsky FG, et al. Antagonistic regulation of growth and immunity by the Arabidopsis basic helix-loop-helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiol. 2014;164:1443–1455. doi: 10.1104/pp.113.234625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.