Significance
TRPM7 (transient receptor potential cation channel subfamily M member 7) is required for normal organ development but also mediates anoxic neuronal death. TRPM7 contains a channel that conducts cations into the cytosol and C-terminal kinase that can phosphorylate multiple substrates. The kinase is cleaved to regulate gene expression and apoptosis. The link between TRPM7’s channel and its organismal function remains the least understood aspect of TRPM7. Here, we identify intracellular Zn2+ storage vesicles that contain the majority of TRPM7 protein. TRPM7 senses reactive oxygen species (ROS) to release Zn2+ from these vesicles. Just as the endoplasmic reticulum sequesters and releases Ca2+, we propose that these vesicles fulfill a similar function for Zn2+ and that TRPM7 coordinates fluctuations in cellular [Zn2+] and ROS during development and injury.
Keywords: TRPM7, zinc, vesicles
Abstract
TRPM7 (transient receptor potential cation channel subfamily M member 7) regulates gene expression and stress-induced cytotoxicity and is required in early embryogenesis through organ development. Here, we show that the majority of TRPM7 is localized in abundant intracellular vesicles. These vesicles (M7Vs) are distinct from endosomes, lysosomes, and other familiar vesicles or organelles. M7Vs accumulate Zn2+ in a glutathione-enriched, reduced lumen when cytosolic Zn2+ concentrations are elevated. Treatments that increase reactive oxygen species (ROS) trigger TRPM7-dependent Zn2+ release from the vesicles, whereas reduced glutathione prevents TRPM7-dependent cytosolic Zn2+ influx. These observations strongly support the notion that ROS-mediated TRPM7 activation releases Zn2+ from intracellular vesicles after Zn2+ overload. Like the endoplasmic reticulum, these vesicles are a distributed system for divalent cation uptake and release, but in this case the primary divalent ion is Zn2+ rather than Ca2+.
TRPM7 (transient receptor potential cation channel subfamily M member 7), an ion channel and cytoplasmic kinase, is ubiquitously expressed and essential in early embryonic development (1–4) but also may mediate oxidative stress-induced anoxic neuronal death in adults (5, 6). As an ion channel, TRPM7 conducts Zn2+>Mg2+∼ Ca2+ and monovalent cations (7–10) and contributes to labile cytosolic and nuclear Zn2+ concentrations (8). TRPM7’s C-terminal kinase can phosphorylate multiple substrates (11–13) and is cleaved to release a proapoptotic, chromatin-modifying enzyme (8, 14). Zn2+ regulates TRPM7’s kinase activity (11) and binding to transcription factors (8). It is a potent signal for many cellular processes previously related to TRPM7 function, including gene expression, mitosis, and cell survival, but is also proapoptotic at extreme concentrations (15–18).
TRPM7 has been proposed to regulate intracellular Mg2+ levels (19). However, as we have shown, Trpm7−/− cells have normal total magnesium concentrations (3, 14), with the many abundant magnesium transporters (20) probably accounting for normal Mg2+ homeostasis. Under physiological conditions, plasma membrane TRPM7’s inward conductance is miniscule (<10 pA/pF at −100 mV), but it increases over minutes if the free cytosolic Mg2+ concentration falls below its normal level of ∼0.5–1 mM (21). Because in most cells the primary buffers for Mg2+ are phosphonucleotides (22), the metabolic state potentially regulates TRPM7 activity as ATP levels rise and fall around 0.5 mM. Under normal circumstances, the driving force for Mg2+ entry into cells is relatively low ([Mg2+]o = 1 mM vs. [Mg2+]i ∼1 mM; EMg = 0 mV) compared with Ca2+ ([Ca2+]o = 2 mM vs. [Ca2+]i ∼100 nM; ECa = +125 mV) or Zn2+ ([Zn2+]o = 1–300 µM vs. [Zn2+]i ∼0.5 nM; EZn = +100 mV, with 1 µM [Zn2+]o). Few Zn2+-permeant channels are known, however, and the many putative Zn2+ transporters (>20 ZnT/SLC30 and ZiP/SLC39 in humans) primarily regulate [Zn2+] in organelles and the cytosol (23). TRPM7’s Zn2+ conductance suggests that it could support rapid changes in cytoplasmic Zn2+ levels under the right circumstances, either across the plasma membrane or from intracellular compartments.
Oxidative stress increases inward divalent ion permeation through TRPM7 (5, 9), whereas acute and chronic oxidative stress in culture (24–26) or during ischemia–reperfusion in vivo (27, 28) induces TRPM7 expression. The resulting increase in cytosolic Zn2+ could result in toxic cytosolic concentrations (9). Conversely, reduction of TRPM7 expression protects animals from postischemia reperfusion (5), a condition typically accompanied by increased reactive oxygen species (ROS) and Zn2+ release from intracellular stores (29). Physiological redox transitions required for stem cell differentiation and gene expression during embryonic development (30, 31) may also be sensed by TRPM7 and transduced via Zn2+-dependent signals (32).
Because TRPM7 patch-clamp recording is limited to the plasma membrane, the assumption has been that TRPM7’s main function is on the plasma membrane. However, we have previously shown that TRPM7 is also on intracellular membranes (33–35). Indeed, ROS activation of divalent ion influx through TRPM7 (5, 9) is reminiscent of ROS activation of TRPM2, which releases Ca2+ and Zn2+ from lysosomes (36, 37). Here we show that TRPM7 is an intracellular Zn2+ release channel located on previously uncharacterized vesicles (M7Vs) that are distinct from familiar organelles. These vesicles sequester Zn2+ during cytosolic overload. We show that the intravesicular M7V environment is reducing and that oxidation induces TRPM7-dependent Zn2+ release from these vesicles. We hypothesize that TRPM7-mediated Zn2+ release from intracellular stores regulates ROS signaling events during embryonic development or postnatal stress/injury.
Results
TRPM7 Is Most Abundant on Intracellular Vesicles.
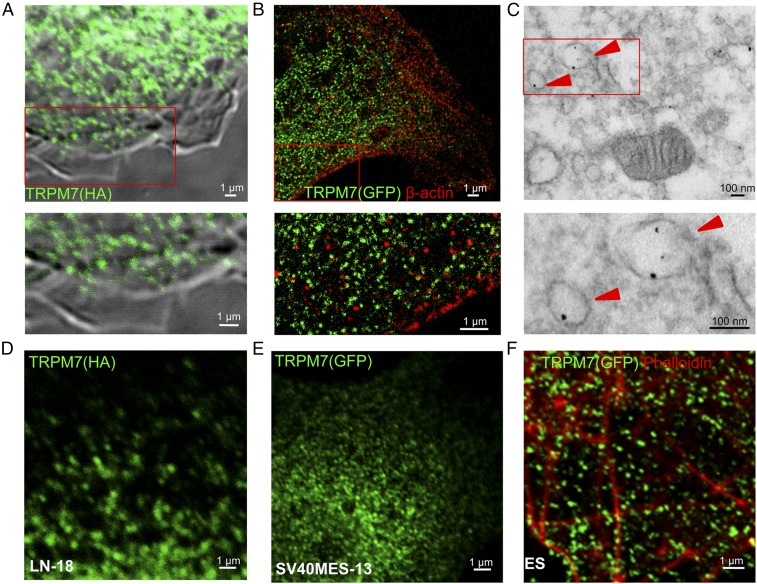
Whole-cell patch-clamp measurements of endogenous and ectopically expressed TRPM7 establish that it is present in the plasma membrane (1, 2). As with many ion channels that are relatively sparse, ectopically expressed TRPM7 was undetected at the plasma membrane by immunocytochemistry (Fig. 1A). Instead TRPM7 was detected largely in intracellular vesicles (Fig. 1 A–E) in multiple cell types. To rule out TRPM7 mislocalization artifacts caused by ectopic overexpression, we used a gene-trap strategy to insert GFP at the N terminus of endogenous TRPM7 in mouse ES cells. Expression of full-length GFP-TRPM7 was confirmed by immunoprecipitation of TRPM7 using either anti-GFP or anti-TRPM7, followed by Western blotting (Fig. S1A). Endogenous GFP-TRPM7, visualized by immunostaining with anti-GFP antibody, was abundant in intracellular vesicles similar in size and distribution to TRPM-positive vesicles in cells ectopically expressing TRPM7 (Fig. 1F). We also coimmunostained cells for GFP (TRPM7) and β-actin, which lines the inner leaflet of plasma membrane (38), and imaged the localization of both proteins by stimulated emission depletion (STED) microscopy. β-Actin was concentrated at the cell periphery in a punctate distribution at the plasma membrane, whereas TRPM7 was detected in ∼100-nm vesicles located only on the cytoplasmic side relative to β-actin (Fig. 1B).
Fig. 1.
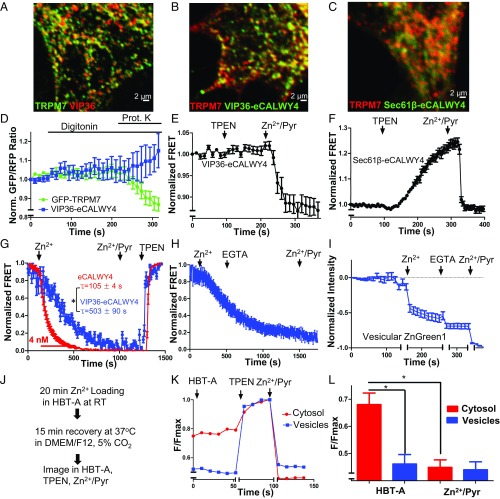
Vesicular localization of TRPM7. (A) Immunofluorescence and differential interference contrast (DIC) images of an HEK293 cell expressing TRPM7-HA. (B and C) Superresolution (B) and transmission electron micrographs (C) of an HEK293 cell expressing TRPM7 tagged with GFP positioned on intravesicular residues (arrowheads indicate anti-GFP immunogold-labeled vesicles). (D and E) Vesicular localization of TRPM7 stably expressed in LN-18 glioma cells (D) and in SV40MES-13 glomerular mesangial cells (E). (F) Endogenous TRPM7 in mouse ES cells genetically tagged with GFP (Fig. S1 and Movie S1).
Fig. S1.
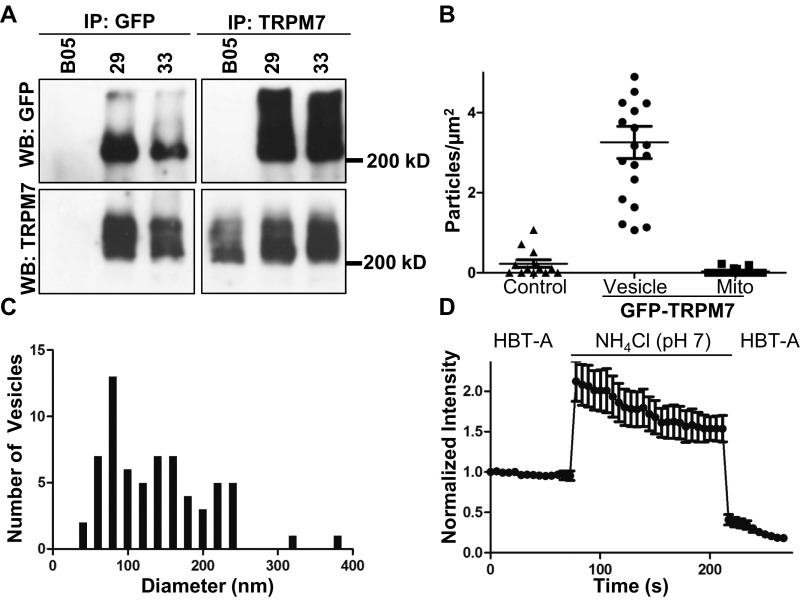
(Related to Fig. 1) TRPM7 is in acidic intracellular vesicles. (A) Confirmation of genetic tagging of endogenous TRPM7 with GFP. Reciprocal anti-GFP (Left) and anti-TRPM7 (Right) immunoprecipitations (IP) of TRPM7 from ES clones expressing endogenous WT (BO5 parental ES cell line) or GFP-TRPM7 (clones 29 and 33). Immunoprecipitates were analyzed by Western blotting for GFP or TRPM7. (B and C) HEK293 cells expressing TRPM7 tagged with pHluorin (placed in the S1/2 loop) were fixed and immunolabeled with anti-GFP and 5 mM gold-conjugated protein A. Quantification of immunogold labeling in untransfected control cells or cells expressing pHluorin-TRPM7 (vesicular vs. mitochondrial) (B) and M7V size distributions (C). Diameters of vesicular structures were quantified from 35 images. (D) HEK293 cells expressing pHluorin-TRPM7 were treated with 50 mM NH4Cl (pH 7.0) to alkalinize intracellular compartments, resulting in an increase in vesicular pHluorin fluorescence.
To determine the intracellular localization of TRPM7 in detail, we carried out transmission electron microscopy (TEM) of anti-GFP–labeled HEK293 cells expressing TRPM7 with a pH-sensitive GFP (pHluorin) (39) inserted into the S1/2 loop. Insertion of tags into the S1/2 loop did not compromise TRPM7 channel function (Fig. S2). TEM revealed unilamellar vesicles with an average diameter of ∼150 nm specifically labeled with anti-GFP (Fig. 1C and Fig. S1 B and C). The luminal anti-GFP labeling demonstrates the expected membrane orientation of TRPM7 polypeptide with the S1/2 loop in the lumen and the N and C termini in the cytosol (Fig. 1C). Imaging of live cells expressing the pHluorin-tagged TRPM7 established that the vesicles are acidic (Fig. S1D). Total internal reflection (TIRF) microscopy of GFP-tagged TRPM7 in live SV40MES-13 cells also revealed TRPM7 localization in vesicles (Movie S1). Thus, under all conditions examined, TRPM7 channels are located primarily on abundant intracellular vesicles with comparatively fewer numbers on the plasma membrane.
Fig. S2.
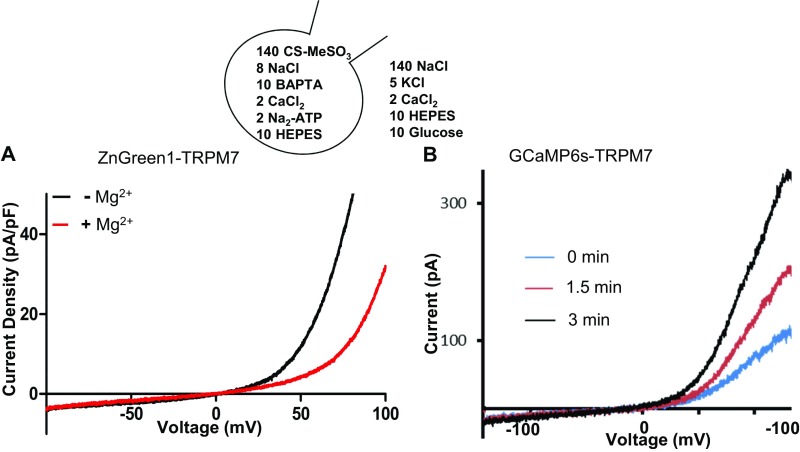
(Related to Figs. 3 and 4) Electrophysiological characterization of TRPM7 function after insertion of fluorescent protein sensors in the S1/S2 loop. (A) TRPM7 currents recorded in HEK293 cells expressing ZnGreen1 in the S1/S2 linker with or without extracellular Mg2+. (B) A TRPM7 DNA construct containing GCaMP6s in the S1/S2 linker was transfected in HEK293 cells, and TRPM7 currents were recorded in Mg2+-free intracellular solutions. Current/voltage (I/V) plots at break-in and the current run-up after Mg2+ chelation are shown. Traces are representative of three to five cells.
M7Vs Are Distinct from Well-Characterized, Membrane-Bound Intracellular Compartments.
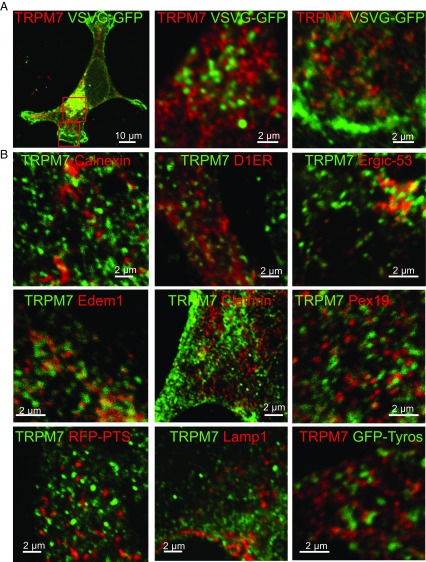
To determine whether M7Vs are simply in route to the plasma membrane via classical secretory pathways, we assessed the colocalization of TRPM7 and GFP-tagged vesicular stomatitis virus protein (VSVG-GFP) (40). TRPM7 did not colocalize with VSVG-GFP in transport vesicles in the cytoplasm or at the plasma membrane (Fig. 2A). Moreover, the vesicles did not colocalize with markers of several known cellular compartments, including calnexin, D1ER, Sec61β (endoplasmic reticulum; ER), PEX19, RFP-PTS (immature and mature peroxisomes), lamp1 (lysosomes), clathrin (endosomes), edem1 [EDEMosomes, ER-derived ERAD-tuning vesicles (41)], ergic-53 (ER–Golgi intermediate compartments), and tyrosinase (melanosomes) (Fig. 2B). M7Vs are also smaller and more abundant than zincosomes, ∼1-µm-wide vesicles that fluorescence brightly upon cellular Zn2+ overload (42). From their morphology, lack of markers, and lack of fusion with the plasma membrane by various stimuli (34), we surmise that M7Vs are previously uncharacterized intracellular membrane compartments.
Fig. 2.
M7Vs are distinct from well-characterized intracellular vesicular compartments. (A) Immunocytochemistry of VSVG-GFP and TRPM7-HA vesicles in HEK293 cells. (B) Absence of colocalization of TRPM7-HA and markers of the ER (Calnexin), EDEMosomes (Edem1), the tyrosinase ER–Golgi intermediate compartment, peroxisomes (RFP-PTS and Pex19), lysosomes (Lamp1), melanosomes (Tyrosinase or Tyros), or clathrin-coated endosomes. See Table S1 for additional description of markers.
Table S1.
Probes used to label various compartments
| Reagent | Target protein | Organelle | Related figure(s) |
| Markers/antibodies | |||
| Anti-calnexin | Calnexin | ER | 2B |
| Anti-ERGIC-53 | ER-Golgi intermediate compartment 53 kDa protein | ER–Golgi intermediate compartment | 2B |
| Anti-Lamp1 | Lysosomal associated membrane protein 1 | Lysosome | 2B |
| Anti-clathrin | Clathrin-coated endosomes | Endosomes | 2B |
| Anti-Pex19 | Peroxisomal biogenesis factor 19 | Immature peroxisomes | 2B |
| Anti–β-actin | β-Actin | Cytoskeleton, subplasma membrane cytoskeleton | 1B |
| Anti-PDI | Protein disulfide isomerase | ER | 3 A and D |
| Anti–cyt-C | Cytochrome C | Mitochondria | 3D |
| VSVG-GFP | Vesicular stomatitis virus G glycoprotein | Transport vesicles | 2A |
| D1ER (used here as a marker, not Ca2+ sensor) | CFP/Citrine FRET pair coupled with M13/calmodulin | ER | 2B |
| Cell Light Peroxisomes-RFP BacMam (RFP-PTS) | RFP containing peroxisome targeting sequence, PTS (Ser-Lys-Leu) | Peroxisomes | 2B |
| GFP-Tyrosinase | Tyrosinase | Melanosomes | 2B |
| VIP36 | Vesicular integral membrane 36 | Post-ER vesicles | 5A |
| Edem1 | ER degradation-enhancing α-mannosidase–like protein 1 | EDEMosomes | 2B |
| GFP-TRPM7 | TRPM7 | M7V | 1E and Movie S1 |
| TRPM7-HA | TRPM7 | M7V | 1D |
| Sensors | |||
| Phluorin-TRPM7 | TRPM7 (pH sensor in S1–S2 linker) | M7V | 1D |
| GCaMP6s | NA (GFP-based Ca2+ sensor) | Cytosol/nucleus | S4 |
| R-GECO1 | NA (RFP-based Ca2+ sensor) | Cytosol/nucleus | S4 |
| RCEPIAer | NA (RFP-based low-affinity Ca2+ sensor) | ER | S4 |
| GCaMP6S-TRPM7 | TRPM7 (Ca2+ sensor in S1-S2 linker) | M7V | S4 and S2 |
| ZnGreen1-TRPM7 | TRPM7 (Zn2+ sensor in S1-S2 linker) | M7V | 5 I–L and S5 C, D, and F |
| ZnGreen1-TRPM7 NT. | TRPM7 N-terminal | Cytosol | 5 K and L and S5 D and E |
| eCALWY4 | CFP/citrine FRET pair coupled with ATOX/WD4 Zn2+ binding domains | Cytosol/nucleus | 5G and 6 D–H |
| VIP36-eCALWY4 | VIP36 | M7V | 5 B, D, E, G, and H, 7 A–C and S5 A and B |
| Sec61β-eCALWY4 | Sec61β | ER-lumen | 5 C and F |
| roGFP2 | NA (ratiometric redox sensor based on cysteines inserted in GFP) | Cytosol/nucleus | 6 B and C |
| roGFP2-TRPM7 | M7V (redox sensor in S1-S2 linker) | M7V | 6 B and C |
| Indicators | |||
| Fluo4-AM | NA | Multiple | S4 |
| MagFluo4-AM | NA | Multiple | S4 |
| Fluozin-3-AM | NA | Multiple | 3, 6D, and 7 |
Sources of reagents not made in this study are described in Methods.
Purification of M7Vs.
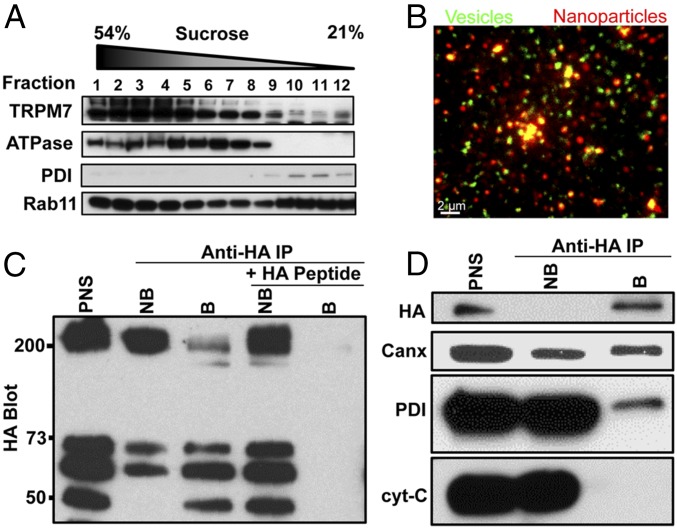
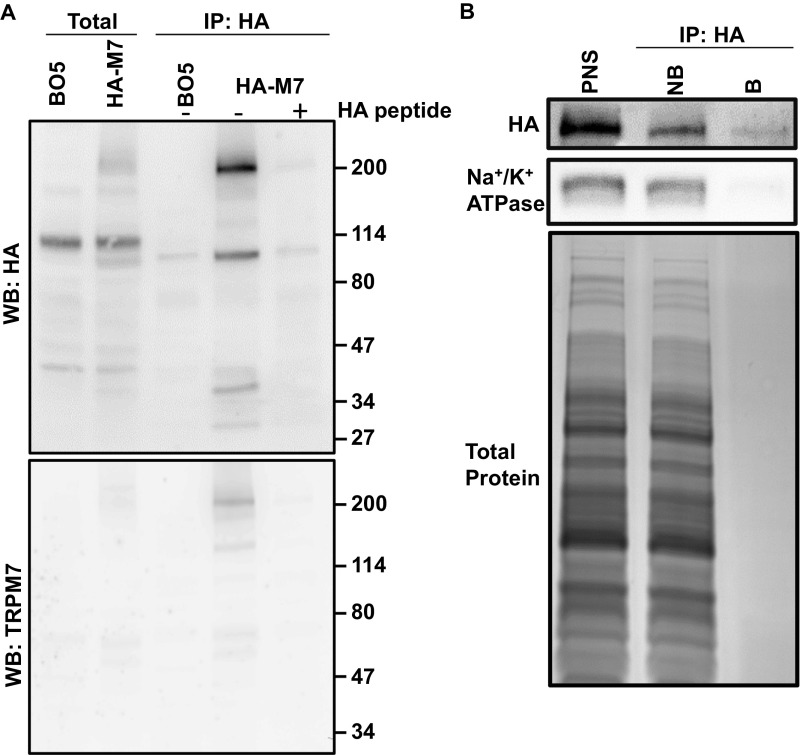
Sucrose gradient fractionation confirms that endogenous TRPM7 is predominantly intracellular because it migrates as a peak distinct from the plasma membrane marker Na+/K+ ATPase (Fig. 3A). To probe the cellular function of TRPM7 vesicles, we isolated TRPM7-containing vesicles from postnuclear supernatants (PNS). We first tagged the C terminus of TRPM7 with an HA epitope and pulled down M7Vs with anti-HA–conjugated magnetic nanoparticles. The pulldown appears specific, because excess HA peptide blocked vesicle binding, the vesicles colocalized with nanoparticles, and the vesicle preparation was depleted of contaminating mitochondrial and endoplasmic reticulum markers (Fig. 3). We performed a second affinity purification of vesicles by N-terminal FLAG tag after elution of anti-HA–bound vesicles for proteomic analysis (Fig. S3A). The affinity binding of the vesicles via tags located on the N and C terminals supports our earlier conclusion that the termini of TRPM7 face the cytoplasm. The substantial reduction of ER and mitochondria (Fig. 3D and Fig. S3A) supports the conclusion that TRPM7 vesicles are distinct from these structures.
Fig. 3.
Purification of M7Vs. (A) Gradient centrifugation of endogenous M7Vs. PNS from SV40MES-13 cells was first separated on a sucrose gradient and then Western blotted for TRPM7, plasma membrane (Na+/K+ ATPase), ER (PDI: protein disulfide isomerase), and recycling endosome (Rab11) markers. (B) Postnuclear supernatants of HEK293 cells stably expressing TRPM7-HA (with GCaMP6s in the S1/2 loop) were isolated using mouse anti-HA–conjugated magnetic nanoparticles. Vesicles were visualized by incubation with Ca2+, and nanoparticles were detected with Alexa Fluor 546-conjugated anti-mouse secondary antibodies. (C) Anti-HA Western blot: HA-peptide blocks nanoparticle binding to vesicles. (D) Western blot of isolated vesicles: enrichment of vesicles and depletion of ER (Calnexin; Canx or PDI) and mitochondria (cyt-C). B, Bound; NB. Not bound. See also Fig. S3A.
Fig. S3.
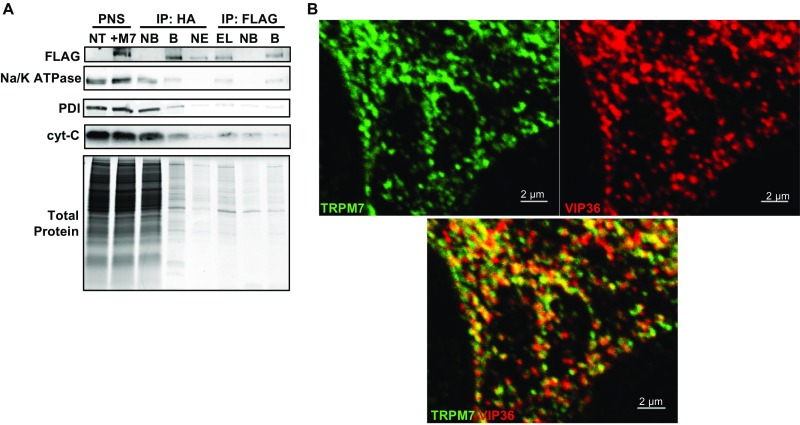
(Related to Figs. 2A and 3 and Dataset S1) Tandem affinity purification (TAP) and proteomic analysis of M7Vs. (A) Proteins of HEK293 cells expressing TRPM7 with an N-terminal FLAG-tag and cleavable C-terminal HA-tag were labeled with heavy isotopes of l-arginine and l-lysine (SILAC). After labeled cells were mixed with equal amounts of unlabeled, nontransfected wild-type (NT) cells, vesicles were isolated from the PNS using anti-HA–conjugated magnetic nanoparticles. Bound [immunoprecipitation (IP): HA, B] vesicles were eluted (IP: HA, EL) from nanoparticles using HRV-3C protease and were immunoprecipitated again with anti-FLAG–conjugated magnetic nanoparticles (IP FLAG: B). NB, not bound. TAP quality was monitored by Western blot of equal amounts of fractions for Na+/K+ ATPase, protein disulfide isomerase (PDI), cytochrome C (cyt-C), and Coomassie Blue protein staining. TAP vesicles then were analyzed by LC-MS-MS. (B) Images in Fig. 2A are presented separately to depict colocalization of VIP36-V5 and TRPM7-HA in HEK293 cells.
The M7V Proteome.
TRPM7 was enriched in the vesicle proteome, with a SILAC (stable isotope labeling with amino acids in cell culture) ratio of 56 in HEK293 cells and 98 in SV40MES-13 cells (Dataset S1). Proteins in known cellular compartments such as mitochondria, Golgi, and ER were present in the vesicle proteome, but ∼60% of the proteome was not classified in the Panther System (Dataset S1). We then screened the unclassified candidate proteins as potential markers for M7Vs and observed colocalization of M7Vs and vesicular integral membrane protein-36 (VIP36, also known as “LMAN2”) (Fig. S3B). VIP36 is an intracellular lectin of unknown function and has been observed in the ER–Golgi intermediate compartment and post-Golgi vesicles (43–45). VIP36 contains a single transmembrane domain with a luminal N terminus (46) that is ideal for targeting sensors into M7Vs (see below).
Purified M7Vs Accumulate Zn2+.
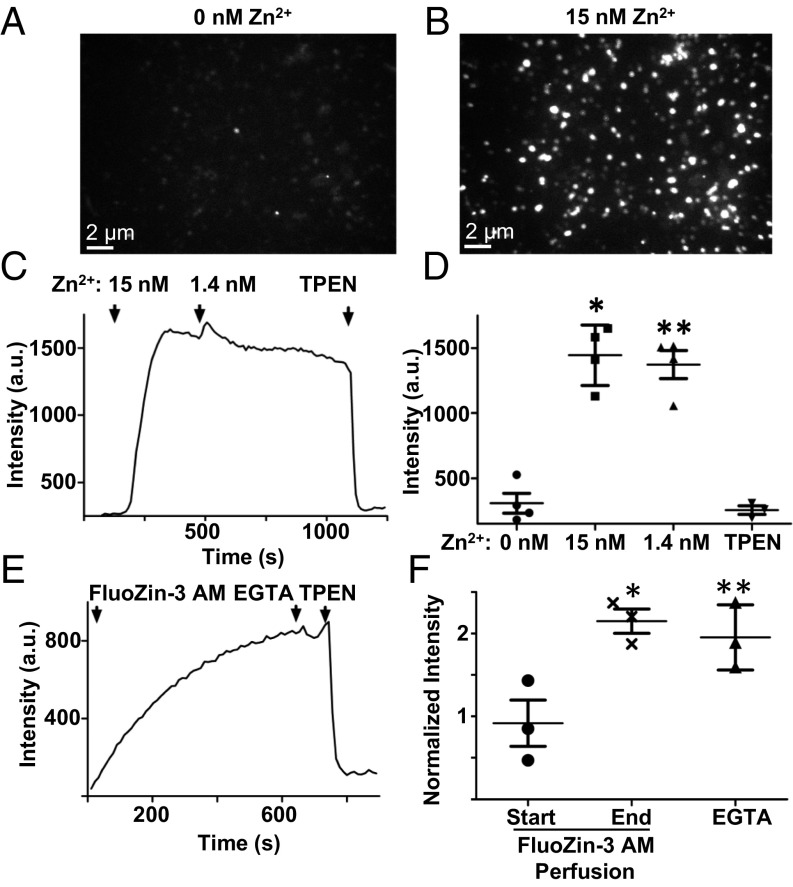
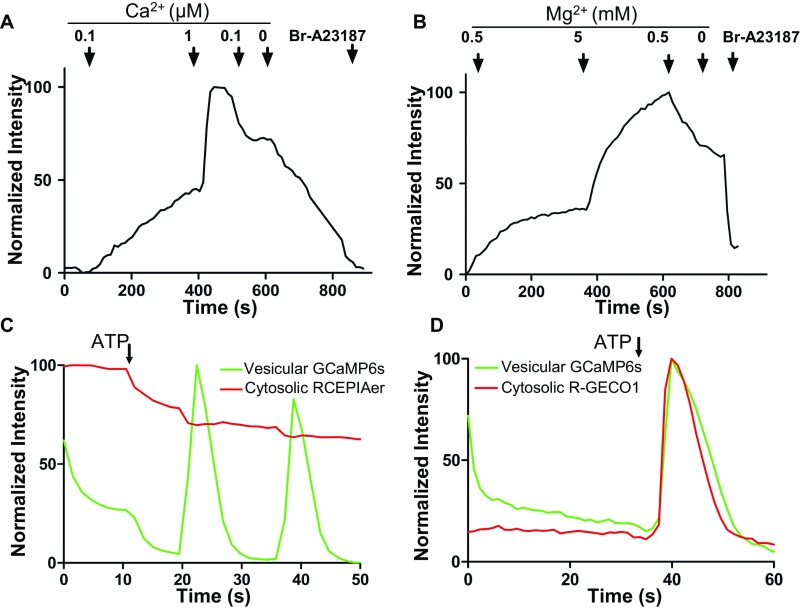
TRPM7’s extracellular domains face the lumen of the M7V (Figs. 1C and 3 B–D), and thus inward cationic current should flow from the vesicular lumen into the cytoplasm under ionic conditions identical to those at the plasma membrane. Purified M7Vs immobilized on Cell-Tak–coated coverslips were loaded with the fluorescent divalent indicators Fluo4-AM (Kd, Ca2+ = 335 nM), MagFluo4-AM (Kd, Mg2+ = 4.7 mM), or FluoZin-3-AM (Kd, Zn2+ = 15 nM) (Methods). These vesicles were permeant to Ca2+, Mg2+, or Zn2+ applied in the bath, but only Zn2+ remained trapped in the vesicles after extravesicular divalent ions were washed away (Fig. 4 A–D and Fig. S4 A and B). Accumulated intravesicular Zn2+ then was chelated with membrane-permeant TPEN [N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine], decreasing indicator fluorescence (Fig. 4 C and D). To rule out the possibility that Zn2+ was trapped by FluoZin-3, we loaded the vesicles with Zn2+ in the absence of FluoZin-3, washed out extravesicular Zn2+, and loaded the vesicles with FluoZin-3-AM. FluoZin-3 fluorescence increased to saturation over 10 min and remained at these levels after the extravesicular fluorophore was washed out (Fig. 4 E and F). These data show that purified M7Vs are capable of selectively accumulating Zn2+.
Fig. 4.
Zn2+ accumulation in purified vesicles. (A and B) M7Vs isolated from SV40MES-13 cells were loaded with FluoZin-3-AM and imaged in medium containing EGTA (A) or 15 nM Zn2+ (B). (C and D) Vesicles were perfused first with 15 nM Zn2+ and then with 1.4 nM Zn2+ or 50 µM TPEN. The mean, SD, and ANOVA statistics from four experiments are shown. *P < 0.001 compared with initial fluorescence; **P < 0.001 after TPEN addition. (E and F) Zn2+ trapping in vesicles is not caused by fluorophore accumulation: Zn2+-loaded vesicles were incubated with FluoZin-3-AM without Zn2+; then extravesicular dye was washed off, and remaining vesicular fluorescence was reduced by Zn2+ chelation by membrane-permeant TPEN but not membrane-impermeant EGTA. Fluorescence intensities in F were normalized to those in TPEN. Results are shown as the mean and SD from three experiments are shown; *P < 0.05; ANOVA. See also Fig. S4 A and B.
Fig. S4.
(Related to Fig. 5) M7Vs do not sequester Ca2+ or Mg2+. (A) M7Vs isolated from SV40MES-13 cells were loaded with Fluo4-AM (A) or MagFluo4 (B) and were perfused with the indicated concentrations of Ca2+ or Mg2+. Then, 5 µM Bromo-A23187 in 1 mM EDTA was used to deplete cations at the end of the time course. (C and D) HEK293 cells coexpressing GCaMP6s in the intravesicular loop of TRPM7 (green) and ER-targeted (RCEPIAer, red, C) or cytosolic (R-GECO1, red, D) Ca2+ sensors were stimulated with 100 µM ATP in HBSS. ATP-induced vesicular Ca2+ spikes (C and D, green) and synchronous Ca2+ efflux from the ER (C, red) or influx into the cytosol (D, red) were monitored simultaneously. Traces are representative of at least three experiments.
M7Vs Accumulate Zn2+ in Intact Cells.
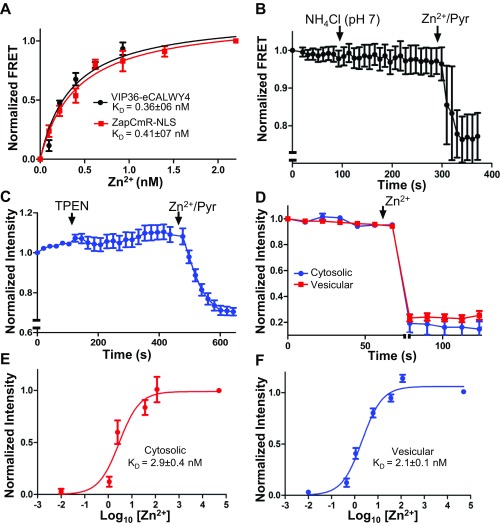
To study vesicular Zn2+ in intact cells, we targeted two genetically encoded Zn2+ sensors to the M7V lumen. We created an intravesicular Zn2+ sensor by targeting eCALWY4 (47) to the TRPM7 vesicle lumen by fusion of VIP36’s N-terminal 36 amino acids (including the signal peptide) to eCALWY4’s N terminus (Fig. 5B). Resistance to proteinase-K digestion indicated that the sensor was inside the vesicle (Fig. 5D). The intravesicular sensor did not respond to membrane-permeable TPEN but was responsive to increased luminal [Zn2+] (Fig. 5E), indicating low intravesicular free [Zn2+]. The Zn2+-binding constant of the vesicular VIP36-eCALWY4 sensor (0.36 ± 06 nM) was determined simultaneously with that of the nuclear Zn2+ sensor, ZapCmR2-NLS (48) (0.41 ± 07 nM), as a reference. Both binding constants were comparable to the Zn2+-binding constants of eCALWY4 (0.63 nM) (47) and Zap1 (0.81 nM) (49) (Fig. S5A). The calculation of Zn2+ concentrations using the binding constant suggests that the vesicles contain <0.1 nM free Zn2+ under resting conditions. To rule out any possible effects of vesicular pH on the sensor’s Zn2+ affinity, we showed that neutralization of the vesicular lumen with NH4Cl did not increase FRET (Fig. S5B). In contrast to M7Vs, ER-localized Sec61β- eCALWY4 (Fig. 5C) was saturated with Zn2+ under resting conditions (Fig. 5F), consistent with previously reported storage of Zn2+ in the ER (50).
Fig. 5.
Zn2+ sequestration by M7Vs in intact cells. (A) Colocalization of VIP36-V5 and TRPM7-HA in HEK293 cells. (B) Colocalization of immunostained VIP36-eCALWY4 intravesicular Zn2+ sensor and TRPM7-HA in HEK293 cells. (C) Lack of colocalization of ER-targeted eCALWY4 and TRPM7-HA. (D) ER lumen-targeted RCEPIA1er was coexpressed with cytosol-facing GFP (located on the N terminus of TRPM7) or VIP36-eCALWY4. Then the plasma membrane was permeabilized with 20 µM digitonin, and proteinase K was added to digest cytosolic fluorescent proteins. ER lumen-targeted RCEPIA1er was used as a proteinase K-inaccessible control. (E) HEK293 cells expressing VIP36-eCALWY4 were incubated with 50 µM TPEN or 50 µM Zn2+/20 µM pyrithione. Shown are means and SEMs of seven cells in more than four representative time-course experiments. (F) eCALWY4 was targeted into the ER lumen using Sec61β, and cells were imaged in 50 µM TPEN or 50 µM Zn2+/20 µM pyrithione. Shown are the mean and SEM of seven cells in a representative time-course experiment. (G) HEK293 cells were transfected with either VIP36-eCALWY4 or eCALWY4 cDNA and then were incubated with 500 µM Zn2+, followed by 50 µM Zn2+/20 µM pyrithione and TPEN. Shown are time constants of Zn2+ entry and 95% CIs of 10–12 cells representative of multiple similar experiments; *P < 0.0001 after an extra sum of squares F-test comparison of the two curves. (H) Vesicular Zn2+ loading was initiated with 500 µM extracellular Zn2+, which then was replaced with 250 µM EGTA to chelate Zn2+. The mean and SEM of data from 19 cells from three experiments are plotted. (I) Cells expressing TRPM7 (ZnGreen1 inserted between the S1 and S2 domains of TRPM7) were perfused with Zn2+ and EGTA as in H. Plots show means and SEMs of cells from one experiment representative of more than three; axis breaks are 5 min. (J) The method for loading and releasing Zn2+ from M7Vs with luminal ZnGreen1. (K and L) After cytosolic and M7V-luminal ZnGreen1 were saturated with Zn2+ and cells were allowed to extrude cytosolic Zn2+ as described in J, residual cytosolic and vesicular Zn2+ were imaged in 50 µM HBT-A or 50 µM TPEN Zn2+/20 µM pyrithione. *P < 0.05, ANOVA after Tukey’s multiple comparison test of 24–36 cells from three experiments. See also Figs. S2–S5 and Dataset S1.
Fig. S5.
(Related to Fig. 5) Characterization of genetically encoded Zn2+ sensors. (A) Vesicular and nuclear Zn2+ were monitored concurrently in a mixture of HEK293 cells expressing VIP36-eCALWY4 or ZapCmR2-NLS and titrated with increasing Zn2+ concentrations in 20 µM pyrithione. (B) VIP36-eCALWY4 FRET was monitored in HEK293 cells before and after neutralization of vesicular pH with NH4Cl. (C) HEK293 cells expressing intravesicular ZnGreen1 (in the TRPM7 S1/2 loop) were incubated with 50 µM TPEN or 50 µM Zn2+/20 µM pyrithione. (D) Cytosolic and vesicular ZnGreen1 sensors were saturated (indicated by the axis break) 20 min after loading with 500 µM extracellular Zn2+ at room temperature. (E and F) Cytosolic (E) and vesicular (F) ZnGreen1 were titrated with increasing concentrations of Zn2+ in 20 µM pyrithione to determine Zn2+ affinities of the sensors. The Kds of the TRPM7-conjugated sensors (21–2.9 nM) indicate higher affinity than that reported for the naked sensor (633 nM) (51). Note: We have inverted eCALWY-4 FRET changes to overlay the calibration curve of eCALWY-4 and that of a conventional FRET-based sensor (ZapCmR2) in Fig. S5A. To keep the panels consistent, intensity changes of ZnGreen1 were also inverted.
Next, we tested M7V cytosolic Zn2+ sequestration. Increasing extracellular [Zn2+] increased cytosolic [Zn2+] to >4 nM (τ = 104 ± 4 s) (Fig. 5G; see also ref. 49), followed by slower vesicular uptake (τ = 503 ± 90 s) (Fig. 5G). Vesicular Zn2+ entry continued after influx was stopped by extracellular Zn2+ chelation with EGTA (Fig. 5H). We also targeted a recently developed intensiometric Zn2+ sensor, ZnGreen1 (51), to the M7V lumen by inserting it into the S1/2 intraluminal loop of TRPM7. M7Vs containing ZnGreen1 did not contain TPEN-chelatable Zn2+ (Fig. S5C) but took up Zn2+ after cytosolic Zn2+ elevation (Fig. 5I and Fig. S5D); cytosolic and M7V-luminal ZnGreen1 have similar Zn2+ equilibrium binding constants of 2.1–2.9 nM (Fig. S5 E and F). ZnGreen1-tagged TRPM7 channels were also functional (Fig. S2A).
To test whether vesicles in intact cells retain loaded Zn2+, we took advantage of the low Zn2+ affinity and wide dynamic range of ZnGreen1 (Fig. S5 E and F), enabling us to monitor changes in Zn2+ that otherwise would saturate eCALWY sensors (47). As shown in Fig. S5 D–F, elevation of extracellular [Zn2+] to 500 µM elevated cytosolic and M7V [Zn2+] to >40 nM, saturating ZnGreen1. When cells were allowed to recover for 15 min in culture medium at 37 °C (Fig. 5J), cytosolic ZnGreen1 fluorescence was no longer saturated, but the vesicular sensor remained saturated (Fig. 5 K and L). These results indicate that Zn2+ was enriched in M7Vs relative to the cytoplasm.
To examine whether divalent cation retention in M7Vs was selective for Zn2+, we also imaged Ca2+ uptake and release from M7Vs in situ using GCaMP6s (52). This probe was also inserted in the S1/2 loop of TRPM7 (Fig. S4 C and D) and did not alter channel function (Fig. S2B). The addition of 100 µM ATP to intact cells triggered Ca2+ spikes in M7Vs that were synchronous with ER Ca2+ release (simultaneously monitored with the red fluorescent, ER-targeted Ca2+ sensor, RCEPIAer) (Fig. S4C) (53) or cytosolic spikes (simultaneously monitored with cytosolic red fluorescent R-GECO1) (Fig. S4D) (54). The equilibration of Ca2+ between the cytosol and M7Vs is consistent with the lack of Ca2+ trapping in vesicles in vitro (Fig. S4A) and supports our earlier immunostaining observation that the vesicles are distinct from the ER. Thus, TRPM7 localizes to vesicles that selectively sequester Zn2+ upon cytosolic Zn2+ overload.
M7Vs Are Enriched in Glutathione.
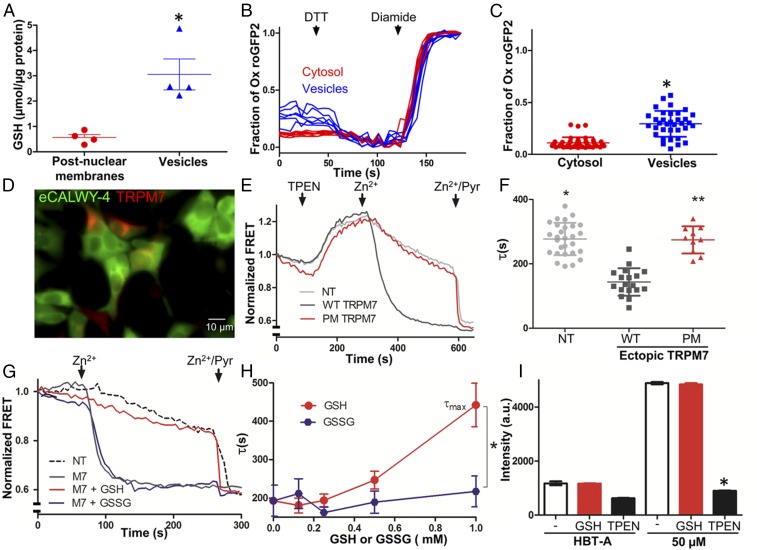
To search for clues about M7Vs’ function, we identified metabolites in isolated M7Vs by HPLC-MS. Only glutathione (GSH) was substantially enriched in M7Vs affinity-purified from HEK293 or HEK293T cells, compared with total postnuclear membranes (Dataset S2). We verified the GSH enrichment using a GSH luciferase assay in endogenous vesicles tagged with an HA-epitope inserted at the N terminus of endogenous TRPM7 (Methods). After HA-TRPM7 expression in the ES cells was validated with immunoprecipitation and Western blotting (Fig. S6A), vesicles were affinity purified with anti-HA nanoparticles (Fig. S6B) using the optimized method shown in Fig. 3 B and D. Total GSH was enriched sevenfold in M7Vs compared with total postnuclear membranes (Fig. 6A). Given the central role of GSH in cellular redox reactions, we next determined the redox state of M7Vs in situ using the redox sensor roGFP2 (55) targeted to M7Vs. Although ∼10% of cytosolic roGFP2 was oxidized (Fig. 6 B and C), close to the ∼5% reported previously (56), ∼30% of roGFP2 was oxidized in M7Vs (Fig. 6 B and C). This percentage is significantly greater than the percentage of oxidized roGFP in the cytosol or in mitochondria (∼11%) (55) but is much lower than the 96–97% observed in the ER lumen (57, 58) or endolysosomes (94–97%) (57).
Fig. S6.
Isolation of vesicles containing endogenous TRPM7. (A) Mouse ES cells containing a gene-trapped TRPM7 exon 1 (BO5) or their derivatives expressing HA-tagged endogenous TRPM7 were solubilized for anti-HA immunoprecipitation (IP) and Western blotting (WB) with anti-HA (Upper) or anti-TRPM7 (Lower). (B) Endogenous M7Vs were isolated from PNSs and were analyzed by Western blotting for HA and Na+/K+ ATPase or by Coomassie Blue (total protein) staining. B, bound; NB, not bound.
Fig. 6.
Regulation of TRPM7-mediated Zn2+ entry by the glutathione redox state. (A) Luciferase assay of GSH in postnuclear membranes and M7Vs purified from ES cells expressing HA-tagged endogenous TRPM7. The mean and SD of four replicates are plotted and compared by Student’s t test; *P < 0.01. (B and C) Cytosolic or intravesicular roGFP2 was maximally reduced with 10 mM DTT and maximally oxidized with 1 mM diamide in HBT-A. *P < 0.001 by Student’s t test, n = 35–42 cells. (D) HEK293 cells expressing eCALWY4 without (green only) or with mCherry-TRPM7 (green and red). (E) Time-course of Zn2+ entry into nontransfected HEK293 cells (NT, gray line), HEK293 cells overexpressing WT mCherry-TRPM7 (WT, black line), or loss-of-function pore-mutant mCherry-TRPM7 (PM, red line). After baseline imaging in HBT-A, cells were perfused with the Zn2+ chelator TPEN, followed by 50 µM Zn2+ and 50 µM Zn2+/20 µM pyrithione (Zn2+/Pyr) at the time points indicated by black arrows. (F) Time constants of Zn2+ entry into nontransfected and TRPM7-overexpressing HEK293 cells. Means and SD are plotted along with results of ANOVA with Kruskal–Wallis post test; asterisks indicate P < 0.05 compared with WT TRPM7-transfected cells. (G) Comparison of the effects of GSH (red line) and GSSG (purple line) on Zn2+ entry into HEK293 cells expressing eCALWY4 without (dashed line) or with (solid lines) mCherry-TRPM7. (H) Dose-dependence of GSH/GSSG effects on Zn2+ entry. τmax is the maximum time constant for Zn2+ entry (no inhibition); n = 3–10 cells; error bars indicate SEM. *P < 0.001. (I) Responses of Calcium Green-1 (low-affinity Zn2+ indicator), to Zn2+ in 100 µM TPEN or 1 mM GSH. The means and SEMs of three to six replicates are plotted; *P < 0.05 by ANOVA followed by a Kruskal–Wallis post test; a.u., arbitrary units. See Figs. S6 and S7 and Dataset S2.
Reduced GSH Inhibits Zn2+ Passage Through TRPM7.
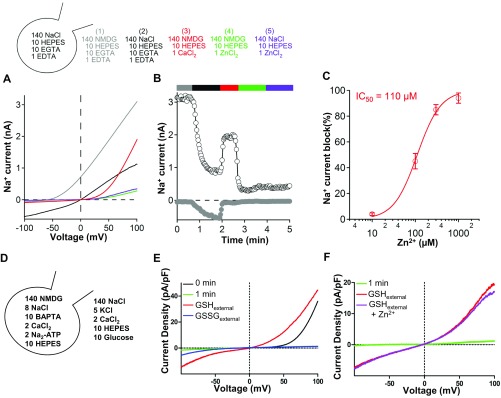
The observation of GSH enrichment in M7Vs motivated us to test whether GSH affects ion conductance through TRPM7. We measured plasma membrane TRPM7 because the extracellular medium can be altered readily to model the M7V lumen. First, Zn2+ entry was measured using cytosolic eCALWY4 (Fig. 6D). Overexpression of TRPM7 tripled the rate of Zn2+ influx from the extracellular medium, whereas a control, nonconducting TRPM7 pore mutant (33) did not enhance Zn2+ influx (Fig. 6 E and F). The addition of 1 mM GSH, but not glutathione disulfide (GSSG), blocked the TRPM7-dependent Zn2+ influx (Fig. 6 G and H) in a dose-dependent manner. To rule out the possibility that the GSH block was caused by Zn2+ chelation, we assayed Zn2+ binding to Calcium Green-1 [Kd = 10 µM (59)]. As shown in Fig. 6I, 1 mM GSH did not alter the binding of background Zn2+ (∼5 µM) or 50 µM Zn2+ to Calcium Green-1. Zn2+ not only permeates TRPM7 (Fig. 6 E–H) but also blocks both inward and outward monovalent TRPM7 currents with an IC50 of ∼110 µM (Fig. S7 A–C). We conclude that GSH likely prevents Zn2+ entry into the TRPM7 pore, an inference supported by patch-clamp experiments demonstrating that GSH, but not GSSG, enhances monovalent TRPM7 currents (Fig. S7E) even in the presence of Zn2+ (Fig. S7 E and F).
Fig. S7.
Relief of Zn2+ block of TRPM7 currents by glutathione. (A and B) The current/voltage (I/V) relationship of monovalent TRPM7 currents (A) and time course of currents (B) in HEK293 cells stably overexpressing GFP-TRPM7, with or without 1 mM Zn2+. (C) Dose-dependence of TRPM7 current inhibition by Zn2+. (D) Compositions of intracellular and extracellular recording solutions for E and F. (E) Enhancement of monovalent TRPM7 currents by reduced, but not oxidized, glutathione. (F) Enhancement of monovalent TRPM7 currents by GSH despite 20 mM Zn2+. Time elapsed just after (0 min) and 1 min after break-in with 140 mM NMDG intracellular solution is indicated. Traces are representative of 5–14 cells.
Oxidation Releases Zn2+ from M7Vs in a TRPM7-Dependent Manner.
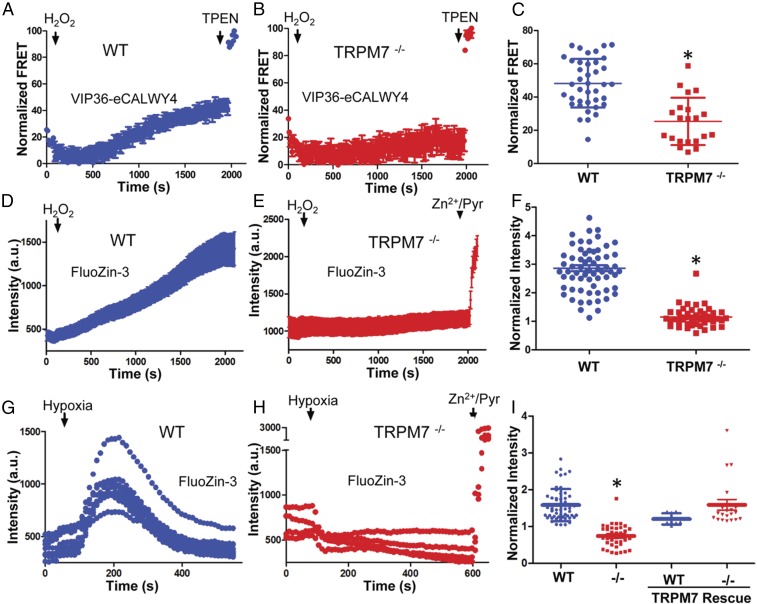
GSH’s inhibition of TRPM7’s Zn2+ conductance suggests that intravesicular reduced GSH enables Zn2+ retention in vesicles and that changes in the intravesicular redox state, reflected in the degree of GSH oxidation (60), may regulate Zn2+ release. To test oxidation-induced Zn2+ release from M7Vs, we transfected WT and TRPM7−/− HEK293T cells with VIP36-eCALWY4, loaded the cells with Zn2+ (as in Fig. 5), and monitored the increase in the mCitrine:mCerulean FRET ratio upon H2O2 treatment in Zn2+-free extracellular medium. Exposure to 100 µM H2O2 resulted in a gradual increase of FRET (i.e., a reduction in Zn2+) in the M7V lumen, which reached a plateau in ∼30 min (Fig. 7A). The FRET increase was substantially reduced in TRPM7−/− cells compared with WT cells (Fig. 7 B and C), indicating that TRPM7 mediates oxidation-induced Zn2+ release from vesicles.
Fig. 7.
TRPM7 is required for ROS-induced Zn2+ release from intracellular stores. (A–C) Zn2+ release from M7Vs was monitored in WT (A and C) or TRPM7−/− (B and C) HEK293T cells expressing the VIP36-eCALWY4 intravesicular Zn2+ sensor. Cells first were incubated with 500 µM Zn2+ and then were perfused with 100 µM H2O2 in Zn2+-free HBT-A containing 250 µM EGTA and 4 mM MgCl2. WT and TRPM7−/− cells were compared by Student’s t test; *P < 0.0001, n = 21–39 cells. (D–F) FluoZin-3–loaded WT (D and F) or TRPM7−/− (E and F) HEK293T cells were similarly loaded with Zn2+ and then were treated with H2O2 in Zn2+-free extracellular medium. The means and SEMs of 44–67 cells are plotted; *P < 0.0001 compared with WT, Student’s t test. (G–I) Experiments were performed as in A–F, but 10 mM sodium dithionite, instead of H2O2, was applied to induce Zn2+ release from intracellular stores, and glucose was omitted from HBT-A. WT mCherry-TRPM7 was overexpressed to rescue the Zn2+ release in TRPM7−/− cells. The means and SEMs of 8–30 cells are plotted; *P < 0.0001 compared with WT, Student’s t test. a.u., arbitrary units.
The initial reduction of the FRET ratio of the Zn2+-saturated sensor by H2O2 (Fig. 7 A and B) raised the possibility that eCALWY was modified by oxidation. We therefore used FluoZin-3 in place of genetically encoded protein sensors to monitor cytosolic Zn2+ in subsequent experiments, because previous studies have shown that FluoZin-3 responses to Zn2+ are not altered by H2O2 or ROS (61, 62). H2O2 increased Zn2+ (FluoZin-3 fluorescence) in Zn2+-loaded WT cells but not in TRPM7−/− cells (Fig. 7 D–F). Because the extracellular solution did not contain significant Zn2+ (<0.2 nM), we conclude that the source of the TRPM7-dependent, H2O2-induced increase in cytosolic Zn2+ was the M7V.
Finally, we tested whether endogenous ROS generation triggered by oxygen–glucose deprivation (OGD) (63, 64) elicits Zn2+ release into the cytosol through TRPM7. OGD with glucose-free sodium dithionite resulted in a wave of Zn2+ release, as recently reported (62), in WT but not in TRPM7−/− HEK293T cells (Fig. 7 G–I). The OGD-induced Zn2+ release was rescued by ectopic TRPM7 expression in TRPM7−/− cells (Fig. 7I). Again, because the extracellular solution did not contain significant Zn2+, we conclude that M7Vs were the source of TRPM7-dependent, OGD-induced increase in cytosolic Zn2+. In conclusion, TRPM7 forms functional intracellular channels that mediate Zn2+ release from the lumen of a previously unrecognized population of vesicles during oxidative stress.
Discussion
Our prior observations of intracellular TRPM7 on 50- to 250-nm vesicles led us to investigate the existence of an additional and perhaps more important function of TRPM7 as an intracellular channel. Here we show that most TRPM7 forms intracellular Zn2+-release channels in a previously unrecognized type of Zn2+-storage vesicle in multiple cell types. Oxidizing conditions activate intracellular Zn2+ release via TRPM7, likely by converting GSH in M7Vs to GSSG. We anticipate these findings will provide insights into the cellular function of TRPM7.
Several transient receptor potential (TRP) channels function in various intracellular compartments. For example, TRPML1 and TRPM2 release lysosomal Ca2+ and Zn2+, whereas TRPM8 may release Ca2+ from the ER in some cell types (65, 66). The Drosophila TRPM channel, proposed to release Zn2+ from intracellular stores during larval development, exhibits TRPM7-like currents (67). We now include TRPM7 in the class of functional intracellular TRP channels. However, the intracellular function of TRPM7 is unique in two ways. First, TRPM7 functions in vesicles that are distinct from known compartments. Second, unlike ER and lysosomes that store both Ca2+ and Zn2+, M7Vs store only Zn2+.
Under resting conditions, M7Vs do not contain free Zn2+, unlike intracellular Zn2+ stores such as mitochondria, ER, lysosomes, synaptic vesicles, and zincosomes (42). Although GSH is enriched in M7Vs and does not chelate micromolar [Zn2+] in physiological solutions, it may still bind Zn2+ under some conditions that may exist in M7Vs (68). The complex dynamics of cellular redox status and M7V GSH content, cytoplasmic free Mg2+, and ATP (as well as other phosphonucleotides) will require careful examination in the future.
M7Vs are uniformly distributed in the cell and thus are an ideal ion storage system. We hypothesize that M7Vs sequester Zn2+ to protect other organelles, but because Zn2+ binds many proteins, DNA, and RNA, the vesicles also could release Zn2+ to alter many cellular processes. We are exploring the possibility that Zn2+ released from M7Vs regulates TRPM7 kinase cleavage, activity, or trafficking. Furthermore, the proteomics data in this study suggest that dozens of proteins are localized, at least partially, in M7Vs. In this study, we focused on identifying probes for the lumens of M7Vs, but verification of the full complement of M7V proteins will elucidate their biogenesis and function. The presence of vesicle trafficking proteins (e.g., vesicle-associated membrane protein A, VAPA) and fusion proteins (e.g., vesicle-associated membrane protein 1, VAMP1, and extended syntaptotagmin-1, ESYT1) in the M7V proteome is consistent with the previously reported M7V exocytosis during shear stress and cholinergic synaptic transmission (33, 34). Enrichment of ferritin light chain in vesicles isolated from SV40MES cells also suggests the possibility of iron storage by M7Vs. Our M7V proteome did not contain transporters for divalent cations (e.g., ZnTs, ZiPs, or other divalent metal transporters) or GSH, likely because these proteins are not highly expressed, but targeted proteomic analysis may reveal their identities.
Total Zn2+ increases by 50% during meiotic maturation of oocytes; about half of this increase is released by vesicular exocytosis within minutes of fertilization (69), and the rest persists through preimplantation (70). Because TRPM7 is required for preimplantation development (71), it will be important to determine if the Zn2+-containing vesicles in oocytes and early embryos are M7Vs and how changes in GSH/redox during early development (31, 72, 73) may regulate Zn2+ uptake and release from M7Vs. In postnatal animals, extracellular free [Zn2+] can rise to several hundred micromolar during ischemia or seizures (16, 17, 74), comparable to the levels required to fill M7Vs in these studies. Zn2+ also may be redistributed from other intracellular sites into M7Vs. These include mitochondria and NADPH oxidase at the plasma membrane, major sites of ROS that liberate Zn2+ from the mitochondrial matrix and from metallothioneins (16, 17, 74). Thus, M7Vs may comprise a sink or reservoir buffering cytosolic Zn2+.
Experimental Procedures
Detailed methods are available in SI Experimental Procedures.
Immunofluorescence Microscopy.
Cells were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton-X 100 at room temperature. Nonspecific binding was blocked with 10% goat serum in PBS, and cells were incubated in primary antibodies for 2 h at room temperature or overnight at 4 °C, followed by detection with fluorophore-conjugated secondary antibodies. Cells then were imaged by confocal or STED microscopy.
TEM.
Transfected cells were fixed in 1% paraformaldehyde in PBS, permeabilized, blocked, and incubated with anti-GFP antibody overnight. After rinsing, cells were labeled with protein-A–conjugated 10-nm colloidal gold (Aurion) or 1.4-nm FluoroNanogold-conjugated secondary antibodies (Nanoprobes); the latter were silver enhanced. Cells then were postfixed in 1% glutaraldehyde, and 10-nm-thin slices were imaged by TEM.
Tagging of Endogenous TRPM7.
TRPM7 was tagged as described in ref. 75. Briefly, ES cells with gene-trapped TRPM7 were purchased from the European Mammalian Mutant Cell Repository (EuMMCR) and verified by genotyping the regions flanking exon 1 of TRPM7. The cells then were electroporated with plasmids encoding GFP or HA-SNAP tags and flippase (FLP) recombinase. ES cell clones with tagged TRPM7 were selected with hygromycin.
Affinity Purification of TRPM7-Containing Vesicles.
SV40MES-13 cells stably expressing GFP-TRPM7-HA or HEK293 cells stably expressing GCaMP6s-TRPM7-HRV3C-HA were homogenized and centrifuged to obtain PNS. The PNS was incubated with mouse anti-HA–conjugated magnetic nanoparticles (Miltenyi Biotec) and were loaded onto LS columns (Miltenyi Biotec). After rinsing, immobilized vesicles were eluted in PBS. For proteomic analysis eluted vesicles were isolated again with anti-FLAG–conjugated magnetic nanoparticles.
Metabolomics.
M7Vs were isolated from HEK293 or HEK293T cells stably expressing GCaMP6s-TRPM7-HA using anti-HA nanoparticles as described above. They then were vortexed in 0.9% NaCl and centrifuged; supernatants were used for LC-MS sample extractions.
Imaging of Zn2+ Flux with Genetically Encoded Zn2+ Sensors.
HEK293 cells transfected with eCALWY4 or with eCALWY4 and mCherry-TRPM7 were imaged as described in ref. 47. To study Zn2+ flux in intracellular compartments, HEK293 cells expressing intravesicular Zn2+ sensors were imaged in HBT-A medium (20 mM Hepes, 120 mM NaCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose, pH 7.5) with or without extracellular Zn2+.
Live Imaging of Vesicular Redox Environment.
A ratiometric redox sensor, roGFP2, was targeted to the lumen of M7Vs by insertion between the S1 and S2 domains of TRPM7. After baseline image acquisition, the sensor was maximally reduced with 10 mM DTT (Bio-Rad) and then maximally oxidized in 1 mM diamide (Sigma).
Zn2+ Release from Intracellular Stores.
Untransfected (FluoZin-3–loaded) or VIP36-eCALWY4–transfected WT and TRPM7−/− HEK293T cells were incubated with 500 µM Zn2+ in Mg2+- and Ca2+-free solution for 10–20 min. The cells then were perfused with 10 mM sodium dithionite (in glucose-free) or 100 µM H2O2 (in normal) HBT-A medium containing 0.25 mM EGTA to chelate Zn2+ and 4 mM Mg2+ to block plasma membrane-localized TRPM7.
TRPM7 Deletion in HEK293T Cells.
A variant of the CRISPR/Cas9 method (76) was used to insert puromycin- and blasticidin-coding sequences into the second exon of Trpm7. The donor DNA contained the predicted coding sequence of Trpm7’s first 12 amino acids followed by an antibiotic resistance cassette, multiple stop codons, and SV40-polyA. Oligonucleotides encoding the genomic RNA (gRNA) sequence were annealed and cloned into BPK1520 (77). Donor DNA containing puromycin-SV40pA and blasticidin-SV40pA cassettes were amplified from pPur (Clontech) and pcDNA6-V5-His (Invitrogen), respectively, with primers flanked with a target site on exon 2 of TRPM7 and were ligated into a pJET1.2 cloning vector (Thermo Fisher). HEK293T cells then were transfected with the BPK1520 construct and the puromycin resistance cassette donor template using Lipofectamine LTX (Thermo Fisher). Puromycin-resistant cells were cloned and genotyped, and knockout of Trpm7 was verified by immunoprecipitation, Western blotting, and electrophysiology.
Statistical Analyses.
Data were plotted and analyzed in Prism 5 (GraphPad) by Student’s t tests, ANOVA with Tukey’s post hoc tests, or extra sum of squares F-tests (for nonlinear regressions).
SI Experimental Procedures
Constructs.
GCaMP6s-TRPM7, pHluorin-TRPM7, and ZnGreen1-TRPM7 (all with sensors inserted into the S1/2 loop between I896 and P897 of mouse TRPM7) were made in the original pcDNA4TO-FLAG-mouse TRPM7 construct (1). GFP-TRPM7 and mCherry-TRPM7 (WT or with pore mutations N1090F, L1091A, and L1092P) were previously described (33–35). GCaMP6s-TRPM7 contained a C-terminal HRV3C protease cleavage site before an HA epitope. VIP36-eCALWY4, VIP36-V5, and edem1-V5 were in pcDNA6V5HisB (Life Technologies) and Sec61β-eCALWY4 was in pCMV (Clontech). Cell Light Peroxisomes-RFP BacMam (RFP-peroxisome targeting sequence or RFP-PTS vector) was purchased from Life Technologies/Thermo Scientific. The following constructs were from Addgene: pDTnLAPP2A (plasmid no. 28226), pCAG-Flpo (plasmid no. 60662), VSVG-GFP (plasmid no. 11912), R-GECO1(plasmid no. 32444), RCEPIA1er (plasmid no. 58216), GFP-Tyrosinase (plasmid no. 32781), ZapCmR-NLS (plasmid no. 59012), GCaMP6s (plasmid no. 40753), D1ER (plasmid no. 36325), and roGFP2 (plasmid no. 49435).
Antibodies.
The following antibodies were obtained commercially: mouse anti-FLAG and anti–FLAG-HRP (Sigma); rat anti–HA-HRP (Roche); rabbit anti-GFP (Life Technologies or Genetex); chicken anti-GFP (Aves Labs); rabbit anti-Na+/K+ ATPase (Cell Signaling Technologies), mouse anti-protein disulfide isomerase, mouse anti-cytochrome C, rabbit anti-clathrin heavy chain, and mouse anti-calnexin (Abcam); mouse anti-TRPM7 (NeuroMab); and rabbit anti-lamp1, rabbit Ergic-53, rabbit anti-pex19, and rabbit anti-rab11 (Santa Cruz Biotechnologies). The rabbit anti-TRPM7 antibody developed in our laboratory was described previously (21).
Immunofluorescence.
Cells were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton-X 100 at room temperature. Nonspecific binding was blocked with 10% goat serum in PBS, and cells were incubated in primary antibodies for 2 h at room temperature or overnight at 4 °C, followed by detection with fluorophore-conjugated secondary antibodies. Nuclei were stained with DAPI in PBS before the cells were mounted on slides using Prolong Gold (Life Technologies). Images were acquired on an Olympus FluoView 1000 confocal microscope.
STED Microscopy.
Immunocytochemistry for STED was performed as above but at higher concentrations of secondary antibodies (1:250). The mounting medium was cured for at least 48 h in Prolong-Gold before imaging. STED images were acquired using a 100×, 1.4 NA oil objective on a Leica SP8 X STED microscope. A white-light laser source was used to excite Alexa Fluo488 and Alexa Fluor 546 dyes; dyes were depleted with a 592-nm and 660-nm laser, respectively. Emitted photons were detected using a GaAsP/HyD hybrid detector.
TIRF Microscopy.
Cells were plated on 3.5-cm glass-bottomed dishes (In Vitro Scientific) and imaged with a 60×, 1.45 NA oil objective (Olympus) excited with a 488-nm solid-state diode laser and a 532-nm diode laser. A dual band dichroic mirror (Z488/532RPC; Chroma Tech) reflected excitation light onto the specimen, and emitted light, filtered by HQ515/30(Chroma) or D620/60 (Chroma), was detected with a cooled CCD camera (ORCA II; Hamamatsu). SlideBook software (3i) was used for instrument control and data acquisition.
TEM.
Transfected cells were plated on coverslips for 18 h and were fixed in 1% paraformaldehyde in PBS for 20 min at room temperature. Cells then were permeabilized in 0.05% saponin for 10 min, blocked, and incubated with anti-GFP antibody overnight at 4 °C. After rinsing, cells were labeled with protein A-conjugated 10-nm colloidal gold (Aurion) or 1.4-nm FluoroNanogold-conjugated secondary antibodies (Nanoprobes); the latter were silver enhanced. Cells then were postfixed in 1% glutaraldehyde, and 10-nm-thin slices were imaged by TEM. Diameters of immunogold-labeled vesicular structures were measured manually using Fiji ImageJ.
Tagging of Endogenous TRPM7.
TRPM7 was tagged according to ref. 75. Briefly, ES cells with gene-trapped TRPM7 were purchased from EUMMCR and were verified by genotyping the regions flanking exon 1 of TRPM7. The cells were cultured in gelatin-coated dishes in knockout-DMEM supplemented with 10% FBS, glutamate, nonessential amino acids (all from Gibco/Thermo-Fisher Scientific), 2-mercaptoethanol, and 100 U/mL leukemia inhibitory factor (LIF) (Millipore/GE Healthcare). The cells then were electroporated with 30 µg of pDTnLAPP2A and 70 µg pCAG-Flpo plasmids at 240 V and 500 µF capacitance. After 48 h, the medium was replenished daily with fresh medium containing 200 µg/mL hygromycin for 10 d. Single clones were selected, expanded, and characterized by genotyping for GFP and Western blotting. For insertion of HA into the N terminus of TRPM7, the GFP DNA sequence was first excised from pDTnLAPP2A using BstXI and BsrGI. An HA-SNAP-V5 DNA sequence then was amplified with primers overlapping with the restriction sites and was ligated using a GeneArt cloning kit (Thermo Fisher). After sequencing, the construct was electroporated into ES cells with pCAG-Flpo, as above, and positive clones were selected with hygromycin. TRPM7 tagging was verified by anti-HA and anti-TRPM7 immunoprecipitation and Western blotting.
Determination of Vesicular Acidity.
pHluorin-coding cDNA (a generous gift of G. Miesenbock, Sloan-Kettering Cancer Center, New York) was inserted into TRPM7 as described above and was transfected into HEK293 cells. Cells were first imaged in HBT-A, a modified Hepes-buffered Tyrode’s solution containing 20 mM Hepes, 120 mM NaCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose (pH 7.5), and then 50 mM NaCl was replaced with 50 mM NH4Cl to alkalinize all intracellular compartments, thus increasing vesicular pHluorin fluorescence, as in ref. 35.
Affinity Purification of TRPM7-Containing Vesicles.
SV40MES-13 cells stably expressing GFP-TRPM7-HA or HEK293 cells stably expressing GCaMP6s-TRPM7-HRV3C-HA were homogenized in a Dounce homogenizer and centrifuged at 1,000 × g for 5 min at 4 °C in PBS with protease inhibitors and 5 mM EDTA vesicle immunoprecipitation (VIP) buffer. The supernatants were centrifuged again at 1,300 × g for 5 min to yield postnuclear supernatants (PNS). The PNS was incubated with mouse anti-HA–conjugated magnetic nanoparticles (Miltenyi Biotec) for 2 h at 4 °C, with or without 20 µg/mL of HA-blocking control peptide. The mixture then was loaded onto LS columns (Miltenyi Biotec) and washed twice with PBS. Immobilized vesicles were eluted in 2 mL of VIP buffer. For Western blotting, the eluate was separated by SDS/PAGE, transferred to PVDF membranes, and detected with HRP-conjugated anti-HA antibodies. To visualize isolated but intact vesicles, Alexa Fluor 546-conjugated anti-mouse secondary antibody (Life Technologies/Thermo Fisher Scientific) was added to the PNS during vesicles isolation, and vesicles were immobilized on Cell-Tak–coated 12-mm coverslips in PBS. The Alexa Fluor 546-labeled nanoparticles and bound vesicles then were imaged in 1 mM Ca2+ to enhance GCaMP6s fluorescence.
Metabolomics.
HEK293 or HEK293T cells stably expressing GCaMP6s-TRPM7-HA were grown in 15-cm dishes and induced with 1 µg/mL doxycycline for 24 h. Vesicles were isolated using anti-HA nanoparticles as described above. The PNS and isolated vesicles then were centrifuged at 35,000 × g for 1 h in weighed tubes, and the pellets were rinsed twice in PBS. After the PBS was aspirated, the tubes were reweighed to determine the weight of the pellet, and the pellet was frozen at −80 °C. Thawed pellets were vortexed in 0.9% NaCl and centrifuged at 15,000 × g for 20 min. Supernatants were aliquoted for LC-MS sample extractions. LC-MS analyses were conducted using targeted measurement of polar metabolites in the negative-ion mode, nontargeted measurement of polar metabolites in the positive-ion mode, C18-negative, nontargeted analysis of metabolites of intermediate polarity in the negative-ion mode, and C8-positive nontargeted analysis of lipids in the positive-ion mode.
Luciferase GSH Assay.
Vesicles were isolated from mouse ES cells expressing HA-tagged endogenous TRPM7 as above, except that the PNS and pellets were assayed for GSH with a GSH/GSSG-GLO kit (Promega), using the manufacturer’s protocol. The GSH concentration was normalized to the total protein concentration.
Imaging of Zn2+ Flux with Genetically Encoded Zn2+ Sensors.
HEK293 cells were transfected with eCALWY4 or with mCherry-TRPM7 and then were split onto coverslips for 12 h before imaging in HBT-A solution. eCALWY4 FRET was imaged as described in ref. 47 on an Olympus IX70 microscope equipped with a DG-4 excitation light source (Sutter). Excitation light was filtered with a 440AF21 filter (Omega) and reflected onto the sample with a 455DRLP filter (Omega). 480AF30 (Omega) and HQ535/30m (Chroma) filters were alternated using a Lambda 10-3 filter changer (Sutter Instruments) to filter emissions from mCerulean and mCitrine, respectively. A cooled CCD camera (Hamamatsu Photonics) was used to detect emitted photons. Again, SlideBook software (3i) was used for instrument control and data acquisition. The time constants of Zn2+ entry were determined from single exponential decay fits of mCitrine/mCerulean fluorescence intensity ratios, with or without GSH. To study Zn2+ flux in intracellular compartments, HEK293 cells expressing intravesicular Zn2+ sensors were imaged with or without extracellular Zn2+. Cells expressing the intensiometric TRPM7-ZnGreen1 Zn2+ sensor were imaged using a GFP filter cube (472/40×, BS495LP, E525/50m; Olympus). ZapCmR2 was imaged with red and green exciter filters, a dual-band dichroic mirror (Z488/532RPC; Chroma Tech), and HQ515/30(Chroma) or D620/60 (Chroma) filters. To test responses of all sensors to Zn2+, sensors were Zn2+-depleted with 50 µM TPEN or were saturated with 50 µM Zn2+/20 µM pyrithione. To study Zn2+ fluxes, vesicles were loaded with 100–500 µM extracellular Zn2+, and then the Zn2+ was chelated with 0.25 mM EGTA. Zn2+ concentrations were calculated as described in ref. 78.
Sensor Titrations.
To determine the Zn2+-binding constants of VIP36-eCALWY4, ZapCmR2, and ZnGreen1-TRPM7, transfected cells were perfused with 20 µM pyrithione in HBT-A solution containing 1 mM EGTA and varying [Zn2+]. Strontium chloride (1 mM) was included for titration of ZnGreen1 to increase the buffering range of EGTA. The cytoplasmic ZnGreen1 construct consists of the N-terminal domain of TRPM7 conjugated to the N terminus of ZnGreen1, and the resulting protein is localized diffusely in both the nucleus and cytoplasm. All [Zn2+] were calculated using MaxChelator (Stanford University).
Determination of Zn2+ Chelation by GSH.
Calcium Green (1 µM) was diluted in HBT-A in 96-well plates, with or without 1 mM GSH. Then 50 µM Zn2+ or 100 µM TPEN was added, and the resulting changes in fluorescence were determined with a FlexStation 3 plate reader (Molecular Probes).
Live Imaging of Vesicular Redox Environment.
A ratiometric redox sensor, roGFP2, was targeted to the lumen of M7Vs by insertion between the S1 and S2 domains of TRPM7. Cells transfected with the vesicular redox sensor were excited with light filtered through ET380x or 472/40x filters (Chroma) and a 74000v2 Fura2/Fluo3 filter cube (Chroma), and emitted light was filtered through an HQ535/30m filter (Chroma). After baseline image acquisition in HBT-A, the sensor was maximally reduced with 10 mM DTT (Bio-Rad) and then was maximally oxidized in 1 mM diamide (Sigma). The degree of oxidation of roGFP2 was calculated as described in ref. 58.
In Vitro Vesicular Ion Uptake and Release.
M7Vs were isolated as previously described and then immobilized on Cell-Tak–coated 12-mm coverslips. The vesicles then were incubated with 40 µM FluoZin-3-AM at 37 °C for 20 min in cytosol-like medium (CLM) containing 30 mM Hepes (pH 7.5), 120 mM KCl, 5 mM NaCl, and 1 mM EGTA. FluoZin-3 fluorescence was imaged on an Olympus IX70 microscope equipped with a DG-4 excitation source using a GFP filter cube. Vesicles were first perfused with 15 nM Zn2+ and then with 50 µM TPEN. In an alternate protocol, we reversed the order of FluoZin-3 and Zn2+ loading: vesicles were first incubated with Zn2+, the excess Zn2+ was washed off, and FluoZin-3-AM then was added to the vesicles. To monitor Ca2+ and Mg2+ entry into TRPM7-containing vesicles, 40 µM Fluo4-AM or MagFluo4-AM, respectively, was loaded into vesicles at 37 °C for 20 min, followed by loading with CLM containing 1 µM Ca2+ or 5 mM Mg2+. Total and free ion concentrations were calculated using MaxChelator (Stanford University).
Determination of Orientation of VIP36-eCALWY4.
RCEPIAer was coexpressed with GFP-TRPM7 or VIP36-eCALWY4. RCEPIAer and GFP fluorescence were imaged before and after the addition of 20 µM digitonin. Proteinase K (50 µg/mL) was subsequently added to digest cytoplasmic fluorescent proteins, as described in ref. 79.
Zn2+ Release from Intracellular Stores.
Untransfected (Fluozin3-AM–loaded) or VIP36-eCALWY4–transfected WT and TRPM7−/− HEK293T cells were plated on poly-l-lysine–coated coverslips for 12 h (TRPM7−/− cells were grown in 10 mM extra MgCl2). The medium was replaced by medium containing normal Mg2+ (0.8 mM) for 6–12 h before imaging. To fill vesicles with Zn2+, transfected or FluoZin3-loaded cells were rinsed once in HBT-A solution and then were incubated with 500 µM Zn2+ in Mg2+ and Ca2+-free HBT-A solution for 10–20 min. The cells then were perfused with 10 mM sodium dithionite (in glucose-free) or 100 µM H2O2 (in normal) HBT-A medium containing 0.25 mM EGTA to chelate extracellular Zn2+ and 4 mM Mg2+ to block plasma membrane-localized TRPM7.
Gradient Fractionation of Endogenous M7Vs.
PNSs from SV40MES-13 cells were prepared in Hepes-buffered saline [20 mM Hepes (pH 7.5), 150 mM NaCl, 2 mM EGTA, 2 mM MgCl2 and protease inhibitors] and were loaded on a 21–54% linear sucrose gradient buffered with 20 mM Hepes. The PNS then was centrifuged for 3 h at 40,000 × g in a Beckman L2 Ultracentrifuge, SW-41 rotor. One-milliliter fractions were collected and analyzed by SDS/PAGE and Western blotting.
Calcium Imaging.
HEK293 cells were transfected to express GCaMP6s-TRPM7 either alone or with cytoplasmic R-GECO1 or ER-targeted RCEPIAer. The cells then were treated with 100 µM ATP in HBSS, and images were acquired at 0.2 Hz in epifluorescence mode on the previously described TIRF microscopy system.
Proteomic Analysis of M7Vs.
HEK293 cells were stably transfected to express inducible FLAG-GCaMP6s-TRPM7-HRV3C-HA and were passaged at least five times in medium containing l-arginine-10 and l-lysine-6 heavy isotopes. After labeled cells were mixed with equal amounts of unlabeled, untransfected WT cells, vesicles were isolated from PNS using anti-HA–conjugated magnetic nanoparticles, as described above. Bound vesicles were incubated with HRV-3C protease (0.03 U/µL) in cleavage buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10 mM DTT] for 12–18 h at 4 °C to elute nanoparticles. Eluates were immuno-isolated again with anti-FLAG–conjugated magnetic nanoparticles. For proteomic analysis of vesicles isolated from SV40MES-13 cells stably expressing GFP-TRPM7-HA, a single purification with anti-HA was performed, and untransfected control cells were labeled with arginine-10 and lysine-8. After Western blots were performed to verify selective enrichment of TRPM7, vesicular proteins were separated by SDS/PAGE, digested, and analyzed by LC-MS-MS. Proteins with SILAC ratios of at least 1.3 were classified using the Panther System (Gene Ontology Consortium, Geneontology.org) (80).
TRPM7 Deletion in HEK293T Cells.
A variant of the CRISPR/Cas9 method (76) was used to insert puromycin- and blasticidin-coding sequences into the second exon of Trpm7. The donor DNA contained the predicted coding sequence of Trpm7’s first 12 amino acids followed by an antibiotic resistance cassette, multiple stop codons, and SV40-polyA. Oligonucleotides encoding the gRNA sequence flanked with BsmBI-recognition sequences (forward: 5′-CACCGGAAAGCACTTTGACCAAGA, reverse: 5′- TTTGAGAACCAGTTTCACGAAAGG; Trpm7 sequences are underlined) were annealed and cloned into pBK1520 (77). Donor DNA containing puromycin-SV40pA and blasticidin-SV40pA cassettes were amplified from pPur (Clontech) and pcDNA6-V5-His (Invitrogen), respectively, with primers flanked with a target site on exon 2 of TRPM7 (Puro-F primer: CTTTGACCATGACCGAGTACAAGCCCAC and SV40_pA R-primer: ATTCCCTCTGATGAGTTTGGACAAACCACAAC) and were ligated into a pJET1.2 (Thermo Fisher). HEK293T cells then were transfected with the pBK1520 construct and the puromycin resistance cassette donor template using Lipofectamine LTX (Thermo Fisher). Puromycin-resistant cells (grown in medium containing 11 mM Mg2+ and 1 µg/mL puromycin) were cloned using limited dilution and were genotyped using forward and reverse primers situated on exon 2 of Trpm7 (Exon2F: TGACGTTTTGGGGTGCCATC and puromycin coding sequence PuroR: CGTGGGCTTGTACTCGGTCAT). All clones with puromycin inserts were heterozygous. One of the clones with the correct sequence-verified puromycin insertion was used to insert a blasticidin resistance cassette into the exon 2 of Trpm7 locus on the second chromosome, followed by blasticidin (3 µg/mL) selection. Genomic DNA of clonal cells containing antibiotics in both alleles was sequenced, and two clones with donor incorporation were selected. Knockout of Trpm7 was verified by immunoprecipitation, Western blotting, and electrophysiology.
Electrophysiological Recordings.
HEK293 cells were transiently transfected with Trpm7 constructs or stably incorporated doxycycline-inducible TRPM7 cDNA. The cells were voltage-clamped with an Axopatch 200B controlled by a Digidata 1440A (Molecular Devices). Pipettes with 3- to 5-MΩ resistance were used. The pipette electrode solution contained (in mM): 120 Cs-MeSO3, 8 NaCl, 10 BAPTA [1,2-bis(2-Aminophenoxy)ethane-N,N,N',N' tetraacetic acid], 2 CaCl2, 2 ATP-Na2, and 10 Hepes. The bath solution was a modified Hepes-buffered Tyrode’s solution, HBT-B, which contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 10 Hepes, 10 glucose, pH 7.4. In experiments with GSH, Cs-MeSO3 was replaced with N-methyl-d-glucamine (NMDG), and the pH of the GSH-containing solution was adjusted to 7.4 before perfusion. Cells were held at 0 mV, and 200-ms ramps from −100 mV to +100 mV were applied every 2 s. Currents were digitized at 10 kHz and low-pass filtered at 2 kHz.
Statistical Analyses.
Data were plotted and analyzed in Prism 5 (GraphPad) by Student’s t tests, ANOVA with Tukey’s post hoc tests, or extra sum of squares F tests (for nonlinear regressions).
Supplementary Material
Acknowledgments
We thank Maria Ericsson for EM, Clary Clish for metabolomics, Eric Spooner for proteomics studies, Bayush Dinegde for DNA purification, Svetlana Gapon for cell culture, and Luba Krapivinsky for TRPM7 antibody purification. This work was supported in part by NIH Institutional Research Training (T32) Grants 5T32HL007572-31 (to S.A.A.) and K99HL124070 (to D.C.) at Boston Children’s Hospital.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707380114/-/DCSupplemental.
References
- 1.Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 2.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 3.Jin J, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin J, et al. The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci USA. 2012;109:E225–E233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarts M, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 6.Bae CY, Sun HS. Current understanding of TRPM7 pharmacology and drug development for stroke. Acta Pharmacol Sin. 2013;34:10–16. doi: 10.1038/aps.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteilh-Zoller MK, et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krapivinsky G, Krapivinsky L, Manasian Y, Clapham DE. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell. 2014;157:1061–1072. doi: 10.1016/j.cell.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo B, Pörzgen P, Penner R, Horgen FD, Fleig A. Development and optimization of a high-throughput bioassay for TRPM7 ion channel inhibitors. J Biomol Screen. 2010;15:498–507. doi: 10.1177/1087057110368294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 12.Clark K, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su LT, et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai BN, et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell. 2012;22:1149–1162. doi: 10.1016/j.devcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 17.Frederickson CJ, Koh J-Y, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 18.McCord MC, Aizenman E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front Aging Neurosci. 2014;6:77. doi: 10.3389/fnagi.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz C, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 20.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol. 2010;298:C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 21.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 22.Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 24.Baldoli E, Castiglioni S, Maier JA. Regulation and function of TRPM7 in human endothelial cells: TRPM7 as a potential novel regulator of endothelial function. PLoS One. 2013;8:e59891. doi: 10.1371/journal.pone.0059891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuensch T, et al. High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes. 2010;59:844–849. doi: 10.2337/db09-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, et al. Role of TRPM7 channels in hyperglycemia-mediated injury of vascular endothelial cells. PLoS One. 2013;8:e79540. doi: 10.1371/journal.pone.0079540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demir T, et al. Evaluation of TRPM (transient receptor potential melastatin) genes expressions in myocardial ischemia and reperfusion. Mol Biol Rep. 2014;41:2845–2849. doi: 10.1007/s11033-014-3139-0. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, et al. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol Brain. 2015;8:11. doi: 10.1186/s13041-015-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae CY, Sun HS. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin. 2011;32:725–733. doi: 10.1038/aps.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Mori Y, et al. Redox-sensitive transient receptor potential channels in oxygen sensing and adaptation. Pflugers Arch. 2016;468:85–97. doi: 10.1007/s00424-015-1716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 35.Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci USA. 2008;105:8304–8308. doi: 10.1073/pnas.0800881105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange I, et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manna PT, et al. TRPM2-mediated intracellular Zn2+ release triggers pancreatic β-cell death. Biochem J. 2015;466:537–546. doi: 10.1042/BJ20140747. [DOI] [PubMed] [Google Scholar]
- 38.Hoock TC, Newcomb PM, Herman IM. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991;112:653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 40.Presley JF, et al. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 41.Noack J, Bernasconi R, Molinari M. How viruses hijack the ERAD tuning machinery. J Virol. 2014;88:10272–10275. doi: 10.1128/JVI.00801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 43.Füllekrug J, Scheiffele P, Simons K. VIP36 localisation to the early secretory pathway. J Cell Sci. 1999;112:2813–2821. doi: 10.1242/jcs.112.17.2813. [DOI] [PubMed] [Google Scholar]
- 44.Hara-Kuge S, et al. Involvement of VIP36 in intracellular transport and secretion of glycoproteins in polarized Madin-Darby canine kidney (MDCK) cells. J Biol Chem. 2002;277:16332–16339. doi: 10.1074/jbc.M112188200. [DOI] [PubMed] [Google Scholar]
- 45.Shimada O, et al. Localization of VIP36 in the post-Golgi secretory pathway also of rat parotid acinar cells. J Histochem Cytochem. 2003;51:1057–1063. doi: 10.1177/002215540305100809. [DOI] [PubMed] [Google Scholar]
- 46.Shirakabe K, Hattori S, Seiki M, Koyasu S, Okada Y. VIP36 protein is a target of ectodomain shedding and regulates phagocytosis in macrophage Raw 264.7 cells. J Biol Chem. 2011;286:43154–43163. doi: 10.1074/jbc.M111.275586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinkenborg JL, et al. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda JG, et al. New alternately colored FRET sensors for simultaneous monitoring of Zn2+ in multiple cellular locations. PLoS One. 2012;7:e49371. doi: 10.1371/journal.pone.0049371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci USA. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chabosseau P, et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+ ACS Chem Biol. 2014;9:2111–2120. doi: 10.1021/cb5004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Ai HW. Single fluorescent protein-based indicators for zinc ion (Zn(2+)) Anal Chem. 2016;88:9029–9036. doi: 10.1021/acs.analchem.6b01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki J, et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, et al. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanson GT, et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 56.Dooley CT, et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 57.Austin CD, et al. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci USA. 2005;102:17987–17992. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birk J, et al. Endoplasmic reticulum: Reduced and oxidized glutathione revisited. J Cell Sci. 2013;126:1604–1617. doi: 10.1242/jcs.117218. [DOI] [PubMed] [Google Scholar]
- 59.Martin JL, Stork CJ, Li YV. Determining zinc with commonly used calcium and zinc fluorescent indicators, a question on calcium signals. Cell Calcium. 2006;40:393–402. doi: 10.1016/j.ceca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 61.Huang YZ, McNamara JO. Neuroprotective effects of reactive oxygen species mediated by BDNF-independent activation of TrkB. J Neurosci. 2012;32:15521–15532. doi: 10.1523/JNEUROSCI.0755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slepchenko KG, Lu Q, Li YV. Zinc wave during the treatment of hypoxia is required for initial reactive oxygen species activation in mitochondria. Int J Physiol Pathophysiol Pharmacol. 2016;8:44–51. [PMC free article] [PubMed] [Google Scholar]
- 63.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Georgiev P, et al. TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab. 2010;12:386–397. doi: 10.1016/j.cmet.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Krezel A, Bal W. Studies of zinc(II) and nickel(II) complexes of GSH, GSSG and their analogs shed more light on their biological relevance. Bioinorg Chem Appl. 2004;2:293–305. doi: 10.1155/S1565363304000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong BY, et al. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev Dyn. 2015;244:935–947. doi: 10.1002/dvdy.24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvacho I, et al. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep. 2016;6:34236. doi: 10.1038/srep34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gardiner CS, Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod. 1994;51:1307–1314. doi: 10.1095/biolreprod51.6.1307. [DOI] [PubMed] [Google Scholar]
- 73.Nasr-Esfahani MH, Johnson MH. Quantitative analysis of cellular glutathione in early preimplantation mouse embryos developing in vivo and in vitro. Hum Reprod. 1992;7:1281–1290. doi: 10.1093/oxfordjournals.humrep.a137843. [DOI] [PubMed] [Google Scholar]
- 74.Jeong J, et al. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc Natl Acad Sci USA. 2012;109:E3530–E3538. doi: 10.1073/pnas.1211775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnütgen F, et al. Resources for proteomics in mouse embryonic stem cells. Nat Methods. 2011;8:103–104. doi: 10.1038/nmeth0211-103. [DOI] [PubMed] [Google Scholar]
- 76.Nakade S, et al. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat Commun. 2014;5:5560. doi: 10.1038/ncomms6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kleinstiver BP, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J Biol Chem. 2009;284:16289–16297. doi: 10.1074/jbc.M900501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White C, Nixon A, Bradbury NA. Determining membrane protein topology using fluorescence protease protection (FPP) J Vis Exp. 2015;98:e52509. doi: 10.3791/52509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.