Significance
Autism spectrum disorder (ASD) is characterized by social deficits. Emerging evidence suggests that the neuropeptide oxytocin, which regulates mammalian social functioning, may be a promising treatment for ASD. However, prior oxytocin treatment trials in ASD patients have produced equivocal results, perhaps because of variability in patients’ underlying neuropeptide biology. Here we provide evidence that oxytocin treatment improves social abilities in children with ASD and that individuals with the lowest pretreatment blood oxytocin concentrations benefit the most from oxytocin administration. These findings reveal a personalized component to oxytocin treatment which may have important implications for accurately testing oxytocin’s therapeutic potential, both for ASD and for a broad range of developmental and psychiatric disorders in which patients exhibit social impairments.
Keywords: autism, biomarkers, clinical trial, oxytocin, social functioning
Abstract
Autism spectrum disorder (ASD) is characterized by core social deficits. Prognosis is poor, in part, because existing medications target only associated ASD features. Emerging evidence suggests that the neuropeptide oxytocin (OXT) may be a blood-based biomarker of social functioning and a possible treatment for ASD. However, prior OXT treatment trials have produced equivocal results, perhaps because of variability in patients’ underlying neuropeptide biology, but this hypothesis has not been tested. Using a double-blind, randomized, placebo-controlled, parallel design, we tested the efficacy and tolerability of 4-wk intranasal OXT treatment (24 International Units, twice daily) in 32 children with ASD, aged 6–12 y. When pretreatment neuropeptide measures were included in the statistical model, OXT compared with placebo treatment significantly enhanced social abilities in children with ASD [as measured by the trial’s primary outcome measure, the Social Responsiveness Scale (SRS)]. Importantly, pretreatment blood OXT concentrations also predicted treatment response, such that individuals with the lowest pretreatment OXT concentrations showed the greatest social improvement. OXT was well tolerated, and its effects were specific to social functioning, with no observed decrease in repetitive behaviors or anxiety. Finally, as with many trials, some placebo-treated participants showed improvement on the SRS. This enhanced social functioning was mirrored by a posttreatment increase in their blood OXT concentrations, suggesting that increased endogenous OXT secretion may underlie this improvement. These findings indicate that OXT treatment enhances social abilities in children with ASD and that individuals with pretreatment OXT signaling deficits may stand to benefit the most from OXT treatment.
Autism spectrum disorder (ASD) is a brain disorder of early childhood onset. ASD is characterized by core social communication impairments as well as restricted, repetitive behaviors, which jeopardize the development of appropriate social skills and the maintenance of social relationships (1). Despite being one of the most devastating childhood disorders in terms of prevalence [1 in 68 US children (2)] and societal cost [$236 billion expended annually in the United States (3)], ASD pathophysiology remains poorly understood. Consequently, there are no approved medications that enhance social abilities in individuals with ASD.
Neurobiological systems that support normative social behavior are one of the most promising signaling pathways for the discovery of ASD therapeutic targets (4). One such candidate is the neuropeptide oxytocin (OXT). OXT is primarily synthesized in the hypothalamus and released into the brain via distributed neural pathways and into systemic circulation via the posterior pituitary (5). OXT binds to four receptors: OXTR, V1AR, V1BR, and V2R (6, 7); its prosocial effects are largely mediated via OXTR and V1AR. OXT is critical for the expression of mammalian social behavior (8–10), and targeted disruption of OXT signaling through pharmacologic or genetic manipulation produces social deficits in rodents (11).
Studies of rodent models of human syndromes with high ASD penetrance (e.g., fragile-X syndrome, Prader–Willi syndrome, cortical dysplasia, and focal epilepsy syndrome modeled using CNTNAP2-knockout mice) have reported social impairments and diminished numbers of hypothalamic OXT-producing cells (12–14). This reduction in brain OXT has been associated with lower blood OXT concentrations in transgenic vs. wild-type animals, with social impairments ameliorated in transgenic animals following OXT treatment (13, 15). These preclinical findings suggest that the OXT signaling pathway may be a promising therapeutic target for improving social abilities in patients with ASD, particularly in those with OXT signaling deficits.
Multiple studies have shown that single doses of OXT administered to individuals with ASD improve processing of social information (16), emotion recognition (17), and social learning (18). However, evidence from OXT treatment trials in ASD patients is more equivocal: Several studies have reported that OXT administration improves social abilities in individuals with ASD (19, 20), but others have found no improvement in the trial’s primary outcome measure (21–24). Interestingly, many OXT administration studies have documented significant variability in responses to OXT (18, 19, 21), highlighting the need to identify specific factors that contribute to OXT efficacy.
Because ASD is an extremely heterogeneous disorder, one possible explanation for these ambiguous outcomes in OXT treatment trials is that individual differences in endogenous neuropeptide biology may influence the response to OXT treatment. Specifically, individuals with ASD who have pronounced OXT signaling deficits may benefit the most from OXT treatment. Studies have shown that some (18, 25, 26) but not all (27–29) individuals with ASD have lower plasma OXT concentrations than controls. Plasma OXT concentrations also positively predict social functioning in children with ASD, such that children with the lowest plasma OXT concentrations show the greatest social deficits (27). However, no prior trial has tested whether individual differences in pretreatment neuropeptide biology contribute to how and to what extent individuals with ASD respond to OXT treatment, nor has any prior trial directly tested whether a statistical model’s explanatory power is improved by the inclusion of such biomarkers. The present study was designed to address these critical gaps in knowledge. Using a double-blind, randomized, placebo-controlled, parallel design, we investigated whether 4-wk intranasal OXT treatment improves social abilities in children with ASD [as measured by the trial’s primary outcome measure, the Social Responsiveness Scale (SRS) Total Raw Score] and whether pretreatment blood measures of neuropeptide biology (i.e., OXT concentrations and OXTR and V1AR gene expression) predict the response to OXT treatment. As secondary outcome measures, we also tested whether OXT ameliorates other core (i.e., repetitive behaviors) or associated (i.e., anxiety) symptoms of ASD and whether OXT is well tolerated in children with ASD, because limited safety and tolerability data are available in pediatric populations.
Results
Participants.
Thirty-two children with ASD (27 male, 5 female), aged 6–12 y, completed this clinical trial (see the CONSORT diagram, Fig. 1). Participant demographic characteristics are presented in Table 1. Participants’ stable concomitant medications, which did not differ by treatment condition, are presented in Table S1.
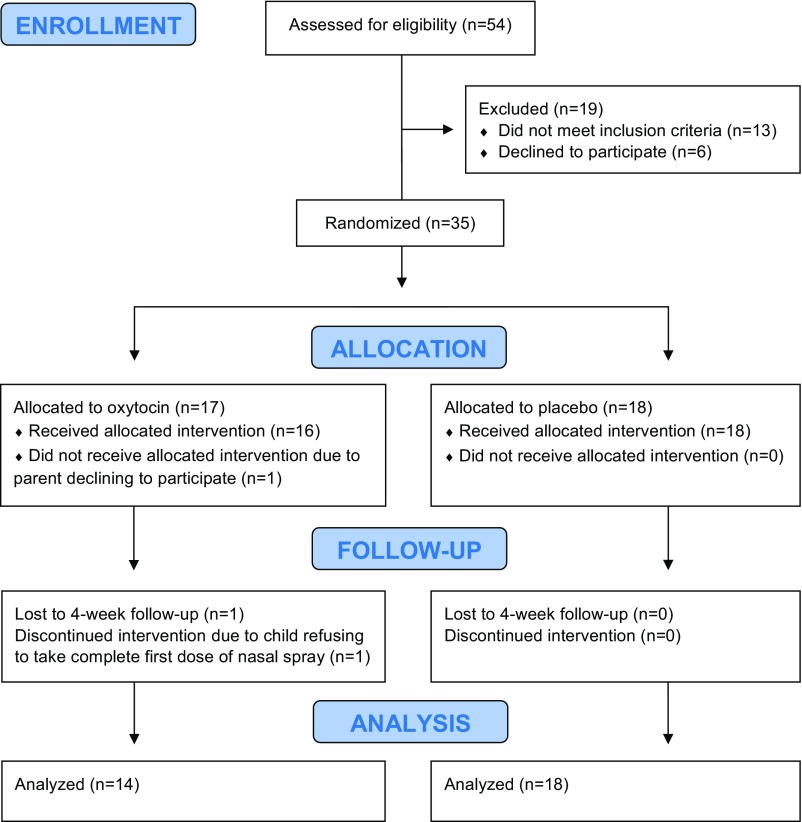
Fig. 1.
The CONSORT flow diagram details the progress of participants through the double-blind, randomized, placebo-controlled 4-wk oxytocin treatment trial.
Table 1.
Participant characteristics
| Sex | Ethnicity | ||||||||
| Treatment | N | Female | Male | Caucasian | Asian | Other | Age, y | Full-scale IQ | Pretreatment SRS Total Raw Score |
| Oxytocin | 14 | 1 | 13 | 6 | 4 | 4 | 9.35 ± 2.34 | 65.21 ± 28.91 | 106.61 ± 30.65 |
| Placebo | 18 | 4 | 14 | 8 | 5 | 5 | 8.13 ± 1.87 | 67.39 ± 26.43 | 106.33 ± 25.00 |
χ2 was used to test whether the distribution of individuals randomized to the treatment conditions differed by sex and by ethnicity; no significant effects were found. For age, IQ, and pretreatment SRS Total Raw Score, differences between groups were tested with a simple one-way general linear model; no significant effects were discerned. The values are reported as mean ± SD.
Table S1.
Participants’ stable concomitant medications during the treatment trial
| Medication | OXT (%), n = 14 | Placebo (%), n = 18 |
| SSRI | 2 (14) | 3 (17) |
| Antipsychotic | 1 (7) | 2 (11) |
| Benzodiazepine | 1 (7) | 0 (0) |
| Stimulant | 1 (7) | 1 (6) |
| Anti-epileptic | 0 (0) | 2 (11) |
| NMDAR antagonist | 0 (0) | 1 (6) |
| Guanfacine | 1 (7) | 0 (0) |
Fisher’s Exact Test was used to test for differences in concomitant medications between OXT-treated and placebo-treated individuals. No significant differences were discerned. Medication classes are reported as counts and percentages. NMDAR, N-methyl-d-aspartate receptor; SSRI, selective serotonin reuptake inhibitor.
Evaluation of Biomarker Inclusion in the Statistical Model.
To test our a priori hypothesis that endogenous pretreatment neuropeptide measures would enhance our ability to detect OXT treatment effects, we first directly compared the R2 of two statistical models for our primary outcome measure (i.e., the SRS Total Raw Score). Failure to include the biomarker measures in our model resulted in an R2 of only 51%, with OXT-treated individuals showing only marginal (and nonsignificant) improvement in social abilities compared with placebo-treated individuals (F1,27 = 3.716; P = 0.0645). However, when the biomarker measures were included in the model (Results below), the R2 increased significantly, from 51 to 73% (F6,21 = 3.131; P = 0.0237), corresponding to an improvement of 33.89 points in the small-sample-size–corrected version of the Akaike information criterion (AICc). Thus, the inclusion of patients’ endogenous pretreatment neuropeptide measures increased the explained variation in SRS improvement significantly, by 43%. Consequently, the biomarker measures were included in all subsequent analyses detailed below.
Effects of OXT Treatment on Social Abilities.
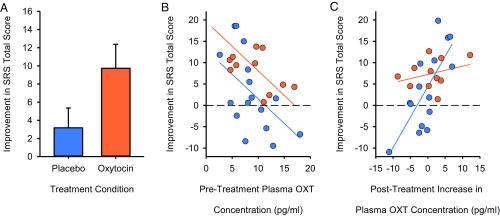
OXT-treated individuals showed greater improvement in social abilities (as measured by the SRS Total Raw Score) than placebo-treated individuals following completion of the 4-wk trial (F1,21 = 5.6083; P = 0.0275) (Fig. 2A). As illustrated in Fig. 2B, no OXT-treated individual’s social abilities worsened, but 6/16 (37.5%) of placebo-treated individuals’ did so. Pretreatment OXT concentration also negatively predicted the magnitude of the improvement in the SRS Total Raw Score (F1,21 = 11.4577; P = 0.0028) (Fig. 2B), such that individuals with the lowest pretreatment OXT concentrations improved the most following 4-wk treatment (Fig. 2B). No effect of pretreatment neuropeptide receptor gene expression on improvement in social abilities (or any other measures) was discerned, but inclusion of this measure in the model did increase R2 and AICc (by 3.06 points), indicating that it contributes explanatory power to the analyses.
Fig. 2.
OXT treatment efficacy and biomarkers of social improvement. (A) OXT compared with placebo treatment enhances social abilities in children with ASD as measured by improvement in the trial’s primary outcome measure, the SRS. Data are presented as least squares means ± SE. (B) The pretreatment blood OXT concentration robustly predicts treatment response, such that individuals with the lowest pretreatment OXT concentrations show the greatest SRS Total Raw Score improvement. (C) Study placebo responders are identifiable by a robust posttreatment increase in blood OXT concentrations that accompany their SRS Total Raw Score improvement. Data are corrected for the blocking factors in the analysis. Placebo-treated children are depicted in blue; OXT-treated children are depicted in orange.
Unexpectedly, pretreatment blood OXT concentration did not differ in the prediction of treatment outcomes between OXT- and placebo-treated individuals (F1,19 = 0.5980; P = 0.4488). In other words, the pretreatment blood OXT concentration predicted treatment efficacy in both groups. To ensure that this finding was not an artifact of insufficient power to detect an interaction, we performed a follow-up analysis. We hypothesized that the observed social improvement in some placebo-treated participants might coincide with increased endogenous OXT secretion. If so, the change in pretreatment to posttreatment endogenous plasma OXT concentration should predict the magnitude of the placebo response, but no such relationship should exist in OXT-treated participants (whose measured OXT concentrations would comprise both endogenous and also variable quantities of exogenous OXT). Accordingly, we tested whether the posttreatment increase in plasma OXT concentration predicted greater improvement in the SRS Total Raw Score. As predicted, we observed a treatment condition × OXT concentration interaction (F1,18 = 6.0333; P = 0.0244) (Fig. 2C), whereby larger increases in posttreatment plasma OXT concentrations predicted larger SRS improvement in placebo-treated (F1,18 = 16.5280; P = 0.0007) but not in OXT-treated (F1,18 = 0.3067; P = 0.5865), individuals.
Effects of OXT Treatment on Other Core and Associated Symptom Measures.
OXT’s effects were specific to social functioning, because OXT compared with placebo treatment did not significantly reduce repetitive behaviors [as measured by the Repetitive Behaviors Scale-Revised (RBS-R) Total Score (F1,20 = 0.0010; P = 0.9757) (Fig. S1A) or any of the RBS-R subscales], nor did pretreatment plasma OXT concentrations predict posttreatment RBS-R Total Score either as an interaction with treatment condition (F1,20 = 0.0168; P = 0.8983) or as a main effect (F1,20 = 0.0359; P = 0.8517). Additionally, no relationships were discerned between pretreatment plasma OXT concentrations and any RBS-R subscales. Similarly, OXT compared with placebo treatment did not reduce anxiety symptoms (as measured by the Spence Children’s Anxiety Scale Total Score, hereafter “Spence”) (F1,18 = 0.9626; P = 0.3395) (Fig. S1B), nor did pretreatment plasma OXT concentrations predict posttreatment Spence Total Score either as an interaction with treatment condition (F1,16 = 1.5400; P = 0.2325), or as a main effect (F1,18 = 0.4176; P = 0.5263).
Fig. S1.
OXT compared with placebo treatment did not ameliorate nonsocial core or associated features of autism spectrum disorder. (A) No significant difference was observed between posttreatment repetitive behaviors as measured by the RBS-R Total Score. (B) No significant difference was observed between posttreatment anxiety symptoms as measured by the Spence Children’s Anxiety Scale Total Score. Data are corrected for the blocking factors in the analysis and are presented as least squares mean ± SE.
Effects of OXT Treatment on Safety and Tolerability Measures.
OXT treatment in children with ASD was well tolerated with minimal side effects. There were no significant differences in the adverse event rates reported in the OXT-treated and the placebo-treated groups as assessed by parent ratings on the Dosage Record Treatment Emergent Symptom Scale (DOTES) (Table S2). Two individuals randomized to OXT treatment did not complete 4-wk assessments. One participant refused to take the nasal spray at the time of the first dose, and one participant was provided with a supply of OXT but did not return to the clinic following 4-wk treatment. (Attempts to bring the latter participant back to the clinic were unsuccessful.)
Table S2.
Reported adverse events during 4-wk treatment assessed using the DOTES
| Adverse event | OXT (%), n = 14 | Placebo (%), n = 18 |
| General | ||
| Cold symptoms | 0 (0) | 1 (6) |
| Fever | 0 (0) | 1 (6) |
| Cough | 1 (7) | 0 (0) |
| Neurological/psychiatric | ||
| Headache | 1 (7) | 0 (0) |
| Insomnia | 1 (7) | 1 (6) |
| Excitement/agitation | 1 (7) | 2 (11) |
| Depressive affect | 0 (0) | 1 (6) |
| Labile mood | 1 (7) | 1 (6) |
| Silly behavior | 1 (7) | 0 (0) |
| More distractible | 1 (7) | 0 (0) |
| HEENT | ||
| Nasal congestion | 3 (21) | 0 (0) |
| Epistaxis | 1 (7) | 0 (0) |
| Sneezing | 0 (0) | 1 (6) |
| Mouth pain | 0 (0) | 1 (6) |
| Intranasal swelling | 1 (7) | 0 (0) |
| Runny nose | 1 (7) | 0 (0) |
| Blinking eyes | 0 (0) | 1 (6) |
| Earache | 0 (0) | 1 (6) |
| Nasal discomfort | 0 (0) | 1 (6) |
| Gastrointestinal | ||
| Loose stool | 1 (7) | 0 (0) |
| Constipation | 1 (7) | 0 (0) |
| Stomach discomfort | 0 (0) | 1 (6) |
| Dermatological | ||
| Skin cut | 0 (0) | 2 (11) |
Fisher’s Exact Test was used to test for differences in adverse events between OXT-treated and placebo-treated individuals. No significant effects were discerned. Adverse events are reported as counts and percentages. HEENT, head, ears, eyes, nose, throat.
No significant changes from baseline in height or weight or in the majority of vital signs measurements were observed after 4-wk treatment (Table S3). Systolic standing blood pressure did show an increase in OXT-treated but not in placebo-treated participants (P = 0.0218). However, this effect was not significant once multiple comparisons were considered (i.e., six blood pressure measurements were performed, so the adjusted significance level was set at P < 0.0083). Change in systolic blood pressure (standing–sitting) did show a significant treatment condition × time point interaction (P = 0.0004) that remained significant once multiple comparisons were considered. This finding was unrelated to OXT treatment, however, because placebo-treated participants showed a decrease in this measurement over the 4-wk treatment period. Thus the treatment-condition groups differed at baseline but not following treatment.
Table S3.
Physiological assessments at pretreatment baseline and following 4-wk treatment
| OXT | Placebo | ||||
| Measure | Baseline | After 4-wk treatment | Baseline | After 4-wk treatment | P value |
| Height, inches | 134 (126, 141) | 134 (127, 142) | 129 (122, 135) | 129 (122, 136) | P = NS |
| Weight, kilograms | 32.7 (27.0, 39.5) | 33.5 (27.7, 40.4) | 28.0 (23.5, 33.2) | 28.4 (23.9, 33.7) | P = NS |
| BP, systolic, sitting, mmHg | 104.2 (98.7, 109.8) | 111.1 (105.5, 116.6) | 101.0 (95.6, 106.4) | 110.9 (105.6, 116.3) | P = NS |
| BP, diastolic, sitting, mmHg | 64.3 (59.0, 69.6) | 71.4 (66.1, 76.7) | 64.6 (59.4, 69.7) | 68.9 (63.8, 74.1) | P = NS |
| BP, systolic, standing, mmHg | 101.6 (95.2, 108.1) | 111.6 (105.2, 118.1) | 107.7 (101.5, 113.9) | 105.6 (99.4, 111.9) | P = NS |
| BP, diastolic, standing, mmHg | 63.3 (58.0, 68.6) | 70.9 (65.6, 76.2) | 68.2 (63.1, 73.3) | 69.8 (64.7, 74.9) | P = NS |
| BP change systolic (standing–sitting) | −2.62a (−6.96, 1.73) | 0.54ab (−3.81, 4.88) | 6.71b (2.53, 10.90) | −5.29a (−9.47, −1.10) | P = 0.0004 |
| BP change diastolic (standing–sitting) | −1.00 (−4.02, 2.02) | −0.46 (−3.48, 2.56) | 3.64 (0.73, 6.55) | 0.86 (−2.05, 3.77) | P = NS |
| Pulse sitting (beats per minute) | 92.5 (83.0, 101.9) | 97.1 (87.7, 106.5) | 92.7 (83.6, 101.8) | 101.6 (92.5, 110.6) | P = NS |
| Pulse standing (beats per minute) | 95.5 (87.3, 103.6) | 102.3 (94.1, 110.5) | 102.6 (94.7, 110.4) | 107.3 (99.4, 115.2) | P = NS |
| Pulse change | 3.00 (−2.69, 8.69) | 5.23 (−0.46, 10.92) | 9.86 (4.38, 15.34) | 5.71 (0.23, 11.19) | P = NS |
| Temperature, °F | 98.3 (98.1, 98.6) | 98.2 (97.9, 98.5) | 98.3 (98.0, 98.5) | 98.3 (98.1, 98.6) | P = NS |
Data were analyzed as a repeated-measures restricted maximum likelihood (REML) mixed model. The significance of the treatment condition × time point interaction is reported. Tukey post hoc tests are reported with standard notation, so that means with the same lettered superscript do not differ significantly. Measures are reported as least squares means with 95% CIs. Fourteen OXT-treated and 17 placebo-treated participants tolerated repeated height and weight assessments; and 13 OXT-treated and 14 placebo-treated participants tolerated repeated vital sign assessments. BP, blood pressure; NS, not significant.
Discussion
Here we studied whether intranasal OXT treatment improved social abilities in children with ASD when accounting for pretreatment variation in neuropeptide biology and tested whether pretreatment blood OXT concentrations predicted OXT treatment response. Despite preclinical evidence indicating that OXT may be a promising therapeutic for human social impairments, prior intranasal OXT treatment trial findings for ASD have been equivocal; some trials have reported that OXT improves social abilities (19, 20), but other trials have not (21, 22). However, the preponderance of preclinical evidence demonstrating OXT rescue of social phenotypes has been gleaned from animals with known OXT signaling impairments (13, 15). Because idiopathic ASD is characterized by enormous heterogeneity, including substantial variability in blood OXT concentrations (27), we hypothesized that the prior ambiguous OXT clinical trial findings might be attributable, at least in part, to variability in participants’ pretreatment neuropeptide biology.
Using a double-blind, randomized, placebo-controlled, parallel design, we found that 4-wk OXT compared with placebo treatment significantly enhanced social abilities in children with ASD. We also found that pretreatment blood OXT concentrations predicted treatment response, such that individuals with the lowest pretreatment OXT concentrations showed the greatest social improvement. Importantly, we also confirmed that failure to include participants’ endogenous pretreatment neuropeptide measures in our statistical model would have resulted in a negative trial, whereas inclusion of these biomarker measures improved the explanatory power of our model by 43%. Results from the present study indicate that OXT administration enhances social abilities in children with ASD and that a priori stratification of ASD patients into treatment conditions based on known pretreatment blood OXT concentrations will be essential to test the full range of OXT’s therapeutic potential.
Placebo responses are frequently reported in psychiatric clinical trials (30), including trials in ASD (19, 22, 31). Placebo responses can compromise trial outcomes, rendering medications that otherwise might have been deemed efficacious as “failed” interventions. Placebo responses occur for a variety of reasons; for example, in one recent negative OXT treatment trial (22), improvement in child ASD symptoms was most strongly associated with caregiver belief that the participant was on the active drug (whether or not this was the case). In other instances, the reason for improvement following placebo treatment is less clear. The present study provided the opportunity to begin exploring the biological basis of social improvement in placebo-treated participants, because we unexpectedly found that pretreatment blood OXT concentrations predicted treatment outcomes in both OXT- and placebo-treated children. In a follow-up analysis, we found that a greater increase in plasma OXT concentration posttreatment predicted the magnitude of SRS improvement in placebo-treated (but not in OXT-treated) individuals. This finding is consistent with the notion that increased endogenous OXT secretion (perhaps caused by enhanced social interactions during the trial) may underlie the social improvement in placebo-treated participants. Although of a correlational and preliminary nature, this finding raises several intriguing ideas that merit further research. For example, increased endogenous OXT release could contribute to observed placebo responses in clinical trials with social behavior end points or could serve as a biological mediator of effective behavioral interventions with significant social learning components (e.g., Pivotal Response Treatment).
There has been recent discussion about the prudence of pediatric OXT use when its long-term consequences are unknown (32). Although longitudinal studies to address this concern are needed, chronic OXT administration is generally well tolerated in children with ASD (19, 22, 33). Tolerability and safety data from the present trial support this assertion, because the adverse event rate did not differ between treatment conditions, and there were no significant changes from baseline in height or weight or in vital sign measurements attributable to OXT treatment. Additionally, although OXT treatment did not ameliorate nonsocial core or associated ASD symptoms, it did not worsen them.
The present study has several limitations. First, although we made a concerted effort to recruit female participants, our final sample was 84% male and was not powered to detect sex differences in treatment response. Second, participants were permitted to take other medications during the intervention. These medications are not known to interact with intranasal OXT, were stable before study entry, and did not differ between groups. Thus the observed social improvement in OXT-treated participants was unlikely to have been caused by other medications or by their interactions with intranasal OXT. Finally, many of our outcome measures relied on parent reporting to ascertain OXT-related changes. Although we used gold-standard instruments, these measures are nevertheless subjective in nature. Development of well-validated objective measures, including biologically based methods such as resting-state EEG, to assess participants’ behavioral change during interventions is urgently needed.
In summary, there are currently no pharmacotherapies that effectively ameliorate core ASD symptoms. Hearteningly, the present clinical trial showed that OXT treatment enhances social abilities in children with ASD and that individuals with pretreatment OXT signaling deficits may stand to benefit most from OXT administration. Although confirmatory evidence from larger-scale, biomarker-stratified OXT treatment trials is needed, our findings suggest that OXT treatment has the potential to reduce suffering in ASD patients by enhancing quality of life through improved social abilities.
Materials and Methods
Study Regulatory Approval.
This study was conducted in the Autism and Developmental Disabilities Clinic (ADDC) in the Division of Child and Adolescent Psychiatry at Stanford University. Recruitment began in June 2012 and ended in April 2016. Before initiating this trial, an Investigational New Drug application (no. 114664) was filed with the Federal Drug Administration, and this study was approved by the Internal Review Board of the Stanford University School of Medicine. This trial was also registered on ClinicalTrials.gov (NCT01624194). Parents and/or legal guardians of the study’s participants provided informed written consent before the initiation of any experimental procedures. If the child was deemed intellectually capable of understanding the study, written assent was also obtained from the child. Finally, this study was overseen by an independent Data Safety Monitoring Board comprised of clinicians with expertise in clinical trials, ASD, and/or pediatric medical care. The trial protocol is available upon request.
Participant Recruitment and Eligibility Criteria.
Children with a diagnostic history of ASD were recruited to participate in this study. Participants were recruited through (i) the Autism and Developmental Disorders Research Registry at Stanford University, (ii) flyers posted in the ADDC or in the surrounding community (e.g., pediatrician offices), (iii) advertisements posted online (e.g., parent listservs), or (iv) special events (e.g., the Bay Area Autism Speaks Walk). Participants were telephone-screened for initial study eligibility and then underwent a medical assessment (including ECG, heart rate, and blood pressure measurement) as well as a comprehensive psychiatric evaluation. The psychiatric evaluation included determination of the accuracy of the child’s previous ASD diagnosis based on Diagnostic and Statistical Manual of Mental Disorders IV Text Revision (DSM-IV-TR) (34) or DSM-5 (1) criteria, which was confirmed with research diagnostic methods [i.e., the Autism Diagnostic Instrument-Revised (35) and the Autism Diagnostic Observation Schedule using the revised algorithms (36)] conducted by research staff trained by a research-reliable clinician.
In addition to meeting diagnostic criteria for ASD, other study inclusion criteria included (i) medically healthy outpatients between 6 and 12.92 y of age with (ii) an IQ >40 [as determined by the Stanford-Binet Intelligence Scales, fifth edition (37)], (iii) a Clinical Global Impression severity rating of ≥4 (38), (iv) a care provider who could reliably bring the participant to clinic visits, provide trustworthy ratings, and interact with the participant on a regular basis, (v) stable medications for at least 4 wk, (vi) no planned changes in psychosocial interventions during the trial, and (vii) willingness to provide blood samples.
Study exclusion criteria included (i) prior or current use of OXT; (ii) DSM-IV-TR or DSM-5 diagnosis of schizophrenia, schizoaffective disorder, or psychotic disorder; (iii) regular nasal obstruction or nosebleeds; (iv) active medical problems: unstable seizures or significant physical illness (e.g., serious liver, renal, or cardiac pathology); (v) sensitivity to preservatives (e.g., chlorobutanol hemihydrate); (vi) evidence of a genetic mutation known to cause ASD (e.g., fragile X Syndrome); (vii) significant hearing or vision impairments; (viii) habitual consumption of large volumes of water; (ix) pregnancy, breastfeeding, or child birth within the last 6 mo; or (x) sexually active females not using a reliable method of contraception.
Pharmacological Intervention.
Syntocinon nasal spray (Novartis) was purchased from a Swiss pharmacy (Dr. Noyer Apotheke). The placebo solution was prepared by Koshland Pharm and consisted of all the ingredients used in the active solution except the OXT compound. A pharmacist next transferred 35 mL of Syntocinon [40 International Units (IU)/mL] or placebo into a disposable nasal applicator to ensure that the drug and placebo applicators were visually indistinguishable to the research team. These applicators were coded and given to the Stanford Health Care’s Investigational Drug Service (IDS) for refrigerated storage (2–8 °C) and subsequent dispensing.
After the screening phase, pretreatment baseline measures were obtained from participants continuing to meet inclusion and exclusion criteria. These measures included assessments to determine participants’ symptom severity and phenotype, evaluations for safety/tolerability monitoring, and blood sample collection for later biomarker quantification. One to four weeks later, participants were randomly assigned (1:1) to a treatment condition (i.e., OXT or placebo), stratified by gender. Randomization was performed by an IDS pharmacist using www.randomization.com, which allocates each participant to an intervention by using the method of randomly permuted blocks. This practice allowed the research team to remain blinded throughout the trial’s duration.
Parents were trained in the clinic by research staff to administer the nasal spray to their child. The first dose, three puffs per nostril (4 IU per puff), for a total dose of 24 IU OXT or placebo was administered in the ADDC. Vital signs were measured before and 30 min after initial single-dose nasal spray administration to monitor for acute, unanticipated reactions to the drug. Participants’ parents then were provided with a 4-wk drug supply and were responsible for their child’s continued twice daily dosing (24 IU per dose, 48 IU/d) at home. Parents were instructed to keep the drug refrigerated with only brief room temperature excursions (i.e., for dosing). On completion of the 4-wk treatment period, participants returned with their parent to the clinic, and behavioral data, safety/tolerability data, and blood samples were again collected.
Blood Sample Collection and Processing Procedures.
Twenty milliliters of whole blood was drawn from the child’s antecubital region by a pediatric phlebotomist using standard protocols at Lucile Packard Children’s Hospital outpatient laboratory. Whole blood was collected into chilled EDTA-treated vacutainer tubes and was placed on wet ice immediately. Samples were centrifuged promptly (1,600 × g at 4 °C for 15 min), and the plasma fraction was aliquoted into polypropylene tubes and flash-frozen on dry ice. Whole blood was also collected into PAXgene RNA tubes (Qiagen) and processed per the manufacturer’s instructions. All samples were stored at −80 °C until quantification.
Plasma OXT Quantification.
Plasma OXT concentrations were quantified using a commercially available enzyme immunoassay kit (Enzo Life Sciences, Inc.). This kit is highly specific and selectively recognizes OXT but not related peptides (i.e., cross-reactivity with vasopressin is 0.6%). A technician blinded to treatment condition performed sample preparation and OXT quantification following established procedures (27). Briefly, plasma samples (1,000 µL per participant) were extracted using Strata-X columns (Phenomenex Inc.) and evaporated using compressed nitrogen. Each evaporated sample was reconstituted in 250 µL of assay buffer before OXT quantification to provide a sufficient sample volume to run each participant’s sample in duplicate wells (100 µL per well). This practice ensured that the plated samples contained sufficiently high OXT quantities to be read above the limit of detection (11.7 pg/mL). Samples were assayed with a tunable microplate reader (Molecular Devices) for the 96-well format per the manufacturer’s instructions.
Quantification of OXTR and V1AR Gene-Expression Levels.
Total RNA was isolated and purified using a PAXgene blood RNA kit from blood stabilized in PAXgene RNA tubes (Qiagen). The first-strand cDNA synthesis reaction was carried out with the QuantiTect reverse transcription kit (Qiagen) with a starting RNA quantity of 1 µg in a 20-µL final volume. The primer sequence information for OXTR and V1AR genes was obtained from published studies and was designed as follows: OXTR forward 5′-CTGAACATCCCGAGGAACTG-3′ and reverse 5′-CTCTGAGCCACTGCAAATGA-3′ (39); V1AR forward 5′-CTTTTGTGATCGTGACGGCTTA-3′ and reverse 5′-TGATGGTAGGGTTTTCCGATTC-3′ (40). Two housekeeping genes, hypoxanthine phosphoribosyltransferase 1 [HPRT1; forward 5′-GGACAGGACTGAACGTCTTGC-3′ and reverse 5′-ATAGCCCCCCTTGAGCACAC-3′ (40)] and ubiquitin C [UBC; forward 5′-GCTGCTCATAAGACTCGGCC-3′ and reverse 5′-GTCACCCAAGTCCCGTCCTA-3′ (40)] were selected for normalization using geNorm. qPCR was performed on the StepOnePlus Real-Time PCR System (Life Technologies) with SYBR Green (Thermo Fisher Scientific). cDNA was PCR amplified in triplicate, and cycle threshold (Ct) values from each sample were obtained using StepOnePlus software. Analyses were conducted using the comparative Ct method (2−ΔΔCt) (41).
Outcome Measures.
The trial’s primary outcome measure was improvement in social abilities as measured by the SRS. The SRS is a norm-referenced questionnaire developed to measure social functioning in both clinical and nonclinical populations (42, 43). The SRS is a sensitive measure (i.e., it strongly correlates with DSM criterion scores) with high reliability.
The trial’s secondary outcome measures included treatment efficacy in nonsocial symptom domains and OXT safety/tolerability. Treatment effect generalizability was evaluated using (i) the RBS-R (44), which measures a comprehensive list of repetitive and stereotyped behaviors, and (ii) The Spence Children’s Anxiety Scale (45), which assesses the severity of trait anxiety symptoms broadly in line with DSM dimensions of anxiety disorder. Anxiety was selected as an outcome measure because of its significant comorbidity with ASD (46), its negative relationship with endogenous OXT concentrations in humans (47), and OXT’s anxiolytic effects in mice (48). Drug safety and tolerability were evaluated using (i) the DOTES, a rating scale that assesses the presence, frequency, and severity of side effects (38), and (ii) change from baseline in vital signs (i.e., blood pressure, heart rate, temperature) and in height and weight.
Statistical Analyses.
Data were managed using REDCap (49) and analyzed using Least-Squares General Linear Models (LS-GLM) in JMP Pro-13 (SAS Institute Inc.). To identify a robust model for our primary outcome measure, initial analyses included IQ, age, and ethnicity as blocking factors. Gender was also included as a blocking factor to enable the use of the SRS Total Raw Score rather than the gender-normed T-Score, which has lower resolution. The pretreatment SRS Total Raw Score was included as a blocking factor to account for the range of possible social ability improvement and thus to reduce possible floor or ceiling effects. Finally, we included treatment condition (i.e., OXT or placebo) as a main effect to test our primary outcome measure and pretreatment blood OXT concentration and neuropeptide receptor gene expression level (expressed as a V1AR:OXTR ratio to account for within-individual differences in baseline expression) as biomarkers hypothesized to affect treatment efficacy. We also tested for treatment condition × biomarker interactions, because a predictive biomarker generally should predict treatment outcome only in drug-treated individuals.
Initial analyses showed that age and ethnicity introduced collinearities and did not improve the R2 of the model; therefore these factors were removed following best practice for linear models (50). Similarly, nonsignificant biomarker interactions were removed to avoid confounds of marginality for the main effects (but see Table S4 for tested interactions). The final model contained IQ, gender, and pretreatment SRS Total Raw Score as blocking variables and treatment condition, pretreatment blood OXT concentration, and V1AR:OXTR ratio as hypothesis-driven main effects (Table S5). To assess the impact of the biomarkers, we also ran the same model excluding the biomarkers (Table S6). The two models yield R2 values and residual sum of squares and degrees of freedom that can be used to calculate the difference in mean squared error (MSE) between the models, and create an F-ratio that allows us to test the difference in MSE (i.e., unexplained variance) against the MSE of the full model, giving a formal test of the difference between the R2 values. The AICc is reported in addition to R2 as a measure of model goodness of fit where appropriate. Differences of two points or more in AICc are considered to represent a meaningful improvement in model fit.
Table S4.
Statistical model for the primary outcome measure with biomarker measures and interaction terms included
| Source | df | Sum of squares | Mean square | F ratio | Probability >F |
| Effect summary | |||||
| Model | 8 | 2,892.2331 | 361.529 | 6.6232 | |
| Error | 19 | 1037.1241 | 54.585 | ||
| Total | 27 | 3929.3571 | |||
| Effect tests | |||||
| Gender | 1 | 53.7194 | 0.9841 | 0.3336 | |
| Pretreatment SRS Total Raw Score | 1 | 1,388.4038 | 25.4354 | <0.0001 | |
| IQ | 1 | 315.3158 | 5.7766 | 0.0266 | |
| Drug | 1 | 287.7586 | 5.2717 | 0.0332 | |
| Pretreatment OXT concentration | 1 | 602.6659 | 11.0408 | 0.0036 | |
| Drug × pretreatment OXT concentration | 1 | 32.6437 | 0.598 | 0.4488 | |
| Pretreatment V1AR:OXTR ratio | 1 | 28.5371 | 0.5228 | 0.4785 | |
| Drug × pretreatment V1AR:OXTR ratio | 1 | 4.8916 | 0.0896 | 0.7679 |
Table S5.
Statistical model for the primary outcome measure with biomarker measures included and interaction terms removed
| Source | df | Sum of squares | Mean square | F ratio | Probability >F |
| Effect summary | |||||
| Model | 6 | 2,858.0891 | 476.348 | 9.3378 | |
| Error | 21 | 1,071.2681 | 51.013 | ||
| Total | 27 | 3,929.3571 | |||
| Effect tests | |||||
| Gender | 1 | 63.3242 | 1.2413 | 0.2778 | |
| Pretreatment SRS Total Raw Score | 1 | 1,608.9667 | 31.5405 | <0.0001 | |
| IQ | 1 | 351.4972 | 6.8904 | 0.0158 | |
| Drug | 1 | 286.0964 | 5.6083 | 0.0275 | |
| Pretreatment OXT concentration | 1 | 584.4907 | 11.4577 | 0.0028 | |
| Pretreatment V1AR:OXT ratio | 1 | 35.7054 | 0.6999 | 0.4122 |
Table S6.
Statistical model for the primary outcome measure with biomarker measures removed
| Source | df | Sum of squares | Mean square | F ratio | Probability >F |
| Effect summary | |||||
| Model | 4 | 2,076.3496 | 519.087 | 6.9053 | |
| Error | 27 | 2,029.6426 | 75.172 | ||
| Total | 31 | 4,105.9922 | |||
| Effect tests | |||||
| Gender | 1 | 3.727 | 0.0496 | 0.8255 | |
| Pretreatment SRS Total Raw Score | 1 | 1,704.896 | 22.68 | <0.0001 | |
| IQ | 1 | 431.1381 | 5.7354 | 0.0238 | |
| Drug | 1 | 279.371 | 3.7164 | 0.0645 |
Once the model inclusive of biomarker measures was identified for our primary outcome measure, the same model was applied to the secondary outcome measures, with the exception that the pretreatment behavioral measure was replaced to match the outcome variable. To minimize the risk of false discovery from multiplicity, we tested the total score for the SRS, RBS-R, and Spence. However, because psychometric validity for the RBS-R Total Score is not well established, we also performed the same analyses on each RBS-R subscale but corrected our critical P value to 0.0083 to protect against multiple comparisons and to achieve the same family-level significance as the total score. Similarly, to minimize the risk of false discovery, blocking factors were not tested for significance (50). The assumptions of LS-GLM (linearity, homogeneity of variance, and normality of error) were confirmed post hoc, and suitable transformations were applied as needed. Post hoc tests were performed as planned contrasts and were Bonferroni corrected for multiple comparisons. Least squares means ± SE are reported. Least squares means are model-derived means controlling for other factors in the analysis and correspond to the model coefficients represented by the F-ratio.
Adverse event data were analyzed with a repeated-measures restricted maximum likelihood mixed model using the treatment condition × time point interaction to test whether treatment affected the measures, controlling for any pretreatment baseline differences. Suitable transformations were applied as needed. Significant results were examined and Tukey-corrected for multiple comparisons.
Acknowledgments
We thank members of the K.J.P. and A.Y.H. laboratories; the pharmacists at the Stanford Health Care Investigational Drug Services and Koshland Pharm; Drs. Carl Feinstein and Elliott Sherr for providing helpful feedback on this manuscript; and the study participants and their families for their participation. This research was supported by The Mosbacher Family Fund for Autism Research (K.J.P.), The Child Health Research Institute (K.J.P., A.Y.H., and O.O.), The Yani Calmidis Memorial Fund for Autism Research (K.J.P.), Autism Speaks Meixner Postdoctoral Fellowship in Translational Research 7895 (to D.S.C.), a Stanford University School of Medicine Dean’s Postdoctoral Fellowship (to D.S.C.), National Institute of Mental Health Grant T32MH019908 (to D.S.K.), and Stanford's Department of Psychiatry (A.Y.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.L.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705521114/-/DCSupplemental.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Diagnostic Criteria for Autistic Disorder. 5th Ed Am Psychiatr Assoc; Washington, DC: 2013. [Google Scholar]
- 2.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years–Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 3.Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168:721–728. doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- 4.DiCicco-Bloom E, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Koshimizu TA, et al. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 7.Arrowsmith S, Wray S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26:356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 9.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SW, Platt ML. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 2014;1580:57–68. doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell HK, Aulino EA, Freeman AR, Miller TV, Witchey SK. Oxytocin and behavior: Lessons from knockout mice. Dev Neurobiol. 2017;77:190–201. doi: 10.1002/dneu.22431. [DOI] [PubMed] [Google Scholar]
- 12.Schaller F, et al. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010;19:4895–4905. doi: 10.1093/hmg/ddq424. [DOI] [PubMed] [Google Scholar]
- 13.Peñagarikano O, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis SM, et al. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014;1580:199–218. doi: 10.1016/j.brainres.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meziane H, et al. An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol Psychiatry. 2015;78:85–94. doi: 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Hollander E, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol Psychiatry. 2016;21:1225–1231. doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 21.Dadds MR, et al. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J Autism Dev Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 22.Guastella AJ, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J Child Psychol Psychiatry. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- 23.Munesue T, et al. Oxytocin for male subjects with autism spectrum disorder and comorbid intellectual disabilities: A randomized pilot study. Front Psychiatry. 2016;7:2. doi: 10.3389/fpsyt.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anagnostou E, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modahl C, et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 26.Al-Ayadhi LY. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences (Riyadh) 2005;10:47–50. [PubMed] [Google Scholar]
- 27.Parker KJ, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M, et al. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen LM, et al. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36:891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- 30.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: Culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- 31.Aman MG, et al. Safety and efficacy of memantine in children with autism: Randomized, placebo-controlled study and open-label extension. J Child Adolesc Psychopharmacol. March 15, 2016 doi: 10.1089/cap.2015.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller G. Neuroscience. The promise and perils of oxytocin. Science. 2013;339:267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostou E, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Diagnostic Criteria for Autistic Disorder. 4th Ed Am Psychiatr Assoc; Washington, DC: 2000. [Google Scholar]
- 35.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 36.Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 37.Roid GH. Stanford Binet’s Intelligence Scales, Fifth Edition, Technical Manual. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- 38.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. U.S. Department of Health, Education, and Welfare; Rockville, MD: 1976. [Google Scholar]
- 39.Gregory SG, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: The presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–799, 741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- 41.Lossie AC, et al. ENU mutagenesis reveals that notchless homolog 1 (Drosophila) affects Cdkn1a and several members of the Wnt pathway during murine pre-implantation development. BMC Genet. 2012;13:106. doi: 10.1186/1471-2156-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Constantino JN, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 43.Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 44.Lam KS, Aman MG. The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- 45.Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children's Anxiety Scale with young adolescents. J Anxiety Disord. 2003;17:605–25. doi: 10.1016/s0887-6185(02)00236-0. [DOI] [PubMed] [Google Scholar]
- 46.White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carson DS, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2015;20:1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- 48.Ring RH, et al. Anxiolytic-like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 49.Harris PA, et al. Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grafen A, Hails R. 2002. Modern Statistics for the Life Sciences (Oxford Univ Press, Oxford) p.