McAndrew et al. (1) report the crystal structure of NOV1, a stilbene-cleaving carotenoid cleavage oxygenase (CCO), in substrate-free and substrate/product-bound forms, all of which with dioxygen (O2) bound to the nonheme iron center of the enzyme. In mononuclear nonheme iron enzymes studied to date, the Fe–O2 interaction is strongly promoted by the binding of organic substrate/cofactor at or near the iron center (2), limiting formation of potentially detrimental reactive oxygen species (3). This proposed nonsequential substrate binding in NOV1 deviates from the current paradigm of O2 activation by nonheme iron enzymes.
The substrate-free NOV1 structure features O2 bound side-on to iron, modeled based on an elongated electron density peak. However, the strength of this elongated peak is nonuniform, in contrast to other side-on Fe–O2 complexes (4, 5), as reflected by the disparate B-factors for the two oxygen atoms (44 and 68 vs. 21 Å2 for iron). The oxygen with the shortest iron bond unexpectedly has the highest B-factor. O2 also was modeled in the NOV1–vanillin complex but has weak electron density support and clashes with the carbonyl oxygen of the bound product.
Also reported was a NOV1–resveratrol complex featuring bound organic substrate and O2 that seem poised to react (6). Suppressive effects of crystal packing on catalysis were invoked to rationalize the stability of the ternary complex but without supporting kinetic data. O2 was modeled similarly to the product complex. Despite the assertion that “well-resolved electron density was observed for the entire substrate molecule,” the map shows a break between the resveratrol α-carbon and 3,5-dihydroxy ring. Substrate B-factors are variable and elevated compared with the surrounding protein atoms. Moreover, resveratrol is listed as an electron density outlier in the Protein Data Bank validation report, as is O2. Thus, cleavage reaction products may significantly occupy the active site.
What other evidence supports these unusual iron–oxy structures? The authors state that the complex could be “described as Fe3+-superoxo or Fe(II)-O2,” although the citation provided is not particularly relevant. O2 complexation with Fe(II) is accompanied by electron transfer from the metal to oxygen, providing an EPR-detectable species (7). However, EPR measurements were made only on anaerobic NOV1. Instead, the reactivity of NOV1 was examined with the O2 surrogate, nitric oxide (NO) (8). NOV1 formed an Fe–NO complex in the absence of substrate with similar reactivity toward O2 implied. However, enzymes with known sequential substrate-O2 binding also react with NO in the absence of substrate (9, 10).
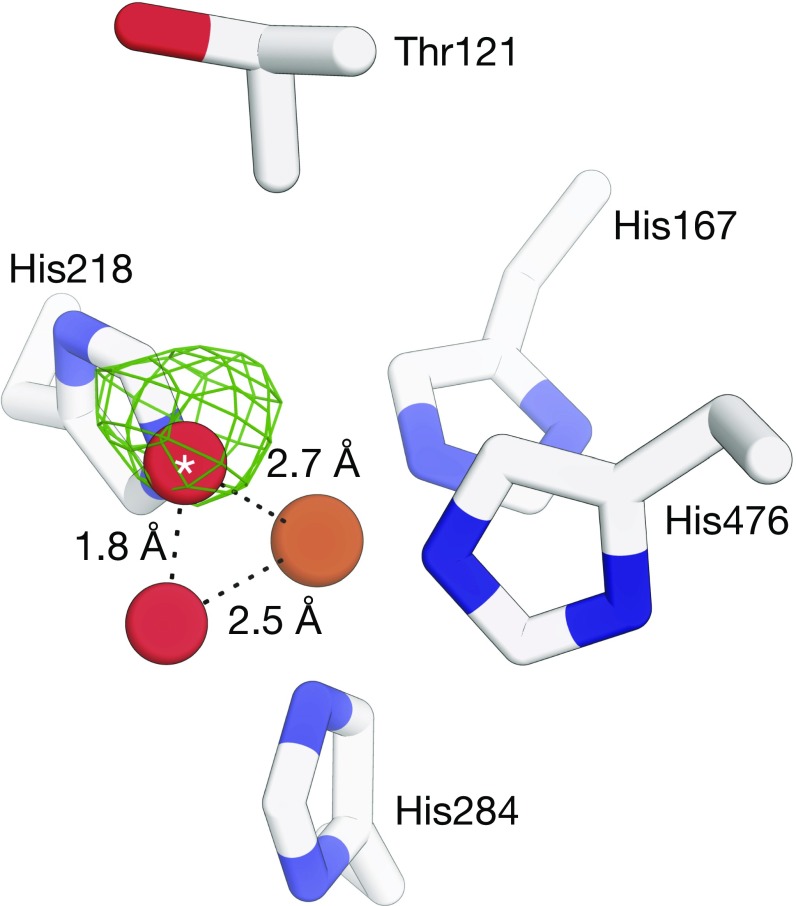
If the density does not represent O2, what are the alternatives? I rerefined the substrate-free NOV1 structure with unrestrained O–O and Fe–O bonds for the Fe–O2 complex (Fig. 1). Interestingly, the O–O bond length refined to a value of ∼1.8 Å, which is inconsistent with a covalent diatomic oxygen species. The density could instead be simply explained by solvent binding to iron at two distinct sites, each with partial occupancy.
Fig. 1.
Structure of the NOV1 iron center refined without O–O and Fe–O bond-length restraints. Shown is an σA-weighted Fo − Fc electron density map (green mesh, contoured at 3 rmsd with a 3-Å carve around the dioxygen atoms) calculated with the oxygen atom of the O2 molecule (red spheres) marked with an asterisk omitted from the model. The 1.8-Å distance between the two oxygen atoms after refinement is inconsistent with a diatomic oxygen species bound to the iron center (brown sphere). Alternative refinements in which two solvent molecules were modeled and repulsive nonbonding interactions between the solvent molecules omitted from the refinement target resulted in a similar but slightly longer O–O distance. Diffraction data and starting atomic coordinates used for the refinements are deposited in the Protein Data Bank under ID code 5J53. Refinement was carried out using REFMAC version 5.8.0158.
In conclusion, the NOV1 iron–oxy complexes should be viewed with some skepticism. More rigorous studies of the Fe–O2 interaction in CCOs with advanced kinetic and spectroscopic techniques are needed.
Acknowledgments
I thank Drs. Krzysztof Palczewski and Leslie T. Webster Jr. for helpful comments on the manuscript. This work was supported by NIH Grant EY009339 and Department of Veterans Affairs Grant IK2BX002683.
Footnotes
The author declares no conflict of interest.
References
- 1.McAndrew RP, et al. Structure and mechanism of NOV1, a resveratrol-cleaving dioxygenase. Proc Natl Acad Sci USA. 2016;113:14324–14329. doi: 10.1073/pnas.1608917113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovaleva EG, Lipscomb JD. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat Chem Biol. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerson JP, Farquhar ER, Que L., Jr Structural “snapshots” along reaction pathways of non-heme iron enzymes. Angew Chem Int Ed Engl. 2007;46:8553–8556. doi: 10.1002/anie.200703057. [DOI] [PubMed] [Google Scholar]
- 4.Jeoung JH, Bommer M, Lin TY, Dobbek H. Visualizing the substrate-, superoxo-, alkylperoxo-, and product-bound states at the nonheme Fe(II) site of homogentisate dioxygenase. Proc Natl Acad Sci USA. 2013;110:12625–12630. doi: 10.1073/pnas.1302144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovaleva EG, Lipscomb JD. Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates. Science. 2007;316:453–457. doi: 10.1126/science.1134697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borowski T, Blomberg MR, Siegbahn PE. Reaction mechanism of apocarotenoid oxygenase (ACO): a DFT study. Chemistry. 2008;14:2264–2276. doi: 10.1002/chem.200701344. [DOI] [PubMed] [Google Scholar]
- 7.Mbughuni MM, et al. Trapping and spectroscopic characterization of an FeIII-superoxo intermediate from a nonheme mononuclear iron-containing enzyme. Proc Natl Acad Sci USA. 2010;107:16788–16793. doi: 10.1073/pnas.1010015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CA, et al. Spectroscopic and theoretical description of the electronic-structure of S=3/2 iron-nitrosyl complexes and their relation to O-2 activation by nonheme tron enzyme active-sites. J Am Chem Soc. 1995;117:715–732. [Google Scholar]
- 9.Wolfe MD, Parales JV, Gibson DT, Lipscomb JD. Single turnover chemistry and regulation of O2 activation by the oxygenase component of naphthalene 1,2-dioxygenase. J Biol Chem. 2001;276:1945–1953. doi: 10.1074/jbc.M007795200. [DOI] [PubMed] [Google Scholar]
- 10.Wolgel SA, et al. Purification and characterization of protocatechuate 2,3-dioxygenase from Bacillus macerans: A new extradiol catecholic dioxygenase. J Bacteriol. 1993;175:4414–4426. doi: 10.1128/jb.175.14.4414-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]